Quantitative Detection of Thitsiol and Urushiol as Markers from the Gluta usitata Lacquer Tree Using HPLC
Abstract
1. Introduction
2. Results and Discussion
2.1. Quantitative Analysis of Lacquer Using HPLC
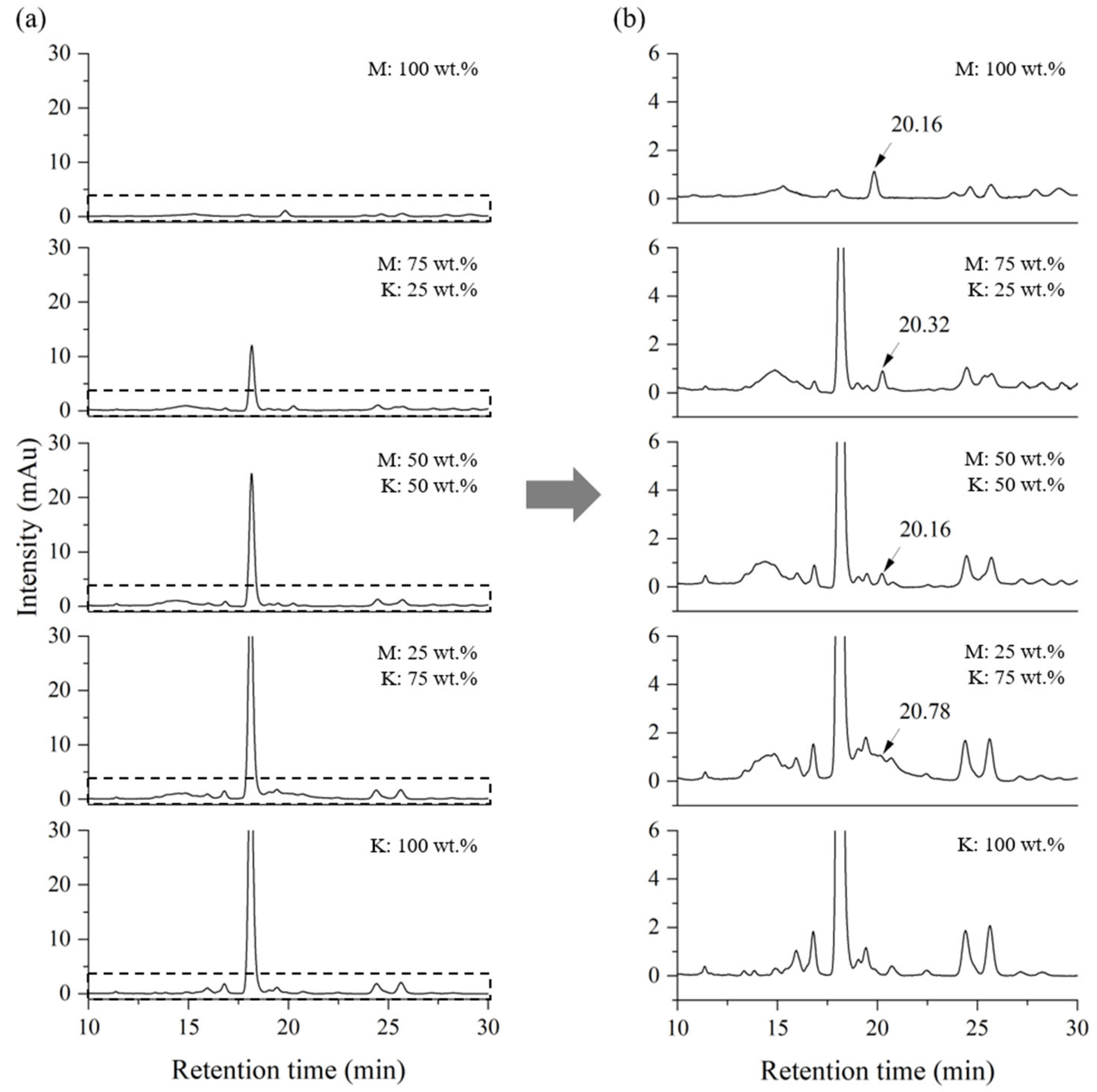
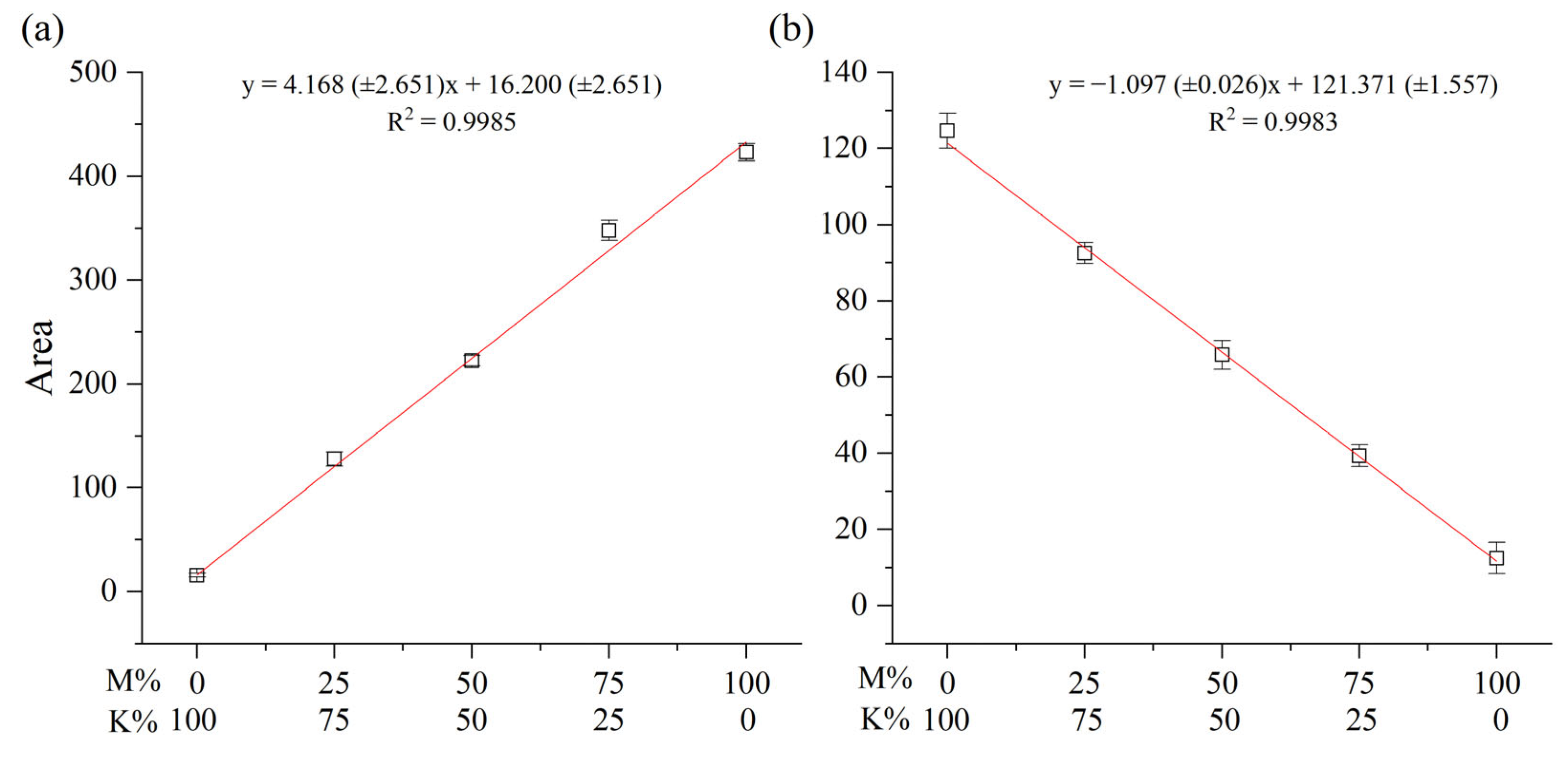
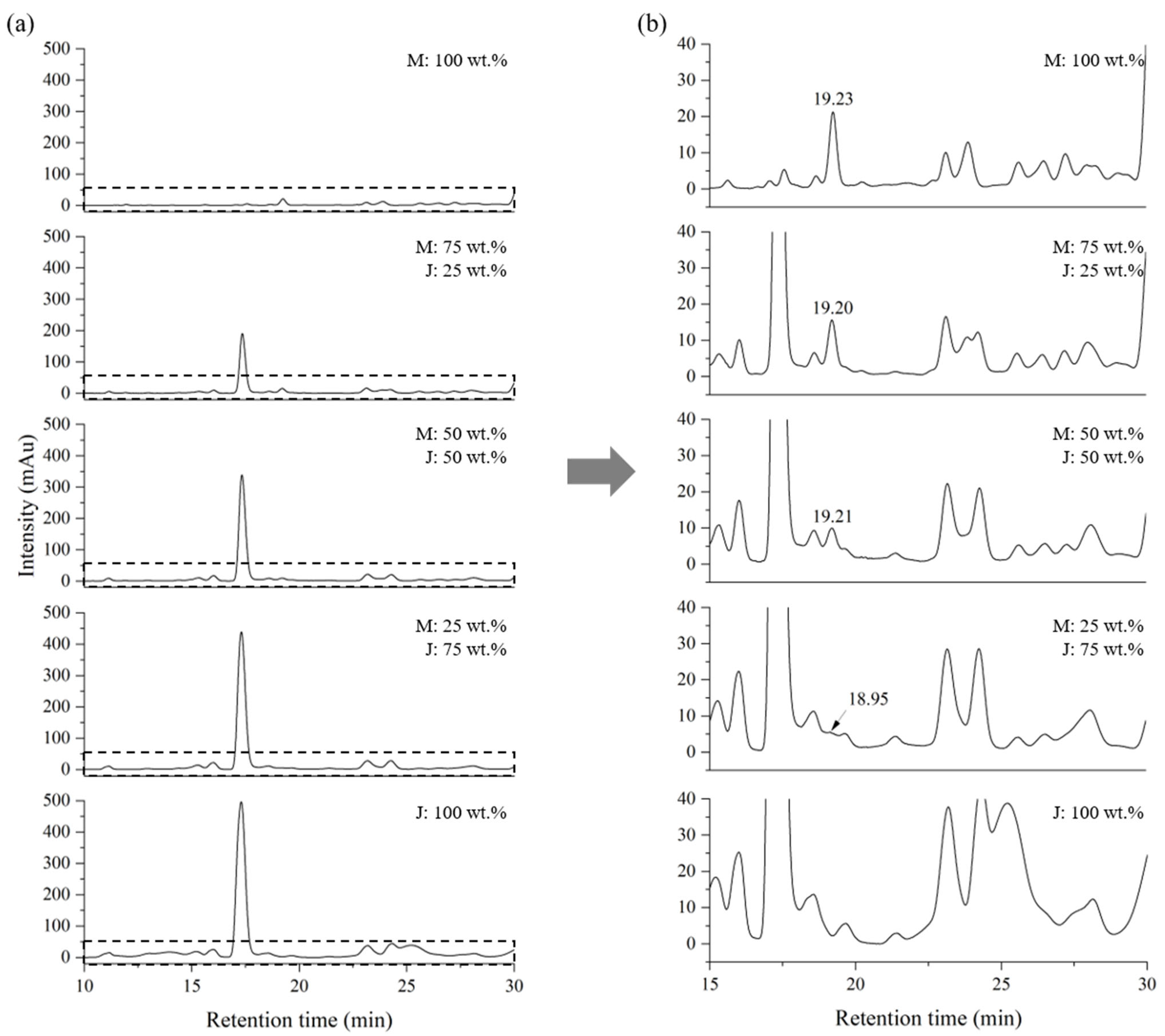
2.2. Blind Test for Quantification of a Mixed Lacquer Sap
3. Materials and Methods
3.1. Materials
3.2. Methods
HPLC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, M. Lacquer: Technology and Conservation; Butterworth-Heinemann: Oxford, UK, 2000. [Google Scholar]
- Lu, R.; Miyakoshi, T. Lacquer Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Impey, O. A Brief Account of Japanese Export Lacquer of the Seventeenth Century, and its Use in Europe. In Japanische und Europäïsche Lackarbeiten: Rezeption, Adaption, Restaurierung/Japanese and European Lacquerware: Adoption, Adaptation, Conservation; Bayerisches Landesamt für Denkmalpflege: Munich, Germany, 2000; pp. 15–30. [Google Scholar]
- Tamburini, D. Analytical pyrolysis applied to the characterisation and identification of Asian lacquers in cultural heritage samples—A review. J. Anal. Appl. Pyrolysis 2021, 157, 105–202. [Google Scholar] [CrossRef]
- Htun, T. Lacquerware Journeys: The Untold Story of Burmese Lacquer, 1st ed.; River Books Press Dist A C: Bangkok, Thailand, 2013; pp. 15–32. [Google Scholar]
- Nagashima, M. Maki-e Production of the Mid-Edo Period as Seen through Historical European Collections in East Asian Lacquer. In Material Culture, Science and Conservation; Rivers, S., Faulkner, R., Pretzel, B., Eds.; Archetype: London, UK, 2011; p. 42. [Google Scholar]
- McSharry, C.; Faulkner, R.; Rivers, S.; Shaffer, M.S.P.; Welton, T. The chemistry of East Asian lacquer: A review of the scientific literature. Stud. Conserv. 2007, 52 (Suppl. S1), 29–40. [Google Scholar] [CrossRef]
- Dong, H.; Wang, C.; Gong, K.; Zhang, F. Advances in the research on the chemical constituents and comprehensive utilization of Rhus verniciflua Stokes. Chem. Ind. For. Prod. 2009, 29, 225–232. [Google Scholar]
- Niimura, N.; Miyakoshi, T. Characterization of natural resin films and identification of ancient coating. J. Mass Spectrom. Soc. Jpn. 2003, 51, 439–457. [Google Scholar] [CrossRef]
- Szczepanowska, H.; Ploeger, R. The Chemical analysis of southeast asian lacquers collected from forests and workshops in Vietnam, Cambodia, and Myanmar. J. Cult. Herit. 2019, 40, 215–225. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, C.; Zheng, G.; Wei, S.; Hu, Z. Comparative studies of bark structure, lacquer yield and urushiol content of cultivated Toxicodendron vernicifluum varieties. N. Z. J. Bot. 2013, 51, 13–21. [Google Scholar] [CrossRef]
- Niimura, N.; Miyakoshi, T.; Miyazato, M.; Nishioka, H.; Onodera, H.; Uetake, Y. Investigation of an ancient eared cup excavated in China using pyrolysis gas chromatography/mass spectrometry, scanning electron microscopy/energy-dispersive X-ray spectrometry, laser Raman spectroscopy, and radiocarbon dating. Surf. Interface Anal. 2021, 53, 681–688. [Google Scholar] [CrossRef]
- Jefferson, A.; Wangchareontrakul, S. Long-chain phenols: Urushiol, laccol, thitsiol and phenylalkyl catechol compounds in Burmese lac from Melanorrhoea usitata. J. Chromatogr. A 1986, 367, 145–154. [Google Scholar] [CrossRef]
- Morais, S.M.; Silva, K.A.; Araujo, H.; Vieira, I.G.; Alves, D.R.; Fontenelle, R.O.; Silva, A.M. Anacardic acid constituents from cashew nut shell liquid: NMR characterization and the effect of unsaturation on its biological activities. Pharmaceuticals 2017, 10, 31. [Google Scholar] [CrossRef]
- Gandhi, T.; Patel, M.; Dholakiya, B.K. Studies on effect of various solvents on extraction of cashew nut shell liquid (CNSL) and isolation of major phenolic constituents from extracted CNSL. J. Nat. Prod. Plant Resour. 2012, 2, 135–142. [Google Scholar]
- Lu, R.; Harigaya, S.; Ishimura, T.; Nagase, K.; Miyakoshi, T. Development of a fast drying lacquer based on raw lacquer sap. Prog. Org. Coat. 2004, 51, 238–243. [Google Scholar] [CrossRef]
- Ishimura, T.; Lu, R.; Yamasaki, K.; Miyakoshi, T. Effects of hybridization of lacquer sap with organic silane on drying properties. Prog. Org. Coat. 2008, 62, 193–198. [Google Scholar] [CrossRef]
- Frade, J.C.; Ribeiro, I.; Graça, J.; Vasconcelos, T.; Rodrigues, J. Chemotaxonomic application of Py-GC/MS: Identification of lacquer trees. J. Anal. Appl. Pyrolysis 2010, 89, 117–121. [Google Scholar] [CrossRef]
- Hao, X.; Schilling, M.R.; Wang, X.; Khanjian, H.; Heginbotham, A.; Han, J.; Auffret, S.; Wu, X.; Fang, B.; Tong, H. Use of THM-PY-GC/MS technique to characterize complex, multilayered Chinese lacquer. J. Anal. Appl. Pyrolysis 2019, 140, 339–348. [Google Scholar] [CrossRef]
- Yu, H.H.; Lim, J.-A.; Ham, S.W.; Lee, K.-B.; Lee, Y. Quantitative analysis of blended Asian lacquers using ToF–SIMS, Py–GC/MS and HPLC. Polymers 2021, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Doh, J.M.; Hahn, H.G.; Lee, K.B.; Lee, Y. Investigation of Asian lacquer films using ToF-SIMS and complementary analytical techniques. Surf. Interface Anal. 2017, 49, 479–487. [Google Scholar] [CrossRef]
- Lee, J.; Jung, S.B.; Terlier, T.; Lee, K.B.; Lee, Y. Molecular identification of Asian lacquers from different trees using Py-GC/MS and ToF-SIMS. Surf. Interface Anal. 2018, 50, 696–704. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Barranco, V. Comparative EIS and XPS studies of the protective character of thin lacquer films containing CR or P salts formed on galvanised steel, galvanneal and galfan substrates. Electrochim. Acta 2004, 49, 951–964. [Google Scholar] [CrossRef][Green Version]
- Ingo, G.; Giorgi, L.; Zacchetti, N.; Azzerri, N. Electrochemical and XPS studies on lacquer—Low tinplated steel adhesion. Corros. Sci. 1992, 33, 361–377. [Google Scholar] [CrossRef]
- Yu, H.H.; Ham, S.W.; Lee, Y. HPLC and ToF‒SIMS Analyses of Toxicodendron vernicifluum Tree Sap Mixed with Other Natural Lacquers. Molecules 2021, 26, 434. [Google Scholar] [CrossRef]
- Kim, J.-B. Analysis of the urushiol in Korean lacquer. Korean J. Food Nutr. 2006, 19, 267–270. [Google Scholar]
- Yamauchi, Y.; Oshima, R.; Kumanotani, J. Configuration of the olefinic bonds in the heteroolefinic side-chains of Japanese lacquer urushiol: Separation and identification of components of dimethylurushiol by means of reductive ozonolysis and high-performance liquid chromatography. J. Chromatogr. A 1982, 243, 71–84. [Google Scholar] [CrossRef]
- Le Hô, A.-S.; Regert, M.; Marescot, O.; Duhamel, C.; Langlois, J.; Miyakoshi, T.; Genty, C.; Sablier, M. Molecular criteria for discriminating museum Asian lacquerware from different vegetal origins by pyrolysis gas chromatography/mass spectrometry. Anal. Chim. Acta 2012, 710, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Yoshida, T.; Miyakoshi, T. Oriental lacquer: A natural polymer. Polym. Rev. 2013, 53, 153–191. [Google Scholar] [CrossRef]
- Oiram Filho, F.; Zocolo, G.J.; Canuto, K.M.; Silva Junior, I.J.D.; de Brito, E.S. Productivity of a preparative high-performance liquid chromatography isolation of anacardic acids from cashew nut shell liquid. Sep. Sci. Plus 2019, 2, 192–199. [Google Scholar] [CrossRef]
- Niimura, N. Determination of the type of lacquer on East Asian lacquer ware. Int. J. Mass Spectrom. 2009, 284, 93–97. [Google Scholar] [CrossRef]
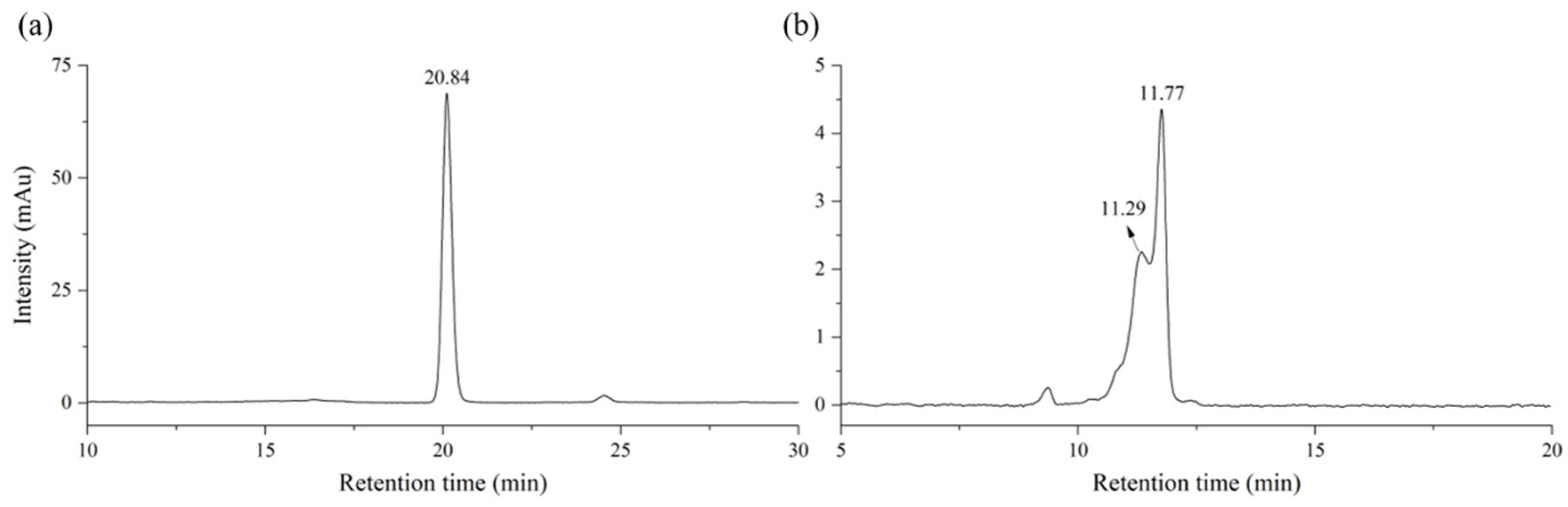


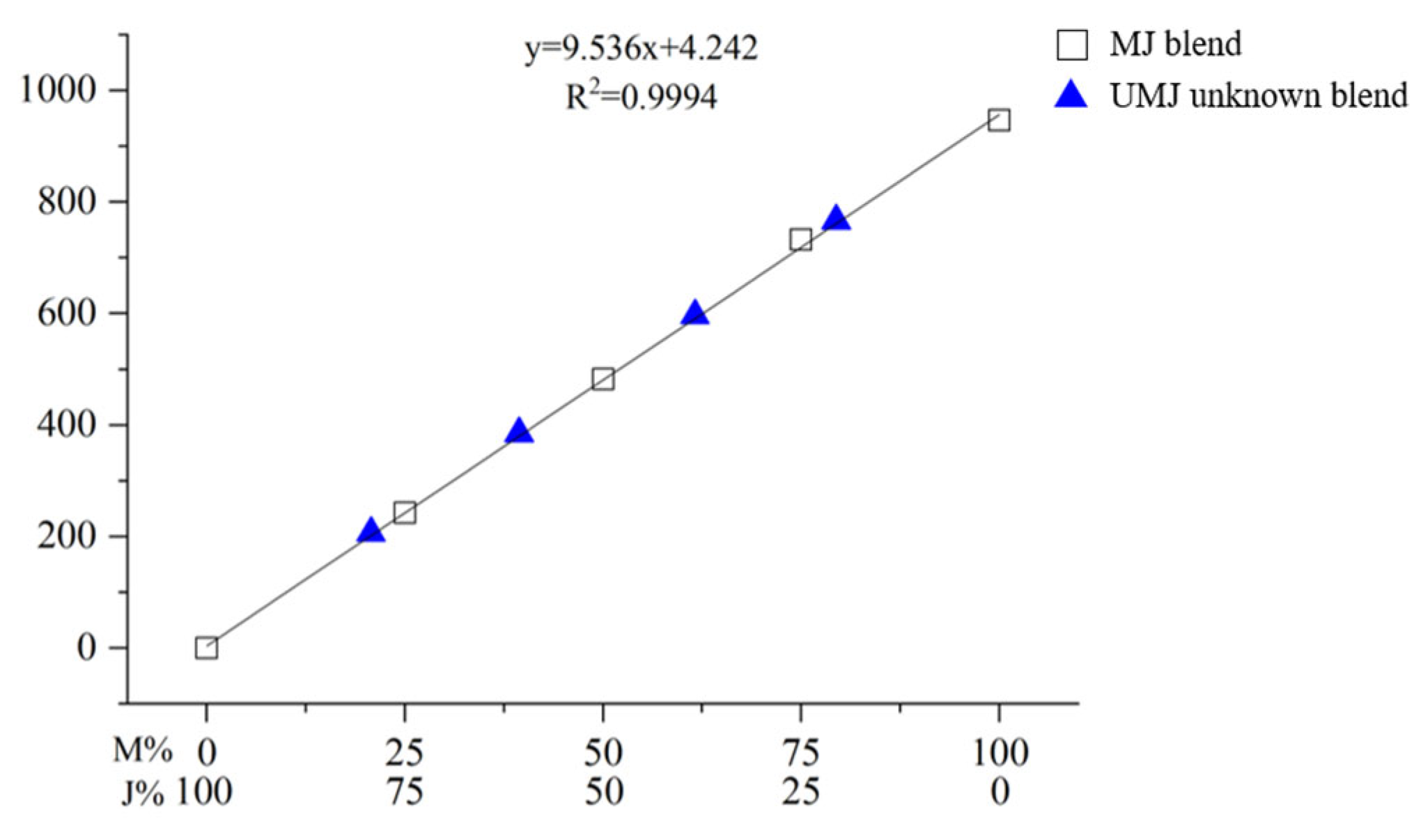
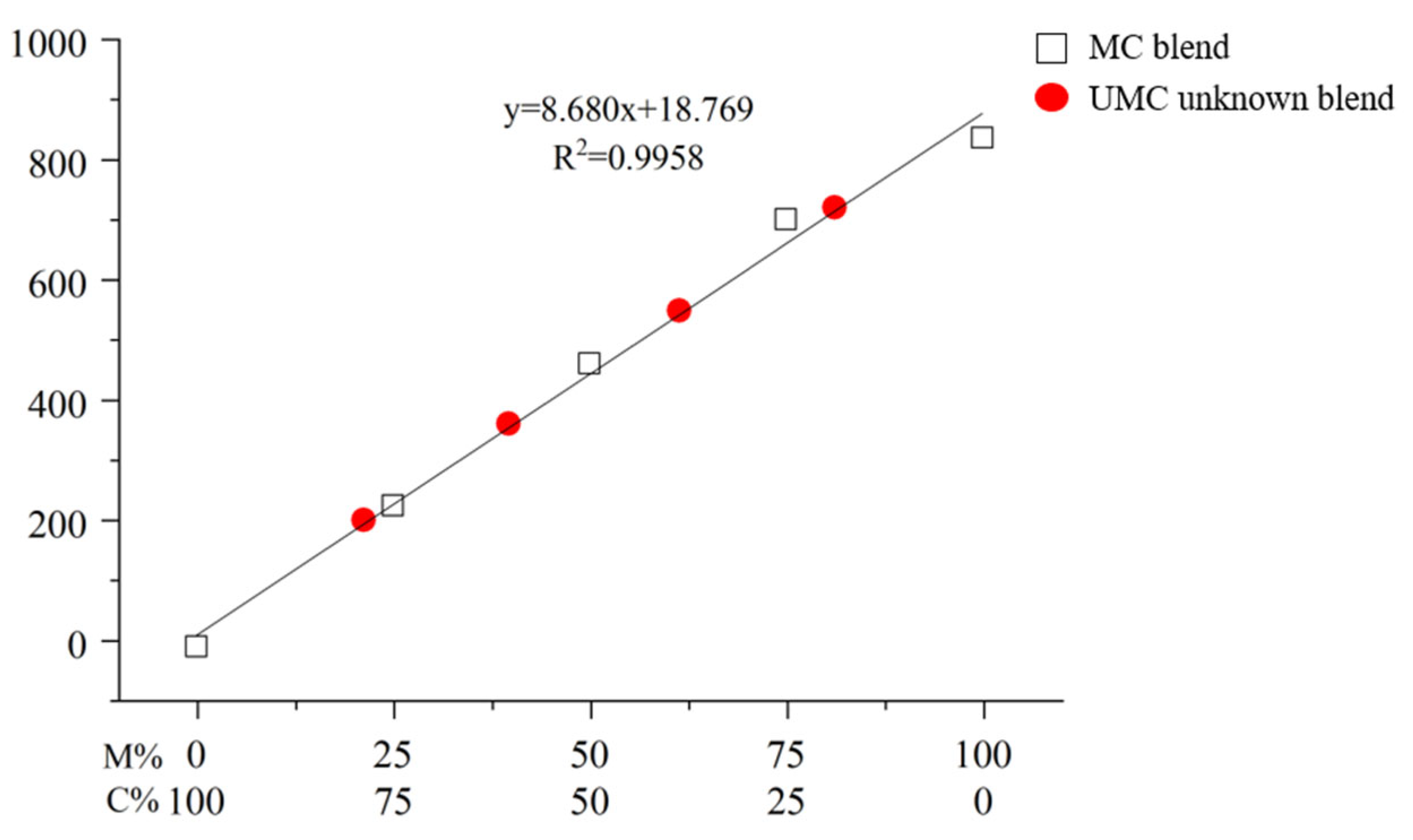
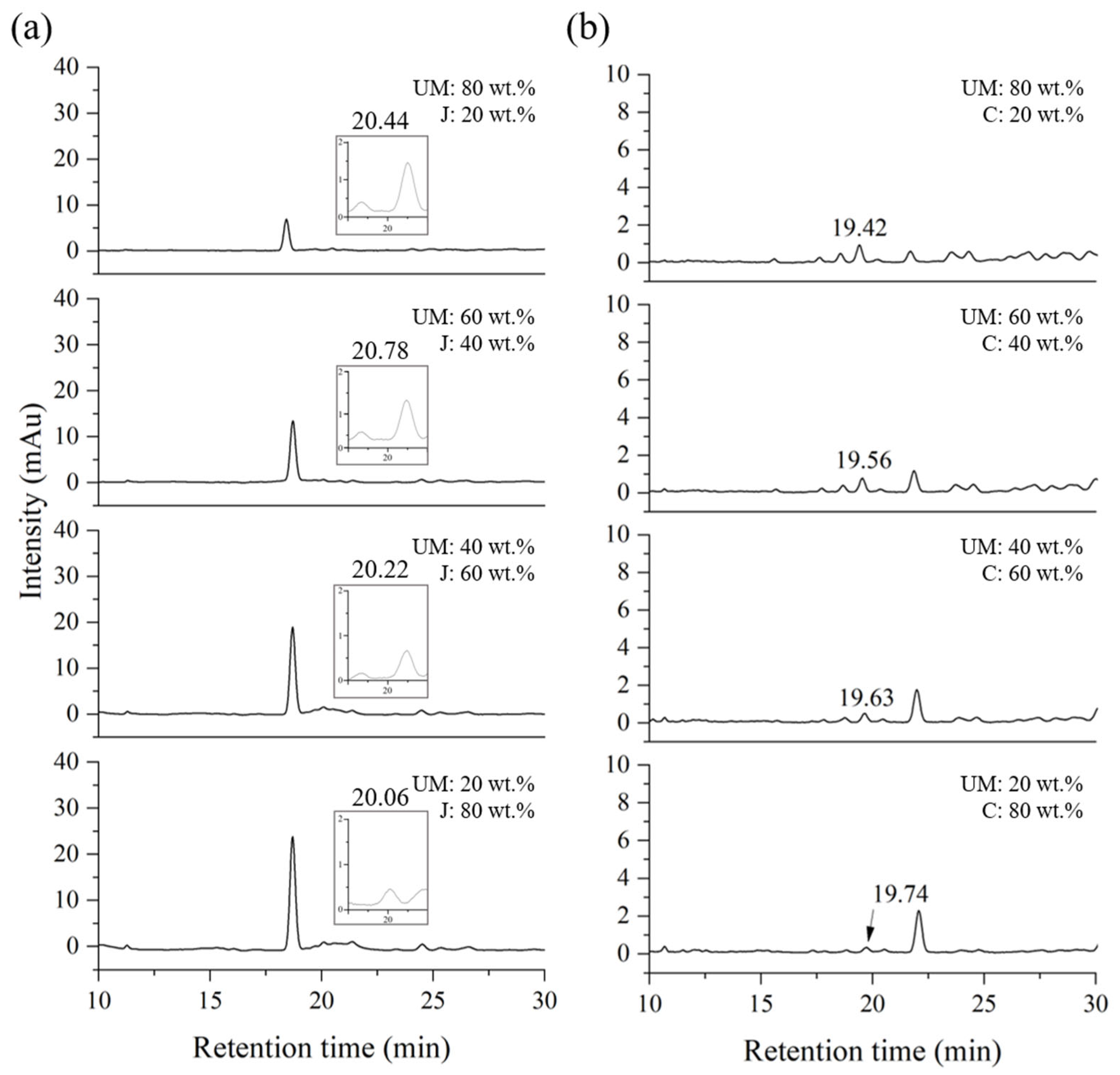
| Compound | Retention Time [RT, min (±SD)] | Response Factor [Rf (±SD)] | LOD (ppm) 1 | LOQ (ppm) 2 |
|---|---|---|---|---|
| Thitsiol 16 | 20.44 (±0.31) | 20.14 (±0.55) | 0.24 | 0.79 |
| Urushiol 15:2 | 11.40 (±0.28) 12.14 (±0.43) | 17.48 (±0.33) | 0.38 | 1.26 |
| Sample | Thitsiol 16 Content [wt.% (±SD)] | Urushiol 15:2 Content [wt.% (±SD)] |
|---|---|---|
| Myanmarese | 7.00 (±0.260) | 0.260 (±0.032) |
| Korean | 0.208 (±0.050) | 1.955 (±0.078) |
| Blend | Myanmarese (wt.%) | Japanese (wt.%) | Blend | Myanmarese (wt.%) | CNSL (wt.%) |
|---|---|---|---|---|---|
| MJ01 | 0 | 100 | MC01 | 0 | 100 |
| MJ02 | 25 | 75 | MC02 | 25 | 75 |
| MJ03 | 50 | 50 | MC03 | 50 | 50 |
| MJ04 | 75 | 25 | MC04 | 75 | 25 |
| MJ05 | 100 | 0 | MC05 | 100 | 0 |
| Myanmarese Composition [wt.% (±SD)] | |||||
|---|---|---|---|---|---|
| Blend | Myanmarese (wt.%) | Actual Content (wt.%) | Blend | Myanmarese (wt.%) | Actual Content (wt.%) |
| UMJ01 | 80.26 (±0.93) | 80.0 | UMC01 | 79.62 (±0.55) | 80.0 |
| UMJ02 | 61.35 (±0.47) | 60.0 | UMC02 | 59.75 (±0.11) | 60.0 |
| UMJ03 | 39.83 (±0.60) | 40.0 | UMC03 | 39.76 (±0.60) | 40.0 |
| UMJ04 | 20.84 (±0.79) | 20.0 | UMC04 | 19.79 (±0.37) | 20.0 |
| IUPAC Name (Trivial Name) | Structural Formula |
|---|---|
| 3-(10-Phenyldecyl) benzene-1,2-diol (Thitsiol 16) C22H30O2 MW: 326.47 | 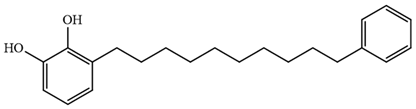 |
| 3-(8Z,11Z-Pentadecadienyl)-1,2-benzenediol (Urushiol 15:2) C21H32O2 MW: 316.48 |  |
| Thitsiol | Urushiol | |
|---|---|---|
| Column | C18 reversed-phase column (YMC-Pack Pro C18, 250 × 4.6 nm I.D. S-5 µm, 12 nm) | C18 reversed-phase column (YMC-Pack Pro C18, 250 × 4.6 nm I.D. S-5 µm, 12 nm) |
| Mobile phase | A: acetonitrile B: aqueous acetic acid, 20 vol% A:B = 9:1 | A: acetonitrile B: aqueous trifluoroacetic acid, 0.1 vol% A:B = 9:1 |
| Flow rate | 0.5 mL/min | 1 mL/min |
| Injection volume | 20 µL | 10 µL |
| Column temperature | 30 °C | 30 °C |
| Detection | UV detector, 280 nm | UV detector, 260 nm |
| Solvent | Acetonitrile | Chloroform |
| Analysis time | 40 min | 20 min |
| Elution mode | Isocratic elution | Isocratic elution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Lee, J.; Lee, K.-B.; Lee, W.-Y.; Lee, Y. Quantitative Detection of Thitsiol and Urushiol as Markers from the Gluta usitata Lacquer Tree Using HPLC. Molecules 2024, 29, 149. https://doi.org/10.3390/molecules29010149
Lee Y, Lee J, Lee K-B, Lee W-Y, Lee Y. Quantitative Detection of Thitsiol and Urushiol as Markers from the Gluta usitata Lacquer Tree Using HPLC. Molecules. 2024; 29(1):149. https://doi.org/10.3390/molecules29010149
Chicago/Turabian StyleLee, Youngseo, Jihye Lee, Kang-Bong Lee, Won-Yong Lee, and Yeonhee Lee. 2024. "Quantitative Detection of Thitsiol and Urushiol as Markers from the Gluta usitata Lacquer Tree Using HPLC" Molecules 29, no. 1: 149. https://doi.org/10.3390/molecules29010149
APA StyleLee, Y., Lee, J., Lee, K.-B., Lee, W.-Y., & Lee, Y. (2024). Quantitative Detection of Thitsiol and Urushiol as Markers from the Gluta usitata Lacquer Tree Using HPLC. Molecules, 29(1), 149. https://doi.org/10.3390/molecules29010149







