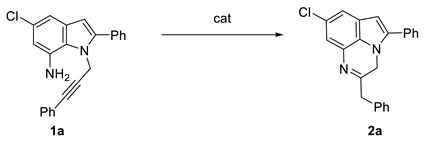
Abstract
A gold-catalyzed protocol to obtain functionalized 3H-pyrrolo [1,2,3-de] quinoxalines from suitable substituted N-alkynyl indoles has been proposed. The mild reaction conditions were revealed to be compatible with different functional groups, including halogen, alkoxyl, cyano, ketone, and ester, allowing the isolation of title compounds with yields from good to high. A reaction mechanism has been proposed, and theoretical calculations have been provided to rationalize the final step of the hypothesized reaction mechanism. As quinoxaline-containing polycyclic compounds, this class of molecules may represent a valuable template in medicinal chemistry and material science.
1. Introduction
Nitrogen-containing heterocycles are a class of compounds of great importance to life science since they are present as scaffolds in several biologically active natural products and synthetic drugs [1]. For this reason, a great deal of attention has been devoted to the development of methods for their preparation and, in particular, to those catalytic protocols that overcome the limitations of traditional C-N bond-forming reactions. In this regard, the transition metal-catalyzed hydroamination assumed great significance [2], with a lot of expedient routes based on gold catalysis [2,3,4,5].
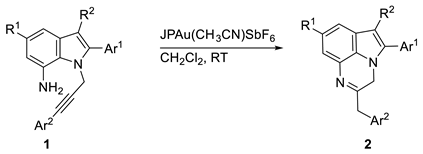
We previously described a domino approach to 4-substituted 1,5-benzodiazepines based on the reactive sequence gold-catalyzed hydroamination/cyclization [6] (Scheme 1a), as well as a stereo and regioselective approach to Z-enamine products via an intermolecular gold-catalyzed reaction of the 2-(arylethynyl)pyridines with anilines [7] (Scheme 1b). Continuing our investigations in this research field, we focused on the intra-molecular gold-catalyzed hydroamination as a tool for the construction of condensed polycyclic structures, envisaging the possibility of obtaining the 3H-pyrrolo-[1,2,3-de] quinoxalines 2 starting from suitable substituted N-alkynyl indoles 1 (Scheme 1c).
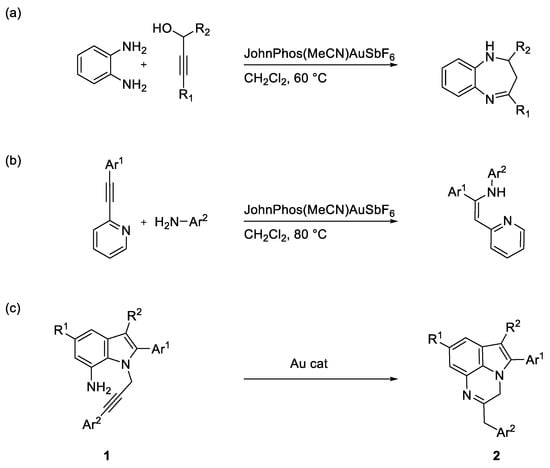
Scheme 1.
(a) Our previous work reporting on the Au-catalyzed synthesis of 1,5-benzodiazepines from o-phenylenediamines and propargylic alcohols. (b) Our previous work reporting on the stereo- and regioselective synthesis of enamines via the Au(I)-catalyzed hydroamination of 2-(Arylethynyl)pyridines with anilines. (c) Our work hypothesis for the gold-catalyzed synthesis of functionalized 3H-pyrrolo-[1,2,3-de] quinoxalines from substituted N-alkynyl indoles.
To the best of our knowledge, the derivatives of which the synthesis was pursued are unknown compounds with a rather infrequently reported heterocyclic core [8,9]. Extensive state-of-the-art studies revealed a lack of methods to achieve their construction, even though their synthesis might be of interest in medicinal chemistry. Indeed, the tricyclic quinoxaline-containing compounds are widespread in a variety of therapeutic agents such as anti-HIV [10], antiparasitic [11,12,13,14], and antitumoral [15] (Figure 1), and make our new condensed cyclic systems promising candidates for diverse uses.
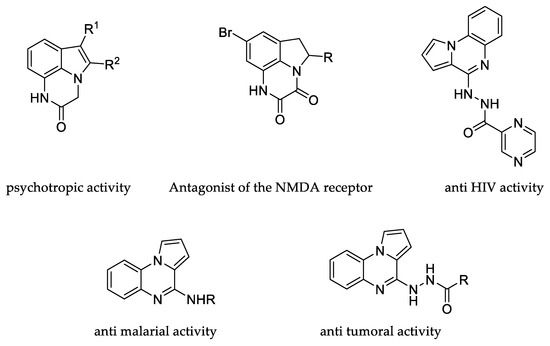
Figure 1.
Bioactive synthetic products containing a tricyclic quinoxaline-containing scaffold.
In addition, the 3H-pyrrolo-[1,2,3-de] quinoxalines 2 may be structurally related to a class of compounds with antiapoptotic activity acting as potent inhibitors of the Mcl-1 protein (Figure 2) [16].
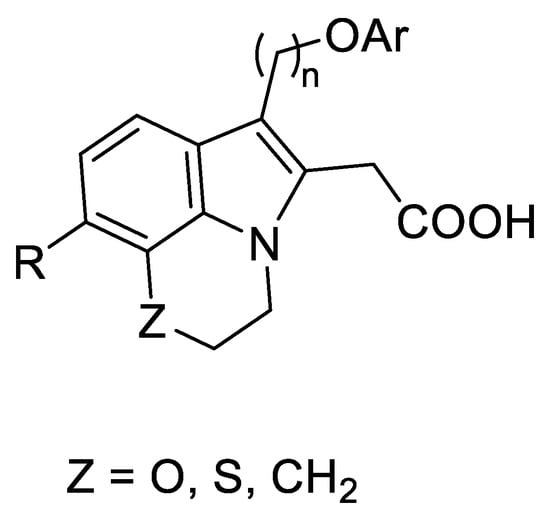
Figure 2.
Structure of known Mcl-1 inhibitors.
As to industrial applications, substituted quinoxalines and their derivatives are known as metal corrosion inhibitors and are often found as constituents of electroluminescent materials [17].
Given this broad range of applications of quinoxaline derivatives and the gap of synthetic methodology for the 3H-pyrrolo-[1,2,3-de] quinoxalines, we decided to study the cyclization of the indole derivatives 1, which were suitably designed to provide an intramolecular gold-catalyzed hydroamination reaction. The proposed methodology is strongly based on the background of the research group, which has been continuously devoted to the construction and functionalization of indoles.
2. Results and Discussion
Substrates for our studies have been synthesized according to slightly modified known procedures depicted in the following Scheme 2 (for a detailed description of the procedures, see Materials and Methods (Section 3)).
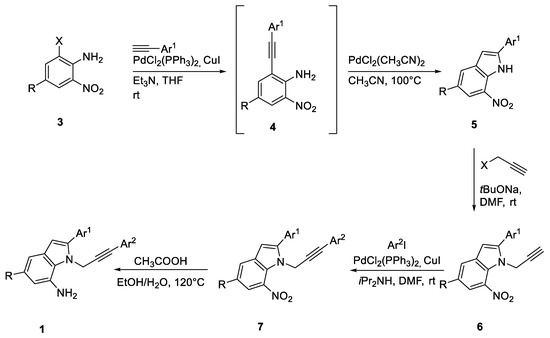
Scheme 2.
Synthesis of substrates 1.
Based on the working hypothesis (Scheme 1c), the reaction of substrate 1a was selected as the model system for a series of preliminary experiments aimed at identifying the best reaction conditions. For our first attempt, we decided to perform the reaction under the same condition previously used for the synthesis of 1,5-benzodiazepines [6]. Pleasingly, a smooth gold-catalyzed intramolecular hydroamination of 1a took place, and the 6-exo-dig cyclization product 2a was isolated in almost quantitative yield after 1 h (Table 1, entry 1).

Table 1.
Synthesis of 2-benzyl-7-chloro-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxaline 2a from 4-chloro-2-phenyl-1-(3-phenylprop-2-yn-1-yl)-1H-indol-7-amine 1a.
Given the high reaction rate, we decided to carry out the reaction at room temperature, obtaining similar results in terms of yield even though with a longer reaction time (Table 1, entry 2). A lower yield (42%) was obtained by switching to the PPh3AuCl/AgSbF6 combination (Table 1, entry 3), with a significant amount of starting material (55%) recovered after 24 h. The reaction was also performed in the presence of other transition metal catalysts leading to worse results in terms of efficiency, time, and harsher reaction conditions. Indeed, the use of PtCl2 resulted in a slower and less efficient cyclization at 80 °C (Table 1, entry 4), while, at the same temperature, using PdCl2(CH3CN)2, the final compound 2a was isolated in 36% yield, along with degradation compounds (Table 1, entry 5). Switching to the Brønsted acid catalyst TsOH, poor results were observed in obtaining a 2 h mixture of degradation compounds (Table 1, entry 6). In this case, very likely, the formation of the imine derivative 2a also occurred, but in the acidic reaction conditions, it was rapidly degraded.
Once we established the best reaction conditions, we investigated this method’s scope. Variously substituted quinoxaline derivatives were obtained in good to excellent yield both in the presence of electron-donating groups and electron-withdrawing groups (Table 2).

Table 2.
Synthesis of functionalized 3H-pyrrolo-[1,2,3-de] quinoxalines 2 from substituted N-propargyl indoles 1 through a gold-catalyzed intramolecular hydroamination.
Notably, in all experiments, compound 2 was the only observed product, with no traces of any 7-endo-dig cyclization product (compounds 8 and 8′, Figure 3).
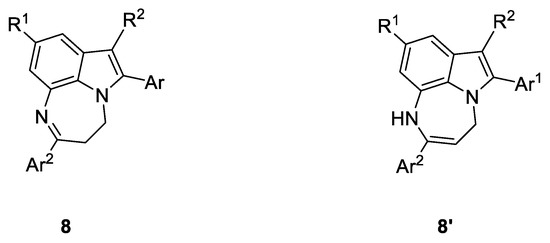
Figure 3.
Structures of two possible isomeric 7-endo-dig cyclization products. The formation of these compounds was never observed.
The formation of 2 may be rationalized based on the general mechanism of the gold-catalyzed hydroamination [18] through the basic steps shown in Scheme 3. Particularly, the hydroamination intermediate IV is formed by admitting the nucleophilic addition of the amine group towards the triple bond activated by the Au(I) coordination (I) followed by a protodeauration step (Scheme 3).
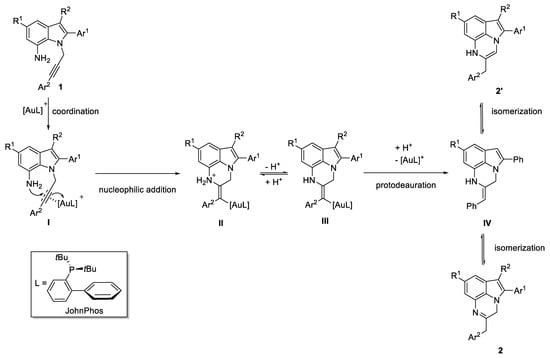
Scheme 3.
Hypothesized mechanism for the formation of 2.
Then, the isomerization of IV can take place in two different modalities providing, alternatively, the imine-derivative 2 or the enamine-derivative 2′. In the reaction conditions, this step proceeds, providing only compound 2, which is very likely the most stable.
To this regard, HF (6-31G**) calculations performed on the two isomeric compounds 2a and 2′a revealed a higher stability of 2a than 2′a by 5.2 kcal/mol (Figure 4) [19] and explained the exclusive formation of imine-derivative 2 in the reaction conditions. In addition, similar isomerization modes are described in the literature [20].

Figure 4.
HF (6-31G**) calculations on (2a) and (2′a).
The 5-aryl-3H-pyrrole [1,2,3-de]quinoxaline derivatives synthesized according to the proposed method are poised for further manipulations in different positions, providing access to compounds with an increased molecular complexity through simple organic reactions. For instance, as reported in Scheme 4, compound 9d can be easily obtained in almost quantitative yield by treating 2d with LiAlH4.
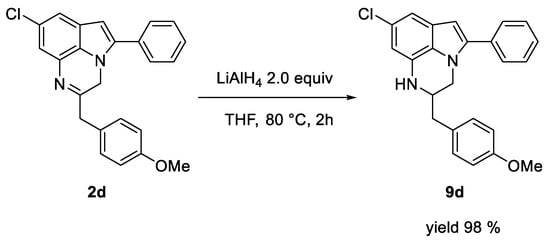
Scheme 4.
Reduction of compound 2d to obtain 9d.
3. Materials and Methods
3.1. General Information
All of the commercially available reagents, catalysts, bases, and solvents were used as purchased without further purification. Starting materials and reaction products were purified by flash chromatography using SiO2 as a stationary phase, eluting with n-hexane/ethyl acetate mixtures. 1H NMR (400.13 MHz), 13C NMR (100.6 MHz), and 19F spectra (376.5 MHz) were recorded with a Bruker Avance 400 spectrometer. Splitting patterns are designed as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), or bs (broad singlet). HRMS of samples were recorded on an Orbitrap Exactive (Thermo Fisher, Waltham, MA, USA). Melting points were determined with a Büchi B-545 apparatus and are uncorrected.
3.2. General Experimental Procedures
3.2.1. Synthetic Procedures for Starting Materials
General Procedure for the Preparation of Substituted 1-(3-Arylprop-2-yn-1-yl)-2-aryl-1H-indol-7-amine 1
Starting materials 1 were prepared according to literature procedures through the four-step sequence of reactions depicted in Scheme 2.
- Typical Procedure for the Preparation of 5-substituted-7-nitro-2-phenyl-1H-indole 5
STEP 1: Synthesis of 5-chloro-7-nitro-2-phenyl-1H-indole 5a.
In a 100 mL two-necked round bottom flask equipped with a magnetic stirring bar, PdCl2(PPh3)2 (0.329 g, 0.469 mmol, 0.04 equiv.) and CuI (0.045 g, 0.234 mmol, 0.02 equiv.) were dissolved in 36.0 mL of THF and 1.56 mL of Et3N at room temperature under a nitrogen atmosphere. Then, 2-iodo-4-chloro-6-nitroaniline (3.5 g, 11.74 mmol, 1.0 equiv.) was added, and, dropwise, phenylacetylene (1.93 mL, 17.61 mmol, 1.5 equiv.). The solution was stirred for 2 h. After this time, the reaction mixture was diluted with Et2O and washed with a saturated solution of NH4Cl, NaHCO3, and brine. The organic layer was separated, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue, containing 4-chloro-2-nitro-6-(phenylethynyl)aniline 4a, was transferred with 60 mL of MeCN in a two-necked 100 mL round bottom flask equipped with a condenser, and a magnetic stirring bar, then, PdCl2(CH3CN)2 was added. The solution was stirred for 2.5 h at 100 °C. After this time, the mixture was cooled to room temperature, concentrated under reduced pressure, purified by chromatography on SiO2 (25–40 μm), eluting with a 92/8 (v/v) n-hexane/ethyl acetate mixture (Rf = 0.26) to obtain 5-chloro-7-nitro-2-phenyl-1H-indole 5a (2,57 g, 80% yield).
The 5-chloro-7-nitro-2-phenyl-1H-indole 5a: yield: 80%; orange solid; mp: 164–166 °C; 1H NMR (400.13 MHz) (CDCl3): δ 10.07 (bs, 1 H), 8.11 (d, J = 1.5 Hz, 1 H), 7.93–7.88 (m, 1 H), 7.74 (d, J = 7.8 Hz, 1 H), 7.53 (t, J = 7.3 Hz, 2 H), 7.45 (t, J = 7.3 Hz, 2 H), 6.90 (d, J = 2.4 Hz, 1 H), 5.05 (d, J = 2.4 Hz, 2 H), and 2.20 (t, J = 2.4 Hz, 1 H); 13C NMR (100.6 MHz) (CDCl3): δ 142.0 (C), 133.7 (C), 133.5 (C), 130.4 (C), 129.34 (CH), 129.27 (CH), 128.8 (C), 127.6 (CH), 125.7 (CH), 125.1 (C), 118.7 (CH), and 100.1 (CH).
- b.
- Typical Procedure for the Preparation of Substituted 7-nitro-2-phenyl-1-(prop-2-yn-1-yl)-1H-indoles 6.
STEP 2: Synthesis of 5-chloro-7-nitro-2-phenyl-1-(prop-2-yn-1-yl)-1H-indole 6a.
A 250 mL round bottom flask, equipped with a magnetic stirring bar, was charged with t-BuONa (1.35 g, 14.02 mmol, 1.5 equiv) and 90 mL of anhydrous DMF. The reaction mixture was cooled at 0 °C, and 5-chloro-7-nitro-2-phenyl-1H-indole (2.4 g, 9.35 mmol, 1.0 equiv) was added dropwise. Then, propargyl bromide (1.21 mL, 14.02 mmol, 1.5 equiv) was added, and the solution was warmed to room temperature and stirred for 6 h. After this time, the reaction mixture was diluted with Et2O and washed with a saturated solution of NaHCO3 and brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by chromatography on SiO2 (25–40 μm), eluting with a 96/4 (v/v) n-hexane/ethyl acetate mixture (Rf = 0.25) to obtain 5-chloro-7-nitro-2-phenyl-1-(prop-2-yn-1-yl)-1H-indole 6a (2.324 g, 80% yield).
The 5-chloro-7-nitro-2-phenyl-1-(prop-2-yn-1-yl)-1H-indole 6a: 80% yield; brown solid; mp 103–104 °C; 1H NMR (400.13 MHz) (CDCl3): δ 7.92 (d, J = 1.9 Hz, 1 H), 7.87 (d, J = 1.9 Hz, 1 H), 7.58–7.50 (m, 5 H), 6.70 (s, 1 H), 5.05 (d, J = 2.4 Hz, 2 H), and 2.20 (t, J = 2.4 Hz, 1 H); 13C NMR (100.6 MHz) (CDCl3): δ 146.8 (C), 137.3 (C), 134.3 (C), 130.6 (C), 129.6 (CH), 129.4 (CH), 129.1 (CH), 127.1 (C), 126.0 (CH), 125.2 (C), 119.8 (CH), 104.1 (CH), 77.1 (C), 74.4 (CH), and 37.1 (CH2).
- c.
- Typical Procedure for the Preparation of Substituted 1-(3-arylprop-2-yn-1-yl)-7-nitro-2-phenyl-1H-indoles 7.
STEP 3: Synthesis of 5-chloro-1-(3-(4-methoxyphenyl)prop-2-yn-1-yl)-7-nitro-2-phenyl-1H-indole 7c.
In a two-necked 50 mL round bottom flask, equipped with a magnetic stirring bar, PdCl2(PPh3)2 (0.084 g, 0.119 mmol, 0.04 equiv.) and CuI (0.011 g, 0.0597 mmol, 0.02 equiv.) were dissolved in 12.3 mL of iPr2NH and 6.1 mL of DMF at room temperature and under nitrogen; then, 4-iodoanisole (0.839 g, 3.584 mmol, 1.2 equiv.) and 5-chloro-7-nitro-2-phenyl-1-(prop-2-yn-1-yl)-1H-indole (0.928 g, 2.98 mmol, 1.0 equiv.) were added, and the resulting mixture was stirred for 24 h. After this time, the mixture was diluted with Et2O and washed with a saturated solution of NH4Cl, a saturated solution of NaHCO3, and with brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by chromatography on SiO2 (25–40 μm), eluting with a 93/7 (v/v) n-hexane/ethyl acetate mixture (Rf = 0.27) to obtain 5-chloro-1-(3-(4-methoxyphenyl)prop-2-yn-1-yl)-7-nitro-2-phenyl-1H-indole 7c (0.860 g, 70% yield).
The 5-chloro-1-(3-(4-methoxyphenyl)prop-2-yn-1-yl)-7-nitro-2-phenyl-1H-indole 7c: 70% yield; yellow solid; mp 133–134 °C; 1H NMR (400.13 MHz) (CDCl3): δ 7.91 (d, J = 1.9 Hz, 1 H), 7.87 (d, J = 1.9 Hz, 1 H), 7.60–7.50 (m, 5 H), 7.21 (d, J = 8.8 Hz, 2 H), 6.78 (d, J = 8.8 Hz, 2 H), 6.70 (s, 1 H), 5.23 (s, 2 H), and 3.78 (s, 3 H); 13C NMR (100.6 MHz) (CDCl3): δ 159.9 (C), 146.7 (C), 138.2 (C), 137.5 (C), 134.2 (C), 133.2 (CH), 130.8 (C), 129.6 (CH), 129.3 (CH), 129.0 (CH), 127.1 (C), 125.8 (CH), 124.9 (C), 119.6 (CH), 113.8 (CH), 103.8 (CH), 86.1 (C), 80.9 (C), 55.2 (CH3), and 38.2 (CH2).
- d.
- Typical Procedure for the Synthesis of Substutited 1-(3-arylprop-2-yn-1-yl)-2-aryl-1H-indol-7-amine 1.
STEP 4: Synthesis of 5-chloro-1-(3-(4-methoxyphenyl)prop-2-yn-1-yl)-2-phenyl-1H-indol-7-amine 1c.
In a 50 mL Carousel Tube Reactor (Radely Discovery Technology), equipped with a magnetic stirring bar, 5-chloro-1-(3-(4-methoxyphenyl)prop-2-yn-1-yl)-7-nitro-2-phenyl-1H-indole (0.180 g, 0.431 mmol, 1.0 equiv.) was added to a solution of EtOH/H2O (3:1) and stirred at 120 °C for 10 min. Then, 51 μL of acetic acid and 72 mg of Fe (0) (0.431 mmol, 1.0 equiv.) were added in three portions every 15 min. The reaction mixture was then stirred for 2 h before being cooled at room temperature and concentrated under reduced pressure. Subsequently, the mixture was diluted with Et2O and washed with a saturated solution of NaHCO3 and with brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by filtration on a pad of Celite eluting with DCM to obtain 5-chloro-1-(3-(4-methoxyphenyl)prop-2-yn-1-yl)-2-phenyl-1H-indol-7-amine 1a (0.140 g, 85% yield).
The 5-chloro-1-(3-(4-methoxyphenyl)prop-2-yn-1-yl)-2-phenyl-1H-indol-7-amine 1c: 85% yield; orange solid; mp 91–92 °C; 1H NMR (400.13 MHz) (CDCl3): δ 7.71–7.68 (m, 2 H), 7.55–7.49 (m, 2 H), 7.48–7.44 (m, 1 H), 7.42 (d, J = 8.8 Hz, 2 H), 7.09 (d, J = 1.8 Hz, 1 H), 6.89 (d, J = 8.8 Hz, 2H), 6.54 (d, J = 1.8 Hz, 1 H), 6.47 (s, 1 H), 5.17 (s, 2 H), 4.35 (bs, 2 H), and 3.84 (s, 3 H); 13C NMR (100.6 MHz) (CDCl3): δ 160.1 (C), 143.2 (C), 134.0 (C), 133.2 (CH), 131.9 (C), 130.8 (C), 129.3 (CH), 128.7 (CH), 128.3 (CH), 127.1 (C), 126.3 (C), 114.1 (CH), 113.8 (C), 111.3 (CH), 110.1 (CH), 102.4 (CH), 86.6 (C), 84.9 (C), 55.3 (CH3), and 36.5 (CH2).
3.2.2. Synthetic Procedures for Final Products
Typical Procedure for the Preparation of Substituted 5-Aryl-3H-pyrrolo [1,2,3-de] Quinoxalines 2: Synthesis of 8-Chloro-2-(4-methoxybenzyl)-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxaline 2c
A 50 mL Carousel Tube Reactor (Radely Discovery Technology), equipped with a magnetic stirring bar, was charged with 5-chloro-1-(3-(4-methoxyphenyl)prop-2-yn-1-yl)-2-phenyl-1H-indol-7-amine 1c (0.140 g, 0.361, 1.0 equiv.) and 2 mL of CH2Cl2 before adding 5.2 mg of (acetonitrile)-[(2-diphenyl)-di-tert-butylphosphine]Au(I) hexafluoroantimonate (0.0072 mmol, 0.02 equiv.). The solution was stirred for 1.5 h at room temperature, monitoring the disappearance of the starting material by TLC. Then, the mixture was concentered under reduced pressure and filtered on a pad of Celite to obtain 8-chloro-2-(4-methoxybenzyl)-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxaline 2c (0.112 g, 80% yield).
The 8-chloro-2-(4-methoxybenzyl)-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxaline 2c: 80% yield; brown oil; 1H NMR (400.13 MHz) (CDCl3): δ 7.87 (d, J = 8.9 Hz, 2 H), 7.41–7.30 (m, 6 H), 7.29 (d, J = 1.9 Hz, 1 H), 6.87 (d, J = 8.8 Hz, 2 H), 6.46 (s, 1 H), 4.32–4.27 (m, 2 H), 3.76 (s, 3 H), and 3.13–3.08 (m, 2 H); 13C NMR (100.6 MHz) (CDCl3): δ 167.1 (C), 161.7 (C), 161.4 (C), 142.8 (C), 134.3 (C), 132.1 (CH), 131.9 (C), 130.8 (C), 129.3 (CH), 129.0 (CH), 128.6 (CH), 128.4 (C), 128.3 (CH), 125.7 (C), 123.7 (CH), 117.9 (CH), 113.9 (CH), 101.9 (CH), 55.4 (CH3), 48.5 (CH2), and 32.6 (CH2). HRMS m/z [M + H]+ calcd for C24H20ClN2O: 387.1259; found: 387.1274.
3.3. Characterization Data of Synthesized Compounds
Characterization data of starting materials and of 9d are reported in Supplementary Materials.
Characterization Data of Final Compounds 2a–i and 2k
The 2-benzyl-8-chloro-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxaline 2a: 98% yield; brown oil; 1H NMR (400.13 MHz) (CDCl3): δ 8.06–7.98 (m, 2 H), 7.58–7.41 (m, 10 H), 6.60 (s, 1 H), 4.50–4.43 (m, 2 H), and 3.34–3.25 (m, 2 H); 13C NMR (100.6 MHz) (CDCl3): δ 167.7 (C), 142.8 (C), 139.7 (C), 134.0 (C), 131.8 (C), 131.1(C), 130.8 (C), 130.4 (CH), 129.3 (CH), 128.67 (CH), 128.64 (CH), 128.3 (CH), 127.2 (CH), 125.7 (C), 124.1 (CH), 118.4 (CH), 102.0 (CH), 48.4 (CH2), and 33.08 (CH2). HRMS m/z [M + H]+ calcd for C23H18ClN2: 357.1153; found: 357.1133.
The 8-chloro-2-(4-chlorobenzyl)-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxaline 2b: 86% yield; brown oil; 1H NMR (400.13 MHz) (CDCl3): δ 7.96 (d, J = 8.7 Hz, 2 H), 7.56–7.42 (m, 10 H), 6.59 (s, 1 H), 4.46–4.48 (m, 2 H), and 3.26–3.20 (m, 2 H); 13C NMR (100.6 MHz) (CDCl3): δ 166.2 (C), 142.8 (C), 138.0 (C), 136.7 (C), 133.7 (C), 131.7 (C), 130.9 (C), 129.3 (CH), 128.8 (CH), 128.7 (CH), 128.6 (CH), 128.4 (CH), 128.3 (CH), 125.7 (C), 124.2 (CH), 118.6 (CH), 102.1 (CH), 48.3 (CH2), and 32.8 (CH2). HRMS m/z [M + H]+ calcd for C15H22N3: 391.0763; found: 391.0754.
The 8-chloro-2-(4-methoxybenzyl)-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxaline 2c: 80% yield; brown oil; 1H NMR (400.13 MHz) (CDCl3): δ 8.00 (d, J = 8.9 Hz, 2 H), 7.59–7.39 (m, 7 H), 7.00 (d, J = 8.9 Hz, 2 H), 7.59 (s, 1 H), 4.46–4.39 (m, 2 H), 3.89 (s, 3 H), and 3.26–3.19 (m, 2 H); 13C NMR (100.6 MHz) (CDCl3): δ 167.1 (C), 161.7 (C), 142.8 (C), 134.3 (C), 132.1 (C), 131.9 (C), 130.8 (C), 129.3 (CH), 129.0 (CH), 128.6 (CH), 128.4 (C), 128.3 (CH), 125.7 (C), 123.6 (CH), 117.9 (CH), 113.9 (CH), 101.9 (CH), 55.4 (CH3), 48.5 (CH2), and 32.6 (CH2). HRMS m/z [M + H]+ calcd for C24H20ClN2O: 387.1259; found: 387.1274.
The 1-(4-((8-chloro-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxalin-2-yl)methyl)phenyl)ethan-1-one 2d: 85% yield; brown solid; mp 77–78 °C; 1H NMR (400.13 MHz) (CDCl3): δ 8.12–8.0 (m, 4 H), 7.57–7.41 (m, 7 H), 6.60 (s, 1 H), 4.50–4.42 (m, 2 H), 3.33–3.25 (m, 2 H), and 2.67 (s, 3 H); 13C NMR (100.6 MHz) (CDCl3): δ 197.6 (C), 166.3 (C), 143.6 (C), 142.9 (C), 138.1 (C), 133.6 (C), 131.7 (C), 130.9 (C), 129.3 (CH), 128.7 (CH), 128.6 (CH), 128.5 (CH), 128.4 (C), 128.3 (C), 127.4 (CH), 125.8 (C), 124.5 (CH), 119.0 (CH), 102.1 (CH), 48.2 (CH2), and 33.0 (CH2). HRMS m/z [M + H]+ calcd for C25H20ClN2O: 399.1259; found: 399.1263.
The 8-chloro-5-(4-methoxyphenyl)-2-(3-(trifluoromethyl)benzyl)-3H-pyrrolo [1,2,3-de]quinoxaline 2e: 80% yield; brown solid; mp 170–171 °C; 1H NMR (400.13 MHz) (CDCl3): δ 8.27 (s, 1 H), 8.21 (d, J = 7.9 Hz, 1 H), 7.76 (d, J = 7.9 Hz, 1 H), 7.62 (t, J = 7.7 Hz, 1 H), 7.53–7.49 (m, 1 H), 7.48–7.40 (m, 3 H), 7.00 (d, J = 8.6 Hz, 2 H), 6.54 (s, 1 H), 4.48–4.40 (m, 2 H), 3.90 (s, 3 H), and 3.34–3.27 (m, 2 H); 13C NMR (100.6 MHz)(CDCl3): δ 165.6 (C), 159.9 (C), 142.8 (C), 140.4 (C), 133.4 (C), 131.1 (q, JCF = 32.0 Hz, C), 131.0 (C), 130.6 (CH), 130.3 (CH), 129.2 (CH), 128.1 (C), 126.8 (q, JCF = 3.6 Hz, CH), 125.7 (C), 124.0 (q, JCF = 273.0 Hz, C), 124.1 (CH), 124.05 (C), 124.00 (q, JCF = 3.6 Hz, CH), 118.8 (CH), 114.2 (CH), 101.4 (CH), 55.4 (CH3), 48.1 (CH2), and 32.9 (CH2); 19 F NMR (376.5 MHz)(CDCl3): δ −63.0. HRMS m/z [M + H]+ calcd for C25H19ClF3N2O: 455.1133; found: 455.1147.
The 8-chloro-2-(4-methoxybenzyl)-5-(4-methoxyphenyl)-3H-pyrrolo [1,2,3-de]quinoxaline 2f: 80% yield; brown solid; mp 150–152 °C; 1H NMR (400.13 MHz) (CDCl3): δ 7.99 (d, J = 8.8 Hz, 2 H), 7.46–7.37 (m, 4 H), 7.05–7.35 (m, 4 H), 6.51 (s, 1 H), 4.44–4.35 (m, 2 H), 3.89 (s, 6 H), and 3.28–3.16 (m, 2 H); 13C NMR (100.6 MHz) (CDCl3): δ 167.0 (C), 161.7 (C), 159.7 (C), 142.7 (C), 134.2 (C), 132.1 (C), 130.8 (C), 130.6 (CH), 129.0 (CH), 128.2.0 (C), 125.6 (C), 124.0 (C), 123.3 (CH), 117.6 (CH), 114.1 (CH), 113.9 (CH), 101.2 (CH), 55.45 (CH3), 55.41 (CH3), 48.4 (CH2), and 32.6 (CH2). HRMS m/z [M + H]+ calcd for C25H22ClN2O2: 417.1364; found: 417.1351.
The 1-(4-((8-methyl-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxalin-2-yl)methyl)phenyl)ethan-1-one 2g: 90% yield; brown solid; mp 141–142 °C; 1H NMR (400.13 MHz) (CDCl3): δ 8.10 (d, J = 8.51 Hz, 2 H), 8.06 (d, J = 8.51 Hz, 2 H), 7.55–7.46 (m, 4 H), 7.45–7.40 (m, 1 H), 7.39 (s, 1 H), 7.35 (s, 1 H), 6.60 (s, 1 H), 4.51–4.46 (m, 2 H), 3.32–3.27 (m, 2 H), 2.67 (s, 3 H), and 2.54 (s, 3 H); 13C NMR (100.6 MHz) (CDCl3): δ 197.7 (C), 164.9 (C), 144.3 (C), 141.7 (C), 137.7 (C), 132.7 (C), 132.3 (C), 130.4 (C), 130.0 (C), 129.3 (CH), 128.6 (CH), 128.5 (CH), 128.1 (C), 128.0 (CH), 127.3 (CH), 126.4 (CH), 120.0 (CH), 102.0 (CH), 48.4 (CH2), 33.1 (CH2), 26.8, (CH3), and 21.1 (CH3); HRMS m/z [M + H]+ calcd for C26H23N2O: 379.1805; found: 379.1796.
The methyl 4-(2-(4-chlorobenzyl)-8-methyl-3H-pyrrolo [1,2,3-de]quinoxalin-5-yl)benzoate 2h: 80% yield; yellow solid; mp 215–216 °C; 1H NMR (400.13 MHz) (CDCl3): δ 8.14 (d, J = 8.0 Hz, 2 H), 7.97 (d, J1 = 8.3 Hz, 2 H), 7.60 (d, J = 8.0 Hz, 2 H), 7.46 (d, J = 8.3 Hz, 2 H), 7.37 (s, 1 H), 7.33 (s, 1 H), 6.68 (s, 1 H), 4.54–4.44 (m, 2 H), 3.98 (s, 3 H), 3.30–3.21 (m, 2 H), and 2.42 (s, 3 H); 13C NMR (100.6 MHz) (CDCl3): δ 166.7 (C), 165.0 (C), 140.6 (C), 138.3 (C), 136.7 (C), 136.4 (C), 133.0 (C), 132.9 (C), 133.2 (C), 129.8 (CH), 129.3 (C), 128.9 (CH), 128.8 (CH), 128.5 (CH), 126.6 (CH), 119.8 (CH), 103.4 (CH), 52.3 (CH3), 48.9 (CH2), 32.7 (CH2), and 21.1 (CH3). HRMS m/z [M + H]+ calcd for C26H22ClN2O2: 429.1364; found: 429.1375.
The 2-(4-chlorobenzyl)-8-methyl-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxaline 2i: 79% yield; brown solid; mp 128–129 °C; 1H NMR (400.13 MHz) (CDCl3): δ 7.96 (d, J = 8.6 Hz, 2 H), 7.59–7.39 (m, 7 H), 7.37 (s, 1 H), 7.32 (s, 1 H), 6.60 (s, 1 H), 4.48–4.42 (m, 2 H), 3.27–3.20 (m, 2 H), and 2.53 (s, 3 H); 13C NMR (100.6 MHz) (CDCl3): δ 164.9 (C), 141.7 (C), 138.6 (C), 136.2 (C), 132.8 (C), 132.4 (C), 130.3 (C), 129.9 (C), 129.3 (CH), 128.9 (C), 128.7 (CH), 128.6 (CH), 128.5 (CH), 128.2 (C), 128.0 (CH), 126.1 (CH), 119.6 (CH), 102.0 (CH), 48.5 (CH2), 32.8 (CH2), and 21.1 (CH3). HRMS m/z [M + H]+ calcd for C24H20ClN2: 371.1310; found: 371.1321.
The 1-(4-((6-(4-methoxyphenyl)-8-methyl-5-phenyl-3H-pyrrolo [1,2,3-de]quinoxalin-2-yl)methyl)phenyl)ethan-1-one 2k: 84% yield; orange solid; mp 133–134 °C; 1H NMR (400.13 MHz) (CDCl3): δ 8.10 (d, J = 8.4 Hz, 2 H), 8.06 (d, J = 8.4 Hz, 2 H), 7.46 (s, 1 H), 7.42–7.31 (m, 6 H), 7.24 (d, J = 8.4 Hz, 2 H), 6.88 (d, J = 8.4 Hz, 2 H), 7.37 (s, 1 H), 7.32 (s, 1 H), 6.60 (s, 1 H), 4.41–4.34 (m, 2 H), 3.83 (m, 3 H), 3.38–3.31 (m, 2 H), and 2.68 (s, 3 H); 13C NMR (100.6 MHz) (CDCl3): δ 197.7 (C), 164.8 (C), 157.8 (C), 144.4 (C), 137.7 (C), 137.3 (C), 132.6 (C), 131.5 (C), 131.1 (CH), 130.2 (C), 129.8 (C), 128.6 (CH), 128.4 (CH), 128.0 (CH), 127.29 (C), 127.27 (CH), 127.1 (C), 127.0 (CH), 119.2 (CH), 115.2 (C), 113.8 (CH) (overlapping), 55.2 (CH3), 47.9 (CH2), 32.2 (CH2), 26.8 (CH3), and 21.1 (CH3). HRMS m/z [M + H]+ calcd for C33H29N2O2: 485.2224; found: 485.2239.
4. Conclusions
A protocol for the synthesis of functionalized 3H-pyrrolo-[1,2,3-de] quinoxalines from substituted N-alkynyl indoles has been developed. The reaction proved to be highly selective in promoting the exclusive formation of the 6-exo-dig cyclization product, which, after isomerization, affords the final compound. As to the isomerization mode, theoretical calculations were provided to support the experimental data indicating that differences in terms of stability between the two possible isomers determine the formation of the imine-type product. The mild reaction conditions in which the reaction takes place led to the synthesis of derivatives with useful functional groups, including halogen, alkoxyl, cyano, ketone, and ester, with yields from good to high in all the cases reported.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28155831/s1.
Author Contributions
Conceptualization, G.F. and A.S.; methodology, A.I. and A.G.; formal analysis, G.F. and A.S.; investigation, F.M. and K.U.; writing—original draft preparation, A.I. and A.G.; writing—review and editing, G.F. and A.S.; supervision, A.G. and A.I.; project administration, G.F.; funding acquisition, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the MIUR (Ministry of Education University and Research) under Grant number 2017SXBSX4 (PRIN project 2017, “Targeting Hedgehog pathway: virtual screening identification and sustainable synthesis of novel Smo and Gli inhibitors and their pharmacological drug delivery strategies for improved therapeutic effects in tumors”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data contained within the article or Supplementary Materials.
Acknowledgments
We gratefully acknowledge the Sapienza University of Rome and the Catholic University of Sacred Heart, Rome.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.E.; Hultzsch, K.C.; Yus, M.; Foubelo, F.; Tada, M. Hydroamination: Direct Addition of Amines to Alkenes and Alkynes. Chem. Rev. 2008, 108, 3795–3892. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A. Gold-Catalyzed Synthesis of Nitrogen Heterocyclic Compounds via Hydroamination Reactions. In Au-Catalyzed Synthesis and Functionalization of Heterocycles; Bandini, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 53–85. [Google Scholar]
- Widenhoefer, R.A.; Han, X. Gold-Catalyzed Hydroamination of C–C Multiple Bonds. Eur. J. Org. Chem. 2006, 2006, 4555–4563. [Google Scholar] [CrossRef]
- Hartwig, J.F. Organotransition Metal Chemistry: From Bonding to Catalysis; University Science Books: Sausalito, CA, USA, 2010. [Google Scholar]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A. Construction of the 1,5-Benzodiazepine Skeleton from o-Phenylendiamine and Propargylic Alcohols via a Domino Gold-Catalyzed Hydroamination/Cyclization Process. Org. Lett. 2016, 18, 3511–3513. [Google Scholar] [CrossRef] [PubMed]
- Cacchi, S.; Fabrizi, G.; Fochetti, A.; Ghirga, F.; Goggiamani, A.; Iazzetti, A. Stereo- and regioselective gold(i)-catalyzed hydroamination of 2-(arylethynyl)pyridines with anilines. Org. Biomol. Chem. 2019, 17, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Grinev, A.N.; Trofimkin, Y.I.; Lomanova, E.V.; Andreeva, N.I.; Mashkovskii, M.D. Synthesis and biological activity of pyrrolo [1,2,3-de]-quinoxaline derivatives. Pharm. Chem. J. 1978, 12, 895–898. [Google Scholar] [CrossRef]
- Nagata, R.; Tanno, N.; Kodo, T.; Ae, N.; Yamaguchi, H.; Nishimura, T.; Antoku, F.; Tatsuno, T.; Kato, T. Tricyclic Quinoxalinediones: 5,6-Dihydro-1H-pyrrolo [1,2,3-de]quinoxaline-2,3-diones and 6,7-Dihydro-1H,5H-pyrido [1,2,3-de]quinoxaline-2,3-diones as Potent Antagonists for the Glycine Binding Site of the NMDA Receptor. J. Med. Chem. 1994, 37, 3956–3968. [Google Scholar] [CrossRef] [PubMed]
- Campiani, G.; Aiello, F.; Fabbrini, M.; Morelli, E.; Ramunno, A.; Armaroli, S.; Nacci, V.; Garofalo, A.; Greco, G.; Novellino, E.; et al. Quinoxalinylethylpyridylthioureas (QXPTs) as potent non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors. Further SAR studies and identification of a novel orally bioavailable hydrazine-based antiviral agent. J. Med. Chem. 2001, 44, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Grellier, P.; Labaied, M.; Sonnet, P.; Léger, J.-M.; Déprez-Poulain, R.; Forfar-Bares, I.; Dallemagne, P.; Lemaître, N.; Péhourcq, F.; et al. Synthesis, Antimalarial Activity, and Molecular Modeling of New Pyrrolo [1,2-a]quinoxalines, Bispyrrolo [1,2-a]quinoxalines, Bispyrido [3,2-e]pyrrolo [1,2-a]pyrazines, and Bispyrrolo [1,2-a]thieno [3,2-e]pyrazines. J. Med. Chem. 2004, 47, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Mouray, E.; Moreau, S.; Mullié, C.; Forfar, I.; Desplat, V.; Belisle-Fabre, S.; Pinaud, N.; Ravanello, F.; Le-Naour, A.; et al. New ferrocenic pyrrolo [1,2-a]quinoxaline derivatives: Synthesis, and in vitro antimalarial activity—Part II. Eur. J. Med. Chem. 2011, 46, 2310–2326. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Forfar, I.; Mamani-Matsuda, M.; Desplat, V.; Saliège, M.; Thiolat, D.; Massip, S.; Tabourier, A.; Léger, J.-M.; Dufaure, B.; et al. Synthesis, analytical behaviour and biological evaluation of new 4-substituted pyrrolo [1,2-a]quinoxalines as antileishmanial agents. Bioorg. Med. Chem. 2007, 15, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Nim, S.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Thivet, E.; Marchivie, M.; Di Pietro, A.; Prasad, R.; Le Borgne, M. Synthesis of new piperazinyl-pyrrolo [1,2-a]quinoxaline derivatives as inhibitors of Candida albicans multidrug transporters by a Buchwald-Hartwig cross-coupling reaction. RSC Adv. 2020, 10, 2915–2931. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Aiello, F.; Grazia, O.D.; Brizzi, A.; Garofalo, A.; Neamati, N. Synthesis and antitumor activities of a series of novel quinoxalinhydrazides. Bioorg. Med. Chem. 2007, 15, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.P.; Bian, Z.; Shaw, S.; Zhao, B.; Goodwin, C.M.; Belmar, J.; Browning, C.F.; Vigil, D.; Friberg, A.; Camper, D.V.; et al. Discovery of tricyclic indoles that potently inhibit Mcl-1 using fragment-based methods and structure-based design. J. Med. Chem. 2015, 58, 3794–3805. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Pessoa, A.M.; Cordeiro, M.N.D.S.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, its derivatives and applications: A State of the Art review. Eur. J. Med. Chem. 2015, 97, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.H.; Baron, M.; Biffis, A. Gold-Catalyzed Intermolecular Alkyne Hydrofunctionalizations—Mechanistic Insights. Catalysts 2020, 10, 1210. [Google Scholar] [CrossRef]
- Calculated by HF, 6-31G** in Titan 1.0.1 2000; Wavefunction Inc.: Irvine, CA, USA, 2000.
- Richardson, A., Jr. The Chemistry of 7-Aminoindoline and Certain Pyrrolo- and Pyrido [1,2,3-de]quinoxalines. J. Org. Chem. 1965, 30, 2589–2593. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).