Abstract
Bisphenol A is one of the most widely used industrial compounds. Over the years, it has raised severe concern as a potential hazard to the human endocrine system and the environment. Developing robust and easy-to-use sensors for bisphenol A is important in various areas, such as controlling and monitoring water purification and sewage water systems, food safety monitoring, etc. Here, we report an electrochemical method to fabricate a bisphenol A (BPA) sensor based on a modified Au nanoparticles/multiwalled carbon nanotubes composite electrocatalyst electrode (AuCu-UPD/MWCNTs/GCE). Firstly, the Au-Cu alloy was prepared via a convenient and controllable Cu underpotential/bulk Au co-electrodeposition on a multiwalled modified carbon nanotubes glassy carbon electrode (GCE). Then, the AuCu-UPD/MWCNTs/GCE was obtained via the electrochemical anodic stripping of Cu underpotential deposition (UPD). Our novel prepared sensor enables the high-electrocatalytic and high-performance sensing of BPA. Under optimal conditions, the modified electrode showed a two-segment linear response from 0.01 to 1 µM and 1 to 20 µM with a limit of detection (LOD) of 2.43 nM based on differential pulse voltammetry (DPV). Determination of BPA in real water samples using AuCu-UPD/MWCNTs/GCE yielded satisfactory results. The proposed electrochemical sensor is promising for the development of a simple, low-cost water quality monitoring system for the detection of BPA in ambient water samples.
1. Introduction
Bisphenol A (BPA) has been widely used in the production of synthetic polycarbonate and epoxy resin consumer goods due to its good optical transparency, ductility and high heat resistance [1], for example, in a wide range of electronic equipment, building materials, medical equipment, paper products, clothing, plastic bottles, food containers, etc. [2,3,4,5,6]. BPA can be easily released from consumer goods into environmental media and cause human exposure through dietary intake or in other ways [7]. The widespread use of BPA can cause health risks through endocrine-disrupting effects, causing abnormal reproductive development, genotoxicity, and immunotoxicity [8,9,10]. Therefore, the development of highly sensitive, simple, and rapid methods for BPA detection is essential for public health, food, and environmental safety. A variety of analytical techniques for BPA detection have been commonly used, such as high-performance chromatography coupled with mass spectrometry [11], chemiluminescence [12], enzyme-linked immunosorbent assay [13], electroanalysis, etc. [14]. The electroanalysis methods are favorable for rapid BPA detection due to their advantages of low cost, high sensitivity, simplicity, and portability.
Au and its nanocomposites are the most-used electrocatalysts for the electrooxidation and electroanalysis of BPA due to their excellent performance and attractive material characteristics, [4,15,16,17,18,19]. Nevertheless, the large-scale commercial application of Au has been severely limited mainly because of its high price. Therefore, it is significant to reduce the usage and improve the electrocatalytic/electroanalysis performance of gold on the electrode surface. Carbon nanotubes and their composites have been exploited broadly for the electrochemical sensing of BPA because of their low cost, feasibility of modification, and favorable electrochemical properties [14,20,21,22,23]. Generally, the current signal, response speed, and sensitivity will be increased during electroanalysis if the electrochemical reaction rate between the analyte and electrode interface can be substantially improved, which is heavily dependent on the development and innovation of electrocatalysts. We believe that it is valuable to invent a simple and efficient method to prepare Au/MWCNT nanocomposites, which will improve the electrocatalytic performance in BPA detection and reduce the usage of Au.
Owing to special interatomic forces, some metallic atomic monolayers (such as Cu, Pb) can form on the surface of specific noble metals (such as Au, Pt) through underpotential deposition (UPD) instead of bulk electrodeposition (BD), which has been widely studied and applied [24,25]. The structure of metals is regulated at the atomic level using the UPD method, which can change the properties of metallic materials. For example, it is a simple and important strategy to construct sub/monolayer noble metal nanofilms through a galvanic replacement reaction (GRR) between the UPD of a Cu or an Pb metal atom monolayer (reductant) and a noble metal salt (oxidant) [24,26,27]. Recently, a PtAu composite electrocatalyst prepared via Bi-underpotential/PtAu-bulk co-electrodeposition and the subsequent chemical dissolution of Bi for the electrooxidation and electroanalysis of formaldehyde were reported [28]. The BD of noble metal nanoparticles can be dynamically regulated and interfere with the UPD of a less-noble metal at the atomic level via the simultaneous UPD of a less-noble metal and the BD of noble metals. Chemical dissolution of UPD metals in the BD of noble metals is uncontrollable and inconvenient compared with electrochemical anodic stripping, and there are only a few reports on the improvement in noble metals’ electrocatalyst performance via the regulation and interference of UPD metals for advanced electroanalysis studies. Thus, it is significant and attractive to further develop such noble metals and composite electrocatalysts with a special nanostructure prepared via metal underpotential/bulk electrodeposition and a subsequent anodic stripping strategy in the electrochemical analysis field.
Herein, we suggest a new and effective electrochemical method for preparing Au nanocomposite electrocatalysts via Cu underpotential/bulk Au co-electrodeposition on a glassy carbon electrode modified with multiwalled carbon nanotubes, followed by the anodic stripping of Cu UPD. We find that the modified electrode (AuCu-UPD/MWCNTs/GCE) shows excellent electrooxidation activity with BPA, and the high-performance electrochemical sensing of BPA is determined via differential pulse voltammetry (DPV). The modified electrode is satisfactorily applied for the electrochemical sensing of BPA in real water samples.
2. Results and Discussion
2.1. Fabrication and Characterization of Modified Electrodes
The three steps for AuCu-UPD/MWCNTs/GCE preparation are briefly given below for clarity (with a detailed description in Section 3). The steps are: (1) preparation of MWCNTs/GCE by drop-casting of MWCNTs on the cleaned GCE; (2) Cu UPD and Au BD simultaneously conducted at the potential of Cu UPD on MWCNTs/GCE; (3) preparation of AuCu-UPD/MWCNTs/GCE via anodic stripping for the removal of Cu UPD. For comparison, the conventional Au-modified MWCNTs/GCE (AuCon/MWCNTs/GCE) was prepared via conventional electrodeposition at the constant potential, and another Au-modified MWCNTs/GCE (AuCu-Con/MWCNTs/GCE) was prepared via conventional Au-Cu bulk co-electrodeposition at the constant potential, and then the BD Cu was removed via anodic stripping.
First, the electrochemical behavior of Cu2+ on the bare Au electrode was investigated using CV and linear sweep anodic stripping voltammetry (LSASV) in 0.2 M aqueous HClO4 (as shown in Figure S1, with details discussed in the Supporting Data). In order to approximate these experimental conditions, the CV experiment was also conducted on the AuCon/MWCNTs/GCE (as shown in Figure S2). The electrochemical behavior of Cu2+ on the AuCon/MWCNTs/GCE is similar to that of Cu2+ on the bare Au electrode according to the CV results. We concluded that the UPD of Cu at 0.1 V can completely avoid the simultaneous BD of Cu on Au and the anodic stripping of Cu UPD can completely finish at 0.6 V. In addition, the UPD of Cu cannot occur on carbon species because the Cu–C interaction is too weak [24,25]. Therefore, only the BD of Au atoms on MWCNTs/GCE occurs, and then the UPD of Cu atoms should happen instantly on the BD of Au here. After a full monolayer of Cu UPD is deposited on the BD Au, the Cu UPD has to stop and only the BD of Au can happen next. Such an alternating process of the BD of Au and then the UPD of Cu continues at the Cu UPD potential for a definite period of time.
Figure 1 shows the cyclic voltammograms of the relevant electrodes in 0.2 M aqueous HClO4. No obvious redox peaks were found for the bare GCE. After the modification of MWCNTs on the GCE, we observed a large background current due to the charging on the MWCNTs with high specific surface area. Anodic peaks at potentials higher than ca. 1.0 V were observed for the AuCon/MWCNTs/GCE, AuCu-Con/MWCNTs/GCE, and AuCu-UPD/MWCNTs/GCE due to the formation of gold oxides (AuOx). Cathodic peaks were observed at ca. 0.9 V, due to the reduction of AuOx. Based on a conversion factor of 390 μC cm−2, the electrochemical real area (SE) and the roughness factor (Rf) of Au on the modified electrodes were calculated as reported [29,30]. Briefly, SE is the ratio of the cathodic charges of the AuOx reduction peak to the conversion factor (390 μC cm−2), and Rf is the ratio of SE to the geometric surface area of the GCE. The cathodic charges under the AuOx reduction peaks follow the order AuCu-UPD/MWCNTs/GCE (366.5 μC) > AuCu-Con/MWCNTs/GCE (74.66 μC) > AuCon/MWCNTs/GCE (30.69 μC). Hence, the corresponding SE and Rf values were calculated as follows: SE = 0.939 cm−2 and Rf = 13.4 for the AuCu-UPD/MWCNTs/GCE, SE = 0.191 cm−2 and Rf = 2.73 for the AuCu-Con/MWCNTs/GCE, and SE = 0.0787 cm−2 and Rf = 1.11 for the AuCon/MWCNTs/GCE. These findings indicate that the surface area of Au nanoparticles prepared using our protocol is increased (vs. Au Cu-Con/MWCNTs/GCE and AuCon/MWCNTs/GCE).
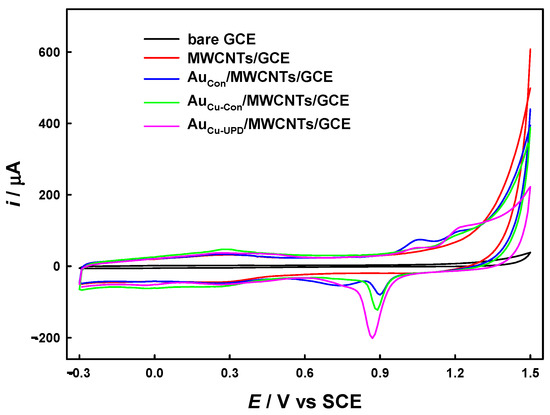
Figure 1.
Cyclic voltammograms for bare GCE, MWCNTs/GCE, AuCon/MWCNTs/GCE, AuCu-Con/MWCNTs/GCE, and AuCu-UPD/MWCNTs/GCE in 0.2 M HClO4. Scan rate: 50 mV s−1.
The electrodes were also characterized using SEM and EDX, as shown in Figure 2. The bare GCE surface was rather smooth, and the corresponding EDX shows the presence of abundant C (Figure 2A,F). After MWCNTs’ modification, uniformly dispersed and slender carbon nanotubes were observed on the MWCNTs/GCE (Figure 2B). On the AuCon/MWCNTs/GCE, many nanoparticles (ca. 80~120 nm) were seen (Figure 2C), and the EDX result confirms the presence of Au (Figure 2G). On the AuCu-Con/MWCNTs/GCE, some smaller nanoparticles (ca. 10~20 nm) and medium-sized nanoparticles (ca. 50~80 nm) were seen (Figure 2E), and the EDX result again confirms the presence of Au (Figure 2H). On the AuCu-UPD/MWCNTs/GCE, many tiny and uniform nanoparticles (ca. 10~20 nm) were seen (Figure 2E), and the EDX result shows more Au load (Figure 2I versus Figure 2G,H) and no signal peak of Cu, which indicate the removal of Cu UPD. Regarding morphology, the SEM results indicate that the Au nanoparticles on the AuCu-UPD/MWCNTs/GCE prepared using our method were smaller and more uniform than those on the AuCon/MWCNTs/GCE and the AuCu-Con/MWCNTs/GCE, which could be due to the dynamic interference due to the UPD of Cu resulting in a decrease in Au nanoparticle aggregation during the BD of Au. The smaller Au nanoparticles could increase the specific surface area and roughness, which is consistent with the results of the electrochemical characterization using CV above.
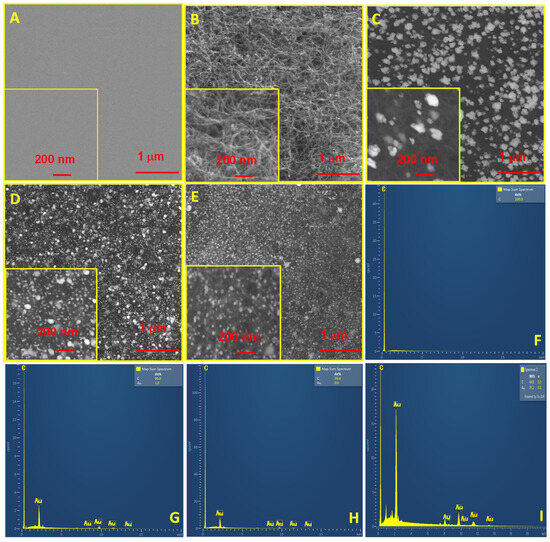
Figure 2.
SEM images and EDX results of bare GCE (A,F), MWCNTs/GCE (B), AuCon/MWCNTs/GCE (C,G), AuCu-Con/MWCNTs/GCE (D,H), and AuCu-UPD/MWCNTs/GCE (E,I). The insets in panels are corresponding enlarged images.
2.2. Electrocatalysis and Electrochemical Sensing of BPA
Figure 3 shows the current responses of the relevant electrodes in 0.1 M PBS (pH 7.5) + 10 μM BPA as measured using CV. The oxidation peaks of BPA appeared at ca. 0.48 V~0.50 V during positive scanning, and no obvious peak current appeared during negative scanning. The AuCu-UPD/MWCNTs/GCE shows the highest anodic peak current of 23.9 µA (background current was deducted), which is ca. 6.10, 2.46, 2.22 and 1.64 times that of bare GCE, MWCNTs/GCE AuCu-Con/MWCNTs/GCE, and AuCon/MWCNTs/GCE, respectively. The substantial increase in the oxidation peak current on the AuCu-UPD/MWCNTs/GCE may be attributed to the high electrochemically active surface area, the excellent electrocatalytic oxidation capability of Au nanoparticles prepared with the intervention of Cu UPD, and the synergistic catalytic effect on BPA between the smaller gold nanoparticles and MWCNTs. Meanwhile, the capacitive current of the modified electrodes also increased due to the double layer capacitance of the electrode being positively correlated with their surface area.
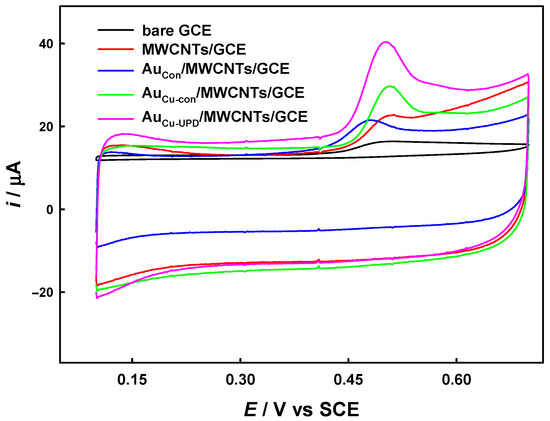
Figure 3.
Cyclic voltammograms for bare GCE, MWCNTs/GCE, AuCon/MWCNTs/GCE, AuCu-con/MWCNTs/GCE, and AuCu-UPD/MWCNTs/GCE in 0.1 M PBS (pH 7.5) containing 10 μM BPA. Scan rate: 50 mV s−1.
To better evaluate the electrochemical behavior of BPA, the relationship between the peak current of BPA on the AuCu-UPD/MWCNTs/GCE and the scanning rate was measured using CV in a 0.1 M PBS solution containing 10 µM BPA. As shown in Figure 4A, the oxidation peak current of BPA was gradually increased in the range of 10 to 300 mV s−1, and the oxidation potential had a positive shift with increasing scan rate. Figure 4B shows that the peak oxidation current of BPA has a good linear relationship with the scan rate, and the linear regression equation is I (μA) = 4.65v + 0.437 (R2 = 0.9901), indicating that the oxidation of BPA on the surface of AuCu-UPD/MWCNTs/GCE was controlled by an adsorption process. Moreover, a plot of the logarithm of peak current versus the logarithm of scan rate is linear, with a slope of 1.12 (as shown in Figure 4C), close to the theoretical value of 1.0 for an adsorption-controlled process. The possible oxidation process of BPA is illustrated in Scheme 1 [20,31].
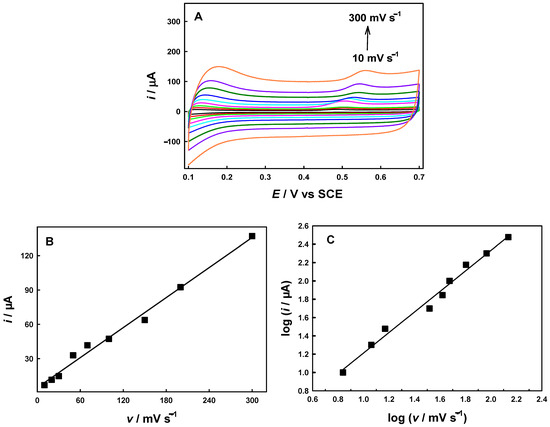
Figure 4.
Cyclic voltammograms for AuCu-UPD/MWCNTs/GCE in 0.1 M PBS (pH 7.5) containing 10 μM BPA at different scan rates (10–300 mV s−1) (A), a plot of peak current versus scan rate (B), and a plot of log of peak current versus log of scan rate (C).
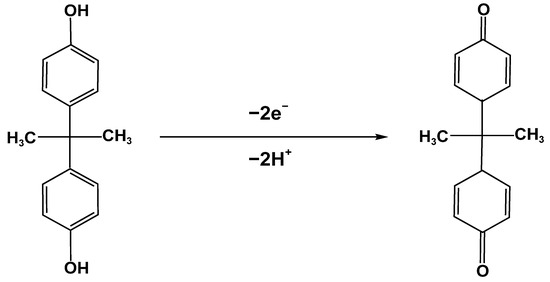
Scheme 1.
The possible electrooxidation of BPA at AuCu-UPD/MWCNTs/GCE.
The detection conditions were optimized to obtain the best sensing performance. First, the effect of co-electrodeposition time for preparing the AuCu-UPD/MWCNTs/GCE on the voltammetry signal was examined. As shown in Figure S3, the oxidation peak current of BPA reached a maximum at 800 s according to the CV results, because a too-short co-electrodeposition time cannot enable enough electrodeposition of Au, and too much time would probably result in superabundant electrodeposited Au with the intervention of Cu UPD, which is unfavorable for the formation of smaller Au nanoparticles with a large specific surface area. The pH of the electrolyte has an important influence on the electrochemical behavior of BPA, and, thus, the effect of pH on the oxidation of BPA with the AuCu-UPD/MWCNTs/GCE was studied using CV (as shown in Figure S4). The peak current reached its maximum value at pH = 7.5, so PBS with a pH of 7.5 was selected as the supporting electrolyte here. Finally, the optimization of the preconcentration time using DPV is shown in Figure S5. Increasing the preconcentration time improved the oxidation peak current of BPA. The oxidation peak current reached its maximum value with a preconcentration time of 300 s, which indicates the enrichment of BPA and a saturated signal, letting us select 300 s as the optimum preconcentration time.
Under the optimized conditions described above, the DPV responses and calibration curves of BPA on the AuCu-UPD/MWCNTs/GCE at different concentrations are shown in Figure 5. The oxidation peak current of BPA increases linearly within two concentration ranges of BPA: from 0.01 to 1.0 μM with a sensitivity of 21.6 μA μM−1 and from 1.0 to 20 μM with a sensitivity of 1.68 μA μM−1, and the linear regression equations are I (μA) = 21.6 c (μM) + 9.36 (R2 = 0.9903) and I (μA) = 1.68 c (μM) + 29.2 (R2 = 0.9901), respectively (the background currents were deducted here). The limit of detection (LOD) was 2.43 nM (0.555 μg L−1). The sensitivity, linear range, and LOD for the electroanalysis of BPA on the AuCu-UPD/MWCNTs/GCE here are better than those of most reported modified electrodes (listed in Table 1), demonstrating the high performance of our developed AuCu-UPD/MWCNTs/GCE BPA sensor.
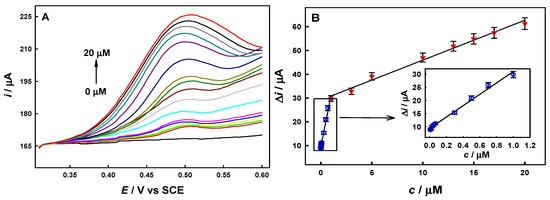
Figure 5.
DPV analysis of BPA at different concentrations on AuCu-UPD/MWCNTs/GCE (A) and the corresponding calibration curves (B) under the optimal conditions.

Table 1.
Comparison of different modified electrodes for BPA electrochemical sensing.
2.3. Anti-Interference Capacity and Stability of the AuCu-UPD/MWCNTs/GCE
In addition, the anti-interference capacity against some interferents was investigated in the presence of 50 nM BPA. Figure 6A,B show that there was very little influence on the DPV response of BPA from the coexistence of 100-fold concentrations of inorganic ions (Zn2+, K+, Ca2+, Pb2+, Mg2+, Cl−, NO3−, and SO42−), 2, 4-nitrophenol, glucose, and ascorbic acid. In general, the change values of the peak current of BPA are below 5%, showing the good anti-interference capacity of the AuCu-UPD/MWCNTs/GCE sensor.
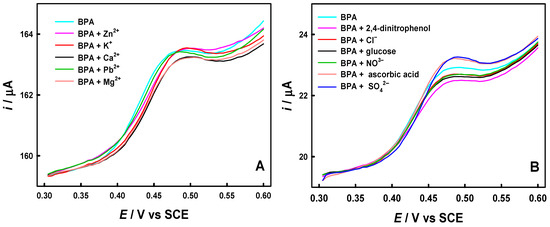
Figure 6.
DPV curves of the AuCu-UPD/MWCNTs/GCE in 0.1 M PBS (pH 7.5) containing 50 nM BPA in the presence of 5 μM Zn2+, K+, Ca2+, Pb2+ or Mg2+ (A) and 5 μM 2, 4-nitrophenol, Cl−, glucose, NO3−, ascorbic acid and SO42− (B): 300 s preconcentration and at preconcentration potential of 0.3 V.
To evaluate the long-term stability of the AuCu-UPD/MWCNTs/GCE, the detection of 5 μM BPA using an identical modified electrode was repeatedly performed every 1–2 days for 30 days using DPV. As shown in Figure S6A, the peak currents of BPA revealed no obvious changes, and the relative standard deviation (RSD) of the peak currents for BPA was 4.2%. The results demonstrate that the AuCu-UPD/MWCNTs/GCE has good long-term stability for the electrochemical sensing of BPA. Moreover, we investigated the reproducibility of five AuCu-UPD/MWCNTs/GCEs prepared under the same conditions (Figure S6B). An RSD of 4.9% was obtained, suggesting the good reproducibility of our modified electrode.
2.4. Practical Water Sample Analysis
Three practical samples (tap water, bottled water, and local Xiangjiang river water) were used for the detection of BPA using the AuCu-UPD/MWCNTs/GCE. The standard addition method was employed here, and the results are listed in Table 2. From the results, no DPV signal response of BPA was found for any of the samples due to the fact that no BPA or only traces of BPA below the LOD were present in the samples. The value of recovery was in the range of 91.5–104.1%. The results are acceptable and demonstrate that our modified electrode has favorable application potential for the practical electrochemical sensing of BPA.

Table 2.
Analysis results of BPA in real water samples via DPV.
3. Materials and Methods
3.1. Instrumentation and Reagents
A CHI660D electrochemical workstation (CH Instrument Co., Shanghai, China) and a conventional three-electrode electrolytic cell were used in all electrochemical experiments. The working electrode was a 3 mm diameter disk glassy carbon electrode (GCE) or its modified electrodes (including MWCNTs/GCE, AuCon/MWCNTs/GCE, AuCu-con/MWCNTs/GCE, and AuCu-UPD/MWCNTs/GCE), the reference electrode was a KCl-saturated calomel electrode (SCE), and the counter electrode was a graphite rod. All potentials are reported with respect to SCE. Scanning electron microscopy (SEM) characterizations with energy-dispersive X-ray spectroscopy (EDX) were collected on a TESCAN-MIRA3 LMH field-emission scanning electron microscope.
HAuCl4·4H2O was purchased from Sinopharm Chemicals Co., Ltd. (Shanghai, China). Bisphenol A, HClO4, CuSO4·5H2O, H2SO4, and K4Fe(CN)6·3H2O were purchased from Chemicals Company of Tianjin (Tianjin, China). Multiwalled carbon nanotubes with carboxylic treatment (MWCNTs) were purchased from Chengdu Organic Chemicals Co., Ltd. (Chengdu, China) Phosphate-buffered saline (PBS) consisting of 0.1 M KH2PO4-K2HPO4 + 0.1 M K2SO4 was used. All chemicals were of analytical grade or better quality, and all the solutions were prepared using Milli-Q ultrapure water (Millipore, Billerica, MA, USA, >18 MΩ cm). All experiments were performed at room temperature (20–25 °C).
3.2. Preparation and Characterization of Modified Electrodes
Cleaning of the GCE was conducted as before [30]. Firstly, the GCE was mechanically polished with alumina powder to a mirror finish. After water rinsing, the polished GCE was ultrasonically treated sequentially in water, ethanol, and water for 5 min each to remove residual alumina powder. The GCE was further treated using cyclic voltammetry (CV, −1.0~1.0 V, 50 mV s−1) in 0.2 M aqueous HClO4 until reproducible cyclic voltammograms were obtained. After water rinsing, the GCE was characterized using CV (0.1~0.6 V, 50 mV s−1) in 0.1 M aqueous Na2SO4 containing 2.0 mM K4Fe(CN)6. The peak-to-peak separation of the Fe(CN)63−/4− redox peaks was below 75 mV, indicating that the GCE had been well cleaned. The resulting MWCNTs were ultrasonically dispersed into N, N-dimethylformamide, and the concentration of MWCNTs was 2 mg mL−1.
The AuCu-UPD/MWCNTs/GCE was fabricated as illustrated in Scheme 2 and is described below: (1) an aliquot of 5 μL of MWCNT suspension was drop-casted on the cleaned GCE and dried in air to obtain the MWCNTs/GCE; (2) Cu underpotential/Au bulk co-electrodeposition on the MWCNTs/GCE was performed at a Cu underpotential deposition potential (0.1 V vs. SCE) for 800 s in aqueous 10.0 mM CuSO4 + 1.0 mM HAuCl4 + 0.2 M HClO4 under the N2 saturation state; (3) Cu UPD was immediately removed as much as possible via anodic stripping at 0.6 V for 600 s in a blank solution containing 0.2 M HClO4; after a quick rinse of the as-prepared modified electrode with ultrapure water, an Au-nanoparticle-modified MWCNTs/GCE (AuCu-UPD/MWCNTs/GCE) was obtained.
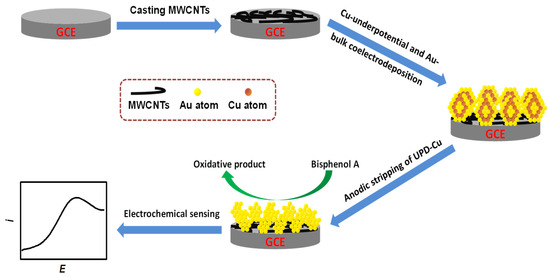
Scheme 2.
Schematic illustration of the sensing and preparation of the modified electrode.
For comparison, the conventional Au-modified MWCNTs/GCE (AuCon/MWCNTs/GCE) was prepared via conventional electrodeposition at a constant potential of 0.1 V for 800 s in aqueous 1.0 mM HAuCl4 + 0.2 M HClO4, and another Au-modified MWCNTs/GCE (AuCu-Con/MWCNTs/GCE) was prepared via conventional Au-Cu bulk co-electrodeposition at −0.1 V for 800 s in 10.0 mM CuSO4 + 1.0 mM HAuCl4 + 0.2 M HClO4, and then the BD Cu was removed via anodic stripping at 0.6 V. The above modified electrodes were also used for electrochemical measurements.
3.3. Electrochemical Measurement of Bisphenol A
Electrochemical investigation of bisphenol A on the electrodes was carried out using CV. The preconcentration of BPA was performed under solution-stirring conditions; DPV was used for the electroanalysis of BPA immediately when solution-stirring stopped. The response current was recorded as the change in value between the oxidation peak current of bisphenol A and the initial background current.
4. Conclusions
In summary, we prepared a new AuCu-UPD/MWCNTs/GCE electrode for the high-performance electrooxidation and electrochemical sensing of bisphenol A. The AuCu-UPD/MWCNTs/GCE was prepared via a simple and controllable Cu underpotential/bulk Au co-electrodeposition and the subsequent electrochemical anodic stripping of Cu UPD. Our new electrochemical sensor shows better analysis performance compared with similar sensors reported previously. Based on the fundamental principles of the electrochemistry of UPD and the anodic stripping of UPD metal, the aggregation through the BD of noble metal nanoparticles can be reduced via the dynamic interference of a less-noble metal at the atomic layer level, and the UPD metal can be effectively removed; thus, the dispersibility and utilization of noble metal electrocatalyst has been considerably improved. The preparation strategy is highly efficient and facile to operate, which can be further extended for the preparation of other noble-metals-based composite nanomaterial and electroanalysis applications in the environmental monitoring of BPA and the detection of other harmful substances.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28248036/s1.
Author Contributions
Conceptualization and methodology, Z.H. and L.C.; formal analysis, investigation, resources, and data curation, Z.C., D.Y. and S.J.; writing—original draft preparation, L.C.; writing, review, and editing, Z.H., T.W. and L.C.; visualization, L.C.; supervision, X.T. and L.N.; project administration, X.J.; funding acquisition, Z.H. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Hunan Province (2020JJ5124), the Natural Science Foundation of Hunan Province (2022JJ50099), the Key Research and Development Program of Hunan Province (2022SK2009), and the Innovation and Entrepreneurship Training Program for College Students of Hunan University of Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.-L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Widespread occurrence of bisphenol a in paper and paper products: Implications for human exposure. Environ. Sci. Technol. 2011, 45, 9372–9379. [Google Scholar] [CrossRef]
- Braver-Sewradj, S.P.d.; Spronsen, R.v.; Hessel, E.V.S. Substitution of bisphenol A: A review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit. Rev. Toxicol. 2020, 50, 128–147. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, L.G.; Freitas, A.A.; Santana, E.R.; Winiarski, J.P.; Dreyer, J.P.; Vieira, I.C. Graphene and gold nanoparticle-based bionanocomposite for the voltammetric determination of bisphenol A in (micro)plastics. Chemosphere 2023, 334, 139016. [Google Scholar] [CrossRef] [PubMed]
- Piña, S.; Sepúlveda, P.; García-García, A.; Moreno-Bárcenas, A.; Toledo-Neira, C.; Salazar-González, R. Fast simultaneous electrochemical detection of Bisphenol-A and Bisphenol-S in urban wastewater using a graphene oxide-iron nanoparticles hybrid sensor. Electrochim. Acta 2023, 248, 143164. [Google Scholar] [CrossRef]
- Priya, T.S.; Chen, T.-W.; Chen, S.-M.; Kokulnathan, T.; Lou, B.-S.; Al-onazi, W.A.; Al-Mohaimeed, A.M.; Elshikh, M.S.; Yu, J. Synthesis of perovskite-type potassium niobate using deep eutectic solvents: A promising electrode material for detection of bisphenol A. Chemosphere 2023, 318, 137948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, Y.; Zhu, H.; Huang, X.; Bai, X.; Kannan, K.; Zhang, T. Concentrations of bisphenol A and its alternatives in paired maternal–fetal urine, serum and amniotic fluid from an e-waste dismantling area in China. Environ. Int. 2020, 136, 105407. [Google Scholar] [CrossRef] [PubMed]
- Catron, T.R.; Keely, S.P.; Brinkman, N.E.; Zurlinden, T.J.; Wood, C.E.; Wright, J.R.; Phelps, D.; Wheaton, E.; Kvasnicka, A.; Gaballah, S.; et al. Host developmental toxicity of BPA and BPA alternatives is inversely related to microbiota disruption in zebrafish. Toxicol. Sci. 2019, 167, 468–483. [Google Scholar] [CrossRef]
- Bonefeld-Jørgensen, E.C.; Long, M.; Hofmeister, M.V.; Vinggaard, A.M. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: New data and a brief review. Environ. Health Persp. 2007, 115, 69–76. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol A and peripheral arterial disease: Results from the NHANES. Environ. Health Persp. 2012, 120, 1297–1300. [Google Scholar] [CrossRef]
- Alnaimat, A.S.; Barciela-Alonso, M.C.; Bermejo-Barrera, P. Determination of bisphenol A in tea samples by solid phase extraction and liquid chromatography coupled to mass spectrometry. Microchem. J. 2019, 147, 598–604. [Google Scholar] [CrossRef]
- Roderick, M.S.; Adcock, J.L.; Terry, J.M.; Smith, Z.M.; Parry, S.; Linton, S.M.; Thornton, M.T.; Barrow, C.J.; Francis, P.S. Chemiluminescence evidence supporting the selective role of ligands in the permanganate oxidation of micropollutants. J. Phys. Chem. A 2013, 117, 10286–10293. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, J.; Wu, C. Determination of endocrine disruption potential of bisphenol a alternatives in food contact materials using in vitro assays: State of the art and future challenges. J. Agric. Food Chem. 2019, 67, 12613–12625. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.U.; Deen, M.J. Bisphenol A electrochemical sensor using graphene oxide and b-cyclodextrin-functionalized multi-walled carbon nanotubes. Anal. Chem. 2020, 92, 5532–5539. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Islam, T.; Imran, A.; Alqahtani, B.; Shah, S.S.; Mahfoz, W.; Karim, M.R.; Alharbi, H.F.; Aziz, M.A.; SalehAhammad, A.J. Mechanistic insights of the oxidation of bisphenol A at ultrasonication assisted polyaniline-Au nanoparticles composite for highly sensitive electrochemical sensor. Electrochim. Acta 2021, 374, 137968. [Google Scholar] [CrossRef]
- Wannapob, R.; Thavarungkul, P.; Limbut, W.; Dawan, S.; Kanatharana, P. A simple and highly stable porous gold-based electrochemical sensor for bisphenol A detection. Electroanalysis 2016, 28, 472–480. [Google Scholar] [CrossRef]
- Zhan, X.; Hu, S.; Wang, J.; Chen, H.; Chen, X.; Yang, J.; Yang, H.; Su, Z. One-pot electrodeposition of metal organic frameworks composite accelerated by gold nanoparticles and electroreduced carbon dots for electroanalysis of bisphenol A in real plastic samples. Sens. Actuat. B Chem. 2021, 346, 130499. [Google Scholar] [CrossRef]
- Su, B.; Shao, H.; Li, N.; Chen, X.; Cai, Z.; Chen, X. A sensitive bisphenol A voltammetric sensor relying on AuPd nanoparticles/graphene composites modified glassy carbon electrode. Talanta 2017, 166, 126–132. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Wu, Z.; Wen, W.; Zhang, X.; Wang, S. Electrochemical sensor based on confined synthesis of gold nanoparticles @ covalent organic frameworks for the detection of bisphenol A. Anal. Chim. Acta 2023, 1239, 340743. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, K.; Liu, C.; Yin, L.; Kang, Q.; Li, L.; Li, B. Electrochemical sensing of bisphenol A based on polyglutamic acid/amino-functionalised carbon nanotubes nanocomposite. Electrochim. Acta 2014, 133, 492–500. [Google Scholar] [CrossRef]
- Zheng, Z.; Du, Y.; Wang, Z.; Feng, Q.; Wang, C. Pt/graphene-CNTs nanocomposite based electrochemical sensors for the determination of endocrine disruptor bisphenol A in thermal printing papers. Analyst 2013, 138, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.; Flower, G.L.; Paudyal, J. Electrochemical sensing of bisphenol A on single-walled carbon nanotube paper electrodes. Electroanalysis 2023, 35, e202200449. [Google Scholar] [CrossRef]
- Verma, D.; Chauhan, D.; Mukherjee, M.D.; Ranjan, K.R.; Yadav, A.K.; Solanki, P.R. Development of MWCNT decorated with green synthesized AgNps-based electrochemical sensor for highly sensitive detection of BPA. J. Appl. Electrochem. 2021, 51, 447–462. [Google Scholar] [CrossRef]
- Cai, B.; Hübner, R.; Sasaki, K.; Zhang, Y.; Su, D.; Ziegler, C.; Vukmirovic, M.B.; Rellinghaus, B.; Adzic, R.R.; Eychmüller, A. Core-shell structuring of pure metallic aerogels towards highly efficient platinum utilization for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2018, 57, 2963–2966. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Xie, F.Y.; Fu, Y.C.; He, Y.; Ma, M.; Xie, Q.J.; Yao, S.Z. Au-supported Pt-Au mixed atomic monolayer electrocatalyst with ultrahigh specific activity for oxidation of formic acid in acidic solution. Chem. Commun. 2012, 48, 12106–12108. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gan, L.; Wei, Y.; Yang, H.; Li, J.; Du, H.; Kang, F. Pt submonolayers on Au nanoparticles: Coverage-dependent atomic structures and electrocatalytic stability on methanol oxidation. J. Phys. Chem. C 2016, 120, 28664–28671. [Google Scholar] [CrossRef]
- Dimitrov, N. Recent advances in the growth of metals, alloys, and multilayers by surface limited redox replacement (SLRR) based approaches. Electrochim. Acta 2016, 209, 599–622. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Z.; Liua, H.; Yuan, Q.; Xie, Q.; Yao, S. Bi-underpotential/PtAu-bulk coelectrodeposition and subsequent Bi dissolution for electrocatalytic oxidation and amperometric analysis of formaldehyde. Analyst 2020, 145, 7546–7550. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real surface area measurements in electrochemistry. Pure Appl. Chem 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Chao, L.; Xiong, X.; Liu, J.; Xu, A.; Huang, T.; He, F.; Xie, Q. Preparation of a porous Au electrode with a sacrificed Prussian blue analogue template for anodic stripping voltammetric analysis of trace arsenic(III). Sens. Actuat. B Chem. 2017, 253, 603–611. [Google Scholar] [CrossRef]
- Messaoud, N.B.; Ghica, M.E.; Dridi, C.; Ali, M.B.; Brett, C.M. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuat. B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Lei, X.; Deng, Z.; Zeng, Y.; Huang, S.; Yang, Y.; Wang, H.; Guo, L.; Li, L. A novel composite of conductive metal organic framework and molecularly imprinted poly (ionic liquid) for highly sensitive electrochemical detection of bisphenol A. Sens. Actuat. B Chem. 2021, 339, 129885. [Google Scholar] [CrossRef]
- Zhou, Y.; She, X.; Wu, Q.; Xiao, J.; Peng, T. Monoclinic WO3 nanosheets-carbon nanotubes nanocomposite based electrochemical sensor for sensitive detection of bisphenol A. J. Electroanal. Chem. 2022, 915, 116355. [Google Scholar] [CrossRef]
- Ali, M.Y.; Alama, A.U.; Howlader, M.M.R. Fabrication of highly sensitive bisphenol A electrochemical sensor amplified with chemically modified multiwall carbon nanotubes and β-cyclodextrin. Sens. Actuat. B Chem. 2020, 320, 128319. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Mahmoud, K.A. Platinum nanoparticles/Ti3C2Tx (MXene) composite for the effectual electrochemical sensing of Bisphenol A in aqueous media. J. Electroanal. Chem. 2021, 880, 114934. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, N.; Sun, X.; Chu, H.; Wang, Y.; Hu, X. Triple-signaling amplification strategy based electrochemical sensor design: Boosting synergistic catalysis in metal-metalloporphyrin-covalent organic frameworks for sensitive bisphenol A detection. Analyst 2021, 146, 4585–4594. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, F.; Xiang, J.; Wang, F.; Liu, Q.; Chen, X. In situ growth of ZIF-8 on gold nanoparticles/magnetic carbon nanotubes for the electrochemical detection of bisphenol A. Anal. Methods 2021, 13, 2338–2344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).