Nicotiana tabacum Leaf Waste: Morphological Characterization and Chemical-Functional Analysis of Extracts Obtained from Powder Leaves by Using Green Solvents
Abstract
1. Introduction
2. Results and Discussion
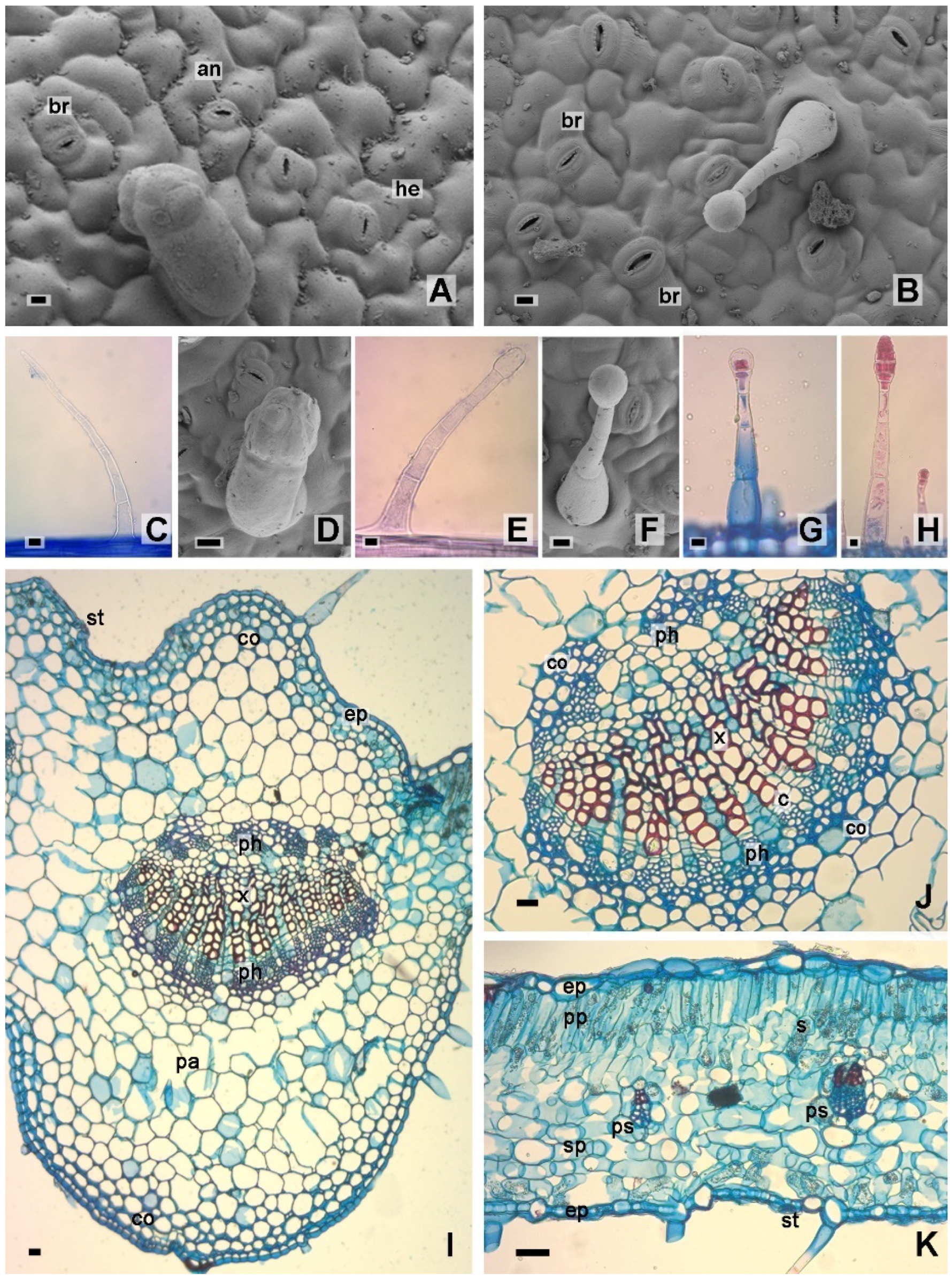
2.1. Leaf Morphology and Anatomy
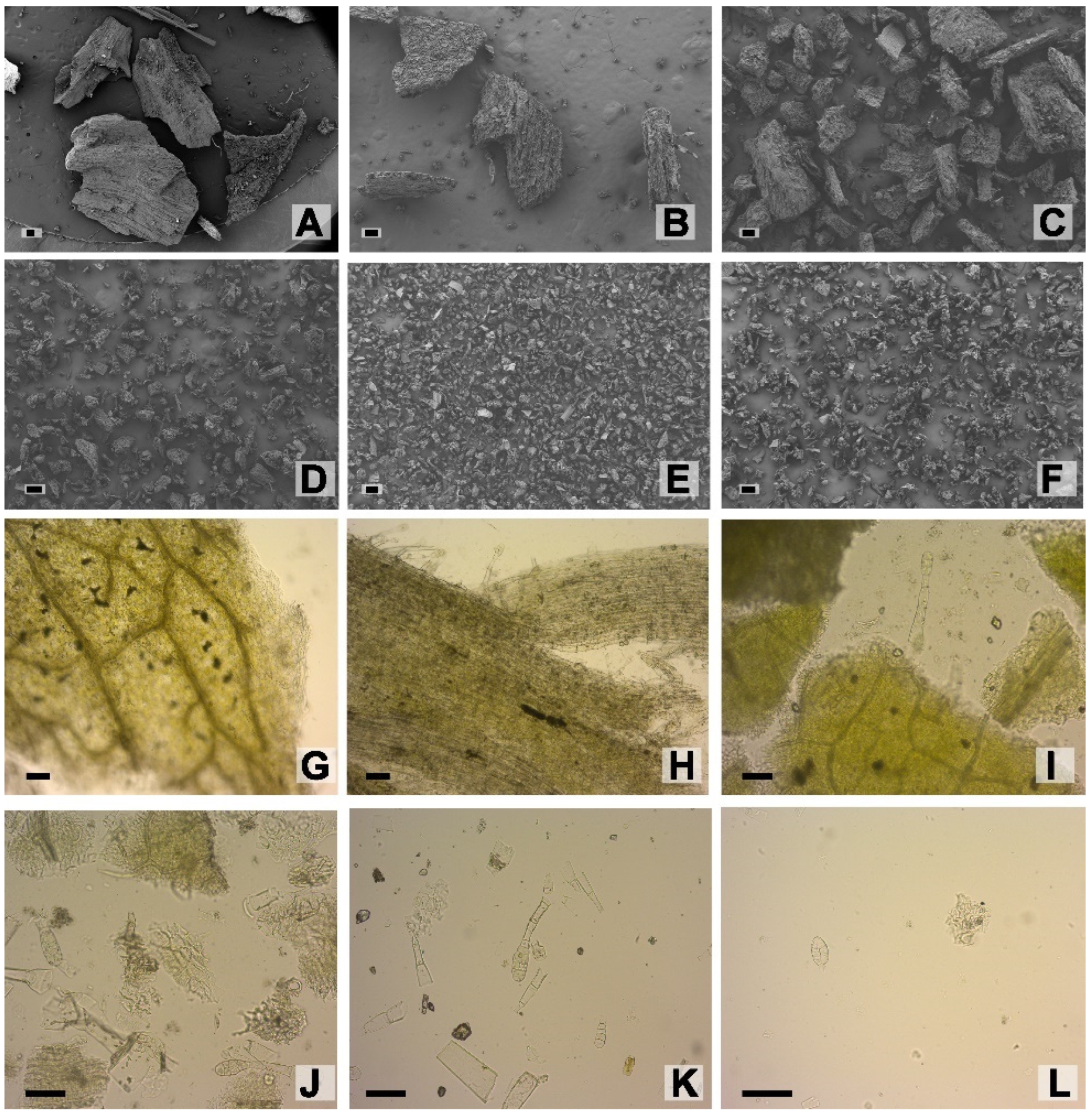
2.2. Tobacco Leaf Powder Characterization
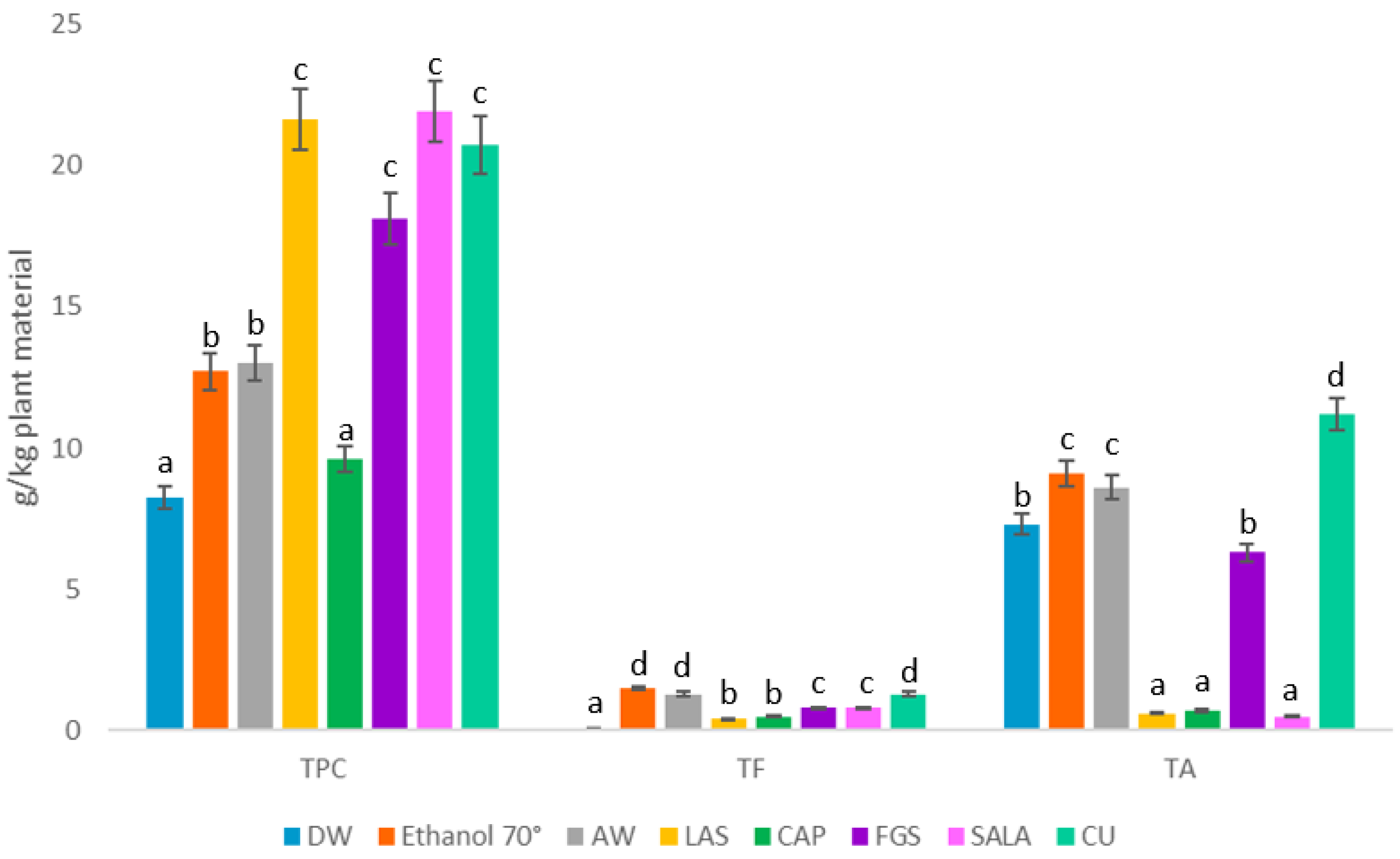
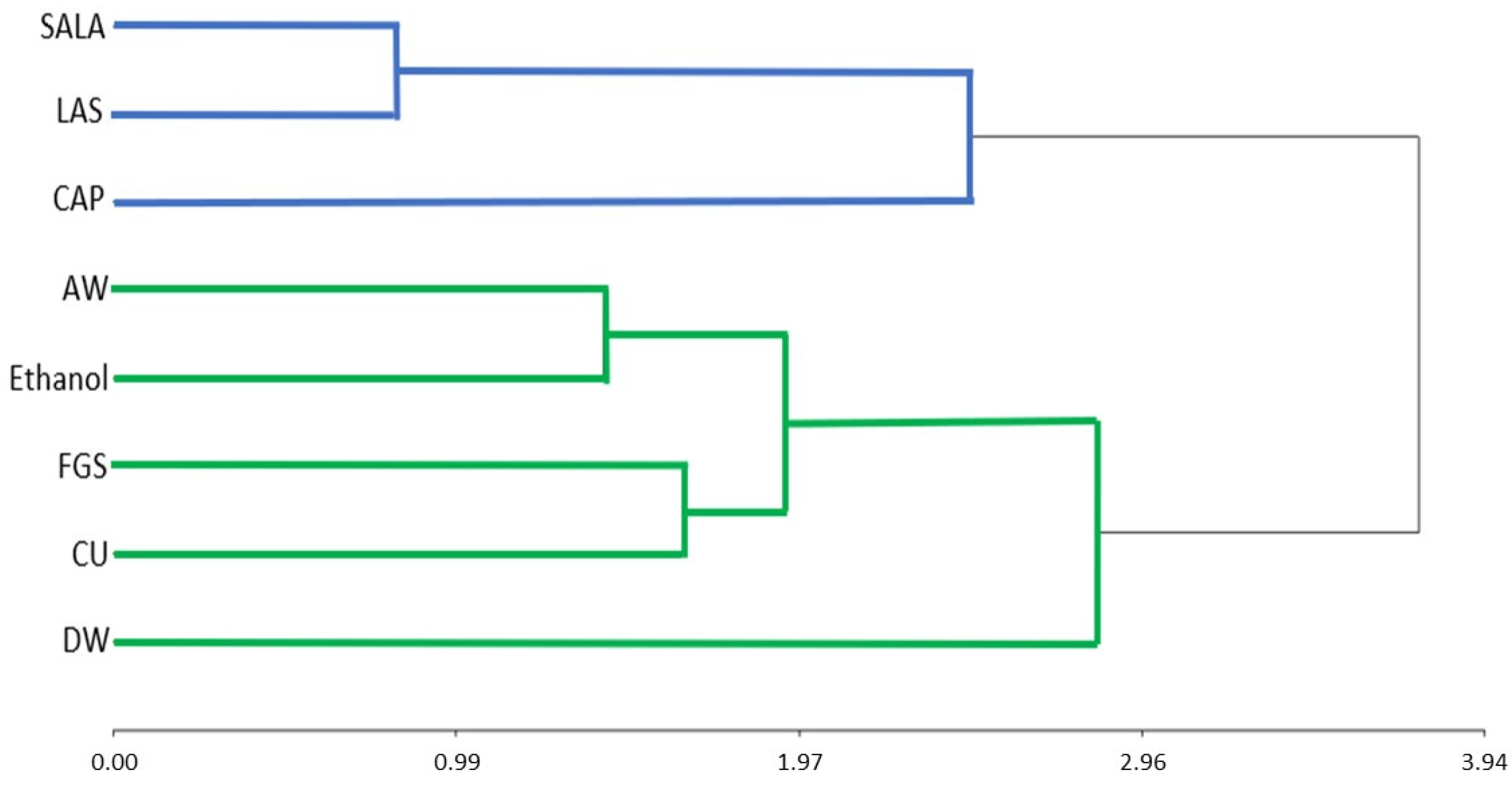
2.3. Phytochemical Characterization by Conventional and Non-Conventional Solvents
2.4. Antioxidant Activity
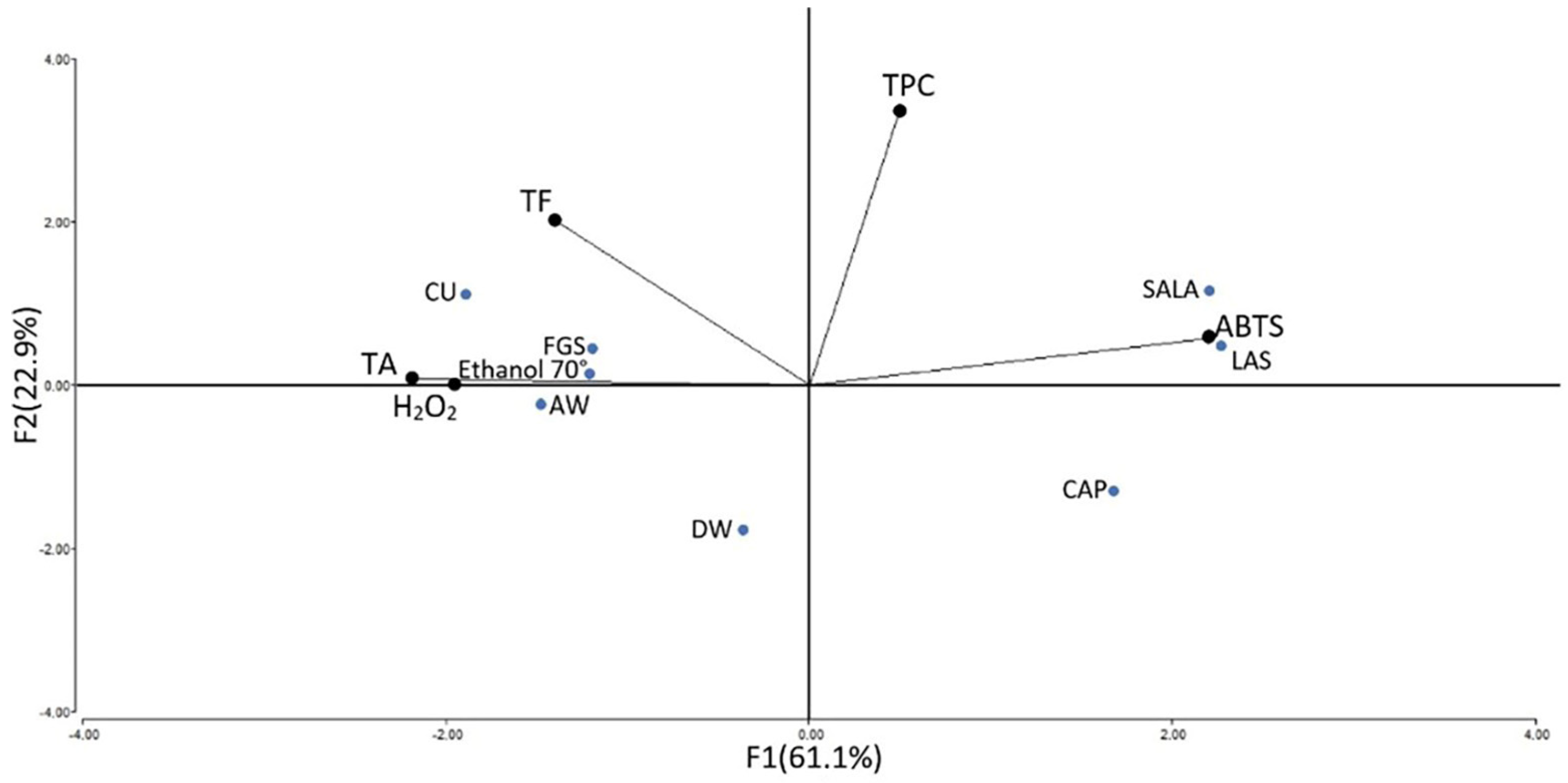
2.5. Correlation between the Total Phenolic Compounds, Total Flavonoid and Total Alkaloid Content, and Antioxidant Activities
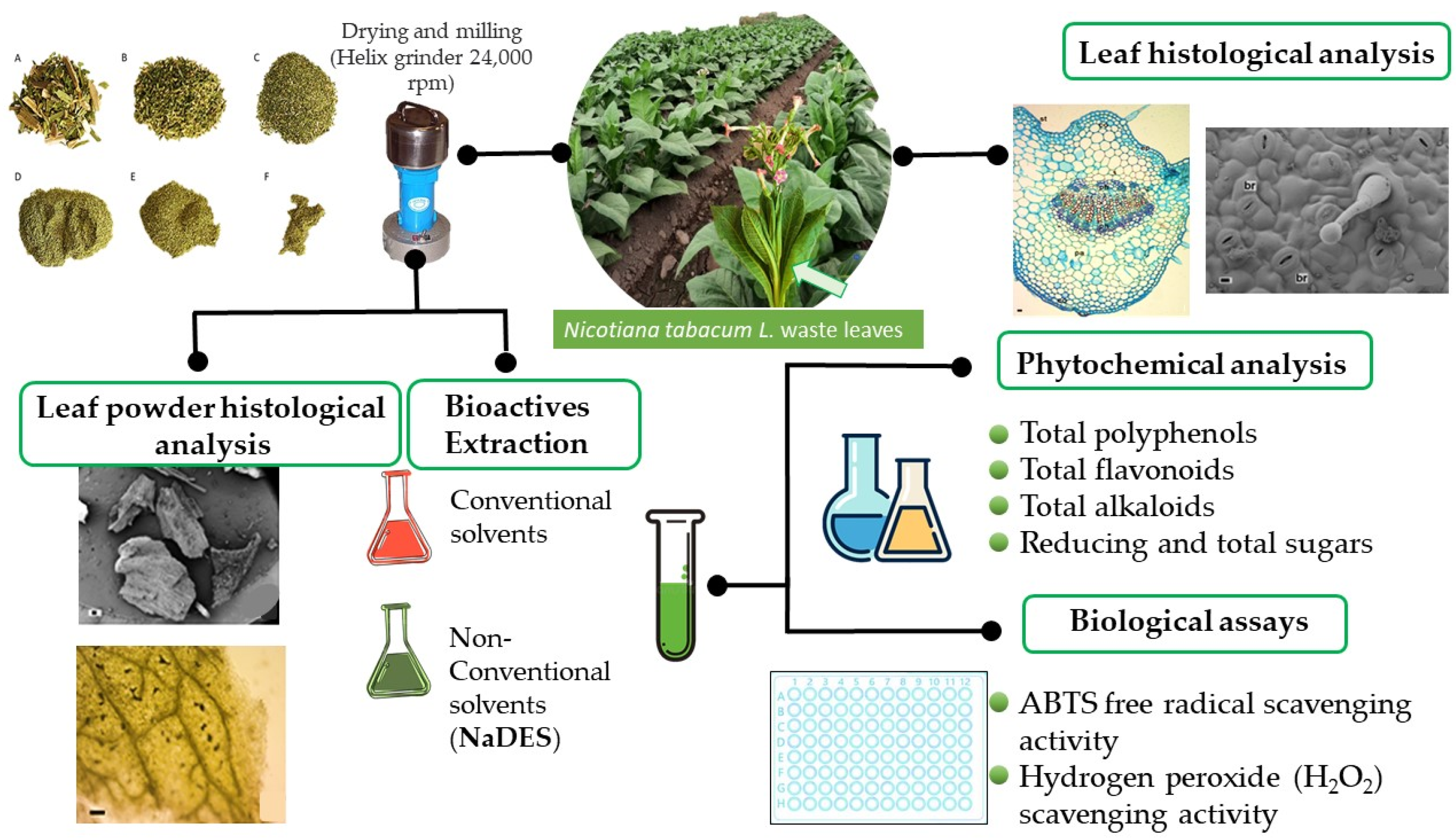
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. Powder Obtention by Milling
3.4. Histological Analysis
3.4.1. Light Microscopy
3.4.2. Scanning Electron Microscopy
3.5. NaDES Preparation
3.6. Powder Extraction
3.7. Determination of Chemical Composition
3.7.1. Total Polyphenol and Flavonoid Quantification
3.7.2. Total Alkaloids
3.7.3. Reducing and Total Sugars
3.8. Antioxidant Activity
3.8.1. ABTS Free Radical Scavenging Activity
3.8.2. Hydrogen Peroxide (H2O2) Scavenging
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hunziker, A.T. Genera Solanacearum. The Genera of Solanaceae Illustrated, Arranged According to a New System; John Wiley and Sons: Hoboken, NJ, USA, 2001; pp. 1–500. [Google Scholar]
- ITGA. International Tobacco Growers Association. Available online: https://atlas.tobaccoleaf.org/argentina/ (accessed on 18 August 2022).
- Cocucci, A.A.; Hunziker, A.T. Solanaceae, Parte 15. Subtribu VIIa. Nicotianinae, Parte B. Nicotiana; Anton, A.M., Zuloaga, F.O., Eds.; Flora Fanerogamica Argentina: Córdoba, Argentina, 2005; Volume 89, pp. 3–15. [Google Scholar]
- Zuloaga, F.O.; Anton, M.A. Flora Argentina. 2022. Available online: http://www.floraargentina.edu.ar/ (accessed on 18 August 2022).
- Zou, X.; Amrit, B.K.; Abu-Izneid, T.; Aziz, A.; Devnath, P.; Rauf, A.; Mitra, S.; Emran, T.; Mujawah, A.; Lorenzo, J.; et al. Current advances of functional phytochemicals in Nicotiana plant and related potential value of tobacco processing waste: A review. Biomed. Pharmacother. 2021, 143, 112191. [Google Scholar] [CrossRef] [PubMed]
- Mumba, P.; Phiri, R. Environmental impact assessment of tobacco waste disposal. Int. J. Environ. Res. 2008, 2, 225–230. [Google Scholar]
- Banožić, M.; Babić, J.; Jokić, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste—A review. Ind. Crops Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Chen, J.X.; Leng, H.Q.; Duan, Y.X.; Zhao, W.; Yang, G.Y.; Guo, Y.D.; Chen, Y.K.; Hu, Q.F. Three new flavonoids from the leaves of oriental tobacco and their cytotoxicity. Phytochem. Lett. 2013, 6, 144–147. [Google Scholar] [CrossRef]
- Chen, Y.K.; Li, X.S.; Yang, G.Y.; Chen, Z.Y.; Hu, Q.F.; Miao, M.M. Phenolic compounds from Nicotiana tabacum and their biological activities. J. Asian Nat. Prod. Res. 2012, 14, 450–456. [Google Scholar] [CrossRef]
- Yang, C.Y.; Geng, C.A.; Huang, X.Y.; Wang, H.; Xu, H.B.; Liang, W.J.; Ma, Y.B.; Zhang, X.M.; Zhou, J.; Chen, J.J. Noreudesmane sesquiterpenoids from the leaves of Nicotiana tabacum. Fitoterapia 2014, 96, 81–87. [Google Scholar] [CrossRef]
- Schwingel, L.; Schwingel, G.; Storch, N.; Barreto, F.; Bassania, V. 3-O-Methylquercetin from organic Nicotiana tabacum L. trichomes: Influence of the variety, cultivation and extraction parameters. Ind. Crops Prod. 2014, 55, 56–62. [Google Scholar] [CrossRef]
- Yang, P.; Tang, S.; Liu, C.; Ye, L.; Zhang, F.; He, P.; Liu, Z.; Chen, Y.; Miao, M.; Shen, Q.; et al. Three new sesquiterpenes from the stems of Nicotiana tabacum and their bioactivities. J. Asian Nat. Prod. Res. 2019, 21, 109–116. [Google Scholar] [CrossRef]
- Yuan, Y.; Qi, L.; Yang, J.; Wu, C.; Liu, Y.; Huang, L. A Scutellaria baicalensis R2R3-MYB gene, SbMYB8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell Tiss. Org. 2015, 120, 961–972. [Google Scholar] [CrossRef]
- Shang, S.; Shi, J.; Tang, J.; Jiang, J.; Zhao, W.; Zheng, X.; Lei, P.; Han, J.; Wang, C.; Yuan, D.; et al. New isolates from leaves of Nicotiana tabacum and their biological activities. Nat. Prod. Res. 2019, 33, 1577–1583. [Google Scholar] [CrossRef]
- Capdesuñer, Y.; García-Brizuela, J.; Mock, H.P.; Hernández, K.V.; de la Torre, M.H.; Santiesteban-Toca, C.E. Accessing to the Nicotiana tabacum leaf antimicrobial activity: In-silico and in-vitro investigations. Plant Physiol. Biochem. 2019, 139, 591–599. [Google Scholar] [CrossRef]
- Zou, X.; Bk, A.; Rauf, A.; Saeed, M.; Al-Awthan, Y.S.; Al-Duais, M.; Bahattab, O.; Khan, M.; Suleria, H.A. Screening of polyphenols in tobacco (Nicotiana tabacum) and determination of their antioxidant activity in different tobacco varieties. ACS Omega 2021, 6, 25361–25371. [Google Scholar] [CrossRef]
- Sifola, M.I.; Carrino, L.; Cozzolino, E.; del Piano, L.; Graziani, G.; Ritieni, A. Potential of pre-harvest wastes of tobacco (Nicotiana tabacum L.) crops, grown for smoke products, as source of bioactive compounds (phenols and flavonoids). Sustainability 2021, 13, 2087. [Google Scholar] [CrossRef]
- Akinpelu, D.A.; Obuotor, E.M. Antibacterial activity of Nicotiana tabacum leaves. Fitoterapia 2000, 71, 199–200. [Google Scholar] [CrossRef]
- Ferchmin, P.A.; Hao, J.; Perez, D.; Penzo, M.; Maldonado, H.M.; Gonzalez, M.T.; Rodriguez, A.D.; de Vellis, J. Tobacco cembranoids protect the function of acute hippocampal slices against NMDA by a mechanism mediated by α4βnicotinic receptors. J. Neurosci. Res. 2005, 82, 631–641. [Google Scholar] [CrossRef]
- Ferchmin, P.A.; Pagán, O.R.; Ulrich, H.; Szeto, A.C.; Hann, R.M.; Eterović, V.A. Actions of octocoral and tobacco cembranoids on nicotinic Nacoulma receptors. Toxicon 2009, 54, 1174–1182. [Google Scholar] [CrossRef]
- Chikezie, P.C.; Akuwudike, A.R.; Chikezie, C.M. Membrane stability and methaemoglobin content of human erythrocytes incubated in aqueous leaf extract of Nicotiana tabacum product. Free Radic. Antioxid. 2012, 2, 5–61. [Google Scholar] [CrossRef]
- Nacoulma, A.P.; Compaoré, M.; De Lorenzi, M.; Kiendrebeogo, M.; Nacoulma, O.G. In vitro Antioxidant and anti-inflammatory activities of extracts from Nicotiana tabacum L. (Solanaceae) leafy galls induced by Rhodococcus fascians. J. Phytopathol. 2012, 160, 617–621. [Google Scholar] [CrossRef]
- Weber, S.; Kaminski, K.; Perret, J.; Leroy, P.; Mazurov, A.; Peitsch, M.; Ivanov, N.; Hoeng, J. Antiparasitic properties of leaf extracts derived from selected Nicotiana species and Nicotiana tabacum varieties. Food Chem. Toxicol. 2019, 132, 110660. [Google Scholar] [CrossRef]
- World Health Organization. Strengthening Tobacco Control. 2022. Available online: https://www.who.int/publications/i/item/strengthening-tobacco-control (accessed on 12 January 2023).
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.-S.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Chia, S.R.; Foo, S.P.; Hew, Y.S.; Loh, Y.J.; Devadas, V.V.; Chew, K.W.; Show, P.L. Extraction of phenolic compounds from fresh and wilt kesum plant using liquid biphasic flotation. Sep. Purif. Technol. 2020, 242, 116831. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.; Li, X.; Luo, Y.; Liu, H. Extraction and HPLC characterization of chlorogenic acid from tobacco residuals. Sep. Sci. Technol. 2007, 42, 3481–3492. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Xu, X.; Lee, F.S.; Wang, X. Rapid determination of total solanesol in tobacco leaf by ultrasound–assisted extraction with RP–HPLC and ESI–TOF/MS. J. Pharm. Biomed. Anal. 2007, 43, 879–885. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Bronze, M.R.; da Ponte, M.N. Supercritical fluid extraction of tobacco leaves: A preliminary study on the extraction of solanesol. J. Supercrit. Fluids 2008, 45, 171–176. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W. Study on supercritical fluid extraction of solanesol from industrial tobacco waste. J. Supercrit. Fluids 2018, 138, 228–237. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, S.T.; Yang, H.J.; Hao, J.; Wang, X.J. Gene expression profile analysis of tobacco leaf trichomes. BMC Plant Biol. 2011, 11, 76. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, M.; Yang, B.; Jiang, Y.; Rao, G. Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 2008, 107, 1399–1406. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, T.; Al-Wahaibi, Y.M.; Hashim, M.A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta 2012, 541, 70–75. [Google Scholar] [CrossRef]
- Ivanović, M.; Razboršek, M.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Takla, S.S.; Shawky, E.; Hammoda, H.M.; Darwish, F.A. Green techniques in comparison to conventional ones in the extraction of Amaryllidaceae alkaloids: Best solvents selection and parameters optimization. J. Chromatogr. A 2018, 1567, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.; Pathak, A.; Yar, M. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Gan, J.; Feng, Y.; He, Z.; Li, X.; Zhang, H. Correlations between antioxidant activity and alkaloids and phenols of maca (Lepidium meyenii). J. Food Qual. 2017, 2017, 3185945. [Google Scholar] [CrossRef]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Granger, C.; Trullàs, C.; Silva, A.; Krutmann, J. Urea in dermatology: A review of its emollient, moisturizing, keratolytic, skin barrier enhancing and antimicrobial properties. Dermatol. Ther. 2021, 11, 1905–1915. [Google Scholar] [CrossRef]
- Smith, W.P. Comparative effectiveness of hydroxy acids on skin properties. Int. J. Cosmet. Sci. 1996, 18, 75–83. [Google Scholar] [CrossRef]
- Dizeo de Strittmater, C. Nueva técnica de diafanización. Bol. Soc. Argent. Bot. 1973, 15, 126–129. [Google Scholar]
- Mercado, M.I.; Ponessa, G.I. Nuevo soporte para obtención de cortes de material vegetal en micrótomo rotativo. Dominguezia 2021, 37, 29–35. [Google Scholar]
- D’Ambrogio de Argüeso, A. Manual de Técnicas en Histología Vegetal; Hemisferio Sur: Buenos Aires, Argentina, 1986; 83p. [Google Scholar]
- Zarlavsky, G.E. Histología Vegetal: Técnicas Simples y Complejas; Sociedad Argentina de Botánica: Buenos Aires, Argentina, 2014; 198p. [Google Scholar]
- Dilcher, D. Approaches to the identification of angiosperm leaves. Bot. Rev. 1974, 40, 1–157. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Önal, A.; Kepekçi, Ş.E.; Öztunç, A. Spectrophotometric methods for the determination of the antidepressant drug Paroxetine hydrochloride in tablets. J. AOAC Int. 2005, 88, 490–495. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Somogyi, M. A new reagent for the determination of sugars. J. Biol. Chem. 1945, 160, 61–68. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Optimized enzymatic colorimetric assay for determination of hydrogen peroxide (H2O2) scavenging activity of plant extracts. MethodsX 2015, 2, 283–291. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2015; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2015. [Google Scholar]

| Stomata | Length (µm) | Width (µm) | Density (mm2) |
| Adaxial epidermis | 39.00 ± 1.42 a | 28.75 ± 0.73 a | 23.25 ± 1.45 a |
| Abaxial epidermis | 42.00 ± 1.42 a | 32.25 ± 0.73 b | 49.25 ± 1.45 b |
| Mesh Size (µm) | Weight after Sieving (g) | Yield in Percentage (%) |
|---|---|---|
| 840 | 4.98 | 51.98 |
| 500 | 1.88 | 19.62 |
| 149 | 2.17 | 22.65 |
| 105 | 0.45 | 4.70 |
| 74 | 0.08 | 0.89 |
| <74 | 0.01 | 0.15 |
| NaDES Code | Components | Molar Ratio | Aspect | pH |
|---|---|---|---|---|
| LAS | sucrose: lactic acid | 0.3:1.2 | Viscous transparent | 4 |
| SALA | sucrose: lactic acid: distilled water | 1:5:7 | Viscous transparent | 1 |
| CAP | citric acid: propylene glycol | 1:4 | Transparent | 5 |
| FGS | glucose: fructose: sucrose: distilled water | 1:1:1:11 | Viscous transparent | 6 |
| CU | choline chloride: urea: distilled water | 1:2:1.5 | Transparent | 6 |
| N. tabacum Leaf Powder | TPC µg GAE/mL | TF µg QE/mL | TA µg ACE/mL | RS mg GE/mL | TS mg GE/mL |
|---|---|---|---|---|---|
| Conventional Solvents | |||||
| DW | 824.0 ± 19.6 a | 14.9 ± 1.8 a | 734.0 ± 2.9 b | 5.2 ± 0.4 e | 7.3 ± 0.5 d |
| Ethanol 70° | 1275.0 ± 14.5 b | 151.3 ± 9.1 d | 919.2 ± 3.6 c | 2.2 ± 0.1 c | 5.7 ± 0.2 c |
| AW | 1300.0 ± 3.0 b | 136.6 ± 6.3 d | 867.5 ± 0.8 c | 3.2 ± 0.2 d | 7.2 ± 0.4 d |
| Non-Conventional Solvents | |||||
| LAS | 2169.0 ± 0.5 c | 46.4 ± 3.1 b | 68.6 ± 0.4 a | 0.40 ± 0.01 b | 0.14 ± 0.01 a |
| CAP | 963.0 ± 9.0 a | 52.2 ± 4.3 b | 78.7 ± 6.0 a | 0.060 ± 0.001 a | 0.020 ± 0.001 a |
| FGS | 1813.0 ± 3.7 c | 89.2 ± 7.5 c | 637.2 ± 1.2 b | N/D | N/D |
| SALA | 2198.0 ± 6.4 c | 80.5 ± 2.5 c | 55.0 ± 3.4 a | 0.50 ± 0.01 b | 0.030 ± 0.001 a |
| CU | 2076.0 ± 3.7 c | 134.0 ± 3.2 d | 1123.0 ± 7.0 d | N/D | 4.7 ± 1.6 b |
| N. tabacum Leaf | ABTS (SC50 µg GAE/mL) | H2O2 (SC50 µg GAE/mL) |
|---|---|---|
| Conventional Extraction Solvents | ||
| DW | 11.5 ± 0.5 d | 38.0 ± 2.4 e |
| Ethanol 70° | 8.9 ± 0.5 cd | 21.0 ± 1.6 d |
| AW | 5.0 ± 0.1 bc | 44.0 ± 2.2 g |
| Non-Conventional Solvents | ||
| LAS | 36.0 ± 0.4 f | 8.4 ± 0.6 b |
| CAP | 26.0 ± 1.8 e | 3.8 ± 0.1 a |
| FGS | 2.9 ± 0.1 ab | 52.0 ± 4.3 h |
| SALA | 41.9 ± 0.2 g | 8.7 ± 0.1 c |
| CU | 1.60 ± 0.05 a | 40.0 ± 3.1 f |
| Quercetin | 1.40 ± 0.0 a | 17.3 ± 0.5 b |
| ABTS | H2O2 | |
|---|---|---|
| TPC | 0.32 * | −0.04 |
| TF | −0.45 * | 0.26 * |
| TA | −0.91 * | 0.77 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, M.; Moreno, M.A.; Albornoz, P.L.; Mercado, M.I.; Zampini, I.C.; Isla, M.I. Nicotiana tabacum Leaf Waste: Morphological Characterization and Chemical-Functional Analysis of Extracts Obtained from Powder Leaves by Using Green Solvents. Molecules 2023, 28, 1396. https://doi.org/10.3390/molecules28031396
Leal M, Moreno MA, Albornoz PL, Mercado MI, Zampini IC, Isla MI. Nicotiana tabacum Leaf Waste: Morphological Characterization and Chemical-Functional Analysis of Extracts Obtained from Powder Leaves by Using Green Solvents. Molecules. 2023; 28(3):1396. https://doi.org/10.3390/molecules28031396
Chicago/Turabian StyleLeal, Mariana, María Alejandra Moreno, Patricia Liliana Albornoz, María Inés Mercado, Iris Catiana Zampini, and María Inés Isla. 2023. "Nicotiana tabacum Leaf Waste: Morphological Characterization and Chemical-Functional Analysis of Extracts Obtained from Powder Leaves by Using Green Solvents" Molecules 28, no. 3: 1396. https://doi.org/10.3390/molecules28031396
APA StyleLeal, M., Moreno, M. A., Albornoz, P. L., Mercado, M. I., Zampini, I. C., & Isla, M. I. (2023). Nicotiana tabacum Leaf Waste: Morphological Characterization and Chemical-Functional Analysis of Extracts Obtained from Powder Leaves by Using Green Solvents. Molecules, 28(3), 1396. https://doi.org/10.3390/molecules28031396









