Progress and Future Potential of All-Small-Molecule Organic Solar Cells Based on the Benzodithiophene Donor Material
Abstract
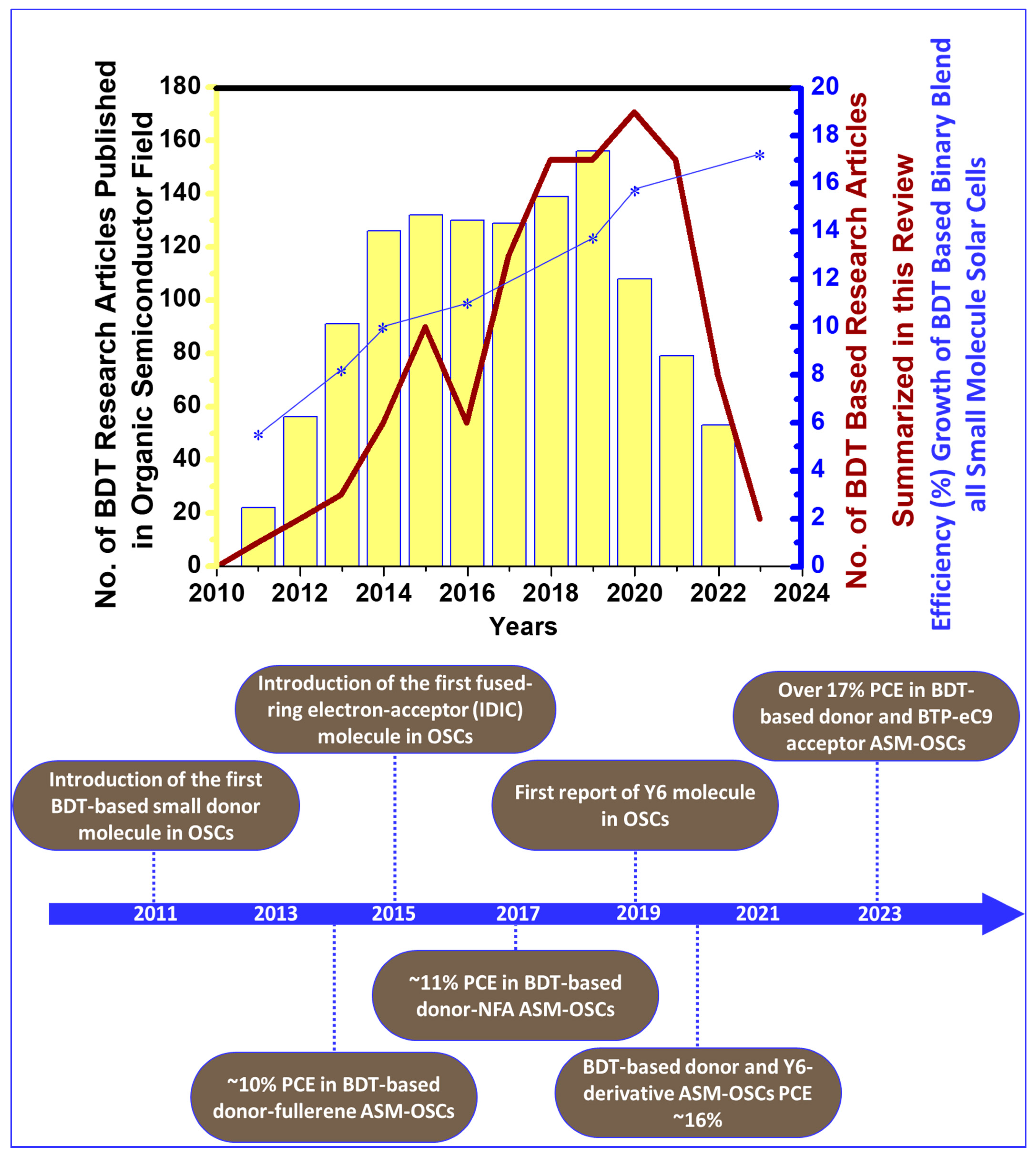
1. Introduction
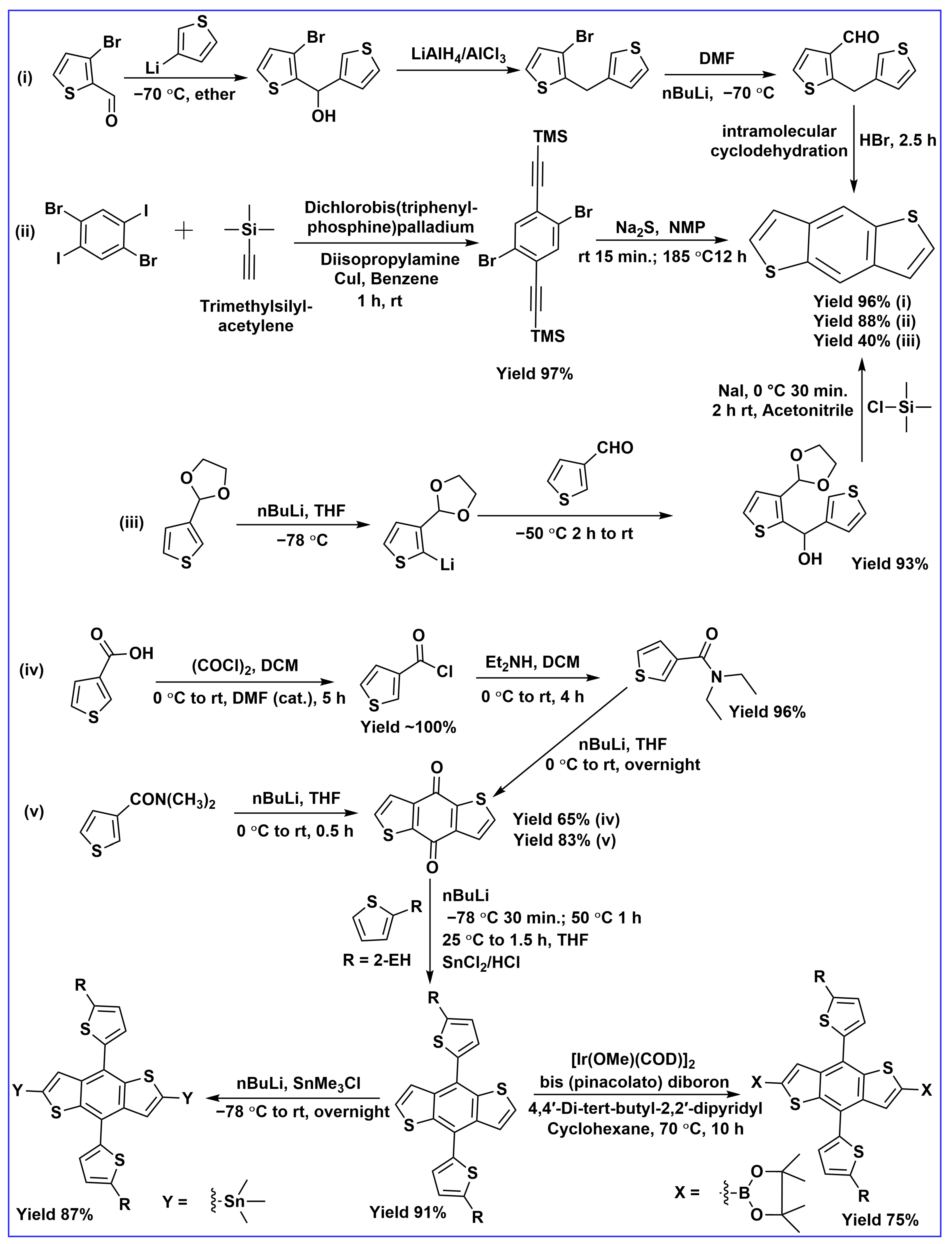
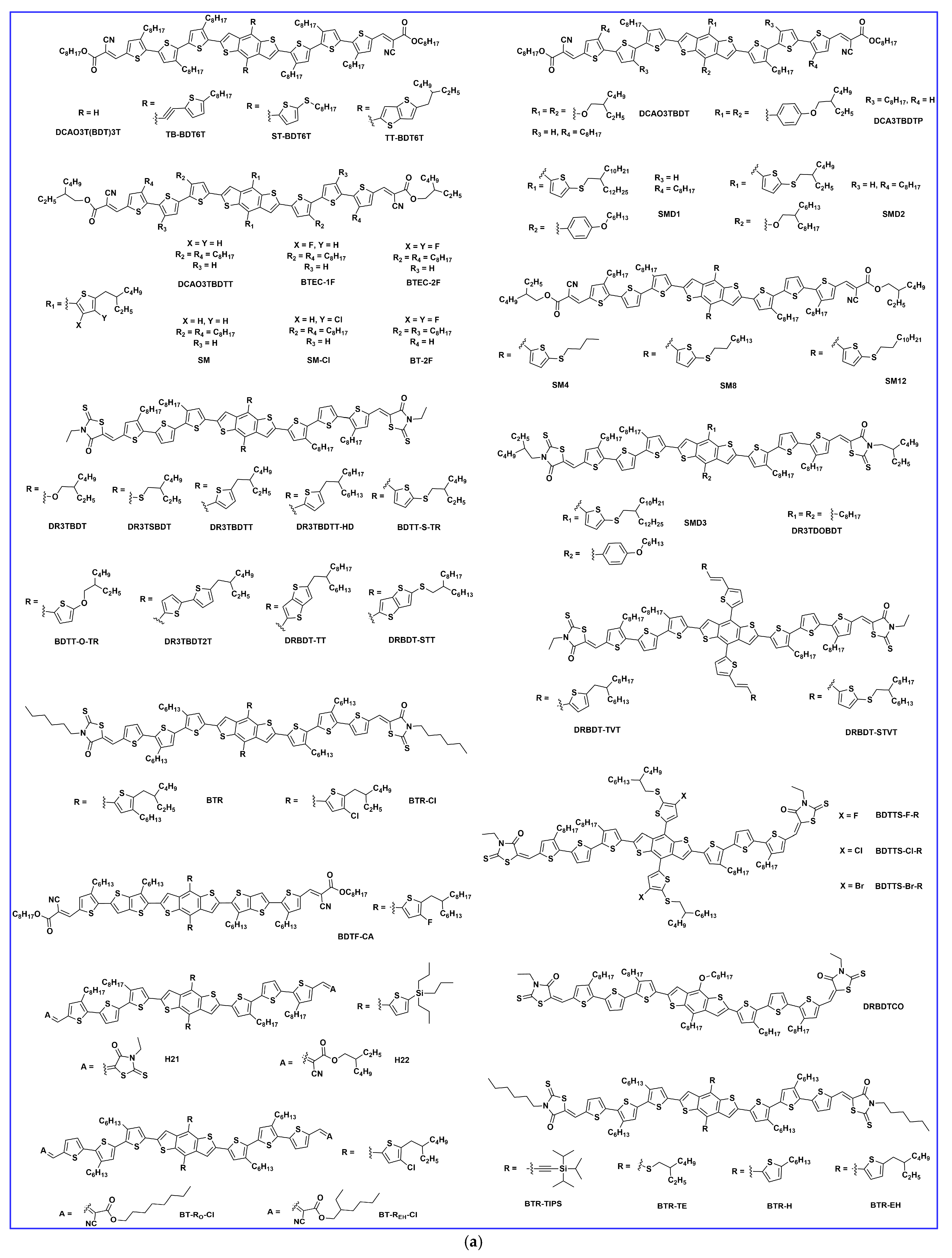
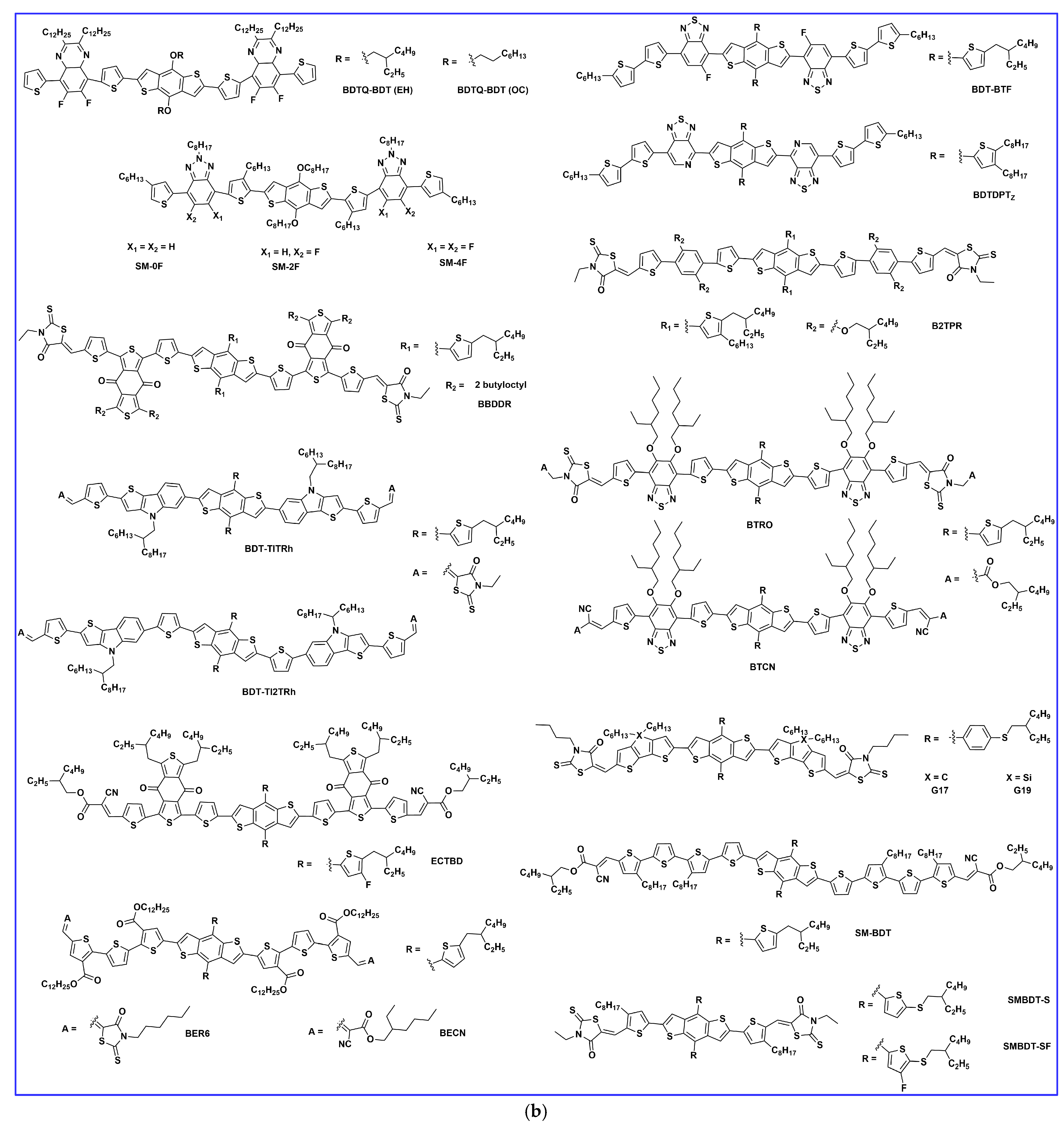
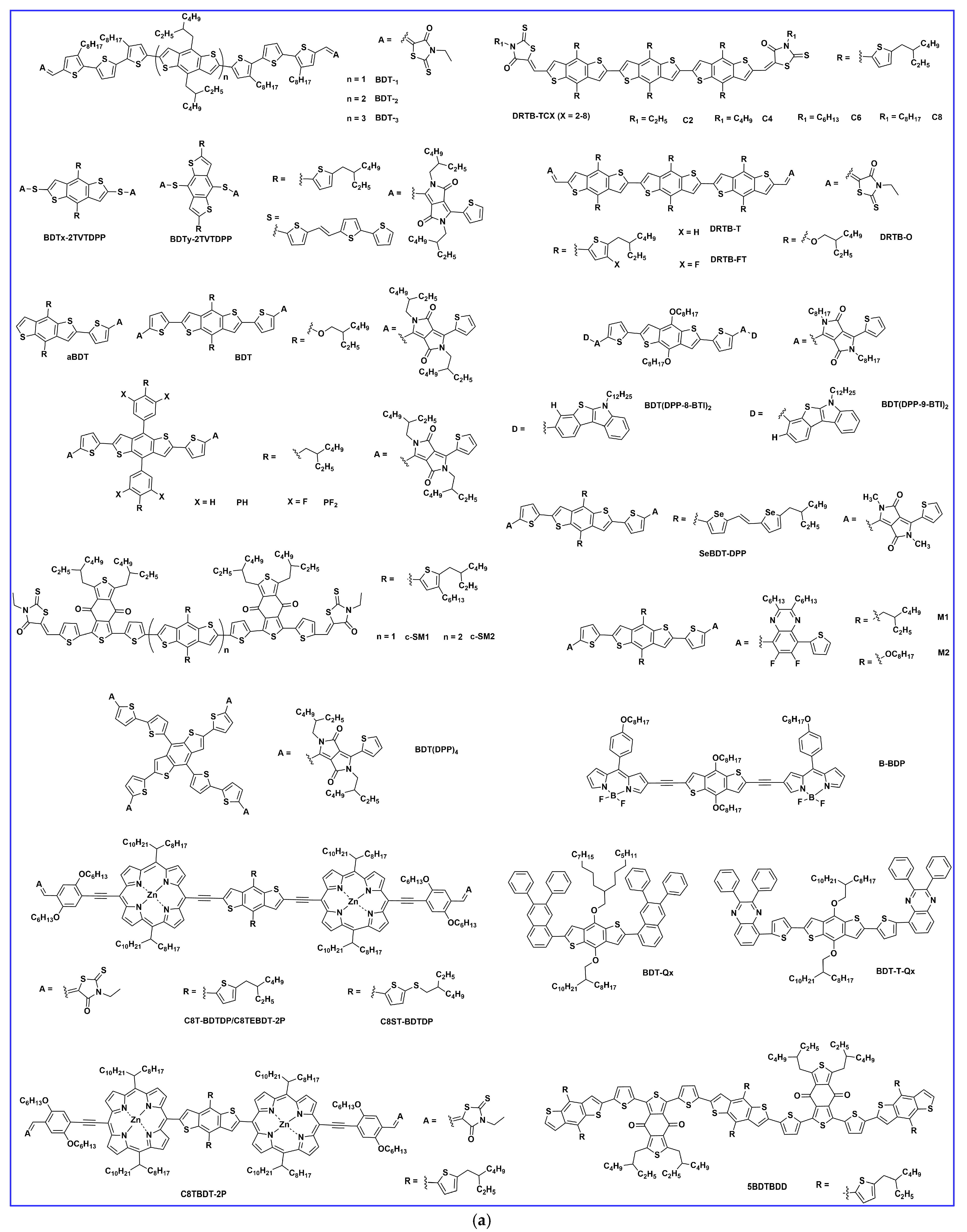
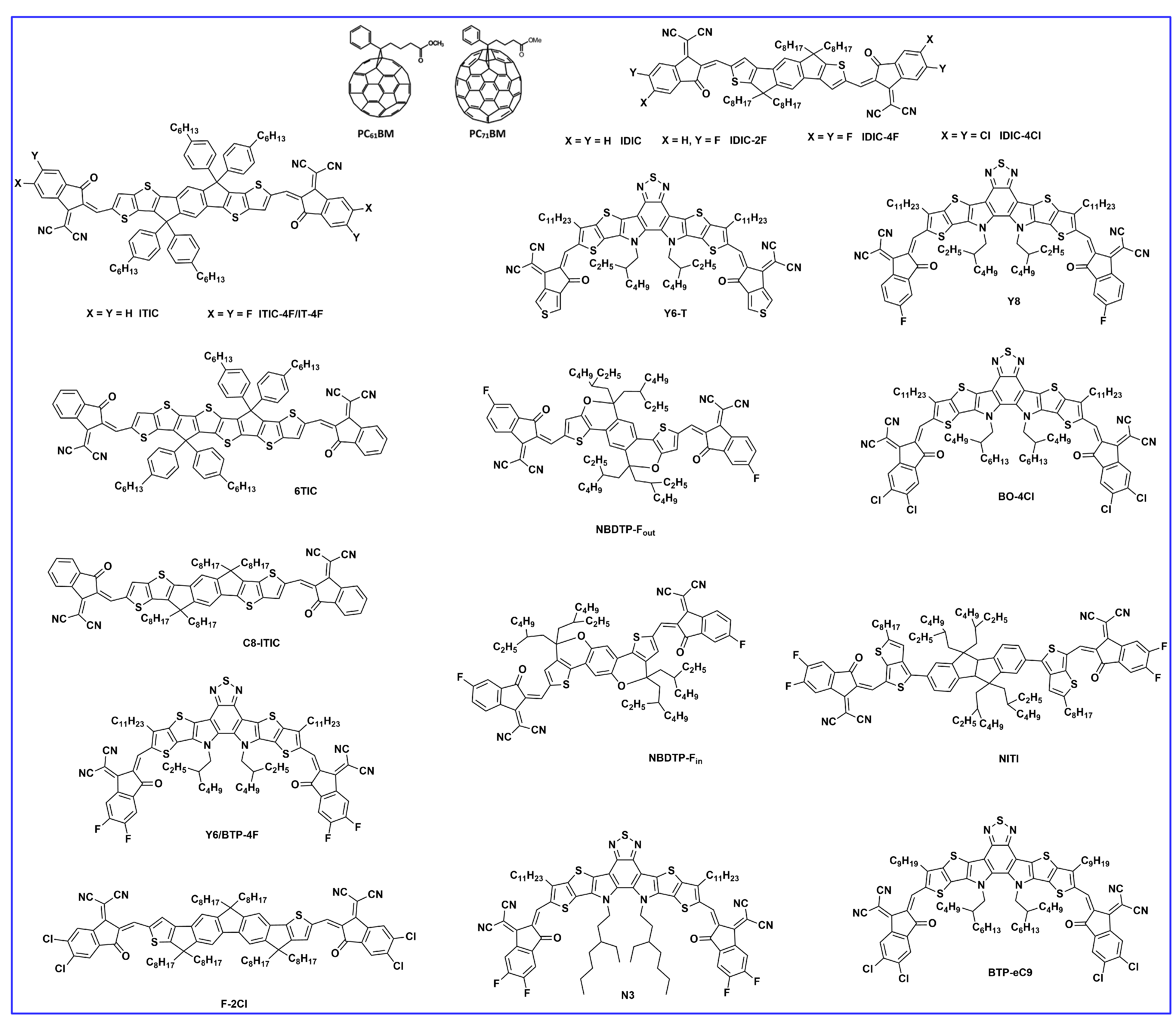
2. Synthesis and Developments of BDT-Based Donor Molecules in OSCs
2.1. Lateral Side Chain Engineering
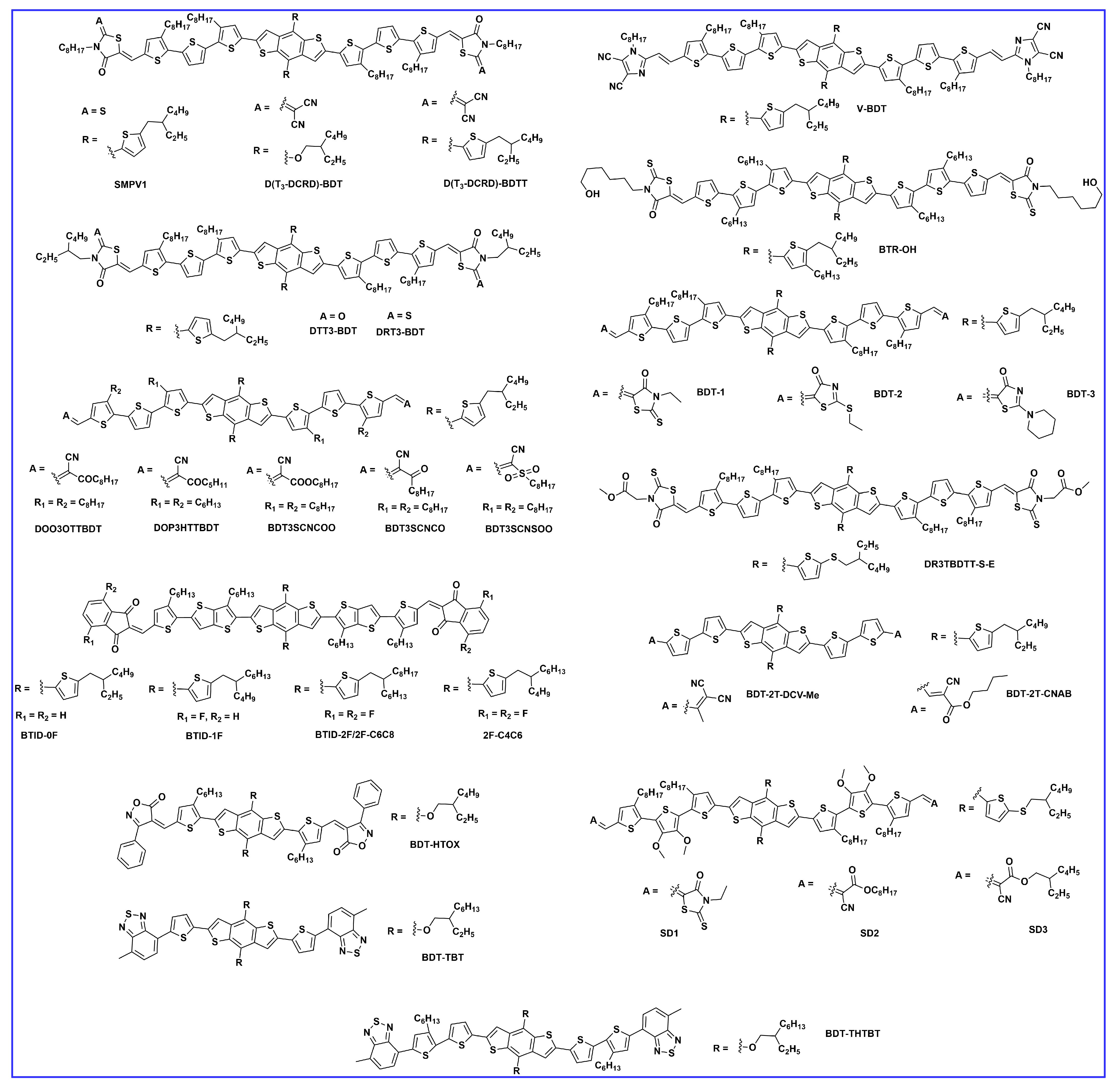
2.2. End Group Engineering
2.3. π-. Bridge Engineering
2.4. Miscellaneous BDT-Based Small Donor Molecules
3. Morphology Optimization Techniques
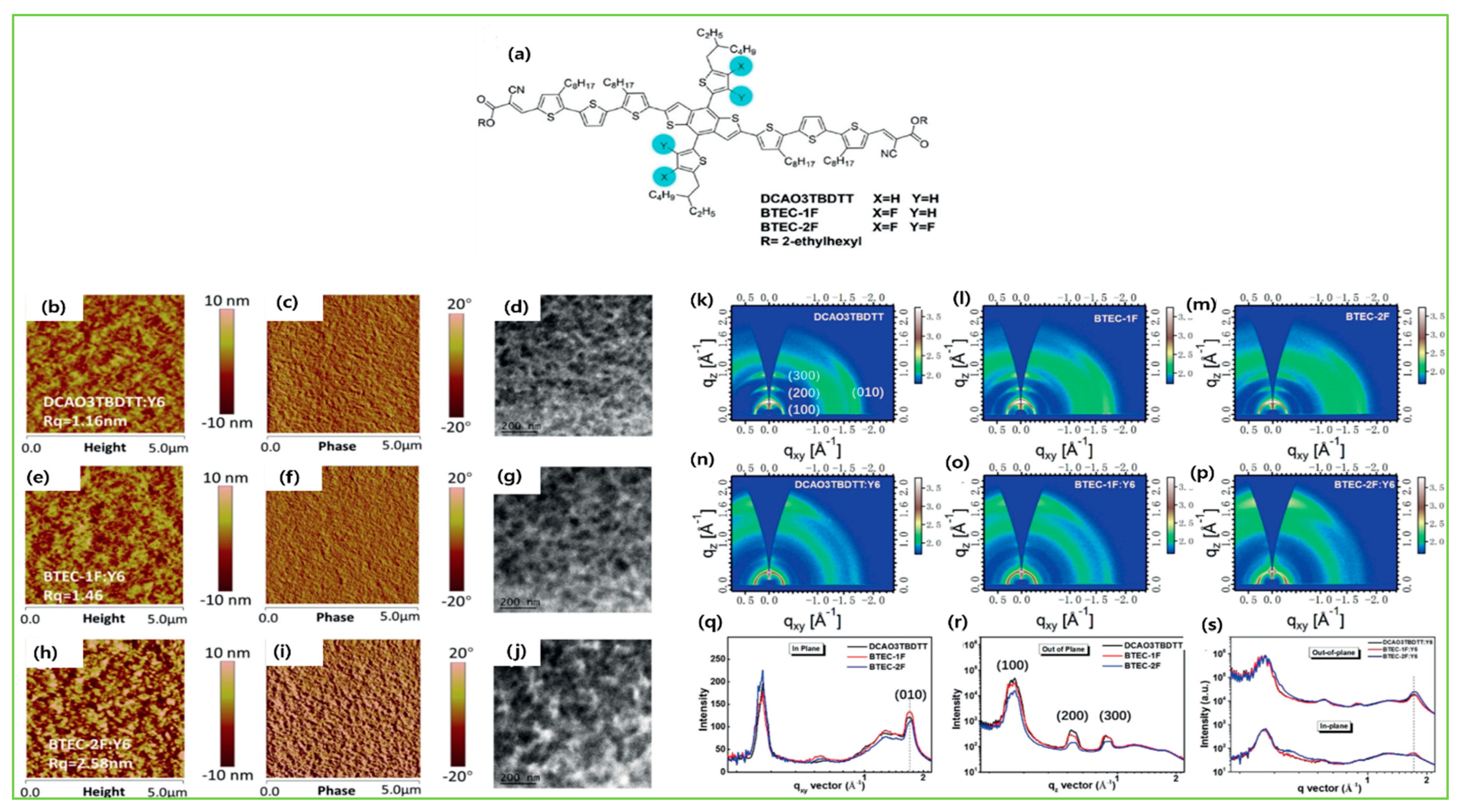
3.1. By Subtle Structure Modification
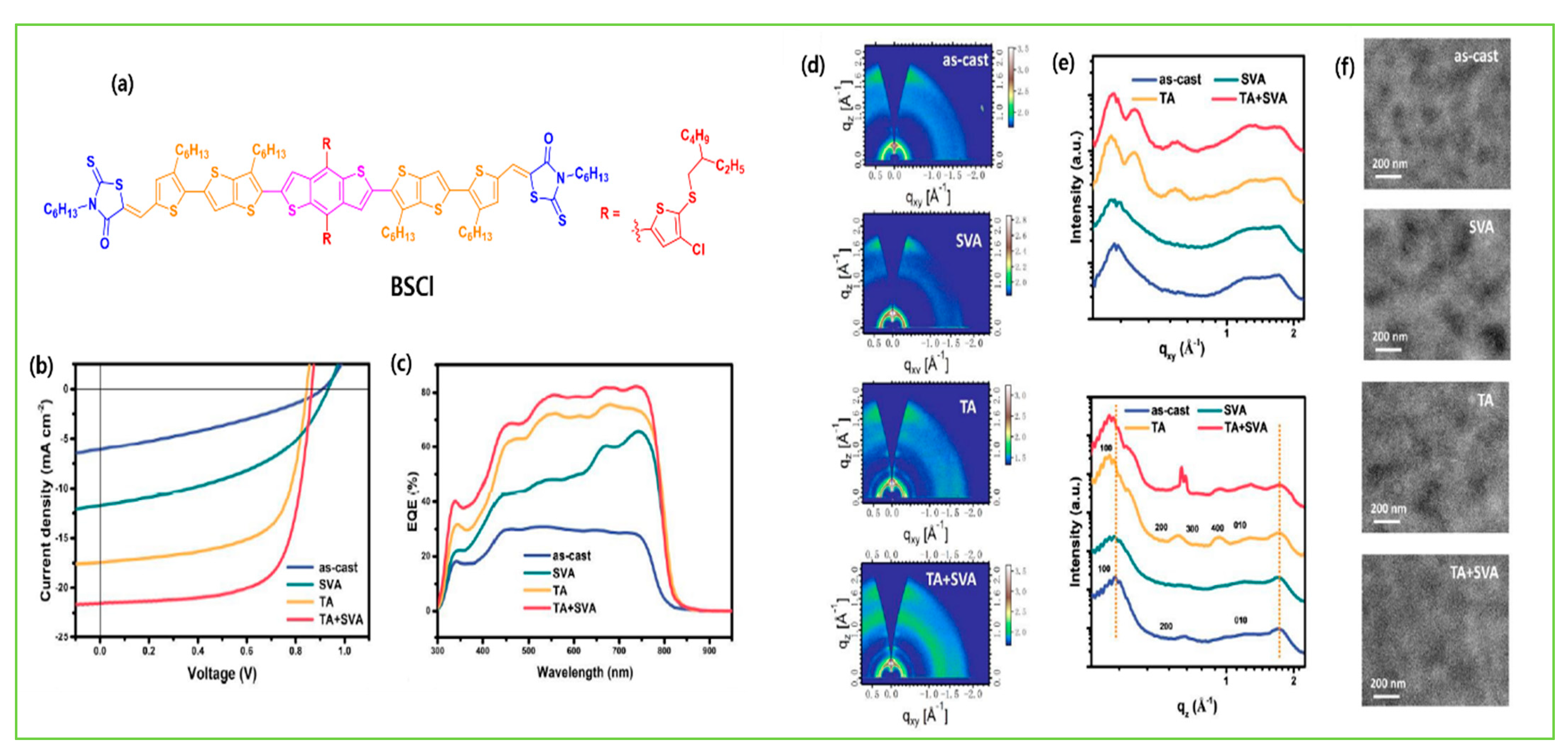
3.2. By Post Treatment (TA, SVA, and Solvent Additives)
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, D.; Jiang, Z.; Tan, W.L.; Zhang, L.; Li, L.; Shan, C.; Mcneill, C.R.; Sonar, P.; Xu, B.; Kyaw, A.K.K. Non-fused Ring Acceptors Achieving over 15.6% Efficiency Organic Solar Cell by Long Exciton Diffusion Length of Alloy-like Phase and Vertical Phase Separation Induced by Hole Transport Layer. Adv. Energy Mater. 2023, 13, 2203402. [Google Scholar] [CrossRef]
- Mumyatov, A.V.; Troshin, P.A. A Review on Fullerene Derivatives with Reduced Electron Affinity as Acceptor Materials for Organic Solar Cells. Energies 2023, 16, 1924. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J.; et al. Single-junction Organic Photovoltaic Cell with 19% Efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Firdaus, Y.; Isikgor, F.H.; Nugraha, M.I.; Yengel, E.; Harrison, G.T.; Hallani, R.; El-Labban, A.; Faber, H.; Ma, C.; et al. Self-assembled Monolayer Enables Hole Transport Layer-free Organic Solar Cells with 18% Efficiency and Improved Operational Stability. ACS Energy Lett. 2020, 5, 2935–2944. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency Organic Solar Cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Han, J.; Huang, D.; Wang, P.; Zhou, L.; Yang, C.; Bao, X.; Yang, R. Over 15% Efficiency All-small-molecule Organic Solar Cells Enabled by a C-shaped Small Molecule Donor with Tailorable Asymmetric Backbone. Nano Energy 2021, 81, 105612. [Google Scholar] [CrossRef]
- Nian, L.; Kan, Y.; Gao, K.; Zhang, M.; Li, N.; Zhou, G.; Jo, S.B.; Shi, X.; Lin, F.; Rong, Q.; et al. Approaching 16% Efficiency in All-small-molecule Organic Solar Cells Based on Ternary Strategy with a Highly Crystalline Acceptor. Joule 2020, 4, 2223–2236. [Google Scholar] [CrossRef]
- Qin, J.; Chen, Z.; Bi, P.; Yang, Y.; Zhang, J.; Huang, Z.; Wei, Z.; An, C.; Yao, H.; Hao, X.; et al. 17% efficiency all-small-molecule organic solar cells enabled by nanoscale phase separation with a hierarchical branched structure. Energy Environ. Sci. 2021, 14, 5903–5910. [Google Scholar] [CrossRef]
- Li, M.; Ni, W.; Wan, X.; Zhang, Q.; Kan, B.; Chen, Y. Benzo [1, 2-b: 4, 5-b′] dithiophene (BDT)-based small molecules for solution processed organic solar cells. J. Mater. Chem. A 2015, 3, 4765–4776. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Shuai, Z.; Wei, Z. A-π-d-π-a Electron-donating Small Molecules for Solution-processed Organic Solar Cells: A Review. Macromol. Rapid Commun. 2017, 38, 1700470. [Google Scholar] [CrossRef]
- Kan, B.; Kan, Y.; Zuo, L.; Shi, X.; Gao, K. Recent Progress on All-small Molecule Organic Solar Cells Using Small-molecule Nonfullerene Acceptors. InfoMat 2021, 3, 175–200. [Google Scholar] [CrossRef]
- Qin, J.; An, C.; Zhang, J.; Ma, K.; Yang, Y.; Zhang, T.; Li, S.; Xian, K.; Cui, Y.; Tang, Y.; et al. 15.3% Efficiency All-small-molecule Organic Solar Cells Enabled by Symmetric Phenyl Substitution. Sci. China Mater. 2020, 63, 1142–1150. [Google Scholar] [CrossRef]
- Hu, D.; Yang, Q.; Chen, H.; Wobben, F.; Le Corre, V.M.; Singh, R.; Liu, T.; Ma, R.; Tang, H.; Koster, L.J.A.; et al. 15.34% Efficiency All-small-molecule Organic Solar Cells with an Improved Fill Factor Enabled by a Fullerene Additive. Energy Environ. Sci. 2020, 13, 2134–2141. [Google Scholar] [CrossRef]
- Guo, J.; Qiu, B.; Yang, D.; Zhu, C.; Zhou, L.; Su, C.; Jeng, U.; Xia, X.; Lu, X.; Meng, L.; et al. 15.71% Efficiency All-small-molecule Organic Solar Cells Based on Low-cost Synthesized Donor Molecules. Adv. Funct. Mater. 2021, 14, 2110159. [Google Scholar] [CrossRef]
- Koßmehl, G.; Beimling, P.; Manecke, G. Über polyarylenalkenylene und polyheteroarylenalkenylene, Synthesen und charakterisierung von poly (thieno [2’, 3’: 1, 2] benzo [4, 5-b] thiophen-2, 6-diylvinylenarylenvinylen)en, poly (4, 8-dimethoxythieno [2’, 3’: 1, 2] benzo [4, 5-b] thiophen-2, 6-diylvinylenarylenvinylen) en und einigen modellverbindungen. Macromol. Chem. Phys. 1983, 184, 627–650. [Google Scholar]
- Yao, H.; Ye, L.; Zhang, H.; Li, S.; Zhang, S.; Hou, J. Molecular Design of Benzodithiophene-based Organic Photovoltaic Materials. Chem. Rev. 2016, 116, 7397–7457. [Google Scholar] [CrossRef]
- Baldoli, C.; Cauteruccio, S.; Licandro, E. Synthesis of Benzo[1,2- B:4,5- B’ ]dithiophene and Benzocondensed Thiaheterocycles. Chem. Sel. 2019, 4, 12680–12682. [Google Scholar] [CrossRef]
- Feng, X. Electronic characters and synthesis method of novel conjugated system based on benzodithiophene groups. Mini Rev. Org. Chem. 2019, 16, 216–227. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, X.; Wang, F.; Zhou, J.; Long, G.; Tian, J.; Chen, Y. High-performance Solar Cells Using a Solution-processed Small Molecule Containing Benzodithiophene Unit. Adv. Mater. 2011, 23, 5387–5391. [Google Scholar] [CrossRef]
- Patra, D.; Budiawan, W.; Huang, T.-Y.; Wei, K.-H.; Wang, P.-C.; Ho, K.-C.; Al-Hashimi, M.; Chu, C.-W. Enhanced Organic Solar Cell Performance by Lateral Side Chain Engineering on Benzodithiophene-based Small Molecules. ACS Appl. Energy Mater. 2018, 1, 3684–3692. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, X.; Liu, Y.; Zuo, Y.; Li, Z.; He, G.; Long, G.; Ni, W.; Li, C.; Su, X.; et al. Small Molecules Based on Benzo[1,2-b:4,5-b’]dithiophene Unit for High-performance Solution-processed Organic Solar Cells. J. Am. Chem. Soc. 2012, 134, 16345–16351. [Google Scholar] [CrossRef]
- Du, Z.; Chen, W.; Qiu, M.; Chen, Y.; Wang, N.; Wang, T.; Sun, M.; Yu, D.; Yang, R. Utilizing Alkoxyphenyl Substituents for Side-chain Engineering of Efficient Benzo[1,2-b:4,5-b′]dithiophene-based Small Molecule Organic Solar Cells. Phys. Chem. Chem. Phys. 2015, 17, 17391–17398. [Google Scholar] [CrossRef]
- Huang, Z.; Kang, X.; Liu, D.; He, Y.; Wu, Y.; Ding, X.; Bao, X.; Yu, L.; Sun, M. Employing Symmetry-breaking Strategy to Reduce Self-aggregation of Donor in Blend Films to Control Morphology for All Small Molecular Organic Solar Cells. Mater. Lett. 2022, 315, 131952. [Google Scholar] [CrossRef]
- Ge, J.; Xie, L.; Peng, R.; Fanady, B.; Huang, J.; Song, W.; Yan, T.; Zhang, W.; Ge, Z. 13.34% Efficiency Non-fullerene All-small-molecule Organic Solar Cells Enabled by Modulating the Crystallinity of Donors via a Fluorination Strategy. Angew. Chem. Int. Ed. 2020, 59, 2808–2815. [Google Scholar] [CrossRef]
- Gao, J.; Ge, J.; Peng, R.; Liu, C.; Cao, L.; Zhang, D.; Fanady, B.; Hong, L.; Zhou, E.; Ge, Z. Over 14% efficiency nonfullerene all-small-molecule organic solar cells enabled by improving the ordering of molecular donors via side-chain engineering. J. Mater. Chem. A 2020, 8, 7405–7411. [Google Scholar] [CrossRef]
- Guo, J.; Hu, K.; Qiu, B.; Zhang, J.; Yang, D.; Zhou, L.; Li, S.; Meng, L.; Zhang, Z.; Li, Y. Fine-tuning Miscibility and π –π Stacking by Alkylthio Side Chains of Donor Molecules Enables High-performance All-small-molecule Organic Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 36033–36043. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, H.; Hu, D.; Xiao, Y.; Fu, J.; Lv, J.; Yu, Q.; Xiao, Z.; Lu, X.; Hu, H.; et al. Design of All-small-molecule Organic Solar Cells Approaching 14% Efficiency via Isometric Terminal Alkyl Chain Engineering. Energies 2021, 14, 2505. [Google Scholar] [CrossRef]
- Bin, H.; Yao, J.; Yang, Y.; Angunawela, I.; Sun, C.; Gao, L.; Ye, L.; Qiu, B.; Xue, L.; Zhu, C.; et al. High-efficiency All-small-molecule Organic Solar Cells Based on an Organic Molecule Donor with Alkylsilyl-thienyl Conjugated Side Chains. Adv. Mater. 2018, 30, 1706361. [Google Scholar] [CrossRef]
- Wu, Q.; Deng, D.; Zhang, J.; Zou, W.; Yang, Y.; Wang, Z.; Li, H.; Zhou, R.; Lu, K.; Wei, Z. Fluorination-substitution Effect on All-small-molecule Organic Solar Cells. Sci. China Chem. 2019, 62, 837–844. [Google Scholar] [CrossRef]
- Ge, J.; Wei, Q.; Peng, R.; Zhou, E.; Yan, T.; Song, W.; Zhang, W.; Zhang, X.; Jiang, S.; Ge, Z. Improved Efficiency in All-small-molecule Organic Solar Cells with Ternary Blend of Nonfullerene Acceptor and Chlorinated and Nonchlorinated Donors. ACS Appl. Mater. Interfaces 2019, 11, 44528–44535. [Google Scholar] [CrossRef]
- Zhou, J.; Zuo, Y.; Wan, X.; Long, G.; Zhang, Q.; Ni, W.; Liu, Y.; Li, Z.; He, G.; Li, C.; et al. Solution-processed and High-performance Organic Solar Cells Using Small Molecules with a Benzodithiophene Unit. J. Am. Chem. Soc. 2013, 135, 8484–8487. [Google Scholar] [CrossRef]
- Kan, B.; Zhang, Q.; Li, M.; Wan, X.; Ni, W.; Long, G.; Wang, Y.; Yang, X.; Feng, H.; Chen, Y. Solution-processed Organic Solar Cells Based on Dialkylthiol-substituted Benzodithiophene Unit with Efficiency Near 10%. J. Am. Chem. Soc. 2014, 136, 15529–15532. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Guo, X.; Min, J.; Guo, B.; Cheng, X.; Zhang, M.; Brabec, C.J.; Li, Y. High-performance Organic Solar Cells Based on a Small Molecule with Alkylthio-thienyl-conjugated Side Chains Without Extra Treatments. Adv. Mater. 2015, 27, 7469–7475. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Cui, C.; Heumueller, T.; Fladischer, S.; Cheng, X.; Spiecker, E.; Li, Y.; Brabec, C.J. Side-chain Engineering for Enhancing the Properties of Small Molecule Solar Cells: A Trade-off Beyond Efficiency. Adv. Energy Mater. 2016, 6, 1600515. [Google Scholar] [CrossRef]
- Kan, B.; Zhang, Q.; Liu, F.; Wan, X.; Wang, Y.; Ni, W.; Yang, X.; Zhang, M.; Zhang, H.; Russell, T.P.; et al. Small Molecules Based on Alkyl/alkylthio-thieno[3,2-b]thiophene-substituted Benzo[1,2-b:4,5-b′]dithiophene for Solution-processed Solar Cells with High Performance. Chem. Mater. 2015, 27, 8414–8423. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Wan, X.; Ni, W.; Kan, B.; Feng, H.; Zhang, Q.; Yang, X.; Wang, Y.; Zhang, Y.; et al. Subtle Balance Between Length Scale of Phase Separation and Domain Purification in Small-molecule Bulk-heterojunction Blends Under Solvent Vapor Treatment. Adv. Mater. 2015, 27, 6296–6302. [Google Scholar] [CrossRef]
- Ni, W.; Li, M.; Wan, X.; Feng, H.; Kan, B.; Zuo, Y.; Chen, Y. A High-performance Photovoltaic Small Molecule Developed by Modifying the Chemical Structure and Optimizing the Morphology of the Active Layer. RSC Adv. 2014, 4, 31977–31980. [Google Scholar] [CrossRef]
- Chen, H.; Hu, D.; Yang, Q.; Gao, J.; Fu, J.; Yang, K.; He, H.; Chen, S.; Kan, Z.; Duan, T.; et al. All-small-molecule Organic Solar Cells with an Ordered Liquid Crystalline Donor. Joule 2019, 3, 3034–3047. [Google Scholar] [CrossRef]
- Tang, H.; Chen, H.; Yan, C.; Huang, J.; Fong, P.W.K.; Lv, J.; Hu, D.; Singh, R.; Kumar, M.; Xiao, Z.; et al. Delicate Morphology Control Triggers 14.7% Efficiency All-small-molecule Organic Solar Cells. Adv. Energy Mater. 2020, 10, 2001076. [Google Scholar] [CrossRef]
- Huo, Y.; Yan, C.; Kan, B.; Liu, X.-F.; Chen, L.-C.; Hu, C.-X.; Lau, T.-K.; Lu, X.; Sun, C.-L.; Shao, X.; et al. Medium-bandgap Small-molecule Donors Compatible with Both Fullerene and Nonfullerene Acceptors. ACS Appl. Mater. Interfaces 2018, 10, 9587–9594. [Google Scholar] [CrossRef]
- Ji, Z.; Xu, X.; Zhang, G.; Li, Y.; Peng, Q. Synergistic Effect of Halogenation on Molecular Energy Level and Photovoltaic Performance Modulations of Highly Efficient Small Molecular Materials. Nano Energy 2017, 40, 214–223. [Google Scholar] [CrossRef]
- Guo, Y.-Q.; Wang, Y.; Song, L.-C.; Liu, F.; Wan, X.; Zhang, H.; Chen, Y. Small Molecules with Asymmetric 4-alkyl-8-alkoxybenzo[1,2-b:4,5-b’]dithiophene as the Central Unit for High-performance Solar Cells with High Fill Factors. Chem. Mater. 2017, 29, 3694–3703. [Google Scholar] [CrossRef]
- Lee, C.; Mitchell, V.D.; White, J.; Jiao, X.; McNeill, C.R.; Subbiah, J.; Jones, D.J.J. Solubilizing core modifications on high-performing benzodithiophene-based molecular semiconductors and their influences on film nanostructure and photovoltaic performance. Mater. Chem. A 2019, 7, 6312–6326. [Google Scholar] [CrossRef]
- Subbiah, J.; Lee, C.J.; Mitchell, V.D.; Jones, D.J. Effect of Side-chain Modification on the Active Layer Morphology and Photovoltaic Performance of Liquid Crystalline Molecular Materials. ACS Appl. Mater. Interfaces 2021, 13, 1086–1093. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, S.; Song, J.; Jin, Y.; Yue, Q.; Qian, Y.; Liu, F.; Zhang, F.; Zhu, X. High-efficiency small-molecule ternary solar cells with a hierarchical morphology enabled by synergizing fullerene and non-fullerene acceptors. Nat. Energy 2018, 3, 952–959. [Google Scholar] [CrossRef]
- Yue, Q.; Wu, H.; Zhou, Z.; Zhang, M.; Liu, F.; Zhu, X. 13.7% Efficiency Small-molecule Solar Cells Enabled by a Combination of Material and Morphology Optimization. Adv. Mater. 2019, 31, 1904283. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fan, H.; Xu, S.; Ye, L.; Guo, Y.; Yi, Y.; Ade, H.; Zhu, X. Isomery-dependent Miscibility Enables High-performance All-small-molecule Solar Cells. Small 2019, 15, 1804271. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, S.; Luo, M.; Liang, J.; Zhou, D.; Zhang, L.; Chen, J. Introducing Siloxane-terminated Side Chains in Small Molecular Donors for All-small-molecule Organic Solar Cells: Modulated Molecular Orientation and Enhanced Efficiency. ACS Appl. Mater. Interfaces 2021, 13, 36080–36088. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Huang, P.; Cai, G.; Lu, X.; Hu, D.; Hu, C.; Lu, S. Symmetrically Fluorinated Benzo[1,2-b:4,5-b’]dithiophene-cored Donor for High-performance All-small-molecule Organic Solar Cells with Improved Active Layer Morphology and Crystallinity. ACS Appl. Mater. Interfaces 2022, 14, 14532–14540. [Google Scholar] [CrossRef]
- Feng, W.; Wu, S.; Chen, H.; Meng, L.; Huang, F.; Liang, H.; Zhang, J.; Wei, Z.; Wan, X.; Li, C.; et al. Tuning Morphology of Active Layer by Using a Wide Bandgap Oligomer-like Donor Enables Organic Solar Cells with over 18% Efficiency. Adv. Energy Mater. 2022, 12, 2104060. [Google Scholar] [CrossRef]
- Wu, S.; Feng, W.; Meng, L.; Zhang, Z.; Si, X.; Chen, Y.; Wan, X.; Li, C.; Yao, Z.; Chen, Y. 15.51% efficiency all-small-molecule organic solar cells achieved by symmetric thiazolyl substitution. Nano Energy 2022, 103, 107801. [Google Scholar] [CrossRef]
- Ma, K.; Feng, W.; Liang, H.; Chen, H.; Wang, Y.; Wan, X.; Yao, Z.; Li, C.; Kan, B.; Chen, Y. Modulation of Alkyl Chain Length on the Thiazole Side Group Enables over 17% Efficiency in All-small-molecule Organic Solar Cells. Adv. Funct. Mater. 2023, 2214926. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.-C.; Hong, Z.; Gao, J.; Yang, Y.; Zhou, H.; Dou, L.; Li, G.; Yang, Y. Solution-processed Small-molecule Solar Cells: Breaking the 10% Power Conversion Efficiency. Sci. Rep. 2013, 3, 3356. [Google Scholar] [CrossRef]
- Fan, Q.; Li, M.; Yang, P.; Liu, Y.; Xiao, M.; Wang, X.; Tan, H.; Wang, Y.; Yang, R.; Zhu, W. Acceptor-donor-acceptor Small Molecules Containing Benzo[1,2- B:4,5- B ‘]dithiophene and Rhodanine Units for Solution Processed Organic Solar Cells. Dye. Pigment. 2015, 116, 13–19. [Google Scholar] [CrossRef]
- Vijay Kumar, C.; Cabau, L.; Koukaras, E.N.; Siddiqui, S.A.; Sharma, G.D.; Palomares, E. Efficient Bulk Heterojunction Solar Cells Based on Solution Processed Small Molecules Based on the Same Benzo[1,2-b:4, 5-b′]thiophene Unit as Core Donor and Different Terminal Units. Nanoscale 2015, 7, 7692–7703. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Yuan, L.; He, C.; Lu, K.; Wei, Z. Effects of Shortened Alkyl Chains on Solution-processable Small Molecules with Oxo-alkylated Nitrile End-capped Acceptors for High-performance Organic Solar Cells. Adv. Energy Mater. 2014, 4, 1400538. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Zhu, L.; Zhang, J.; Lu, K.; Wei, Z. Effects of End-capped Acceptors Subject to Subtle Structural Changes on Solution-processable Small Molecules for Organic Solar Cells. Phys. Chem. Chem. Phys. 2015, 17, 8894–8900. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Zhang, Y.; Zhang, J.; Wang, Z.; Zhu, L.; Fang, J.; Xia, B.; Wang, Z.; Lu, K.; Ma, W.; et al. Fluorination-enabled Optimal Morphology Leads to over 11% Efficiency for Inverted Small-molecule Organic Solar Cells. Nat. Commun. 2016, 7, 13740. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.A.; Zhang, J.; Deng, D.; Wang, Z.; Yang, Y.; Wu, Q.; Wei, Z. Modulation of the Molecular Orientation at the Bulk Heterojunction Interface via Tuning the Small Molecular Donor–nonfullerene Acceptor Interactions. ACS Appl. Mater. Interfaces 2018, 10, 31526–31534. [Google Scholar] [CrossRef]
- Chen, M.; Yi, M.; Yi, J.; Li, M.; Du, C.; Lin, K.; Tong, W.; Ma, C.; Liu, F.; Wang, H. Vinazene End-capped Acceptor-donor-acceptor Type Small Molecule for Solution-processed Organic Solar Cells. Org. Electron. 2017, 44, 11–19. [Google Scholar] [CrossRef]
- Tang, H.; Xu, T.; Yan, C.; Gao, J.; Yin, H.; Lv, J.; Singh, R.; Kumar, M.; Duan, T.; Kan, Z.; et al. Donor Derivative Incorporation: An Effective Strategy Toward High Performance All-small-molecule Ternary Organic Solar Cells. Adv. Sci. 2019, 6, 1901613. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Zhang, Y.; Yang, Y.; Liu, C.-H.; Izquierdo, R.; Xiao, S.S.; Perepichka, D.F. Understanding the Photovoltaic Behavior of A–D–A Molecular Semiconductors Through a Permutation of End Groups. J. Org. Chem. 2020, 85, 52–61. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, Y.; Zhu, X.; Zhou, X.; Yang, C.; Zhang, J.; Lu, K.; Sun, X.; Wei, Z. Constructing High-performance All-small-molecule Ternary Solar Cells with the Same Third Component but Different Mechanisms for Fullerene and Non-fullerene Systems. Adv. Energy Mater. 2019, 9, 1900190. [Google Scholar] [CrossRef]
- Guo, J.; Balakirev, D.O.; Gu, C.; Peregudova, S.M.; Ponomarenko, S.A.; Liu, Z.; Luponosov, Y.N.; Min, J.; Lei, A. End Group Tuning in Small Molecule Donors for Non-fullerene Organic Solar Cells. Dye. Pigment. 2020, 175, 108078. [Google Scholar] [CrossRef]
- Sylvianti, N.; Kim, Y.H.; Marsya, M.A.; Kim, D.G.; Ahn, B.H.; Hong, S.S.; Kim, J.H. New A-D-A type small molecules based on benzodithiophene derivative for organic solar cells. Mol. Cryst. Liq. Cryst. 2018, 660, 66–71. [Google Scholar] [CrossRef]
- Sylvianti, N.; Kim, Y.H.; Kim, D.G.; Maduwu, R.D.; Jin, H.C.; Moon, D.K.; Kim, J.H. Synthesis of Conjugated Materials Based on Benzodithiophene—Benzothiadazole and Their Application of Organic Solar Cells. Macromol. Res. 2018, 26, 552–556. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, X.; Yu, L.; Li, Y.; Li, R.; Peng, Q. Molecular Packing Modulation Enabling Optimized Blend Morphology and Efficient All Small Molecule Organic Solar Cells. Dye. Pigment. 2021, 191, 109387. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, P.; He, C.; Zhang, J.; Shen, P.; Zhang, Y.; Yi, Y.; Zhang, Z.; Li, Z.; Li, Y. Solution-processable Organic Molecule Photovoltaic Materials with Bithienyl-benzodithiophene Central Unit and Indenedione End Groups. Chem. Mater. 2013, 25, 2274–2281. [Google Scholar] [CrossRef]
- Tang, A.; Zhan, C.; Yao, J. Series of Quinoidal Methyl-dioxocyano-pyridine Based π-extended Narrow-bandgap Oligomers for Solution-processed Small-molecule Organic Solar Cells. Chem. Mater. 2015, 27, 4719–4730. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Wang, Z.; Wu, Q.; Ma, W.; Lu, K.; Wei, Z. A Simple but Efficient Small Molecule with a High Open Circuit Voltage of 1.07 V in Solution-processable Organic Solar Cells. Asian J. Org. Chem. 2018, 7, 558–562. [Google Scholar] [CrossRef]
- Komiyama, H.; To, T.; Furukawa, S.; Hidaka, Y.; Shin, W.; Ichikawa, T.; Arai, R.; Yasuda, T. Oligothiophene–indandione-linked Narrow-band Gap Molecules: Impact of Π-conjugated Chain Length on Photovoltaic Performance. ACS Appl. Mater. Interfaces 2018, 10, 11083–11093. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; An, N.G.; Lee, S.M.; Heo, J.; Kim, D.S.; Kim, J.Y.; Yang, C. Influence of the Crystalline Nature of Small Donors Molecules on the Efficiency and Stability of Organic Photovoltaic Devices. Sol. RRL 2018, 2, 1700235. [Google Scholar] [CrossRef]
- Liang, L.; Wang, J.T.; Xiang, X.; Ling, J.; Zhao, F.G.; Li, W.S. Influence of moiety sequence on the performance of small molecular photovoltaic materials. J. Mater. Chem. A 2014, 2, 15396–15405. [Google Scholar] [CrossRef]
- Yao, X.; Shao, W.; Xiang, X.; Xiao, W.-J.; Liang, L.; Zhao, F.-G.; Ling, J.; Lu, Z.; Li, J.; Li, W.-S. Side Chain Engineering on a Small Molecular Semiconductor: Balance Between Solubility and Performance by Choosing Proper Positions for Alkyl Side Chains. Org. Electron. 2018, 61, 56–64. [Google Scholar] [CrossRef]
- Qiu, B.; Chen, S.; Xue, L.; Sun, C.; Li, X.; Zhang, Z.G.; Yang, C.; Li, Y. Effects of alkoxy and fluorine atom substitution of donor molecules on the morphology and photovoltaic performance of all small molecule organic solar cells. Front. Chem. 2018, 6, 413. [Google Scholar] [CrossRef]
- Kim, Y.J.; Baek, J.Y.; Ha, J.J.; Chung, D.S.; Kwon, S.K.; Park, C.E.; Kim, Y.H. A high-performance solution-processed small molecule: Alkylselenophene-substituted benzodithiophene organic solar cell. J. Mater. Chem. C 2014, 2, 4937–4946. [Google Scholar] [CrossRef]
- Duan, R.; Wagner, M.; Müllen, K.; Li, C. D1-A-D2-A-D1-type Constitutional π-conjugated Small Molecular Isomers Bearing Benzodithiophene, Benzothiadiazole, and Thiophene. Dye. Pigment. 2018, 151, 54–63. [Google Scholar] [CrossRef]
- Revoju, S.; Biswas, S.; Eliasson, B.; Sharma, G.D. Effect of Acceptor Strength on Optical, Electrochemical and Photovoltaic Properties of Phenothiazine-based Small Molecule for Bulk Heterojunction Organic Solar Cells. Dye. Pigment. 2018, 149, 830–842. [Google Scholar] [CrossRef]
- Guo, J.; Bin, H.; Wang, W.; Chen, B.; Guo, J.; Sun, R.; Zhang, Z.G.; Jiao, X.; Li, Y.; Min, J. All-small molecule solar cells based on donor molecule optimization with highly enhanced efficiency and stability. J. Mater. Chem. A 2018, 6, 15675–15683. [Google Scholar] [CrossRef]
- Huo, Y.; Gong, X.-T.; Lau, T.-K.; Xiao, T.; Yan, C.; Lu, X.; Lu, G.; Zhan, X.; Zhang, H.-L. Dual-accepting-unit Design of Donor Material for All-small-molecule Organic Solar Cells with Efficiency Approaching 11%. Chem. Mater. 2018, 30, 8661–8668. [Google Scholar] [CrossRef]
- Wan, J.; Xu, X.; Zhang, G.; Li, Y.; Feng, K.; Peng, Q. Highly Efficient Halogen-free Solvent Processed Small-molecule Organic Solar Cells Enabled by Material Design and Device Engineering. Energy Environ. Sci. 2017, 10, 1739–1745. [Google Scholar] [CrossRef]
- Bin, H.; Yang, Y.; Zhang, Z.-G.; Ye, L.; Ghasemi, M.; Chen, S.; Zhang, Y.; Zhang, C.; Sun, C.; Xue, L.; et al. 9.73% Efficiency Nonfullerene All Organic Small Molecule Solar Cells with Absorption-complementary Donor and Acceptor. J. Am. Chem. Soc. 2017, 139, 5085–5094. [Google Scholar] [CrossRef]
- Bin, H.; Angunawela, I.; Qiu, B.; Colberts, F.J.M.; Li, M.; Dyson, M.J.; Wienk, M.M.; Ade, H.; Li, Y.; Janssen, R.A.J. Precise Control of Phase Separation Enables 12% Efficiency in All Small Molecule Solar Cells. Adv. Energy Mater. 2020, 10, 2001589. [Google Scholar] [CrossRef]
- Ma, P.; Wang, C.; Wen, S.; Wang, L.; Shen, L.; Guo, W.; Ruan, S. Small Molecules Based on Tetrazine Unit for Efficient Performance Solution-processed Organic Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 155, 30–37. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.; Wen, S.; Ma, P.; Wang, G.; Wang, C.; Li, H.; Shen, L.; Guo, W.; Ruan, S. Enhanced Photovoltaic Performance of Tetrazine-based Small Molecules with Conjugated Side Chains. ACS Sustain. Chem. Eng. 2017, 5, 8684–8692. [Google Scholar] [CrossRef]
- Agneeswari, R.; Kong, M.; Lee, J.; Kwon, J.H.; Tamilavan, V.; Park, S.S.; Park, S.H.; Jin, Y. Wide band-gap organic molecules containing benzodithiophene and difluoroquinoxaline derivatives for solar cell applications. Mol. Cryst. Liq. Cryst. 2019, 685, 29–39. [Google Scholar] [CrossRef]
- Gu, H.; Qin, Y.; Dai, W.; Zhou, D.; Xie, Y. Fluorination Effects of A-D-A Small Molecule Donors Based Benzotriazole for Organic Solar Cells. Synth. Met. 2019, 251, 95–103. [Google Scholar] [CrossRef]
- Fang, J.; Ye, C.; Wang, X.; Wang, Y.; Guo, X.; Fan, Q.; Ma, W.; Zhang, M. Non-fullerene Organic Solar Cells Based on a Small Molecule with Benzo[1,2-c:4,5-c’]dithiophene-4,8-dione as π-bridge. Org. Electron. 2019, 67, 175–180. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, J.; Huang, H.; Yun, Y.; Li, Z.; You, F.; Zhao, B.; Qin, T.; Gao, D.; Huang, W. Thieno[3,2-b]indole (TI) Bridged A-π-D-π-A Small Molecules: Synthesis, Characterizations and Organic Solar Cell Applications. Dye. Pigment. 2019, 160, 16–24. [Google Scholar] [CrossRef]
- Wang, K.; Guo, B.; Xu, Z.; Guo, X.; Zhang, M.; Li, Y. Solution-processable Organic Molecule for High-performance Organic Solar Cells with Low Acceptor Content. ACS Appl. Mater. Interfaces 2015, 7, 24686–24693. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Z.; Geng, Y.; Li, H.; Lin, C.; Mi, L.; Guo, X.; Zhang, M.; Li, Y. Synthesis of Organic Molecule Donor for Efficient Organic Solar Cells with Low Acceptor Content. Org. Electron. 2019, 64, 54–61. [Google Scholar] [CrossRef]
- Wang, K.; Guo, X.; Ye, C.; Wang, Y.; Meng, Y.; Li, X.; Zhang, M. A New Small-molecule Donor Containing Non-fused Ring π-bridge Enables Efficient Organic Solar Cells with High Open Circuit Voltage and Low Acceptor Content. ChemPhysChem 2019, 20, 2674–2682. [Google Scholar] [CrossRef]
- Meng, W.; Lv, J.; Duan, T.; Kan, Z.; Lu, S.; Dai, X.; Li, Z. Small Molecule Donor Based on Alkoxylated Benzothiadiazole Unit: Synthesis and Photovoltaics Properties. Mater. Chem. Phys. 2020, 247, 122874. [Google Scholar] [CrossRef]
- Chen, S.; Yan, T.; Fanady, B.; Song, W.; Ge, J.; Wei, Q.; Peng, R.; Chen, G.; Zou, Y.; Ge, Z. High Efficiency Ternary Organic Solar Cells Enabled by Compatible Dual-donor Strategy with Planar Conjugated Structures. Sci. China Chem. 2020, 63, 917–923. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, X.; Zhu, M.; Xia, H.; Tang, W.; Peng, W.; Guo, J.; Qian, C.; Zhang, B.; Liu, Y.; et al. Wide-band Gap Small-molecule Donors with Diester-terthiophene Bridged Units for High-efficiency All-small-molecule Organic Solar Cells. ACS Appl. Energy Mater. 2021, 4, 5868–5876. [Google Scholar] [CrossRef]
- Chen, Z.; Song, W.; Yu, K.; Ge, J.; Zhang, J.; Xie, L.; Peng, R.; Ge, Z. Small-molecular donor guest achieves rigid 18.5% and flexible 15.9% efficiency organic photovoltaic via fine-tuning microstructure morphology. Joule 2021, 5, 2395–2407. [Google Scholar] [CrossRef]
- Li, S.; Ma, Q.; Qiu, B.; Meng, L.; Zhang, J.; Wu, Y.; Zhang, Z.; Zhang, Z.-G.; Li, Y. Effects of the Center Units of Small-molecule Donors on the Morphology, Photovoltaic Performance, and Device Stability of All-small-molecule Organic Solar Cells. Sol. RRL 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Lee, E.; Tran, D.K.; Park, J.; Ko, W.; Jenekhe, S.A.; Hwang, Y.-J. Benzodithiophene-based Wide-bandgap Small-molecule Donors for Organic Photovoltaics with Large Open-circuit Voltages. Org. Electron. 2021, 88, 105996. [Google Scholar] [CrossRef]
- Badgujar, S.; Lee, G.-Y.; Park, T.; Song, C.E.; Park, S.; Oh, S.; Shin, W.S.; Moon, S.-J.; Lee, J.-C.; Lee, S.K. High-performance Small Molecule via Tailoring Intermolecular Interactions and Its Application in Large-area Organic Photovoltaic Modules. Adv. Energy Mater. 2016, 6, 1600228. [Google Scholar] [CrossRef]
- Duan, L.; Chen, J.; Liu, B.; Wang, X.; Zhu, W.; Yang, R. The A-D-A Type Small Molecules with Isomeric Benzodithiophene Cores: Synthesis and Influence of Isomers on Photoelectronic Properties. Tetrahedron 2017, 73, 550–557. [Google Scholar] [CrossRef]
- Loser, S.; Lou, S.J.; Savoie, B.M.; Bruns, C.J.; Timalsina, A.; Leonardi, M.J.; Smith, J.; Harschneck, T.; Turrisi, R.; Zhou, N.; et al. Systematic evaluation of structure—Property relationships in heteroacene—Diketopyrrolopyrrole molecular donors for organic solar cells. J. Mater. Chem. A 2017, 5, 9217–9232. [Google Scholar] [CrossRef]
- Liao, J.; Xu, Y.; Zhao, H.; Zong, Q.; Fang, Y. Novel A-D-A Type Small Molecules with Β-alkynylated BODIPY Flanks for Bulk Heterojunction Solar Cells. Org. Electron. 2017, 49, 321–333. [Google Scholar] [CrossRef]
- Eastham, N.D.; Dudnik, A.S.; Harutyunyan, B.; Aldrich, T.J.; Leonardi, M.J.; Manley, E.F.; Butler, M.R.; Harschneck, T.; Ratner, M.A.; Chen, L.X.; et al. Enhanced Light Absorption in Fluorinated Ternary Small-molecule Photovoltaics. ACS Energy Lett. 2017, 2, 1690–1697. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; He, C.; Zhang, J.; Yang, Y.; Zhu, J.; Cui, Y.; Zhao, W.; Zhang, H.; Zhang, Y.; et al. Modulating Molecular Orientation Enables Efficient Nonfullerene Small-molecule Organic Solar Cells. Chem. Mater. 2018, 30, 2129–2134. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, L.; Liu, D.; He, C.; Zhang, J.; Zhang, Y.; Hou, J. Influence of the Replacement of Alkoxyl with Alkylthienyl on Photovoltaic Properties of Two Small Molecule Donors for Organic Solar Cells. Sci. China Chem. 2017, 60, 1340–1348. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, N.; Sun, Y.; Ke, X.; Zhang, H.; Li, C.; Wan, X.; Chen, Y. All-small-molecule organic solar cells based on a fluorinated small molecule donor with high open-circuit voltage of 1.07 V. Front. Chem. 2020, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Xu, X.; Tao, Q.; Zhang, Y.; Zhu, M.; Shao, L.; Zhu, W.; Peng, Q.; Liao, Y. Improving Photovoltaic Performance of the Linear Benzothienoindole-terminated Molecules by Tuning Molecular Framework and Substituted Position of Terminals. Dye. Pigment. 2017, 142, 406–415. [Google Scholar] [CrossRef]
- Shin, Y.; Song, C.E.; Lee, W.-H.; Lee, S.K.; Shin, W.S.; Kang, I.-N. Synthesis and Characterization of a Soluble A-D-A Molecule Containing a 2D Conjugated Selenophene-based Side Group for Organic Solar Cells. Macromol. Rapid Commun. 2017, 38, 1700016. [Google Scholar] [CrossRef]
- He, Q.; Shahid, M.; Panidi, J.; Marsh, A.V.; Huang, W.; Daboczi, M.; Kim, J.-S.; Fei, Z.; Anthopoulos, T.D.; Heeney, M. A Versatile Star-shaped Organic Semiconductor Based on Benzodithiophene and Diketopyrrolopyrrole. J. Mater. Chem. C 2019, 7, 6622–6629. [Google Scholar] [CrossRef]
- Kong, M.; Lee, J.; Song, S.; Lee, W.K.; Kim, J.Y.; Park, S.H.; Jin, Y. Synthesis and photovoltaic properties of organic molecules based on difluoroquinoxaline derivatives for OPVs. Mol. Cryst. Liq. Cryst. 2020, 705, 57–64. [Google Scholar] [CrossRef]
- Tran, H.; Haris, M.; Ahn, T.; Lee, S.K. Structure engineering of small molecules for organic solar cells. Mol. Cryst. Liq. Cryst. 2020, 705, 35–40. [Google Scholar] [CrossRef]
- Piradi, V.; Zhang, G.; Li, T.; Zhang, M.; Peng, Q.; Zhan, X.; Zhu, X. Side-chain Engineering of Benzodithiophene-bridged Dimeric Porphyrin Donors for All-small-molecule Organic Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 41506–41514. [Google Scholar] [CrossRef]
- Piradi, V.; Xu, X.; Yin, H.; Ho, J.K.W.; Yan, F.; Peng, Q.; So, S.K.; Zhu, X. Highly Semitransparent Indoor Nonfullerene Organic Solar Cells Based on Benzodithiophene-bridged Porphyrin Dimers. Energy Technol. 2021, 10, 2100908. [Google Scholar] [CrossRef]
- Lee, S.W.; Jin, H.C.; Kim, J.H.; Chang, D.W. Synthesis of ADA type quinoxaline-based small molecules for organic photovoltaic cells. Mol. Cryst. Liq. Cryst. 2020, 705, 7–14. [Google Scholar] [CrossRef]
- Xia, H.; Xu, X.; Guo, J.; Qian, C.; Zhang, K.; Zhu, M.; Zhang, B.; Peng, W.; Peng, Q.; Zhu, W. Structure Evolution from D-A-D Type Small Molecule Toward D-A-D-A-D Type Oligomer for High-efficiency Photovoltaic Donor Materials. Dye. Pigment. 2021, 186, 108950. [Google Scholar] [CrossRef]
- Yang, D.; Yu, K.; Xu, J.; Zhang, J.; Zhang, J.; Gao, J.; Song, W.; Li, D.; Chen, Z.; Ge, Z. Achieving 10% efficiency in non-fullerene all-small-molecule organic solar cells without extra treatments. J. Mater. Chem. A 2021, 9, 10427–10436. [Google Scholar] [CrossRef]
- Yuan, L.; Zhao, Y.; Zhang, J.; Zhang, Y.; Zhu, L.; Lu, K.; Yan, W.; Wei, Z. Oligomeric Donor Material for High-efficiency Organic Solar Cells: Breaking down a Polymer. Adv. Mater. 2015, 27, 4229–4233. [Google Scholar] [CrossRef]
- Du, Z.; Chen, W.; Wen, S.; Qiao, S.; Liu, Q.; Ouyang, D.; Wang, N.; Bao, X.; Yang, R. New Benzo[1,2-b:4,5-b’]dithiophene-based Small Molecules Containing Alkoxyphenyl Side Chains for High Efficiency Solution-processed Organic Solar Cells. ChemSusChem 2014, 7, 3319–3327. [Google Scholar] [CrossRef]
- Hoang, Q.V.; Song, C.E.; Kang, I.-N.; Moon, S.-J.; Lee, S.K.; Lee, J.-C.; Shin, W.S. Low Band Gap Diketopyrrolopyrrole-based Small Molecule Bulk Heterojunction Solar Cells: Influence of Terminal Side Chain on Morphology and Photovoltaic Performance. RSC Adv. 2016, 6, 28658–28665. [Google Scholar] [CrossRef]
- Tao, Q.; Duan, L.; Xiong, W.; Huang, G.; Wang, P.; Tan, H.; Wang, Y.; Yang, R.; Zhu, W. D(A-A′)2 Architecture: An Efficient Strategy to Improve Photovoltaic Performance of Small Molecules for Solution-processed Organic Solar Cells. Dye. Pigment. 2016, 133, 153–160. [Google Scholar] [CrossRef]
- Yang, L.; Qin, J.; Li, S.; Zhang, J.; Yang, Y.; Cao, B.; He, C.; Hou, J. Terminal Alkyl Chain Tuning of Small Molecule Donor Enables Optimized Morphology and Efficient All-small-molecule Organic Solar Cells. Dye. Pigment. 2022, 200, 110147. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Yang, D.; Yu, K.; Li, D.; Xia, Z.; Ge, Z. Two Star-shaped Small Molecule Donors Based on Benzodithiophene Unit for Organic Solar Cells. Chin. Chem. Lett. 2022, 33, 247–251. [Google Scholar] [CrossRef]
- Wu, Q.; Deng, D.; Zhou, R.; Zhang, J.; Zou, W.; Liu, L.; Wu, S.; Lu, K.; Wei, Z. Modulation of Donor Alkyl Terminal Chains with the Shifting Branching Point Leads to the Optimized Morphology and Efficient All-small-molecule Organic Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 25100–25107. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Q.; Deng, D.; Wu, S.; Sun, R.; Min, J.; Zhang, J.; Wei, Z. The post-treatment effects on open circuit voltages and device performances in a high efficiency all-small-molecule organic solar cell. J. Mater. Chem. C 2020, 8, 15385–15392. [Google Scholar] [CrossRef]
- Morvillo, P.; Parenti, F.; Diana, R.; Fontanesi, C.; Mucci, A.; Tassinari, F.; Schenetti, L. A novel copolymer from benzodithiophene and alkylsulfanyl-bithiophene: Synthesis, characterization and application in polymer solar cells. Sol. Energy Mater. Sol. Cells 2012, 104, 45–52. [Google Scholar] [CrossRef]



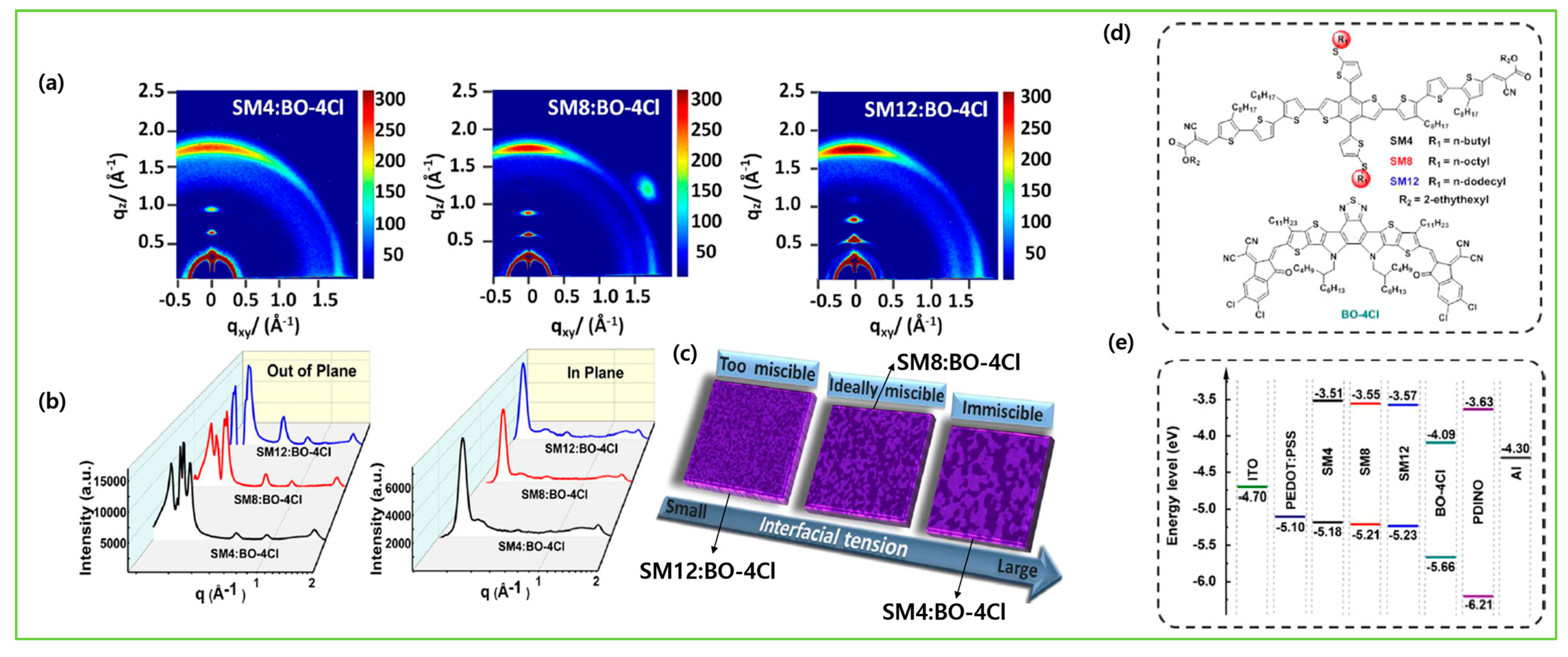
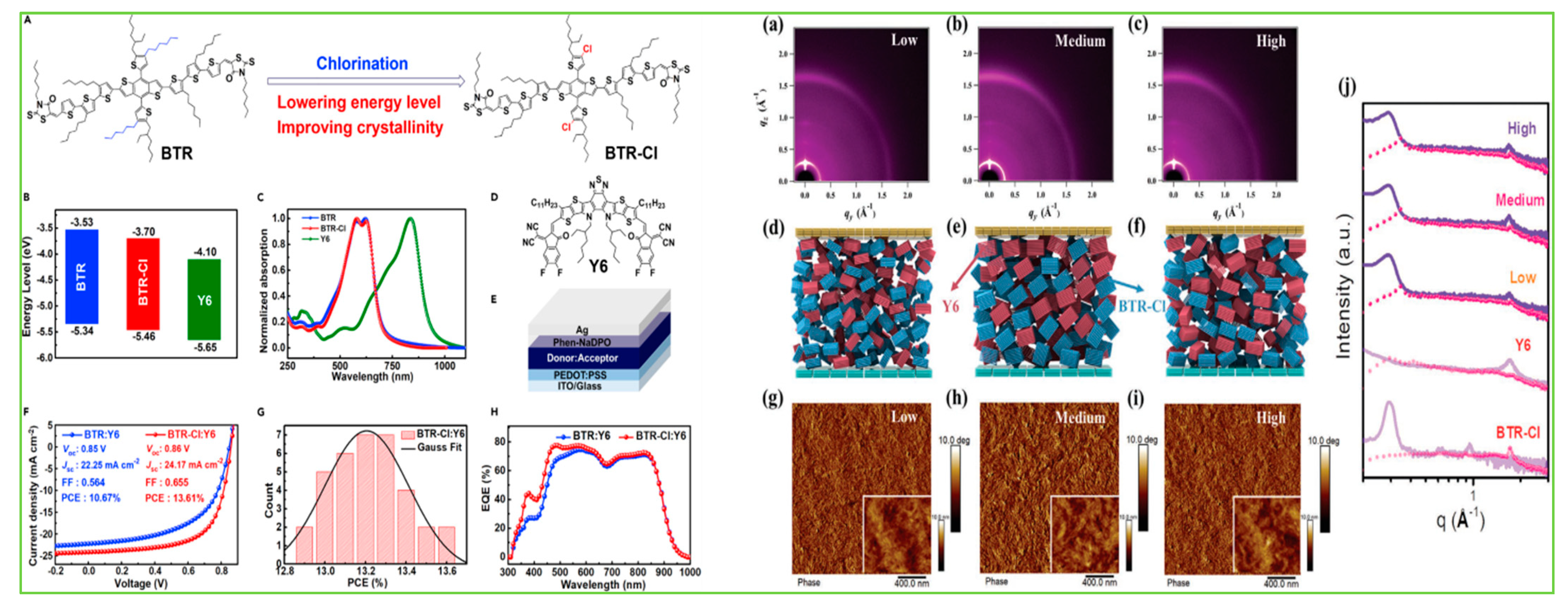
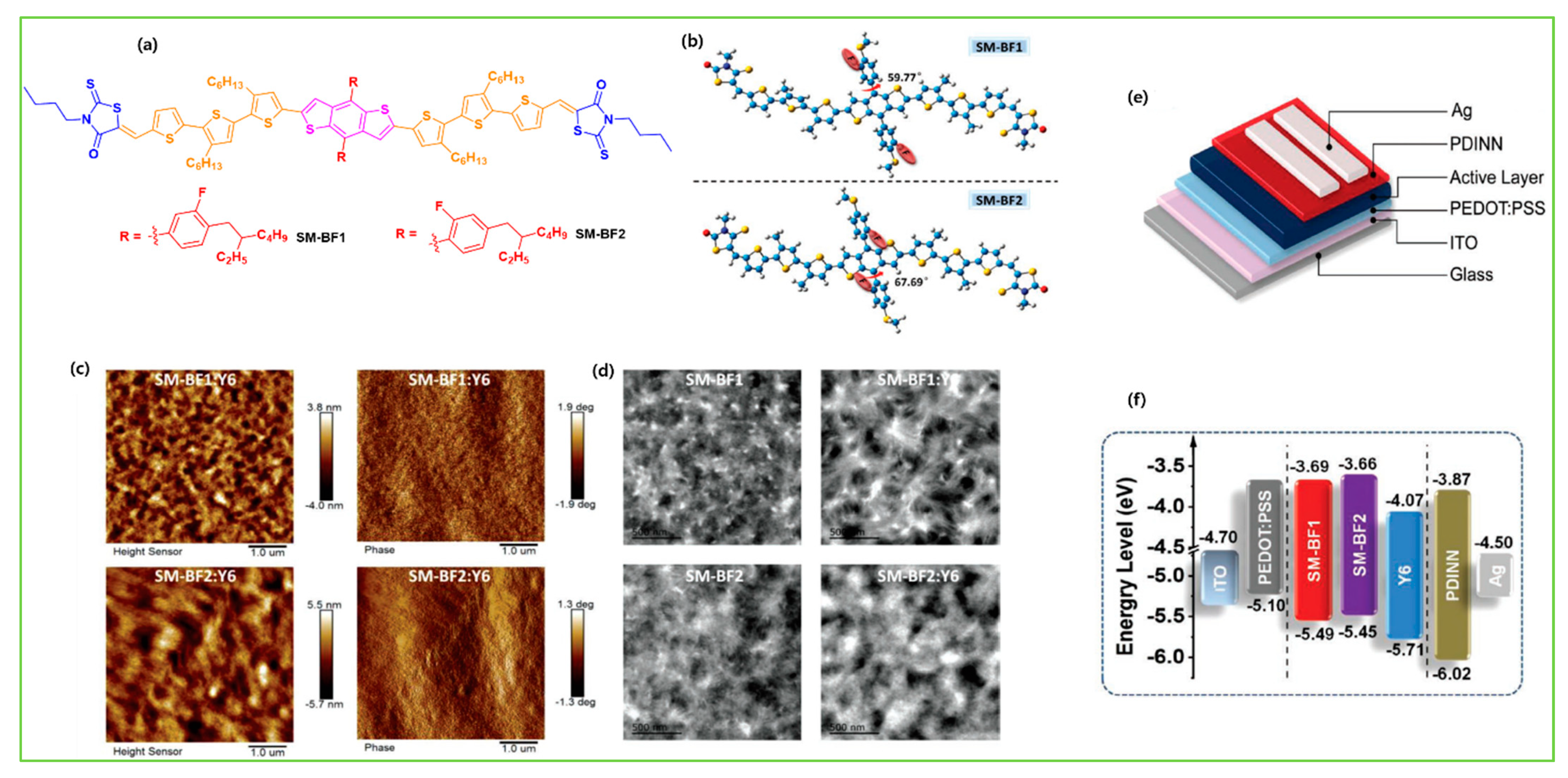
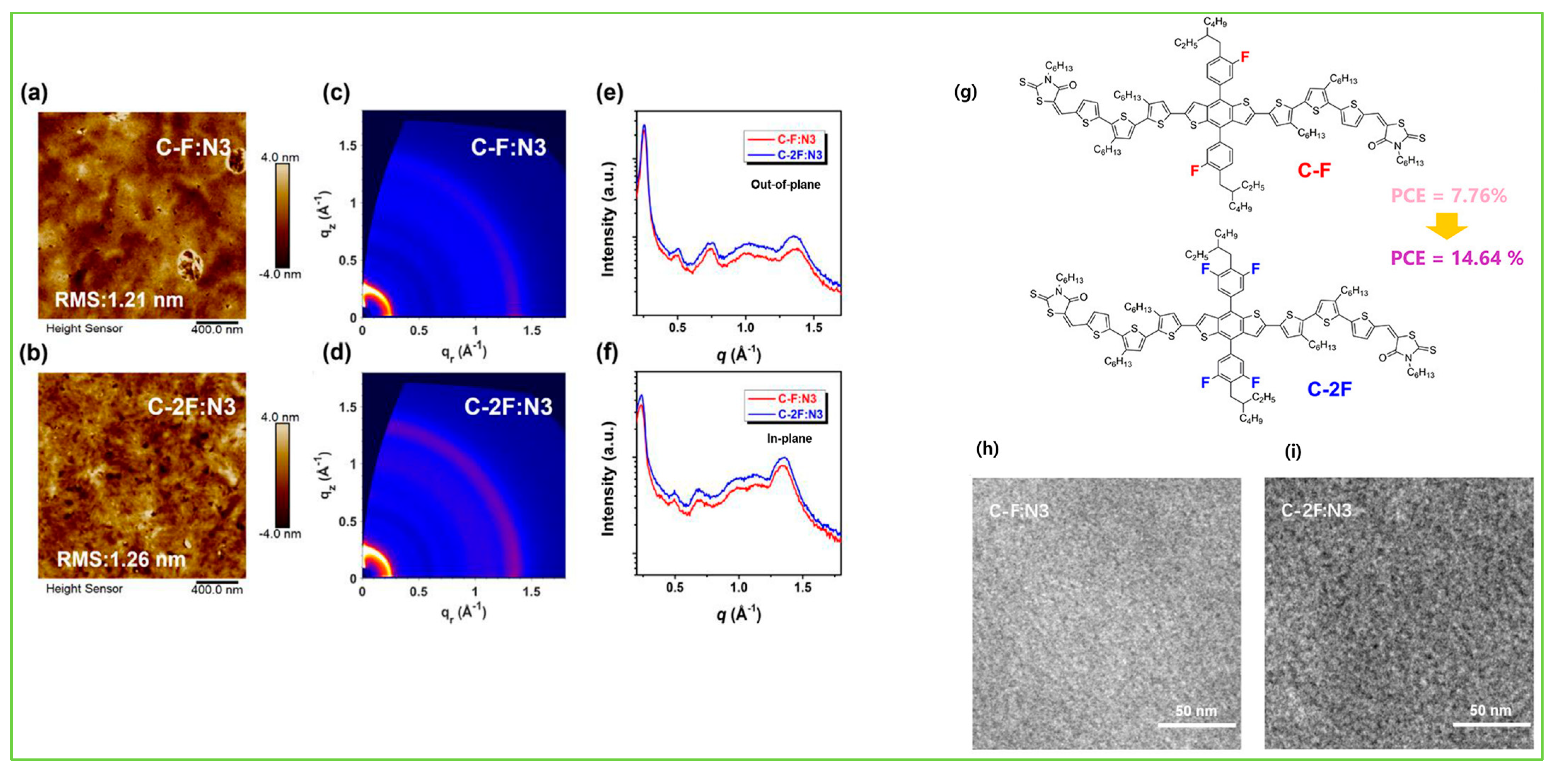
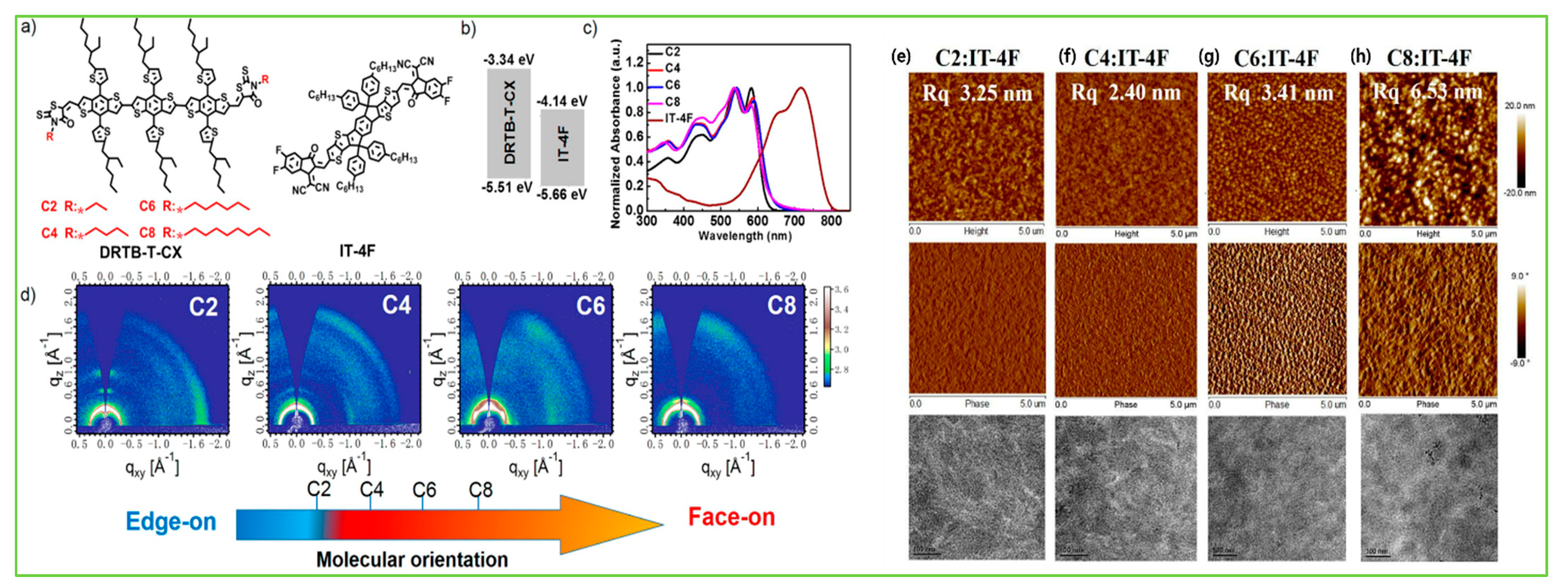
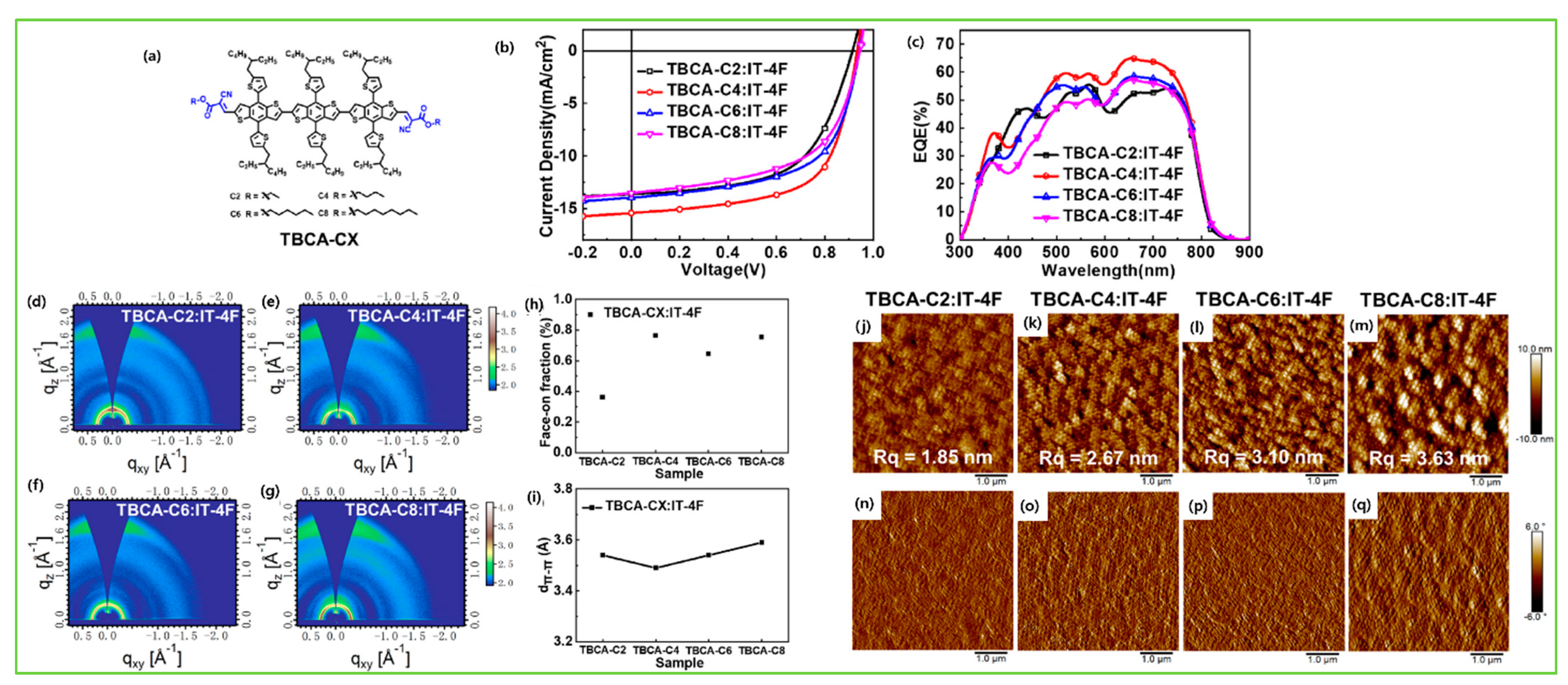
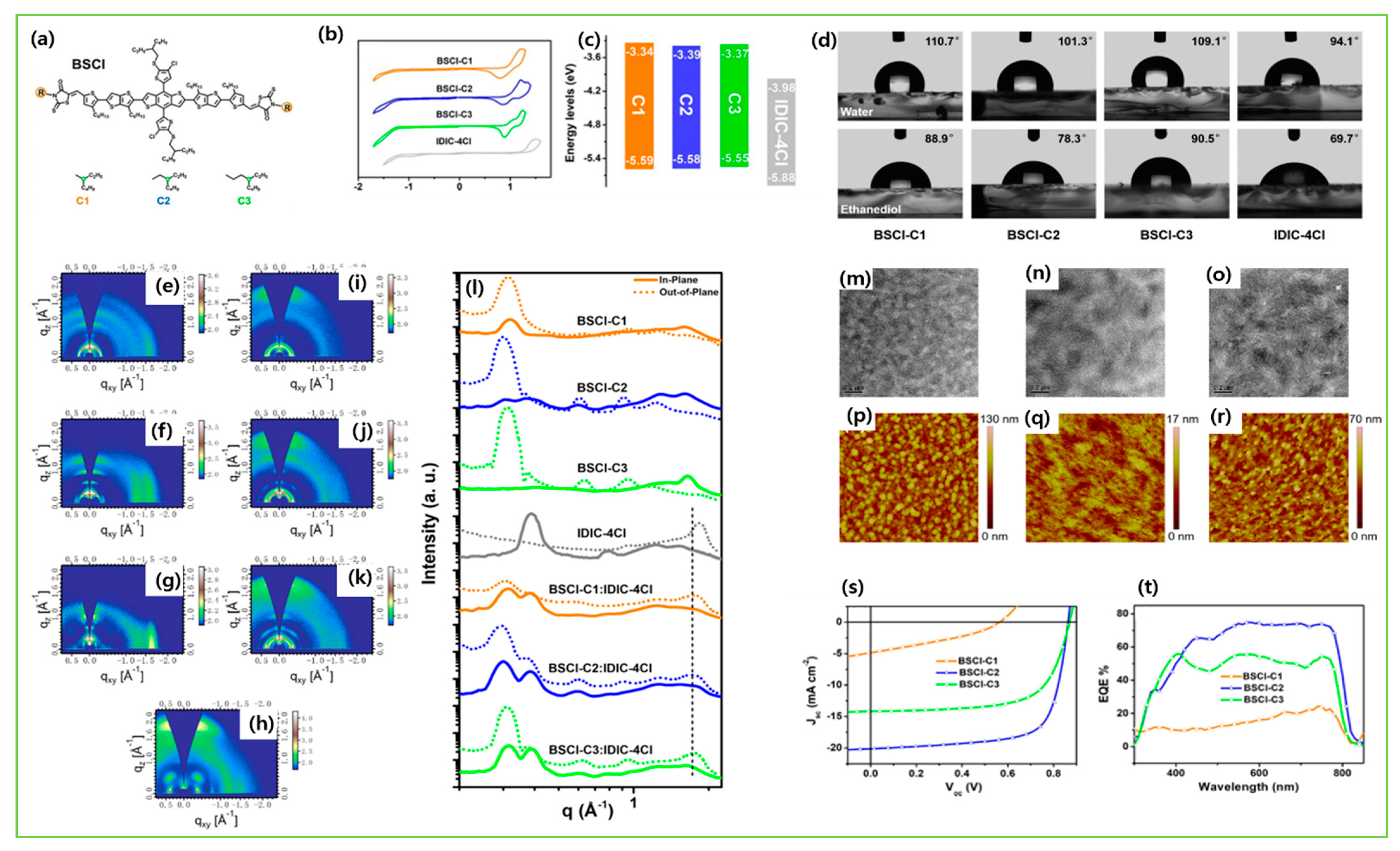
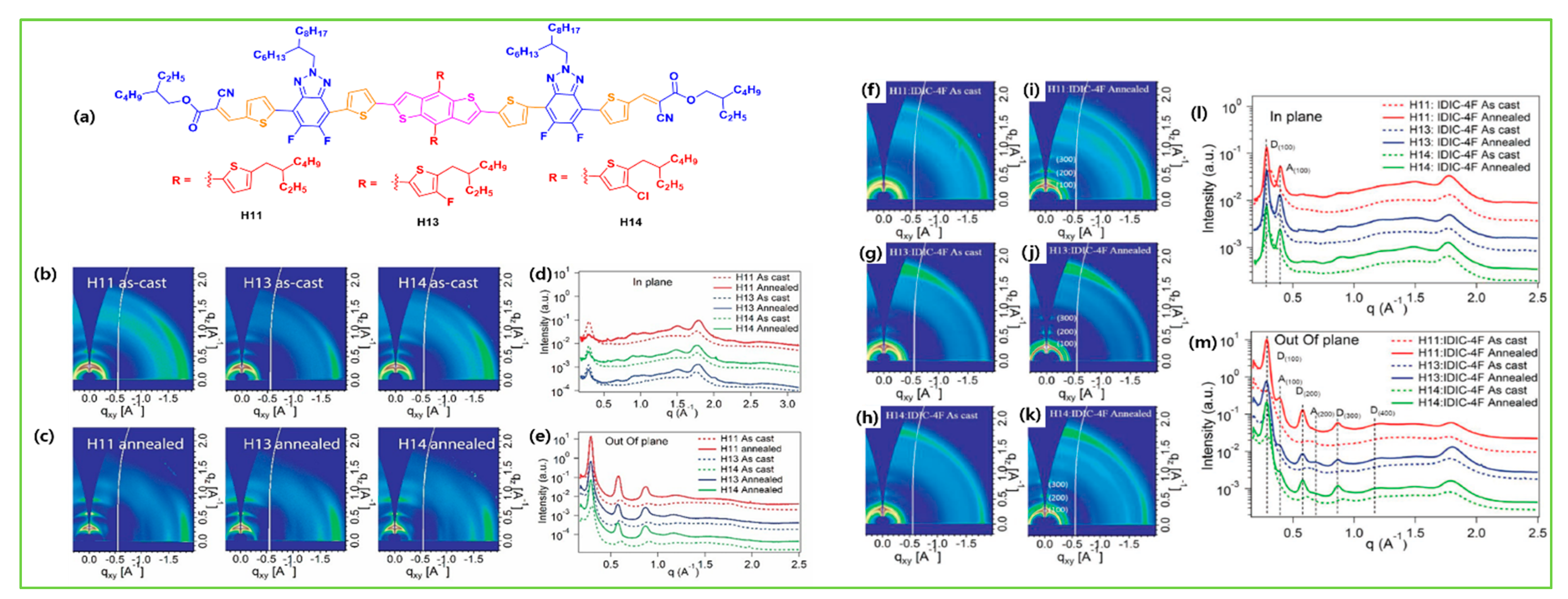

| Donor | Acceptor | Eg (eV) | HOMO/LUMO (eV) | µh (cm2V−1s−1) | VOC (V) | JSC (mAcm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| DCAO3T(BDT)3T | PC61BM | 1.83 | −5.11/−3.54 | 1.51 × 10−4 | 0.93 | 9.77 | 59.9 | 5.44 | [19] |
| TB-BDT6T | PC61BM | 1.76 | −5.31/−3.63 | 5.38 × 10−5 | 0.85 | 6.87 | 58.7 | 3.59 | [20] |
| ST-BDT6T | PC61BM | 1.75 | −5.40/−3.67 | 8.19 × 10−5 | 0.96 | 8.47 | 60.5 | 4.98 | [20] |
| TT-BDT6T | PC61BM | 1.78 | −5.35/−3.64 | 1.94 × 10−4 | 0.97 | 9.40 | 61.8 | 5.79 | [20] |
| DCAO3TBDT | PC61BM | 1.84 | −5.04/−3.24 | 1.38 × 10−4 | 0.95 | 8.00 | 60.0 | 4.56 | [21] |
| DR3TBDT | PC71BM | 1.74 | −5.02/−3.27 | 2.47 × 10−4 | 0.93 | 12.21 | 65.0 | 7.38 | [21] |
| DCA3TBDTP | PC61BM | 1.82 | −5.25/−3.43 | 2.74 × 10−4 | 0.90 | 7.88 | 63.6 | 4.51 | [22] |
| SMD1 | PC71BM | 1.82 | −5.23/−3.47 | 1.40 × 10−4 | 0.96 | 6.95 | 53.8 | 3.59 | [23] |
| SMD2 | PC71BM | 1.83 | −5.19/−3.45 | 1.04 × 10−4 | 0.95 | 5.11 | 64.4 | 3.13 | [23] |
| SMD3 | PC71BM | 1.74 | −5.40/−3.72 | 1.95 × 10−4 | 0.94 | 8.00 | 62.2 | 4.67 | [23] |
| DR3TBDTT | Y6 | 1.92cv | −5.25/−3.33 | 4.60 × 10−4 | 0.80 | 21.71 | 60.9 | 10.64 | [24] |
| BTEC-1F | Y6 | 2.00cv | −5.37/−3.37 | 4.17 × 10−4 | 0.87 | 21.21 | 61.3 | 11.33 | [24] |
| BTEC-2F | Y6 | 2.01cv | −5.39/−3.38 | 5.43 × 10−4 | 0.85 | 21.55 | 72.3 | 13.34 | [24] |
| BT-2F | Y6 | 2.00cv | −5.40/−3.40 | 3.93 × 10−4 | 0.85 | 22.38 | 72.27 | 13.80 | [25] |
| BT-2F | N3 | - | - | - | 0.84 | 23.81 | 70.22 | 14.09 | [25] |
| SM4 | BO-4Cl | 1.83 | −5.18/−3.51 | 3.51 × 10−4 | 0.76 | 18.18 | 46.8 | 6.44 | [26] |
| SM8 | BO-4Cl | 1.82 | −5.21/−3.55 | 3.93 × 10−4 | 0.85 | 21.23 | 72.5 | 13.11 | [26] |
| SM12 | BO-4Cl | 1.82 | −5.23/−3.57 | 2.25 × 10−4 | 0.84 | 19.20 | 65.9 | 10.59 | [26] |
| BT-RO-Cl | Y6 | 1.84 | −5.41/−3.57 | 2.46 × 10−4 | 0.86 | 22.50 | 68.6 | 13.35 | [27] |
| BT-REH-Cl | Y6 | 1.83 | −5.41/−3.58 | 2.81 × 10−4 | 0.86 | 22.93 | 69.8 | 13.90 | [27] |
| H21 | IDIC | 1.81 | −5.38/−3.63 | 2.49 × 10−4 | 0.90 | 13.00 | 65.6 | 7.62 | [28] |
| H22 | IDIC | 1.89 | −5.39/−3.59 | 4.26 × 10−4 | 0.94 | 15.38 | 71.2 | 10.29 | [28] |
| BDTF-CA | IDIC | 1.90 | −5.44/−3.53 | 5.4 × 10−4 | 0.99 | 12.09 | 51.14 | 6.12 | [29] |
| BDTF-CA | IDIC-2F | - | - | 1.9 × 10−4 | 0.94 | 12.69 | 58.07 | 9.11 | [29] |
| BDTF-CA | IDIC-4F | - | - | 6.3 × 10−4 | 0.88 | 14.96 | 63.95 | 8.42 | [29] |
| SM | IDIC | 1.92 | −5.25/−3.33 | 3.39 × 10−4 | 0.91 | 15.18 | 67.8 | 9.39 | [30] |
| SM-Cl | IDIC | 2.08 | −5.42/−3.34 | 2.58 × 10−4 | 0.97 | 12.61 | 63.08 | 7.73 | [30] |
| SM:SM-Cl (1.8:0.2) | IDIC | - | - | 4.01 × 10−4 | 0.92 | 16.05 | 69.58 | 10.29 | [30] |
| DR3TBDTT | PC71BM | 1.72 | −5.02/−3.27 | 2.88 × 10−4 | 0.93 | 13.17 | 66.2 | 8.12 | [31] |
| DR3TBDTT-HD | PC71BM | 1.76 | −5.06/−3.29 | 1.52 × 10−4 | 0.96 | 11.92 | 59.4 | 6.79 | [31] |
| DR3TBDT2T | PC71BM | 1.76 | −5.07/−3.29 | 3.29 × 10−4 | 0.92 | 12.09 | 72.1 | 8.02 | [31] |
| DR3TSBDT | PC71BM | 1.74 | −5.07/−3.30 | 6.13 × 10−4 | 0.91 | 14.45 | 73.0 | 9.95 | [32] |
| BDTT-S-TR | PC70BM | 1.73 | −5.18/−3.25 | 6.57 × 10−4 | 0.97 | 13.45 | 70.5 | 9.20 | [33] |
| BDTT-TR | PC70BM | 1.74 | −5.17/−3.39 | 5.48 × 10−4 | 0.93 | 11.75 | 68.1 | 7.44 | [34] |
| BDTT-O-TR | PC70BM | 1.74 | −5.14/−3.34 | 3.70 × 10−4 | 0.90 | 11.03 | 65.5 | 6.50 | [34] |
| DRBDT-TT | PC71BM | 1.78 | −5.13/−3.33 | 5.41 × 10−4 | 0.92 | 13.12 | 72.0 | 8.70 | [35] |
| DRBDT-STT | PC71BM | 1.80 | −5.15/−3.34 | 4.74 × 10−4 | 0.91 | 12.40 | 71.0 | 8.01 | [35] |
| DR3TBDTT | PC71BM | - | - | 6.57 × 10−4 | 0.88 | 14.21 | 76.0 | 9.58 | [36] |
| DR3TDOBDT | PC71BM | 1.79 | −5.08/−3.27 | 4.08 × 10−4 | 0.94 | 12.56 | 70.0 | 8.26 | [37] |
| BTR | Y6 | 1.78 | −5.34/−3.53 | 3.01 × 10−4 | 0.85 | 22.25 | 56.4 | 10.67 | [38] |
| BTR-Cl | Y6 | 1.78 | −5.46/−3.70 | 2.72 × 10−4 | 0.86 | 24.17 | 65.5 | 13.61 | [38] |
| BTR-Cl | Y6 | - | - | 8.51 × 10−5 | 0.83 | 23.66 | 74.7 | 14.7 | [39] |
| BTR-Cl | PC71BM:Y6 | - | - | 9.78 × 10−4 | 0.84 | 23.75 | 77.1 | 15.34 | [13] |
| DRBDT-TVT | PC71BM | 1.75 | −5.11/−3.41 | 3.6 × 10−4 | 0.88 | 10.73 | 72.76 | 6.87 | [40] |
| DRBDT-STVT | PC71BM | 1.76 | −5.14/−3.43 | 3.4 × 10−4 | 0.91 | 10.25 | 73.61 | 6.84 | [40] |
| DRBDT-TVT | IDIC | - | - | 1.4 × 10−4 | 0.84 | 12.22 | 64.58 | 6.63 | [40] |
| DRBDT-STVT | IDIC | - | - | 5.0 × 10−5 | 0.89 | 10.93 | 67.17 | 6.51 | [40] |
| BDTTS-F-R | PC71BM | 1.76 | −5.28/−2.82 | 3.24 × 10−4 | 0.95 | 14.31 | 68.9 | 9.37 | [41] |
| DTTS-Cl-R | PC71BM | 1.77 | −5.35/−2.84 | 2.79 × 10−4 | 0.96 | 14.92 | 75.3 | 10.78 | [41] |
| BDTTS-Br-R | PC71BM | 1.78 | −5.40/−2.87 | 1.85 × 10−4 | 0.98 | 13.85 | 63.1 | 8.55 | [41] |
| DRBDTCO | PC71BM | 1.73 | −4.94/−3.30 | 6.5 × 10−4 | 0.87 | 12.54 | 75.0 | 8.18 | [42] |
| dDRBDTCO | PC71BM | 1.80 | −5.02/−3.30 | 5.9 × 10−4 | 0.89 | 10.88 | 73.0 | 7.07 | [42] |
| BTR-TIPS | PC71BM | 1.84 | −5.42/−3.13 | 2.8 × 10−4 | 0.94 | 8.4 | 62.0 | 5.0 | [43] |
| BTR-TE | PC71BM | 1.78 | −5.39/−3.33 | 9.4 × 10−4 | 0.90 | 14.0 | 70.0 | 9.0 | [43] |
| BTR-H | PC71BM | 1.76 | −5.32/−3.29 | 3.9 × 10−4 | 0.87 | 10.1 | 58.0 | 5.1 | [43] |
| BTR-EH | PC71BM | 1.73 | −5.32/−3.34 | 7.2 × 10−4 | 0.89 | 11.8 | 67.0 | 7.0 | [43] |
| BTR | Y6 | - | - | 3.81 × 10−4 | 0.83 | 22.1 | 61.0 | 11.0 | [44] |
| BTR-TE | Y6 | - | - | 2.92 × 10−4 | 0.84 | 23.4 | 67.0 | 13.2 | [44] |
| BTR-TIPS | Y6 | - | - | 5.89 × 10−5 | 0.83 | 18.5 | 54.0 | 8.3 | [44] |
| PM6:BTR(0.9:0.1) | Y6 | - | - | - | 0.84 | 24.6 | 76.0 | 15.7 | [44] |
| PM6:BTR-TE(0.9:0.1) | Y6 | - | - | - | 0.84 | 24.5 | 78.0 | 16.1 | [44] |
| PM6:BTR-TIPS(0.9:0.1) | Y6 | - | - | - | 0.82 | 24.1 | 71.0 | 14.0 | [44] |
| BTR | NITI | - | - | 2.91 × 10−4 | 0.95 | 15.02 | 48.69 | 6.82 | [45] |
| BTR | PC71BM | - | - | 4.43 × 10−4 | 0.90 | 13.80 | 72.86 | 9.03 | [45] |
| BTR | PC71BM:NITI | - | - | 6.14 × 10−4 | 0.94 | 19.50 | 73.83 | 13.63 | [45] |
| BSFTR | Y6 | 1.98cv | −5.59/−3.61 | 6.43 × 10−4 | 0.85 | 23.16 | 69.66 | 13.69 | [46] |
| BDT3TR-SF | NBDTP-Fout | 1.81 | −5.37/−2.85 | 1.80 × 10−4 | 0.80 | 21.40 | 64.6 | 11.02 | [47] |
| BDT3TR-SF | NBDTP-Fin | - | - | 3.27 × 10−5 | 0.87 | 0.07 | 32.4 | 0.01 | [47] |
| BTR | BO-4Cl | 1.82cv | −5.34/−3.52 | 1.4 × 10−3 | 0.83 | 18.93 | 72.0 | 11.3 | [12] |
| B1 | BO-4Cl | 1.86cv | −5.37/−3.51 | 2.3 × 10−3 | 0.83 | 24.41 | 75.0 | 15.3 | [12] |
| SM-BF1 | Y6 | 1.75 | −5.49/−3.69 | 1.42 × 10−4 | 0.85 | 26.64 | 69.7 | 15.71 | [14] |
| SM-BF2 | Y6 | 1.77 | −5.45/−3.66 | 4.86 × 10−5 | 0.80 | 20.21 | 63.1 | 10.23 | [14] |
| S35 | Y6 | 1.80 | −5.37/−3.57 | 4.11 × 10−4 | 0.85 | 22.54 | 62.35 | 11.95 | [48] |
| S35−1Si | Y6 | 1.80 | −5.43/−3.63 | 7.00 × 10−4 | 0.86 | 22.93 | 61.84 | 12.19 | [48] |
| S35−2Si | Y6 | 1.81 | −5.38/−3.57 | 8.20 × 10−4 | 0.83 | 23.59 | 68.80 | 13.50 | [48] |
| C-F | N3 | 1.88 | −5.29/−3.53 | 2.81 × 10−4 | 0.79 | 20.51 | 47.52 | 7.76 | [49] |
| C-2F | N3 | 1.90 | −5.36/−3.54 | 2.70 × 10−3 | 0.85 | 24.87 | 69.33 | 14.64 | [49] |
| PM6:Y6 | PC71BM | - | - | 1.04 × 10−3 | 0.85 | 26.37 | 76.0 | 17.00 | [50] |
| PM6:CNS-6-8 | Y6:PC71BM | 1.93cv | −5.55/−3.62 | 1.21 × 10−3 | 0.87 | 26.43 | 78.8 | 18.07 | [50] |
| SW1 | Y6 | 1.79 | −5.42/−3.43 | 4.11 × 10−4 | 0.81 | 25.09 | 63.8 | 12.90 | [51] |
| SW2 | Y6 | 1.81 | −5.47/−3.45 | 3.28 × 10−4 | 0.84 | 25.10 | 74.0 | 15.51 | [51] |
| BO-1 | BTP-eC9 | 1.72 | −5.30/−3.51 | 1.03 × 10−3 | 0.85 | 25.56 | 77.74 | 16.79 | [52] |
| HD-1 | BTP-eC9 | 1.73 | −5.31/−3.46 | 1.14 × 10−3 | 0.84 | 26.04 | 78.46 | 17.19 | [52] |
| OD-1 | BTP-eC9 | 1.73 | −5.36/−3.48 | 0.99 × 10−3 | 0.83 | 25.49 | 71.89 | 15.18 | [52] |
| Donor | Acceptor | Eg (eV) | HOMO/LUMO (eV) | µh (cm2V−1s−1) | VOC (V) | JSC (mAcm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| SMPV1 | PC71BM | 1.77 | −5.51/−3.64 | 3.3 × 10−4 | 0.94 | 12.5 | 69.0 | 8.1 | [53] |
| D(T3-DCRD)-BDT | PC61BM | 1.62 | −5.39/−2.84 | 5.07 × 10−5 | 0.93 | 2.44 | 49.0 | 1.10 | [54] |
| D(T3-DCRD)-BDTT | PC61BM | 1.61 | −5.46/−2.83 | 6.22 × 10−4 | 0.96 | 3.69 | 55.0 | 1.94 | [54] |
| DRT3-BDT | PC71BM | 1.74 | −5.42/−3.54 | 8.68 × 10−5 | 0.90 | 11.92 | 63.0 | 6.76 | [55] |
| DTT3-BDT | PC71BM | 1.84 | −5.38/−3.44 | 2.94 × 10−5 | 0.86 | 10.52 | 58.0 | 5.25 | [55] |
| DOO3OTTBDT | PC71BM | 1.76 | −5.19/−3.46 | 1.4 × 10−4 | 0.94 | 8.0 | 70.0 | 5.26 | [56] |
| DOP3HTTBDT | PC71BM | 1.77 | −5.11/−3.37 | 1.1 × 10−4 | 0.87 | 9.94 | 65.0 | 5.64 | [56] |
| BDT3SCNCOO | PC71BM | 1.84 | −5.08/−3.47 | 1.2 × 10−4 | 0.89 | 9.98 | 72.0 | 6.4 | [57] |
| BDT3SCNCO | PC71BM | 1.76 | −5.18/−3.46 | 4.0 × 10−5 | 0.92 | 10.2 | 65.0 | 6.4 | [57] |
| BDT3SCNSOO | PC71BM | 1.85 | −5.11/−3.46 | 1.4 × 10−6 | 0.93 | 6.1 | 53.0 | 3.0 | [57] |
| BTID-0F | PC71BM | 1.71 | −4.91/−3.20 | 4.70 × 10−4 | 0.93 | 14.0 | 64.0 | 8.30 | [58] |
| BTID-1F | PC71BM | 1.70 | −4.98/−3.28 | 6.4 × 10−4 | 0.94 | 15.3 | 72.0 | 10.4 | [58] |
| BTID-2F | PC71BM | 1.68 | −5.05/−3.37 | 1.4 × 10−3 | 0.95 | 15.7 | 76.0 | 11.3 | [58] |
| 2F-C4C6 | IDIC | 1.82 cv | −5.19/−3.37 | 7.26 × 10−5 | 0.84 | 12.05 | 57.2 | 6.21 | [59] |
| 2F-C6C8 | IDIC | 1.83 cv | −5.24/−3.41 | 8.72 × 10−5 | 0.90 | 13.98 | 65.2 | 8.23 | [59] |
| V-BDT | PC71BM | 2.02 | −5.34/−3.64 | - | 0.89 | 6.88 | 61.0 | 3.73 | [60] |
| BTR-OH | PC71BM | 1.82 | −5.49/−3.45 | 1.4 × 10−5 | 0.90 | 13.56 | 65.3 | 8.0 | [61] |
| BTR:BTR-OH(0.8:0.2) | PC71BM | - | - | 6.8 × 10−5 | 0.93 | 14.62 | 74.2 | 10.14 | [61] |
| BDT-1 | PC71BM | 1.73 | −5.28/−3.25 | 6.9 × 10−5 | 0.90 | 11.4 | 52.9 | 5.46 | [62] |
| BDT-2 | PC71BM | 1.78 | −5.27/−3.22 | 7.0 × 10−6 | 0.82 | 8.3 | 43.9 | 2.99 | [62] |
| BDT-3 | PC71BM | 1.96 | −5.25/−2.90 | 1.0 × 10−8 | 0.55 | 2.6 | 27.0 | 0.38 | [62] |
| DR3TBDTT | PC71BM | 1.77 | −5.02/−3.27 | 3.53 × 10−4 | 0.90 | 13.37 | 74.8 | 9.09 | [63] |
| DCAO3TBDTT | IDIC | 1.88 | −5.24/−2.28 | 5.70 × 10−4 | 0.91 | 15.53 | 66.9 | 9.49 | [63] |
| DR3TBDTT-S-E: DR3TBDTT | PC71BM | 1.73 | −5.33/−3.50 | 6.74 × 10−4 | 0.91 | 14.89 | 76.9 | 10.38 | [63] |
| DCAO3TBDTT: DR3TBDTT-S-E | IDIC | - | - | 7.21 × 10−4 | 0.91 | 16.37 | 67.4 | 10.04 | [63] |
| BDT-2T-DCV-Me | IDIC | 1.90 | −5.56/−3.35 | 3.48 × 10−4 | 1.06 | 4.75 | 29.3 | 1.56 | [64] |
| BDT-2T-CNAB | IDIC | 1.90 | −5.54/−3.31 | 4.67 × 10−3 | 1.04 | 10.10 | 58.8 | 6.17 | [64] |
| BDT-HTOX | PC71BM | 1.72 | −5.44/−3.62 | - | 0.88 | 3.26 | 27.9 | 0.80 | [65] |
| BDT-TBT | PC71BM | 2.14 | −5.43/−3.29 | - | 0.84 | 2.56 | 31.9 | 0.69 | [66] |
| BDT-THTBT | PC71BM | 2.02 | −5.33/−3.31 | - | 0.82 | 4.20 | 30.1 | 1.04 | [66] |
| SD1 | Y6T | 1.87 | −5.08/−3.41 | 8.32 × 10−4 | 0.88 | 18.23 | 63.1 | 10.12 | [67] |
| SD2 | Y6T | 1.88 | −5.11/−3.41 | 5.18 × 10−4 | 0.89 | 17.79 | 52.3 | 8.28 | [67] |
| SD3 | Y6T | 1.89 | −5.10/−3.40 | 3.59 × 10−4 | 0.89 | 14.47 | 45.0 | 5.79 | [67] |
| Donor | Acceptor | Eg (eV) | HOMO/LUMO (eV) | µh (cm2V−1s−1) | VOC (V) | JSC (mAcm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| D1 | PC70BM | 1.61 | −5.19/−3.56 | 2.04 × 10−4 | 1.03 | 10.07 | 54.7 | 5.67 | [68] |
| DO1 | PC70BM | 1.59 | −5.18/−3.56 | 1.71 × 10−4 | 0.91 | 9.47 | 48.2 | 4.15 | [68] |
| D2 | PC70BM | 1.60 | −5.16/−3.54 | 2.82 × 10−2 | 0.92 | 11.05 | 66.4 | 6.75 | [68] |
| DO2 | PC70BM | 1.60 | −5.16/−3.52 | 2.63 × 10−2 | 0.92 | 8.58 | 64.8 | 5.11 | [68] |
| 0TBM | PC71BM | 1.42 | −5.13/−4.11 | 9.06 × 10−6 | 0.64 | 0.10 | 31.5 | 0.02 | [69] |
| 1TBM | PC71BM | 1.46 | −5.11/−3.84 | 6.70 × 10−5 | 0.83 | 3.49 | 33.2 | 0.95 | [69] |
| 2TBM | PC71BM | 1.46 | −5.10/−3.80 | 2.37 × 10−5 | 0.79 | 6.66 | 35.1 | 1.85 | [69] |
| 2TBM (out) | PC71BM | 1.48 | −5.09/−3.75 | 2.37 × 10−5 | 0.81 | 6.33 | 30.4 | 1.56 | [69] |
| 3TBM | PC71BM | 1.42 | −5.10/−3.75 | 7.74 × 10−3 | 0.80 | 13.81 | 56.4 | 6.29 | [69] |
| 4TBM | PC71BM | 1.42 | −5.06/−3.76 | 1.27 × 10−5 | 0.78 | 5.01 | 40.3 | 1.56 | [69] |
| 4TBM out) | PC71BM | 1.56 | −5.12/−3.76 | 1.27 × 10−5 | 0.76 | 2.33 | 25.3 | 0.45 | [69] |
| 5TBM | PC71BM | 1.42 | −5.10/−3.74 | 4.00 × 10−3 | 0.81 | 9.62 | 68.8 | 5.35 | [69] |
| TBDT-2HT-ID | PC71BM | 1.78 | −5.47/−3.68 | 7.6 × 10−5 | 1.07 | 12.4 | 55.5 | 7.36 | [70] |
| BDT-1T-ID | PC71BM | 1.80 | −5.23/−3.43 | 1.7 × 10−3 | 1.06 | 11.0 | 49.0 | 5.9 | [71] |
| BDT-2T-ID | PC71BM | 1.72 | −5.13/−3.41 | 7.1 × 10−3 | 0.96 | 14.0 | 49.0 | 6.9 | [71] |
| BDT-3T-ID | PC71BM | 1.65 | −5.03/−3.38 | 4.2 × 10−3 | 0.93 | 13.5 | 49.0 | 6.2 | [71] |
| BDT-4T-ID | PC71BM | 1.64 | −4.99/−3.35 | 5.4 × 10−3 | 0.88 | 11.3 | 51.0 | 5.1 | [71] |
| BDTTID | PC70BM | 1.74 | −5.36/−3.59 | 1.96 × 10−5 | 1.03 | 10.20 | 53.0 | 5.54 | [72] |
| BDT3TID | PC70BM | 1.67 | −5.16/−3.48 | 1.87 × 10−5 | 0.89 | 9.04 | 59.0 | 4.74 | [72] |
| BDT(ThBTTh)2 | PC61BM | 1.77 | −5.17/−3.40 | 4.7 × 10−4 | 0.89 | 9.33 | 54.5 | 4.53 | [73] |
| BDT(BTTh)2 | PC61BM | 1.77 | −5.11/−3.34 | 0.86 × 10−4 | 0.82 | 4.74 | 40.5 | 1.58 | [73] |
| a-SM1 | PC61BM | 1.66 | −5.15/−3.49 | 9.2 × 10−4 | 0.67 | 4.13 | 50.1 | 1.40 | [74] |
| a-SM2 | PC61BM | 1.66 | −5.13/−3.47 | 1.29 × 10−3 | 0.65 | 6.50 | 60.3 | 2.57 | [74] |
| SM-BT-2OR | IDIC | 1.77 | −5.34/−3.12 | 7.37 × 10−5 | 0.94 | 13.57 | 56.5 | 7.20 | [75] |
| SM-BT-2F | IDIC | 1.66 | −5.36/−3.26 | 1.77 × 10−5 | 0.98 | 6.74 | 41.7 | 2.76 | [75] |
| BDTSe-TTPD | PC71BM | 1.86 | −5.34/−3.48 | 3.04 × 10−6 | 0.90 | 10.5 | 46.3 | 4.37 | [76] |
| M7a | PC71BM | 1.84 | −5.01/−3.36 | - | 0.98 | 7.74 | 51.0 | 3.9 | [77] |
| M7b | PC71BM | 1.88 | −5.12/−3.26 | - | 0.97 | 5.54 | 49.0 | 2.5 | [77] |
| b-SM1 | PC71BM | 1.84 | −5.32/−3.16 | 9.89 × 10−5 | 0.99 | 11.18 | 56.0 | 6.20 | [78] |
| b-SM2 | PC71BM | 1.72 | −5.28/−3.24 | 1.89 × 10−4 | 1.04 | 12.06 | 60.0 | 7.45 | [78] |
| BDT(TVT-SR)2 | IDIC | 1.82 | −5.33/−3.18 | 1.48 × 10−4 | 0.98 | 15.92 | 71.2 | 11.10 | [79] |
| SBDT-BDD | IDIC | 1.77 | −5.25/−3.55 | 3.5 × 10−4 | 0.97 | 15.15 | 62.5 | 9.2 | [80] |
| SBDT-BDD | PC71BM:IDIC | - | - | 3.8 × 10−4 | 0.97 | 16.21 | 69.3 | 10.9 | [80] |
| BDTTNTTR | PC71BM | 1.51 | −5.29/−3.53 | 2.01 × 10−3 | 0.89 | 15.70 | 71.7 | 10.02 | [81] |
| BDTSTNTTR | PC71BM | 1.50 | −5.35/−3.60 | 3.18 × 10−3 | 0.93 | 16.21 | 76.5 | 11.53 | [81] |
| H11 | IDIC | 1.87 | −5.31/−3.03 | 7.7 × 10−5 | 0.98 | 15.21 | 65.5 | 9.73 | [82] |
| H12 | IDIC | 1.87 | −5.28/−3.01 | 7.9 × 10−5 | 0.96 | 10.51 | 54.9 | 5.51 | [82] |
| H13 | IDIC-4F | 1.93 | −5.43/−3.39 | 2.02 × 10−4 | 0.94 | 17.3 | 63.2 | 10.3 | [83] |
| H14 | IDIC-4F | 1.94 | −5.46/−3.47 | 4.41 × 10−4 | 0.94 | 18.3 | 70.2 | 12.1 | [83] |
| BDT(TTzT)2 | PC71BM | 1.91 | −5.51/−3.67 | 8.76 × 10−5 | 0.98 | 9.56 | 53.5 | 5.01 | [84] |
| BDT(TTz2T)2 | PC71BM | 1.77 | −5.30/−3.64 | 2.14 × 10−4 | 0.88 | 10.55 | 57.2 | 5.29 | [84] |
| TBDT(TTzT)2 | PC71BM | 1.99 | −5.59/−3.69 | 4.63 × 10−4 | 1.03 | 9.50 | 61.6 | 6.10 | [85] |
| TBDT(TTz2T)2 | PC71BM | 1.82 | −5.37/−3.65 | 7.25 × 10−4 | 0.94 | 10.65 | 65.1 | 6.56 | [85] |
| BDTQ-BDT(EH) | PC70BM | 2.10 | −5.36/−3.26 | - | 0.83 | 4.50 | 32.0 | 1.20 | [86] |
| BDTQ-BDT(OC) | PC70BM | 2.11 | −5.30/−3.19 | - | 0.79 | 3.52 | 30.0 | 0.83 | [86] |
| SM-0F | PC71BM | 1.56cv | −5.09/−3.53 | 2.10 × 10−4 | 0.73 | 7.3 | 43.4 | 2.56 | [87] |
| SM-2F | PC71BM | 1.49cv | −5.12/−3.63 | 3.52 × 10−4 | 0.75 | 11.0 | 44.9 | 3.94 | [87] |
| SM-4F | PC71BM | 1.55cv | −5.13/−3.58 | 2.35 × 10−4 | 0.77 | 9.1 | 46.7 | 3.48 | [87] |
| BBDDR | IDIC | 1.79 | −5.40/−3.61 | 2.4 × 10−4 | 1.01 | 14.6 | 53.0 | 7.8 | [88] |
| BDT-TITRh | PC71BM | 1.78 | −5.24/−3.74 | 2.1 × 10−5 | 0.80 | 13.05 | 33.8 | 3.52 | [89] |
| BDT-TI2TRh | PC71BM | 1.75 | −5.17/−3.71 | 8.0 × 10−5 | 0.85 | 13.23 | 37.3 | 4.19 | [89] |
| BDT-BTF | PC71BM | 1.78 | −5.20/−3.24 | 3.9 × 10−3 | 0.85 | 10.48 | 66.0 | 5.88 | [90] |
| BDTDPTz | PC71BM | 1.65 | −5.42/−3.65 | 8.20 × 10−5 | 0.87 | 12.83 | 56.4 | 6.28 | [91] |
| B2TPR | PC71BM | 1.68 | −5.23/−3.55 | 1.26 × 10−4 | 0.98 | 11.3 | 64.0 | 7.1 | [92] |
| BTRO | IDIC-4F | 1.79 | −5.30/−3.41 | 4.29 × 10−4 | 0.91 | 11.04 | 41.0 | 4.08 | [93] |
| BTCN | IDIC-4F | 1.82 | −5.31/−3.43 | 6.94 × 10−4 | 0.89 | 11.46 | 45.0 | 4.62 | [93] |
| ECTBD | Y6 | 1.93 | −5.47/−3.54 | - | 0.81 | 6.37 | 30.5 | 1.58 | [94] |
| ECTBD(15%):PM6 | Y6 | - | - | - | 0.85 | 25.54 | 76.2 | 16.51 | [94] |
| BER6 | IDIC | 1.91 | −5.41/−2.90 | 1.47 × 10−4 | 0.97 | 14.86 | 63.0 | 9.03 | [95] |
| BECN | IDIC | 1.85 | −5.44/−2.99 | 6.44 × 10−5 | 0.96 | 11.10 | 51.0 | 5.52 | [95] |
| D18-Cl:G17(0.9:0.1) | Y6 | 1.80 | −5.39/−3.60 | 2.53 × 10−4 | 0.88 | 25.99 | 76.7 | 17.13 | [96] |
| D18-Cl:G19(0.9:0.1) | Y6 | 1.83 | −5.30/−3.53 | 2.77 × 10−4 | 0.87 | 27.36 | 77.7 | 18.53 | [96] |
| SM-BDT | Y8 | 1.84 | −5.10/−2.70 | 2.04 × 10−4 | 0.84 | 21.63 | 58.7 | 10.68 | [97] |
| SMBDT-S | PC71BM | 1.85 | −5.56/−3.56 | - | 1.05 | 5.55 | 50.0 | 2.89 | [98] |
| SM-BDT-SF | PC71BM | 1.86 | −5.72/−3.59 | - | 1.18 | 1.39 | 55.0 | 0.90 | [98] |
| Donor | Acceptor | Eg (eV) | HOMO/LUMO (eV) | µh (cm2V−1s−1) | VOC (V) | JSC (mAcm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| BDT-1 | PC71BM | 1.77 | −5.14/−3.37 | 5.38 × 10−5 | 0.89 | 13.02 | 62.0 | 7.18 | [99] |
| BDT-2 | PC71BM | 1.76 | −5.13/−3.37 | 2.44 × 10−4 | 0.89 | 13.17 | 73.0 | 8.56 | [99] |
| BDT-3 | PC71BM | 1.82 | −5.10/−3.28 | 9.19 × 10−5 | 0.90 | 11.34 | 70.0 | 7.14 | [99] |
| BDTx-2TVTDPP | PC61BM | 1.61 | −5.10/−3.32 | 1.61 × 10−4 | 0.67 | 3.61 | 65.8 | 1.58 | [100] |
| BDTy-2TVTDPP | PC71BM | 1.71 | −5.30/−3.32 | 2.8 × 10−5 | 0.86 | 8.60 | 38.7 | 2.85 | [100] |
| aBDT | PC61BM | 1.96 | −5.23/−3.39 | 3.5 × 10−5 | 0.81 | 1.74 | 27.0 | 0.40 | [101] |
| BDT | PC61BM | 1.85 | −5.16/−3.40 | 2.7 × 10−4 | 0.85 | 8.67 | 49.0 | 3.60 | [101] |
| B-BDP | PC71BM | 1.46 | −5.11/−3.65 | 4.53 × 10−4 | 0.73 | 11.84 | 53.8 | 4.65 | [102] |
| PH | PC61BM | 1.70 | −5.36/−3.66 | 7.45 × 10−6 | 0.83 | 8.36 | 59.6 | 4.15 | [103] |
| PF2 | PC61BM | 1.70 | −5.47/−3.75 | 4.01 × 10−6 | 0.94 | 7.81 | 57.8 | 4.26 | [103] |
| PH:PF2 (0.1:0.9) | PC61BM | - | - | 7.17 × 10−6 | 0.93 | 9.18 | 57.6 | 4.90 | [103] |
| DRTB-T-C2 | IT-4F | 2.0 | −5.51/−3.34 | 3.27 × 10−5 | 0.89 | 16.66 | 64.0 | 9.52 | [104] |
| DRTB-T-C4 | IT-4F | 1.99 | −5.50/−3.32 | 1.74 × 10−5 | 0.91 | 18.27 | 68.0 | 11.24 | [104] |
| DRTB-T-C6 | IT-4F | 1.98 | −5.50/−3.32 | 5.55 × 10−5 | 0.93 | 17.92 | 63.0 | 10.52 | [104] |
| DRTB-T-C8 | IT-4F | 1.97 | −5.52/−3.33 | 3.14 × 10−5 | 0.93 | 16.15 | 61.0 | 9.14 | [104] |
| DRTB-O | PC71BM | 1.90 | −5.50/−3.56 | 5.44 × 10−5 | 1.01 | 7.49 | 65.0 | 4.91 | [105] |
| DRTB-T | PC71BM | 1.90 | −5.48/−3.56 | 1.14 × 10−4 | 1.01 | 10.02 | 70.0 | 7.08 | [105] |
| DRTB-O | IDIC | - | - | 3.74 × 10−7 | 0.99 | 0.57 | 27.0 | 0.15 | [105] |
| DRTB-T | IDIC | - | - | 3.46 × 10−4 | 0.98 | 14.22 | 65.0 | 9.06 | [105] |
| DRTB-FT | F-2Cl | 1.99 | −5.64/−3.61 | 8.56 × 10−5 | 1.07 | 13.46 | 53.2 | 7.66 | [106] |
| BDT(DPP-9-BTI)2 | PC71BM | 1.46 | −5.07/−3.61 | 2.06 × 10−4 | 0.55 | 11.84 | 54.1 | 3.52 | [107] |
| BDT(DPP-8-BTI)2 | PC71BM | 1.43 | −5.20/−3.77 | 2.19 × 10−4 | 0.64 | 11.91 | 63.4 | 4.80 | [107] |
| SeBDT-DPP | PC71BM | 1.63 | −5.42/−3.79 | 5.9 × 10−3 | 0.79 | 10.98 | 58.0 | 5.04 | [108] |
| BDT(DPP)4 | PC71BM | 1.69 | −5.36/−3.33 | - | 0.75 | 8.54 | 39.0 | 2.5 | [109] |
| BDT(DPP)4 | C8-ITIC | - | - | - | 0.86 | 10.1 | 45.0 | 3.9 | [109] |
| M1 | PC61BM | 2.31 | −5.79/−3.49 | - | 0.55 | 3.07 | 32.0 | 0.54 | [110] |
| M2 | PC61BM | 2.10 | −5.66/−3.52 | - | 0.61 | 2.10 | 32.0 | 0.41 | [110] |
| c-SM1 | ITIC-4F | 1.79 | −5.37/−3.58 | - | 0.83 | 2.28 | 21.0 | 0.41 | [111] |
| c-SM2 | ITIC-4F | 1.64 | −5.50/−3.86 | - | 0.71 | 4.77 | 24.0 | 0.82 | [111] |
| C8T-BDTDP | 6TIC | 1.58 | −5.28/−3.70 | 1.66 × 10−4 | 0.75 | 17.75 | 64.0 | 8.73 | [112] |
| C8ST-BDTDP | 6TIC | 1.59 | −5.24/−3.65 | 3.82 × 10−4 | 0.79 | 19.53 | 65.6 | 10.39 | [112] |
| C8TEBDT-2P | IDIC | 1.57 | −5.24/−3.67 | 1.26 × 10−4 | 0.86 | 14.5 | 64.6 | 7.46 | [113] |
| C8TBDT-2P | IDIC | 1.72 | −5.19/−3.47 | 3.75 × 10−5 | 0.80 | 6.83 | 45.8 | 2.68 | [113] |
| BDT-Qx | PC71BM | 2.08 | −5.34/−3.26 | - | 0.75 | 2.20 | 31.4 | 0.52 | [114] |
| BDT-T-Qx | PC71BM | 1.95 | −5.35/−3.40 | - | 0.78 | 2.41 | 31.7 | 0.59 | [114] |
| 3BDTBDD | ITIC | 2.03 | −5.50/−3.38 | 1.73 × 10−5 | 0.90 | 9.51 | 50.6 | 4.33 | [115] |
| 5BDTBDD | ITIC | 1.87 | −5.46/−3.51 | 1.12 × 10−4 | 0.91 | 13.23 | 65.6 | 7.89 | [115] |
| 3BDT-4 | Y6 | 1.83 | −5.14/−3.44 | 8.0 × 10−5 | 0.83 | 17.0 | 41.3 | 5.82 | [116] |
| 3BDT-5 | Y6 | 1.90 | −5.15/−3.40 | 3.64 × 10−4 | 0.84 | 21.3 | 58.1 | 10.4 | [116] |
| O-BDTdFBT | PC71BM | 1.83 | −5.37/−3.52 | 3.1 × 10−5 | 0.97 | 11.48 | 70.0 | 8.10 | [117] |
| BDT-O-DPP | PC61BM | 1.69 | −5.16/−3.47 | 1.54 × 10−4 | 0.88 | 9.54 | 51.1 | 4.28 | [118] |
| BDT-PO-DPP | PC61BM | 1.70 | −5.25/−3.55 | 2.98 × 10−4 | 0.83 | 11.23 | 60.3 | 5.63 | [118] |
| BDT(DPP-TTHex)2 | PC71BM | 1.49 | −5.16/−3.64 | 1.01 × 10−5 | 0.65 | 6.08 | 60.0 | 2.36 | [119] |
| BDT(DPP-TT)2 | PC71BM | 1.53 | −5.15/−3.61 | 1.28 × 10−5 | 0.69 | 13.39 | 56.0 | 5.12 | [119] |
| BDT(DPP)2 | PC61BM | 1.55 | −5.62/−3.47 | - | 0.81 | 3.20 | 49.2 | 1.26 | [120] |
| BDTT(DPP)2 | PC61BM | 1.52 | −5.68/−3.46 | - | 0.78 | 2.83 | 34.9 | 0.77 | [120] |
| BDT(TPD-DPP)2 | PC61BM | 1.55 | −5.67/−3.63 | - | 0.78 | 5.69 | 54.5 | 2.41 | [120] |
| BDTT(TPD-DPP)2 | PC61BM | 1.55 | −5.68/−3.64 | - | 0.77 | 10.83 | 50.9 | 4.25 | [120] |
| TBCA-C2 | IT-4F | 2.03 | −5.51/−3.48 | 1.52 × 10−5 | 0.91 | 13.61 | 59.0 | 7.34 | [121] |
| TBCA-C4 | IT-4F | 2.03 | −5.49/−3.46 | 1.06 × 10−4 | 0.93 | 15.43 | 64.0 | 9.21 | [121] |
| TBCA-C6 | IT-4F | 2.03 | −5.53/−3.50 | 2.42 × 10−5 | 0.94 | 13.97 | 60.0 | 7.91 | [121] |
| TBCA-C8 | IT-4F | 2.03 | −5.52/−3.49 | 1.38 × 10−5 | 0.94 | 13.52 | 57.0 | 7.24 | [121] |
| BDT-3Th | Y6 | 1.97 | −5.04/−3.39 | - | 0.87 | 11.90 | 36.5 | 3.78 | [122] |
| BDT-4Th | Y6 | 2.01 | −5.14/−3.38 | - | 0.84 | 17.20 | 40.3 | 5.83 | [122] |
| BSCl-C1 | IDIC-4Cl | 1.87 | −5.59/−2.25 | 4.17 × 10−5 | 0.56 | 4.90 | 33.9 | 0.90 | [123] |
| BSCl-C2 | IDIC-4Cl | 1.82 | −5.58/−2.19 | 3.83 × 10−4 | 0.86 | 20.10 | 71.3 | 12.40 | [123] |
| BSCl-C3 | IDIC-4Cl | 1.84 | −5.55/−2.18 | 1.58 × 10−4 | 0.87 | 14.20 | 67.0 | 8.25 | [123] |
| BSCl | IDIC-4Cl | 1.58 | −5.55/−3.30 | 5.4 × 10−5 | 0.86 | 21.5 | 70.0 | 13.03 | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, S.; Lee, J. Progress and Future Potential of All-Small-Molecule Organic Solar Cells Based on the Benzodithiophene Donor Material. Molecules 2023, 28, 3171. https://doi.org/10.3390/molecules28073171
Alam S, Lee J. Progress and Future Potential of All-Small-Molecule Organic Solar Cells Based on the Benzodithiophene Donor Material. Molecules. 2023; 28(7):3171. https://doi.org/10.3390/molecules28073171
Chicago/Turabian StyleAlam, Shabaz, and Jaewon Lee. 2023. "Progress and Future Potential of All-Small-Molecule Organic Solar Cells Based on the Benzodithiophene Donor Material" Molecules 28, no. 7: 3171. https://doi.org/10.3390/molecules28073171
APA StyleAlam, S., & Lee, J. (2023). Progress and Future Potential of All-Small-Molecule Organic Solar Cells Based on the Benzodithiophene Donor Material. Molecules, 28(7), 3171. https://doi.org/10.3390/molecules28073171







