Antibacterial and Analgesic Properties of Beta-Caryophyllene in a Murine Urinary Tract Infection Model
Abstract
1. Introduction
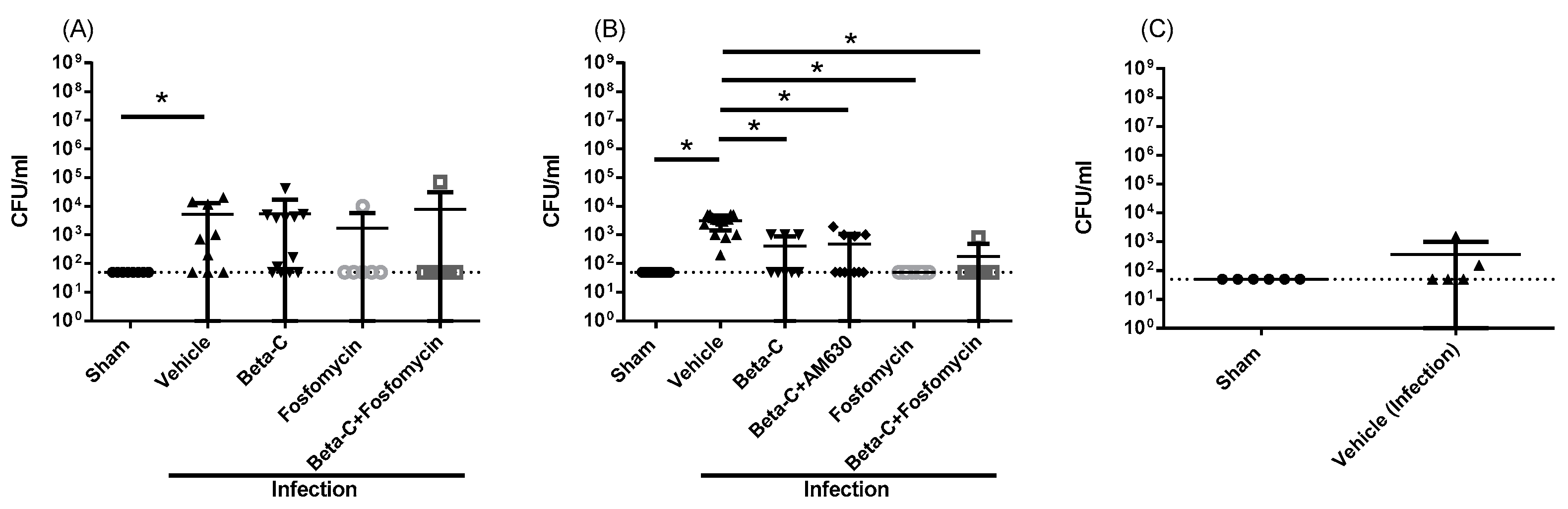
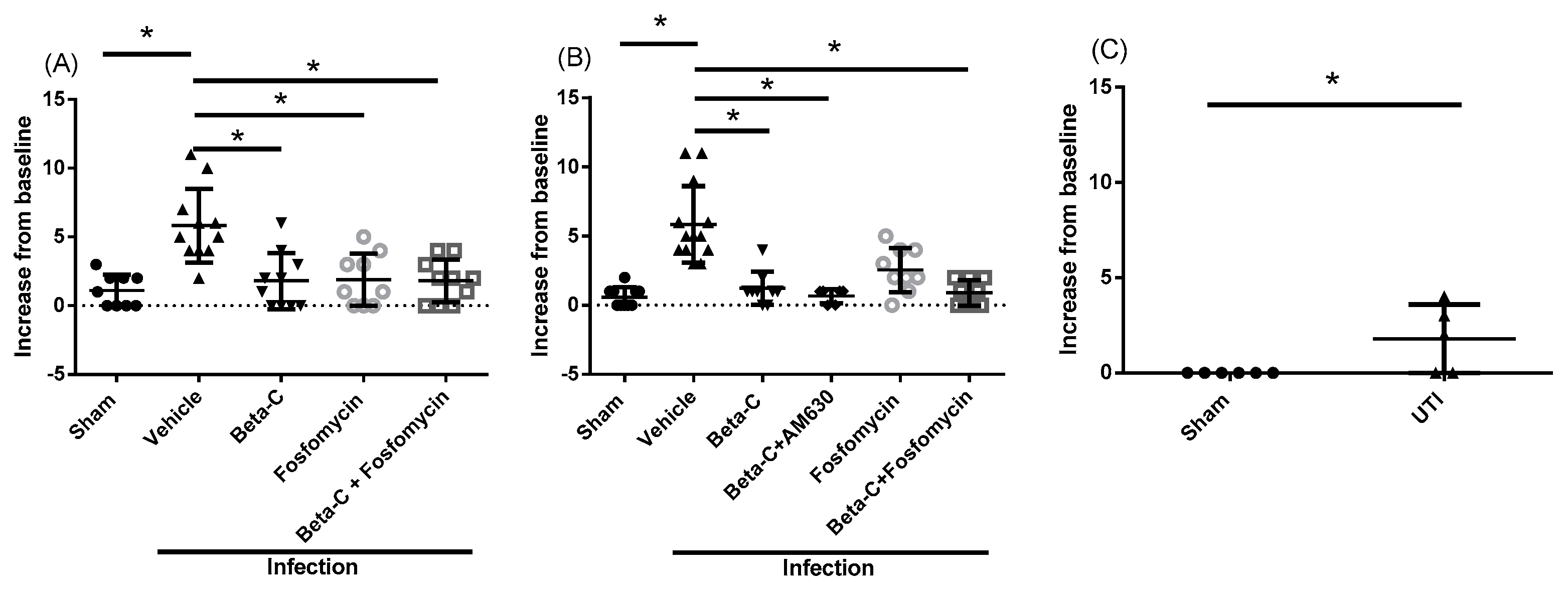
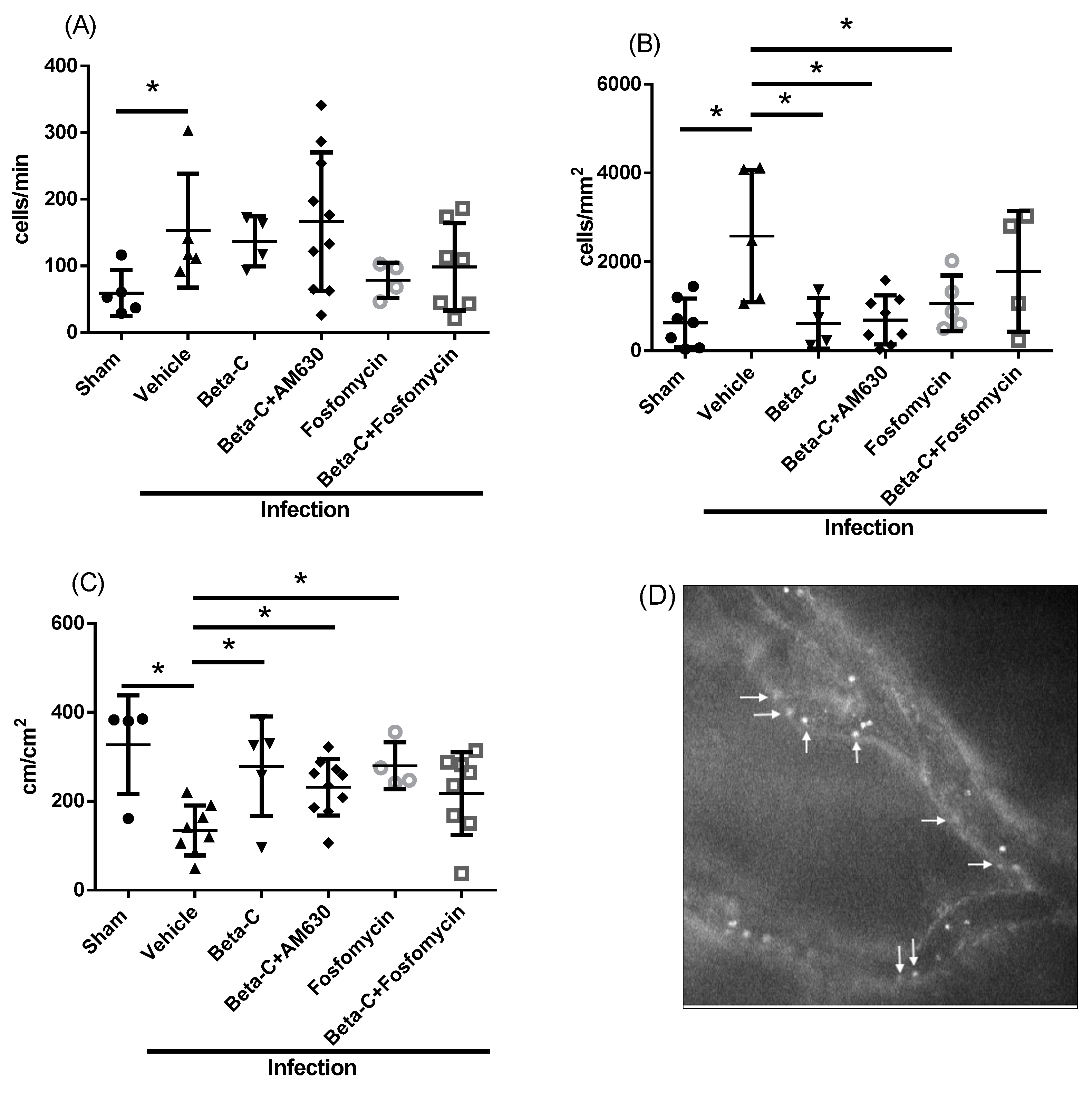
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Culture Conditions
4.2. Murine Model of Lower UTI
4.3. Pain and Behavior Assessment
4.4. Intravital Microscopy
4.5. Bacterial Enumeration
4.6. Analysis of Bladder Tissue Cytokines
4.7. Bladder Histopathology
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhan, J.; Zhang, K.; Chen, H.; Cheng, S. Global, Regional, and National Burden of Urinary Tract Infections from 1990 to 2019: An Analysis of the Global Burden of Disease Study 2019. World J. Urol. 2022, 40, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. The Epidemiology of Urinary Tract Infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Glover, M.; Moreira, C.G.; Sperandio, V.; Zimmern, P. Recurrent Urinary Tract Infections in Healthy and Nonpregnant Women. Urol. Sci. 2014, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, 103–120. [Google Scholar] [CrossRef]
- Ganguly, N.; Giang, P.H.; Gupta, C.; Basu, S.K.; Siddiqui, I.; Salunke, D.M.; Sharma, P. Mycobacterium Tuberculosis Secretory Proteins CFP-10, ESAT-6 and the CFP10:ESAT6 Complex Inhibit Lipopolysaccharide-Induced NF-ΚB Transactivation by Downregulation of Reactive Oxidative Species (ROS) Production. Immunol. Cell Biol. 2008, 86, 98–106. [Google Scholar] [CrossRef]
- Brown, P.D. Impact of Changing Patterns of Anti-Microbial Resistance in Uropathogens: Emerging Treatment and Strategies. Curr. Infect. Dis. Rep. 2003, 5, 499–503. [Google Scholar] [CrossRef]
- DeMarsh, M.; Bookstaver, P.B.; Gordon, C.; Lim, J.; Griffith, N.; Bookstaver, N.K.; Justo, J.A.; Kohn, J.; Al-Hasan, M.N. Prediction of Trimethoprim/Sulfamethoxazole Resistance in Community-Onset Urinary Tract Infections. J. Glob. Antimicrob. Resist. 2020, 21, 218–222. [Google Scholar] [CrossRef]
- Gardiner, B.J.; Stewardson, A.J.; Abbott, I.J.; Peleg, A.Y. Nitrofurantoin and Fosfomycin for Resistant Urinary Tract Infections: Old Drugs for Emerging Problems. Aust. Prescr. 2019, 42, 14–19. [Google Scholar] [CrossRef]
- Critchley, I.A.; Cotroneo, N.; Pucci, M.J.; Mendes, R. The Burden of Antimicrobial Resistance among Urinary Tract Isolates of Escherichia coli in the United States in 2017. PLoS ONE 2019, 14, e0220265. [Google Scholar] [CrossRef]
- Mohsen, S.; Dickinson, J.A.; Somayaji, R. Update on the Adverse Effects of Antimicrobial Therapies in Community Practice. Can. Fam. Physician 2020, 66, 651–659. [Google Scholar] [PubMed]
- Colgan, R.; Williams, M. Diagnosis and Treatment of Acute Uncomplicated Cystitis. Am. Fam. Physician 2011, 84, 771–776. [Google Scholar] [PubMed]
- Pertwee, R.G. The Therapeutic Potential of Drugs That Target Cannabinoid Receptors or Modulate the Tissue Levels or Actions of Endocannabinoids. Drug Addict. From Basic Res. Ther. 2008, 7, 637–686. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Merriam, F.V.; Wang, Z.; Guerios, S.D.; Bjorling, D.E. Cannabinoid Receptor 2 Is Increased in Acutely and Chronically Inflamed Bladder of Rats. Neurosci. Lett. 2008, 445, 130–134. [Google Scholar] [CrossRef]
- Christie, S.; Brookes, S.; Zagorodnyuk, V. Endocannabinoids in Bladder Sensory Mechanisms in Health and Diseases. Front. Pharmacol. 2021, 12, 708989. [Google Scholar] [CrossRef]
- Berger, G.; Arora, N.; Burkovskiy, I.; Xia, Y.; Chinnadurai, A.; Westhofen, R.; Hagn, G.; Cox, A.; Kelly, M.; Zhou, J.; et al. Experimental Cannabinoid 2 Receptor Activation by Phyto-Derived and Synthetic Cannabinoid Ligands in LPS-Induced Interstitial Cystitis in Mice. Molecules 2019, 24, 4239. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Maffei, M.E. Plant Natural Sources of the Endocannabinoid (E)-β-Caryophyllene: A Systematic Quantitative Analysis of Published Literature. Int. J. Mol. Sci. 2020, 21, 6540. [Google Scholar] [CrossRef]
- Smedman, L.Å.; Snajberk, K.; Zavarin, E.; Mon, T.R. Oxygenated Monoterpenoids and Sesquiterpenoid Hydrocarbons of the Cortical Turpentine from Different Abies Species. Phytochemistry 1969, 8, 1471–1479. [Google Scholar] [CrossRef]
- Bastaki, M.; Api, A.M.; Aubanel, M.; Bauter, M.; Cachet, T.; Demyttenaere, J.C.R.; Diop, M.M.; Harman, C.L.; Hayashi, S.M.; Krammer, G.; et al. Dietary Administration of β-Caryophyllene and Its Epoxide to Sprague-Dawley Rats for 90 Days. Food Chem. Toxicol. 2020, 135, 110876. [Google Scholar] [CrossRef]
- Klauke, A.-L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The Cannabinoid CB2 Receptor-Selective Phytocannabinoid Beta-Caryophyllene Exerts Analgesic Effects in Mouse Models of Inflammatory and Neuropathic Pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, C.; Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A. Local Anaesthetic Activity of β-Caryophyllene. Farmaco 2001, 56, 387–389. [Google Scholar] [CrossRef]
- Neta, M.C.S.; Vittorazzi, C.; Guimarães, A.C.; Martins, J.D.L.; Fronza, M.; Endringer, D.C.; Scherer, R. Effects of β-Caryophyllene and Murraya paniculata Essential Oil in the Murine Hepatoma Cells and in the Bacteria and Fungi 24-h Time-Kill Curve Studies. Pharm. Biol. 2017, 55, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Jwa, S.K. Inhibitory Effects of β-Caryophyllene on Streptococcus Mutans Biofilm. Arch. Oral Biol. 2018, 88, 42–46. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Pieri, F.A.; Souza, M.C.d.C.; Vermelho, L.L.R.; Vermelho, M.L.R.; Perciano, P.G.; Vargas, F.S.; Borges, A.P.B.; da Veiga-Junior, V.F.; Moreira, M.A.S. Use of β-Caryophyllene to Combat Bacterial Dental Plaque Formation in Dogs. BMC Vet. Res. 2016, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Rudick, C.N.; Billips, B.K.; Pavlov, V.I.; Yaggie, R.E.; Schaeffer, A.J.; Klumpp, D.J. Host-Pathogen Interactions Mediating Pain of Urinary Tract Infection. J. Infect. Dis. 2010, 201, 1240–1249. [Google Scholar] [CrossRef]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; Landefeld, C.S.; et al. Screening for Asymptomatic Bacteriuria in Adults: US Preventive Services Task Force Recommendation Statement. JAMA-J. Am. Med. Assoc. 2019, 322, 1188–1194. [Google Scholar] [CrossRef]
- Rosen, J.M.; Klumpp, D.J. Mechanisms of Pain from Urinary Tract Infection. Int. J. Urol. 2014, 21, 26–32. [Google Scholar] [CrossRef]
- Rosen, J.M.; Yaggie, R.E.; Woida, P.J.; Miller, R.J.; Schaeffer, A.J.; Klumpp, D.J. TRPV1 and the MCP-1/CCR2 Axis Modulate Post-UTI Chronic Pain. Sci. Rep. 2018, 8, 7188. [Google Scholar] [CrossRef] [PubMed]
- Heblinski, M.; Santiago, M.; Fletcher, C.; Stuart, J.; Connor, M.; McGregor, I.S.; Arnold, J.C. Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Actions of Phytocannabinoids or Endocannabinoids on TRPA1 and TRPV1 Channels. Cannabis Cannabinoid Res. 2020, 5, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.P.; Boi, M.; Carta, A.; Murru, E.; Quartu, M.; Carta, G.; Banni, S. Anti-Inflammatory Effect of Beta-Caryophyllene Mediated by the Involvement of TRPV1, BDNF and TrkB in the Rat Cerebral Cortex after Hypoperfusion/Reperfusion. Int. J. Mol. Sci. 2022, 23, 3633. [Google Scholar] [CrossRef]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.S.; Chun, H.S.; Kwon, S.H.; et al. β-Caryophyllene Potently Inhibits Solid Tumor Growth and Lymph Node Metastasis of B16F10 Melanoma Cells in High-Fat Diet-Induced Obese C57BL/6N Mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Engelsöy, U.; Rangel, I.; Demirel, I. Impact of Proinflammatory Cytokines on the Virulence of Uropathogenic Escherichia coli. Front. Microbiol. 2019, 10, 1051. [Google Scholar] [CrossRef]
- Waldhuber, A.; Puthia, M.; Wieser, A.; Cirl, C.; Dürr, S.; Neumann-Pfeifer, S.; Albrecht, S.; Römmler, F.; Müller, T.; Zheng, Y.; et al. Uropathogenic Escherichia coli Strain CFT073 Disrupts NLRP3 Inflammasome Activation. J. Clin. Investig. 2016, 126, 2425–2436. [Google Scholar] [CrossRef]
- Cirl, C.; Wieser, A.; Yadav, M.; Duerr, S.; Schubert, S.; Fischer, H.; Stappert, D.; Wantia, N.; Rodriguez, N.; Wagner, H.; et al. Subversion of Toll-like Receptor Signaling by a Unique Family of Bacterial Toll/Interleukin-1 Receptor Domain-Containing Proteins. Nat. Med. 2008, 14, 399–406. [Google Scholar] [CrossRef]
- Yadav, M.; Zhang, J.; Fischer, H.; Huang, W.; Lutay, N.; Cirl, C.; Lum, J.; Miethke, T.; Svanborg, C. Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Escherichia coli Virulence. PLoS Pathog. 2010, 6, e1001120. [Google Scholar] [CrossRef]
- Schiwon, M.; Weisheit, C.; Franken, L.; Gutweiler, S.; Dixit, A.; Meyer-Schwesinger, C.; Pohl, J.M.; Maurice, N.J.; Thiebes, S.; Lorenz, K.; et al. Crosstalk between Sentinel and Helper Macrophages Permits Neutrophil Migration into Infected Uroepithelium. Cell 2014, 156, 456–468. [Google Scholar] [CrossRef]
- Zec, K.; Volke, J.; Vijitha, N.; Thiebes, S.; Gunzer, M.; Kurts, C.; Engel, D.R. Neutrophil Migration into the Infected Uroepithelium Is Regulated by the Crosstalk between Resident and Helper Macrophages. Pathogens 2016, 5, 15. [Google Scholar] [CrossRef]
- Szczesniak, A.M.; Porter, R.F.; Toguri, J.T.; Borowska-Fielding, J.; Gebremeskel, S.; Siwakoti, A.; Johnston, B.; Lehmann, C.; Kelly, M.E.M. Cannabinoid 2 Receptor Is a Novel Anti-Inflammatory Target in Experimental Proliferative Vitreoretinopathy. Neuropharmacology 2017, 113, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.; Kianian, M.; Zhou, J.; Kuster, I.; Kuschnereit, R.; Whynot, S.; Hung, O.; Shukla, R.; Johnston, B.; Cerny, V.; et al. Cannabinoid Receptor 2 Activation Reduces Intestinal Leukocyte Recruitment and Systemic Inflammatory Mediator Release in Acute Experimental Sepsis. Crit. Care 2012, 16, R47. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.H.; Haskó, J.; Skuba, A.; Fan, S.; Dykstra, H.; McCormick, R.; Reichenbach, N.; Krizbai, I.; Mahadevan, A.; Zhang, M.; et al. Activation of Cannabinoid Receptor 2 Attenuates Leukocyte-Endothelial Cell Interactions and Blood-Brain Barrier Dysfunction under Inflammatory Conditions. J. Neurosci. 2012, 32, 4004–4016. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, J.; Kelly, M.E.M.; Zhou, J.; Lehmann, C. Experimental Cannabinoid 2 Receptor-Mediated Immune Modulation in Sepsis. Mediat. Inflamm. 2014, 2014, 978678. [Google Scholar] [CrossRef]
- Zhang, M.; Martin, B.R.; Adler, M.W.; Razdan, R.K.; Jallo, J.I.; Tuma, R.F. Cannabinoid CB2 Receptor Activation Decreases Cerebral Infarction in a Mouse Focal Ischemia/Reperfusion Model. J. Cereb. Blood Flow Metab. 2007, 27, 1387–1396. [Google Scholar] [CrossRef]
- Krustev, E.; Reid, A.; McDougall, J.J. Tapping into the Endocannabinoid System to Ameliorate Acute Inflammatory Flares and Associated Pain in Mouse Knee Joints. Arthritis Res. Ther. 2014, 16, 1–12. [Google Scholar] [CrossRef]
- Zeitlinger, M.; Marsik, C.; Steiner, I.; Sauermann, R.; Seir, K.; Jilma, B.; Wagner, O.; Joukhadar, C. Immunomodulatory Effects of Fosfomycin in an Endotoxin Model in Human Blood. J. Antimicrob. Chemother. 2007, 59, 219–223. [Google Scholar] [CrossRef]
- Bajory, Z.; Szabó, A.; Király, I.; Pajor, L.; Messmer, K. Involvement of Nitric Oxide in Microcirculatory Reactions after Ischemia-Reperfusion of the Rat Urinary Bladder. Eur. Surg. Res. 2008, 42, 28–34. [Google Scholar] [CrossRef]
- Ince, C. Hemodynamic Coherence and the Rationale for Monitoring the Microcirculation. Crit. Care 2015, 19 (Suppl. 3), S8. [Google Scholar] [CrossRef]
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef]
- Welch, R.A.; Burland, V.; Plunkett, G.; Redford, P.; Roesch, P.; Rasko, D.; Buckles, E.L.; Liou, S.-R.; Boutin, A.; Hackett, J.; et al. Extensive Mosaic Structure Revealed by the Complete Genome Sequence of Uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 17020–17024. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Pal, K.K.; Bhatt, D.M.; Chauhan, S.M. Growth Promotion and Yield Enhancement of Peanut (Arachis hypogaea L.) by Application of Plant Growth-Promoting Rhizobacteria. Microbiol. Res. 2004, 159, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Hannan, T.J.; Hunstad, D.A. A Murine Model for Escherichia coli Urinary Tract Infection. Methods Mol. Biol. 2016, 1333, 159–175. [Google Scholar] [CrossRef]
- Da Silva Oliveira, G.L.; Machado, K.C.; Machado, K.C.; da Silva, A.P.d.S.C.L.; Feitosa, C.M.; de Castro Almeida, F.R. Non-Clinical Toxicity of β-Caryophyllene, a Dietary Cannabinoid: Absence of Adverse Effects in Female Swiss Mice. Regul. Toxicol. Pharmacol. 2018, 92, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Boucher, M.; Meen, M.; Codron, J.P.; Coudore, F.; Kemeny, J.L.; Eschalier, A. Cyclophosphamide-Induced Cystitis in Freely-Moving Conscious Rats: Behavioral Approach to a New Model of Visceral Pain. J. Urol. 2000, 164, 203–208. [Google Scholar] [CrossRef]
- Hopkins, W.J.; Gendron-Fitzpatrick, A.; Balish, E.; Uehling, D.T. Time Course and Host Responses to Escherichia coli Urinary Tract Infection in Genetically Distinct Mouse Strains. Infect. Immun. 1998, 66, 2798–2802. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickson, K.; Scott, C.; White, H.; Zhou, J.; Kelly, M.; Lehmann, C. Antibacterial and Analgesic Properties of Beta-Caryophyllene in a Murine Urinary Tract Infection Model. Molecules 2023, 28, 4144. https://doi.org/10.3390/molecules28104144
Dickson K, Scott C, White H, Zhou J, Kelly M, Lehmann C. Antibacterial and Analgesic Properties of Beta-Caryophyllene in a Murine Urinary Tract Infection Model. Molecules. 2023; 28(10):4144. https://doi.org/10.3390/molecules28104144
Chicago/Turabian StyleDickson, Kayle, Cassidy Scott, Hannah White, Juan Zhou, Melanie Kelly, and Christian Lehmann. 2023. "Antibacterial and Analgesic Properties of Beta-Caryophyllene in a Murine Urinary Tract Infection Model" Molecules 28, no. 10: 4144. https://doi.org/10.3390/molecules28104144
APA StyleDickson, K., Scott, C., White, H., Zhou, J., Kelly, M., & Lehmann, C. (2023). Antibacterial and Analgesic Properties of Beta-Caryophyllene in a Murine Urinary Tract Infection Model. Molecules, 28(10), 4144. https://doi.org/10.3390/molecules28104144








