Abstract
COVID-19 has had an impact on human quality of life and economics. Scientists have been identifying remedies for its prevention and treatment from all possible sources, including plants. Nigella sativa L. (NS) is an important medicinal plant of Islamic value. This review highlights the anti-COVID-19 potential, clinical trials, inventions, and patent literature related to NS and its major chemical constituents, like thymoquinone. The literature was collected from different databases, including Pubmed, Espacenet, and Patentscope. The literature supports the efficacy of NS, NS oil (NSO), and its chemical constituents against COVID-19. The clinical data imply that NS and NSO can prevent and treat COVID-19 patients with a faster recovery rate. Several inventions comprising NS and NSO have been claimed in patent applications to prevent/treat COVID-19. The patent literature cites NS as an immunomodulator, antioxidant, anti-inflammatory, a source of anti-SARS-CoV-2 compounds, and a plant having protective effects on the lungs. The available facts indicate that NS, NSO, and its various compositions have all the attributes to be used as a promising remedy to prevent, manage, and treat COVID-19 among high-risk people as well as for the therapy of COVID-19 patients of all age groups as a monotherapy or a combination therapy. Many compositions of NS in combination with countless medicinal herbs and medicines are still unexplored. Accordingly, the authors foresee a bright scope in developing NS-based anti-COVID-19 composition for clinical use in the future.
1. Introduction
COVID-19, a pandemic transmissible sickness, is triggered by SARS-CoV-2. As of 1 April 2022, this disease has infected >488 million individuals, led to about 6.16 million mortalities, and affected the quality of life and worldwide economy since its inception in 2019 [1]. COVID-19 cases have been documented around the globe, wherein the United States, India, Brazil, France, and Germany have reported the majority of cases [1]. The clinical features of COVID-19 are similar to normal flu (cough, sore throat, high fever, headache, sneezing, myalgia, breathlessness, etc.), and it mainly targets the respiratory system, leading to acute respiratory distress syndrome. If untreated on time, COVID-19 may be fatal, and the patient may die due to pneumonia, respiratory failure, and damage to other vital organs due to cytokine storm [2]. Some vaccines (Janssen, Moderna, and Pfizer-BioNTech), new drugs (remdesivir, molnupiravir, favipiravir, and a combination of nirmatrelvir with ritonavir), and supportive therapy (a combination of paracetamol, vitamin C, and zinc) have been established to prevent/treat COVID19 [2,3,4]. The drugs used to prevent/treat COVID-19 act on different enzymes/proteins of SARS-CoV-2, including RNA-dependent-RNA-polymerase, helicase, main protease, non-structural proteins (Nsp9 and Nsp15), and RNA replicase [2,3,4]. Many new drugs and their combinations are also in clinical trials [3,4]. Despite the development of many vaccines and some drugs, the battle against COVID-19 is far from over. The identification and the spread of new contagious SARS-CoV-2 strains (α, β, γ, δ, Omicron, etc.) also pose challenges to the existing vaccines and treatment of COVID-19 [5]. Accordingly, the development of alternative and supporting therapies has been perceived as a promising way to combat COVID-19 [6,7,8,9,10]. Many studies have demonstrated the effectiveness of health supplements (zinc, vitamin C, vitamin D, etc.) and herbal products (black seed, honey, ginger, garlic, etc.) to prevent/treat COVID-19 [11,12]. This review highlights the anti-COVID-19 potential of Nigella sativa L. (NS) and its major chemical constituents.
2. Materials and Methods
The non-patent literature search for the titled review was performed on the PubMed database and Sci-finder database, utilizing keywords COVID-19 or SARS-CoV-2 in combination with different keywords related to N. sativa (Black cumin, Black seed, and Kalonji). The relevant non-patent literature citing the prophylactic/therapeutic use of N. sativa against COVID-19 was reviewed and cited in this review. The patent searching was performed utilizing different patent databases (Sci-Finder, Espacenet, Patentscope, and USPTO). The patents/patent applications were segregated according to their patent family, and duplicate patent families were removed. The patent applications that exclusively provide clear information about the use of N. sativa or N. sativa-based inventions to treat COVID-19 have been included in this review. The patent searching results are provided as Supplementary Material (Table S1).
3. N. sativa L.
3.1. Introduction
Phytotherapy is one of the alternative approaches explored by the scientific community to discover and develop preventive and/or therapeutic regimens against the menace of SAR-CoV-2. Amongst thousands of ethnomedicinal plants which are known to be effective against infectious diseases and alleviate acute respiratory symptoms, N. sativa and its phytoconstituents are one of the extensively studied natural substances.
N. sativa belonging to the family Ranunculaceae is a nutrient-rich, annual flowering medicinal plant of significant historical and religious importance. It is usually recognized as black seed or black cumin in English, Habbatul barakah in Arabic, and Kalonji in Hindi and Urdu [13]. Its nutritional value and a wide array of therapeutic properties have been known to mankind since ancient times. Muslims consider black seed as a panacea, i.e., a remedy that can cure all diseases except death, while traditional medicine practitioners believed it to be ‘the herb from heaven’ [14]. Its beneficial uses are also mentioned by the 10th-century physician Avicenna in his book ‘The Canon of medicine’ [15]. Because of its health benefits and a broad spectrum of traditional medicinal uses, this nutraceutical herb has been described in the literature as a miraculous herb [16], prophetic medicine [17], habbat al barakah [18], a holy herb of the Middle East [19], a mine for multi cure [20], a novel beauty care ingredient [21], a complementary drug [22], a universal healer [23], a marvel herb [24], a black seed of miracle [25], blessed seed [26], and a wonder herb [27].
Although all parts of the black seed herbaceous plant are of biological and economic importance, NS seeds and their oil (NSO) are most commonly consumed for their health benefits and to treat diseases in various indigenous systems of medicines [13]. NS seeds, owing to their peculiar aroma, fragrance, and nutritional value, are also used as food additives. The nutritional value of black seed is due to its rich content of vegetable proteins and amino acids, fats, fibers, carbohydrates, minerals, and vitamins. In traditional and indigenous medicine, different dosage forms of black seeds have been employed to manage multiple acute and chronic sicknesses, including respiratory diseases (asthma, bronchitis), pain and inflammation, skin allergy, hypertension, diabetes, liver disorders, rheumatism, mental disorder, malaria, loss of hunger, insomnia, snake bite, indigestion, and dysmenorrhea [28,29]. Numerous preclinical and clinical studies have explored the health-promoting potential of this traditional medicinal plant and provided evidence in favor of its antioxidant, antidiabetic, cardioprotective, neuroprotective, analgesic and anti-inflammatory, antimicrobial, anthelmintic, anti-oxytocic, anti-obesity, anti-cancer, anti-asthmatic, hepatoprotective, gastroprotective, anti-schistosomiasis, immunomodulatory, nephroprotective, and wound healing activities. Traditional uses, nutritional properties, and biological activities of NS have been thoroughly reviewed [13,15,28,29,30,31].
Phytochemical investigations of various plant parts of NS revealed it to contain various classes of secondary bioactive metabolites, such as terpenoids (thymoquinone, p-cymene, trans-anethole, carvacrol, limonene, dithymoquinone), alkaloids (nigellidine, nigellicimine, nigellicine, nigellicimine N-oxide, nigellamines), sterols (sitosterol, stigmasterol, tocopherol, tocotrienol), saponins (alpha-hederin, kalopanoxsaponin, nigella A-D), flavonoids (quercetin, kaempferol, rutin), phenolic acids (gallic acid, vanillic acid, trans-cinnamic acid) [13,28,29,32,33]. Black seeds on extraction with organic solvents or mechanical pressing yield approximately 34–39% of NS fixed oil (NSO) which primarily contains lipids (~98% to 99%) such as triacylglycerol, free fatty acids, sterols, and a small amount of volatile oil (1% to 2%) such as p-cymene and thymol [28,29]. Many preparations of NS seeds, seed oil, and extracts containing varying amounts of thymoquinone (TQ), either alone or in combination with other herbal products in capsule, powder, soft gels, and oil forms, are also available on the market. Black Cummin® of Bixabotanical is a proprietary Ayurvedic medicine for digestion to promote immunity and general health. Black seed oil containing 2% TQ extract, a brand of Health Thru Nutrition (HTN), is a dietary supplement available commercially as soft gels (500 mg).
3.2. Important Biologically Active Chemical Constituents of NS
The pharmacological activities of NS, in general, are credited to the existence of volatile components, especially TQ, but other secondary metabolites (alkaloids, pentacyclic triterpenes, phytosterols, flavonoids), including those present in NSO, have also been shown to exhibit therapeutic activities [34,35,36,37,38] (Figure 1). The chemical structure of selected important bioactive components of NS is presented in Figure 2.
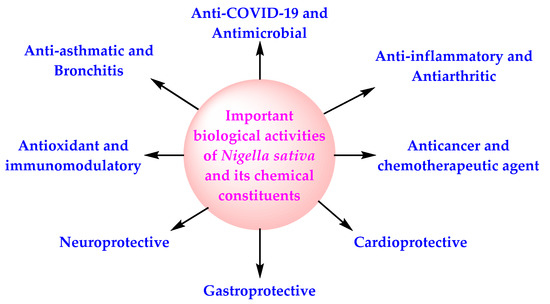
Figure 1.
Therapeutic properties of NS and its chemical components.
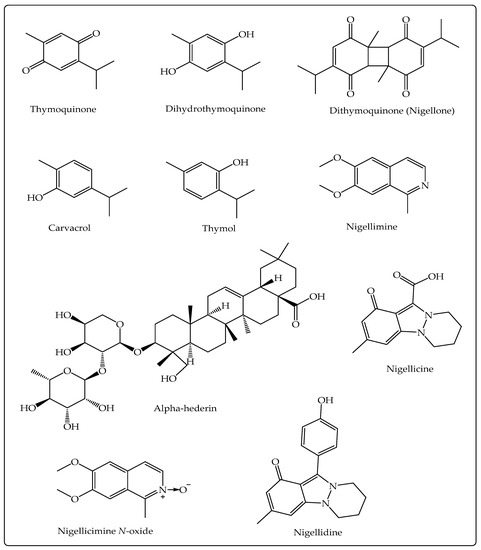
Figure 2.
Chemical structure of the main bioactive constituents of N. sativa L. against COVID-19.
TQ (2-methyl-5-isopropyl-1, 4-benzoquinone; Figure 2) is a monoterpenoid and one of the key volatile components (30%–48%) of the NS volatile oil which exhibits an array of useful bioactivities [39]. It shows anti-inflammatory action by decreasing the appearance of pro-inflammatory cytokines (the COX-2, TNF-α, IL-6) and by upregulating the levels of IL-4 and IL-10, the anti-inflammatory cytokines [40]. Recently, Boskabady et al. demonstrated that TQ is effective in ameliorating lipopolysaccharide (LPS) generated lung inflammation in rats [41]. The immunomodulatory influences of TQ are mediated through activation of T cells, the appearance of CD62L and CD8+ T cells, and by helping in the formation of IFN-γ [42]. It is an effective chemotherapeutic agent against breast, bladder, cervical, colorectal, gastric, lung, prostate, pancreatic cancers, and leukemia in vitro and in vivo studies. It shows anticancer activity by producing apoptosis, autophagia, cell cycle arrest, controlling angiogenesis, and regulating signaling pathways [35,43,44]. Thymohydroquinone (THQ; Figure 2) exhibits stronger antioxidant activity and protects cell damage better than TQ [45]. Some other activities shown by TQ include neuroprotective, cardioprotective, antimicrobial, gastroprotective, antiarthritic, and anti-asthmatic activities [39]. Nigellone has been reported to inhibit trachea contraction along with improved mucociliary clearance and thus is useful in the treatment of bronchitis and asthma [46]. Alpha-Hederin, a water-soluble pentacyclic triterpene of NS, exhibits anticancer activity by inducing apoptosis, anti-inflammatory, and bronchodilatory activity by raising the levels of cAMP that leads to elevated β2-adrenergic responsiveness [47,48,49].
3.3. Anti-COVID-19 Chemical Constituents of NS
Several research publications reviewed the therapeutic activities of NS and TQ viz., antiviral, anti-inflammatory, immunomodulatory, antioxidant, and broncho-dilatory activities and suggested their possible role in combating COVID-19 [50,51,52,53,54]. A summary of the in silico, in vitro, and in vivo investigations done on bioactive phytoconstituents of NS since the break out of the COVID-19 pandemic is presented in Table 1. Data presented in Table 1 indicate that four in silico studies identified α-Hederin as the promising anti-COVID agent while DTQ in one in vitro and three in silico studies emerged as the lead anti-viral candidate. An in vivo study performed in experimental rats similarly exhibited the anti-SARS-CoV-2 efficacy of nigellidine [55]. Surprisingly, TQ was identified as a lead compound in only one in silico and one in vitro study. These phytochemicals have been shown to act at different molecular targets of SARS-CoV-2 (RdRp, 3CLpro, Nsp15, Nsp2, Nsp3, Mpro, and ACE2) (Figure 3).

Table 1.
Summary of several in silico, in vitro, and in vivo investigations carried out on NS chemical constituents against COVID-19.
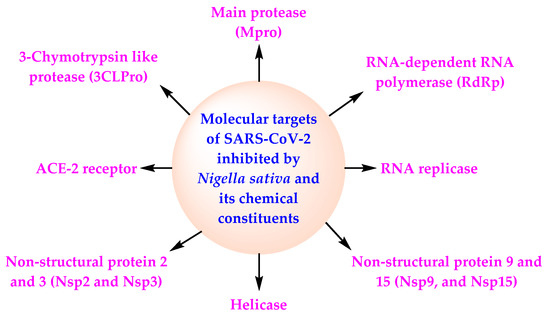
Figure 3.
Mechanism of action of NS and its chemical constituents against SARS-CoV-2.
3.4. Anti-COVID-19 Clinical Trials on NS and Its Chemical Constituents
A search for the clinical studies related to NS was conducted on the clinical trial database [67] utilizing the terms “N. sativa or its synonyms” and the name of its different important chemical constituents (TQ, THQ, DTQ or nigellone, nigellimine, nigellicine, nigellidine, nigellicimine, and nigellicimine N-oxide) on 20 February 2022. This search provided eight clinical studies belonging to NS and TQ to treat/prevent COVID-19. Two CTs have been conducted in Saudi Arabia, two in Pakistan, two in Tunisia, and one each in the United States, and Egypt. Two CTs were related to the prevention of COVID-19, whereas six CTs involved treatment of COVID-19 utilizing NS. Most of the CTs have been conducted employing the capsule (500 mg) containing an effective amount of NS and/or NSO. The CTs have also been done using a combination of NS and honey or Omega 3. The data of this search are summarized in Table 2.

Table 2.
Interventional clinical studies on NS against COVID-19/SARS-CoV-2.
4. Patent Summary
A patent search was carried out on 20 February 2022, using patent databases as mentioned in the literature [68,69,70,71]. The summary of the finalized patents/patent application is provided in Table 3.

Table 3.
Summary of the patent literature.
5. Conclusions
The supplementation of the NS possesses a variety of advantageous effects on human well-being. Several compositions comprising NS and NSO have been tested clinically and have also been claimed in patent applications to prevent/treat COVID-19. The clinical studies and patent filings support the therapeutic efficacy of NS against COVID-19 owing to its immunomodulatory, antioxidant, antiviral, and anti-inflammatory effects. NS can prevent and treat COVID-19 patients with a faster recovery rate. Many compositions of NS in combination with many medicinal herbs and medicines are still unexplored. However, further clinical trials are recommended to establish the anti-COVID-19 potential of the unexplored NS compositions.
6. Discussion and Perspective
NS is one of the Quranic and prophetic medicinal plants with beneficial effects on human health [87]. The literature and clinical studies have demonstrated its efficacy in preventing and treating COVID-19. The summary of eight CTs on NS seeds and NSO is mentioned in Table 1. The data of six CTs have not been published. However, the data of two CTs (NCT04401202 and NCT04347382) involving the use of NS against COVID-19 have been published [52,88].
One of the published clinical studies (NCT04401202) concluded that NSO supplementation provides faster recovery of 62% of the mild COVID-19 patients on day 14 of the treatment. The normal revival time was also briefer than the control group. This study suggested that the reduction in the COVID-19 symptoms (anosmia, chills, runny nose, and loss of appetite) might be owing to the anti-inflammatory properties of NS [52]. The infection of SARS-CoV-2 causes the cytokine storm because of the liberation of leukocytes and pro-inflammatory cytokines [89,90]. The discussion part of the published CTs [52] stated that NS demonstrates immunostimulant and anti-inflammatory effects by restricting the release of pro-inflammatory cytokines [54]. In addition, NS also displays antioxidant and antiviral effects [54]. Accordingly, drugs like NS can stop the cytokine storm and are useful to prevent/treat COVID-19.
The published results of another CT (NCT04347382) affirmed that the combination of honey and NSO improved the COVID-19 symptoms, viral clearance, and mortality among COVID-19 patients. This report also advocated the use of the combination of honey and NS along with other treatments of COVID-19 [88]. According to this study, the combination of honey and NS provided its anti-COVID-19 activity due to their antioxidative/antiviral/immunostimulant chemical constituents (phenolic compounds, flavonoids, and zinc) that attack the multiple sites (lowering the expression of ACE-2 receptor, RdRp, Mpro protease, etc.,) of SARS-CoV-2.
A CT (IRCT20180712040449N2) was conducted in Iran utilizing a combination of 500 mg of capsule-1 containing hydroethanolic extract of Rheum palmatum (root), Glycyrrhiza glabra (rhizome), and Punica granatum (fruit peel) (0.5:1:1), capsule-2 containing 500 mg of NS seed powder, and a polyherbal water decoction prepared from a herbal mixture (1 g each herb in the same sachet) comprising Matricaria chamomilla (flower), Zataria multiflora (aerial part), Glycyrrhiza glabra (root), Ficus carica (fruit), Urtica dioica (leaf), Althaea officinalis (flower), and Nepeta bracteate (flower) [91]. Capsule-1 and capsule-2 were administered to patients two times a day and the polyherbal decoction two times a day along with the standard care. This treatment significantly reduced the hospital dyspnea, accelerated recovery time, and lowered the COVID-19 symptoms. This study implicitly indicates that the chemical constituents of NS (TQ, hederagenin, THQ, nigelledine, and α-hederin) are anti-COVID-19 compounds.
Many in silico, in vitro, and in vivo studies have demonstrated potential anti-COVID-19 activity of the main chemical constituents of NS due to their diverse mechanisms of action against SARS-CoV-2 (Table 1) (Figure 3). TQ is one of the main chemical constituents of NSO possessing antioxidant, anti-inflammatory, and antiviral activities [31,92]. It is disclosed that TQ may bind the hydrophobic component of SARS-CoV-2 because of its lipophilicity. This causes activation of the virus. TQ also reduces the generation of cytokine storm in COVID-19 patients because of its anti-inflammatory potential [93]. TQ has also demonstrated SARS-CoV-2 inhibitory activity and can be an efficacious adjuvant for the therapy of COVID-19 [94].
Many findings have also established the potential of NS seeds and TQ in reducing thrombus formation [95]. It is a well-known fact that thrombus formation causes multiple organ collapse and fatality among COVID-19 patients. Therefore, NS may be used as a therapeutic formulation, including its nano-formulations [96] to treat COVID-19, and may also be used as a supportive therapy with anti-COVID-19 medicines [4,6,97,98].
NS and its oil possess antiviral activity, cardioprotective activity, gastroprotective activity, neuroprotective activity, anticancer activity, antidiabetic activity, antioxidant activity, anti-obesity activity, immunomodulatory activity, antihistaminic activity, and anti-inflammatory activity [99,100] (Figure 1). NS and NSO inhibit the activity of SARS-CoV-2 through the diverse mechanism of action (Figure 3). All these facts, in combination with the data of the CTs based on NS, indicate the potential of NS for the management of COVID-19 in normal COVID-19 patients as well as among the high-risk population.
A patent application is filed for a novel and inventive research of industrial application [101]. Our search revealed several patent applications connected to the practice of different compositions of NS to prevent/treat COVID-19 (Table 2). Most of these patents were published in 2021. Like non-patent literature, the patent literature also cites NS as an immunomodulator (activates natural killer cells, interferon, and dendritic cells), suppresses virus multiplication by its chemical constituents like TQ, protects lungs from pathology by blocking the activities of several inflammatory mediators that perform a major function in the COVID-19 pathology, several chemical constituents of NS, including TQ, nigellidine, and alpha-heridin have displayed anti-COVID-19 activity, the extracts of NS showed BCG like activity, and many NS compositions are in clinical trial against COVID-19 [73,75,77].
NS also has antihistaminic, antioxidant, antiallergic, and anti-asthmatic effects [74]. NS is also a source of zinc [77], which has also demonstrated activity against SARS-CoV-2 [102]. Many publications have also stated the usefulness of NS against chronic diseases like cancer, cardiovascular diseases, and diabetes, as mentioned above [78]. It is acceptably recognized that immunocompromised patients with little levels of natural killer cells, interferon, and dendritic cells are vulnerable to SARS-CoV-2 infection. COVID-19 patients suffer from cytokine storm owing to increased activity of the pro-inflammatory mediators in the body, and the malfunction of the lung among COVID-19 patients lead to severe consequences [73,75,77]. All these facts indicate that NS and its various compositions have all the attributes to be used as a promising remedy for the management of COVID-19 among high-risk individuals as well as to treat normal COVID-19 patients as a monotherapy or a combination therapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27092750/s1, Table S1: Patent searching results.
Author Contributions
M.I., S.A.K., A. and S.M.B.A. conceptualized and wrote the initial draft of the manuscript. M.K.A., S.M.A., F.N.A., M.K., O.A. and A. participated in data curation and literature search. The review and final editing of the manuscript was carried out by M.I., S.M.B.A., A.K.A. and S.J. The supervision of the manuscript writing was performed by M.I. and S.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deputyship for Research & Innovation, Ministry of Education, Saudi Arabia, via grant number “IF-2020-NBU-215”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education, Saudi Arabia, for funding this work through the project number IF-2020-NBU-215. The authors are thankful to Sulaiman Al-Habib Medical Group’s research center for their tremendous support. All the authors of this manuscript are thankful to their respective Institutes/Universities for the successful completion of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Worldometer on COVID-19 Cases. Available online: https://www.worldometers.info/coronavirus/ (accessed on 1 April 2022).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Emergency Use Authorization. Available online: https://www.fda.gov.ph/list-of-fda-issued-emergency-use-authorization/ (accessed on 31 March 2022).
- Alshrari, A.S.; Hudu, S.A.; Imran, M.; Asdaq, S.M.B.; Ali, A.M.; Rabbani, S.I. Innovations and development of COVID-19 vaccines: A patent review. J. Infect. Public Health 2022, 15, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Rajan, A.; Damodaran, A.; Kamath, S.R.; Nair, K.S.; Zachariah, S.M.; Sahu, R.K.; Fattepur, S.; Sreeharsha, N.; Nair, A.; et al. Identifying Mucormycosis Severity in Indian COVID-19 Patients: A Nano-Based Diagnosis and the Necessity for Critical Therapeutic Intervention. Antibiotics 2021, 10, 1308. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Rabbani, S.I.; Alkahtani, M.; Aldohyan, M.M.; Alabdulsalam, A.M.; Alshammari, M.S.; Alajlan, S.A.; Binrokan, A.; Mohzari, Y.; Alrashed, A.; et al. A Patent Review on the Therapeutic Application of Monoclonal Antibodies in COVID-19. Int. J. Mol. Sci. 2021, 22, 11953. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.K.; Grover, P.; Asdaq, S.M.B.; Mehta, L.; Tomar, R.; Imran, M.; Pathak, A.; Jangra, A.; Sahoo, J.; Alamri, A.S.; et al. Potential role of nicotinamide analogues against SARS-COV-2 target proteins. Saudi J. Biol. Sci. 2021, 28, 7567–7574. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Ikbal, A.M.A.; Sahu, R.K.; Bhattacharjee, B.; Paul, T.; Deka, B.; Fattepur, S.; Widyowati, R.; Vijaya, J.; Al Mohaini, M.; et al. Nanotechnology integration for SARS-CoV-2 diagnosis and treatment: An approach to preventing pandemic. Nanomaterials 2021, 11, 1841. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Alshrari, A.S.; Imran, M.; Sreeharsha, N.; Sultana, R. Knowledge, attitude and practices of healthcare professionals of Riyadh, Saudi Arabia towards COVID-19: A cross-sectional study. Saudi J. Biol. Sci. 2021, 28, 5275–5282. [Google Scholar]
- Asdaq, S.M.B.; Rabbani, S.I.; Imran, M.; Alanazi, A.A.; Alnusir, G.Y.; Al-Shammari, A.A.; Alsubaie, F.H.; Alsalman, A.J. A review on potential antimutagenic plants of Saudi Arabia. Appl. Sci. 2021, 11, 8494. [Google Scholar] [CrossRef]
- Villena-Tejada, M.; Vera-Ferchau, I.; Cardona-Rivero, A.; Zamalloa-Cornejo, R.; Quispe-Florez, M.; Frisancho-Triveño, Z.; Abarca-Meléndez, R.C.; Alvarez-Sucari, S.G.; Mejia, C.R.; Yañez, J.A. Use of medicinal plants for COVID-19 prevention and respiratory symptom treatment during the pandemic in Cusco, Peru: A cross-sectional survey. PLoS ONE 2021, 16, e0257165. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Intan, D.; Hananto, J.E.; Harapan, H.; Kurniawan, A. Vitamin D supplementation and COVID-19 outcomes: A 614 systematic review, meta-analysis and meta-regression. Rev. Med. Virol. 2021, 32, e2269. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Tripathi, J.; Manik, S.; Umar, L.; Rabia, J. Preliminary phytochemical studies of the miracle herb of the century, Nigella sativa L. (Black seed). Indo Am. J. Pharm. Res. 2013, 3, 3000–3007. [Google Scholar]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A promising natural remedy for wide range of illnesses. Evid. Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, H. Nigella sativa: The miraculous herb. Pak. J. Biochem. Mol. Biol. 2011, 44, 44–48. [Google Scholar]
- Ijaz, H.; Tulain, U.R.; Qureshi, J.; Danish, Z.; Musayab, S.; Akhtar, M.F.; Saleem, A.; Khan, K.A.; Zaman, M.; Waheed, I.; et al. Nigella sativa (Prophetic Medicine): A review. Pak. J. Pharm. Sci. 2017, 30, 229–234. [Google Scholar]
- Ismail, M.Y.; Yaheya, M. Therapeutic role of prophetic medicine Habbat El Baraka (Nigella sativa L.)—A review. World Appl. Sci. J. 2009, 7, 1203–1208. [Google Scholar]
- Yarnell, E.; Abascal, K. Nigella sativa: Holy herb of the middle East. Altern. Complement. Ther. 2011, 17, 99–105. [Google Scholar] [CrossRef]
- El-Tahir, K.E.; Bakeet, D.M. The black seed Nigella sativa Linnaeus-A mine for multi cures: A plea for urgent clinical evaluation of its volatile oil. J. Taibah. Univ. Med. Sci. 2006, 1, 1–19. [Google Scholar]
- Sudhir, S.P.; Deshmukh, V.O.; Verma, H.N. Nigella sativa seed, a novel beauty care ingredient: A review. Int. J. Pharm. Sci. Res. 2016, 7, 3185. [Google Scholar]
- Dajani, E.Z.; Shahwan, T.G.; Dajani, N.E. Overview of the preclinical pharmacological properties of Nigella sativa (black seeds): A complementary drug with historical and clinical significance. J. Physiol. Pharmacol. 2016, 67, 801–817. [Google Scholar]
- Areefa, A.; Mohd, A.; Shah, C.S. A review on Nigella sativa (Kalonji) seeds: A universal healer. Cellmed 2020, 10, 11.1–11.14. [Google Scholar]
- Begum, S.; Mannan, A. A review on Nigella sativa: A Marvel herb. J. Drug Deliv. Therap. 2020, 10, 213–219. [Google Scholar] [CrossRef]
- Thakur, S.; Kaurav, H.; Chaudhary, G. Nigella sativa (Kalonji): A black seed of miracle. Int. J. Res. Rev. 2021, 8, 342–357. [Google Scholar] [CrossRef]
- Momin, M.; Momin, S.; Kurhade, S.; Butte, K. Nigella sativa: Blessed seed. Int. J. Res. Phytochem. Pharmacol. 2013, 3, 78–84. [Google Scholar]
- Ara, I.; Maqbool, M.; Fekadu, G.; Hajam, T.A.; Dar, M.A. Pharmaceutical significance of Nigella Sativa L., a Wonder herb. J. Appl. Pharm. Sci. Res. 2020, 3, 4–13. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Imran, M.; Ul-Haq, I.; Živković, J.; Abu-Reidah, I.M.; Sen, S.; Taheri, Y.; Acharya, K.; Azadi, H.; et al. Nigella plants-Traditional uses, bioactive phytoconstituents, preclinical and clinical studies. Front. Pharmacol. 2021, 12, 625386. [Google Scholar] [CrossRef]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Torbati, M.; Damirchi, S.A.; Geoffrey, P.; Savage, G.P. A comprehensive review of the physicochemical, quality and nutritional properties of Nigella sativa oil. Food Rev. Int. 2019, 35, 342–362. [Google Scholar] [CrossRef]
- Farooq, J.; Sultana, R.; Taj, T.; Asdaq, S.M.B.; Alsalman, A.J.; Mohaini, M.A.; Al Hawaj, M.A.; Kamal, M.; Alghamdi, S.; Imran, M.; et al. Insights into the protective effects of thymoquinone against toxicities induced by chemotherapeutic agents. Molecules 2021, 27, 226. [Google Scholar] [CrossRef]
- Khan, M.A.; Afzal, M. Chemical composition of Nigella sativa Linn: Part 2 Recent advances. Inflammopharmacology 2016, 24, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ali, M.; Albratty, M.M.; Najmi, A.Y.; Azeem, U.; Khan, S.A.; Rather, M.A. Nigella sativa: From chemistry to medicine. In Black Seeds (Nigella sativa); Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–62. [Google Scholar]
- Gholamnezhad, Z.; Havakhah, S.; Boskabady, M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J. Ethnopharmacol. 2016, 190, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M.; Fu, S.; Fu, J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. A review on possible therapeutic effect of Nigella sativa and thymoquinone in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2018, 17, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Balbaa, M.; El-Zeftawy, M.; Abdulmalek, S.A.; Shahin, Y.R. Health-promoting activities of Nigella sativa fixed oil. In Black Cumin (Nigella sativa) Seeds: Chemistry, Technology, Functionality, and Application; Springer: Cham, Switzerland, 2021; pp. 361–379. [Google Scholar]
- Gheita, T.A.; Kenawy, S.A. Effectiveness of Nigella sativa oil in the management of rheumatoid arthritis patients: A placebo controlled study. Phytother. Res. 2012, 26, 1246–1248. [Google Scholar] [CrossRef]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic potential and pharmaceutical development of thymoquinone: A multitargeted molecule of natural origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar] [CrossRef]
- Kalamegam, G.; Alfakeeh, S.M.; Bahmaid, A.O.; AlHuwait, E.A.; Gari, M.A.; Abbas, M.M.; Ahmed, F.; Abu-Elmagd, M.; Pushparaj, P.N. In vitro Evaluation of the anti-inflammatory effects of thymoquinone in osteoarthritis and in silico analysis of inter-related pathways in age-related degenerative diseases. Front. Cell Dev. Biol. 2020, 8, 646. [Google Scholar] [CrossRef]
- Boskabady, M.; Khazdair, M.R.; Bargi, R.; Saadat, S.; Memarzia, A.; Roshan, N.M.; Hosseini, M.; Askari, V.R.; Boskabady, M.H. Thymoquinone ameliorates lung inflammation and pathological changes observed in lipopolysaccharide-induced lung injury. Evid. Based Complement. Altern. Med. 2021, 2021, 6681729. [Google Scholar] [CrossRef]
- Shaterzadeh-Yazdi, H.; Noorbakhsh, M.F.; Hayati, F.; Samarghandian, S.; Farkhondeh, T. Immunomodulatory and anti-inflammatory effects of thymoquinone. Cardiovasc. Hematol. Disord. Drug Targets 2018, 18, 52–60. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef]
- AbuKhader, M.M.; Khan, S.A. Thymoquinone and nanoparticles: A promising approach for the clinical trials. J. Bionanosci. 2017, 11, 258–265. [Google Scholar] [CrossRef]
- Staniek, K.; Gille, L. Is thymoquinone an antioxidant? BMC Pharmacol. 2010, 10, A9. [Google Scholar] [CrossRef] [Green Version]
- Wienkötter, N.; Höpner, D.; Schütte, U.; Bauer, K.; Begrow, F.; El-Dakhakhny, M.; Verspohl, E.J. The effect of nigellone and thymoquinone on inhibiting trachea contraction and mucociliary clearance. Planta Med. 2008, 74, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Stefanowicz-Hajduk, J.; Ochocka, J.R. Alpha-Hederin, the active saponin of Nigella sativa, as an anticancer agent inducing apoptosis in the SKOV-3 cell line. Molecules 2019, 24, 2958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gepdiremen, A.; Mshvildadze, V.; Süleyman, H.; Elias, R. Acute anti-inflammatory activity of four saponins isolated from ivy: Alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine 2005, 12, 440–444. [Google Scholar] [CrossRef]
- Saadat, S.; Mohammadi, M.; Fallahi, M.; Keyhanmanesh, R.; Aslani, M.R. The protective effect of α-hederin, the active constituent of Nigella sativa, on tracheal responsiveness and lung inflammation in ovalbumin-sensitized guinea pigs. J. Physiol. Sci. 2015, 65, 285–292. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Ghafari, S.; Sadeghi, M. Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19. Pharm. Biol. 2021, 59, 696–703. [Google Scholar] [CrossRef]
- Khan, S.A.; Al-Balushi, K. Combating COVID-19: The role of drug repurposing and medicinal plants. J. Infect. Public Health 2021, 14, 495–503. [Google Scholar] [CrossRef]
- Koshak, A.E.; Koshak, E.A.; Mobeireek, A.F.; Badawi, M.A.; Wali, S.O.; Malibary, H.M.; Atwah, A.F.; Alhamdan, M.M.; Almalki, R.A.; Madani, T.A. Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial. Complement. Ther. Med. 2021, 61, 102769. [Google Scholar] [CrossRef]
- Badary, O.A.; Hamza, M.S.; Tikamdas, R. Thymoquinone: A Promising natural compound with potential benefits for COVID-19 prevention and cure. Drug Des. Dev. Ther. 2021, 15, 1819–1833. [Google Scholar] [CrossRef]
- Islam, M.N.; Hossain, K.S.; Sarker, P.P.; Ferdous, J.; Hannan, M.A.; Rahman, M.M.; Chu, D.T.; Uddin, M.J. Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phytother. Res. 2021, 35, 1329–1344. [Google Scholar] [CrossRef]
- Banerjee, A.; Kanwar, M.; Mohapatra, P.K.D.; Saso, L.; Nicoletti, M.; Maiti, S. Nigellidine (Nigella sativa, black-cumin seed) docking to SARS CoV-2 nsp3 and host inflammatory proteins may inhibit viral replication/transcription and FAS-TNF death signal via TNFR 1/2 blocking. Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duru, C.E.; Duru, I.A.; Adegboyega, A.E. In silico identification of compounds from Nigella sativa seed oil as potential inhibitors of SARS-CoV-2 targets. Bull. Natl. Res. Cent. 2021, 45, 57. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Abbasi, H.W.; Shahid, S.; Gul, S.; Abbasi, S.W. Molecular docking, simulation and MM-PBSA studies of Nigella sativa compounds: A computational quest to identify potential natural antiviral for COVID-19 treatment. J. Biomol. Struct. Dyn. 2021, 39, 4225–4233. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Firoz, A.; Alaidarous, M.; Alshehri, B.; Bin Dukhyil, A.A.; Banawas, S.; Alsagaby, S.A.; Alturaiki, W.; Bhat, G.A.; Kashoo, F.; et al. Identification of SARS-CoV-2 RNA-dependent RNA polymerase inhibitors from the major phytochemicals of Nigella sativa: An in silico approach. Saudi J. Biol. Sci. 2022, 29, 394–401. [Google Scholar] [CrossRef]
- Rizvi, S.M.D.; Hussain, T.; Moin, A.; Dixit, S.R.; Mandal, S.P.; Adnan, M.; Jamal, Q.M.S.; Sharma, D.C.; Alanazi, A.S.; Unissa, R. Identifying the most potent dual-targeting compound(s) against 3CLprotease and NSP15exonuclease of SARS-CoV-2 from Nigella sativa: Virtual screening via physicochemical properties, docking and dynamic simulation analysis. Processes 2021, 9, 1814. [Google Scholar] [CrossRef]
- Baig, A.; Srinivasan, H. SARS-CoV-2 Inhibitors from Nigella sativa. Appl. Biochem. Biotechnol. 2022, 194, 1051–1092. [Google Scholar] [CrossRef]
- Maiti, S.; Banerjee, A.; Nazmeen, A.; Kanwar, M.; Das, S. Active-site Molecular docking of Nigellidine with nucleocapsid- NSP2-MPro of COVID-19 and to human IL1R-IL6R and strong antioxidant role of Nigella-sativa in experimental rats. J. Drug Target. 2022, 30, 511–521. [Google Scholar] [CrossRef]
- Khan, S.L.; Siddiqui, F.A.; Jain, S.P.; Sonwane, G.M. Discovery of potential inhibitors of SARS-CoV-2 (COVID-19) main protease (Mpro) from Nigella sativa (Black seed) by molecular docking study. Coronaviruses 2021, 2, 384–402. [Google Scholar] [CrossRef]
- Mani, R.J.; Sehgal, N.; Dogra, N.; Saxena, S.; Katare, D.P. Deciphering underlying mechanism of Sars-CoV-2 infection in humans and revealing the therapeutic potential of bioactive constituents from Nigella sativa to combat COVID19: In-silico study. J. Biomol. Struct. Dyn. 2022, 40, 2417–2429. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Mazumder, A.; Rana, A.K.; Srivastava, Y. Inhibitory potential of dietary phytocompounds of Nigella sativa against key targets of novel coronavirus (COVID-19). Indian J. Pharm. Educ. Res. 2021, 55, 190–197. [Google Scholar] [CrossRef]
- Esharkawy, E.R.; Almalki, F.; Hadda, T.B. In vitro potential antiviral SARS-CoV-19- activity of natural product thymohydroquinone and dithymoquinone from Nigella sativa. Bioorg. Chem. 2022, 102, 105587. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, B.; Xiao, Z.; Zhou, M.; Ge, L.; Jia, F.; Liu, Y.; Jin, H.; Zhu, X.; Gao, J.; et al. Computational and experimental studies reveal that thymoquinone blocks the entry of coronaviruses into in vitro cells. Infect. Dis. Ther. 2021, 10, 483–494. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/home (accessed on 1 April 2022).
- Imran, M.; Alshrari, S.A.; Thabet, H.K.; Abida Bakht, A.M. Synthetic molecules as DprE1 inhibitors: A patent review. Expert Opin. Ther. Patents 2021, 31, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Alshrari, S.A.; Tauseef, M.; Khan, S.A.; Hudu, S.A.; Abid. Mucormycosis medications: A patent review. Expert Opin. Ther. Patents 2021, 31, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Asdaq, S.M.B.; Khan, S.A.; Unnikrishnan, M.D.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Mohzari, Y.; Alrashed, A.; AlMotairi, M.; et al. Innovations and patent trends in the development of USFDA approved protein kinase inhibitors in the last two decades. Pharmaceuticals 2021, 14, 710. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Alshammari, M.K.; Alreshidi, M.A.; Alreshidi, A.A.; Alghonaim, R.S.; Alanazi, F.A.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Discovery, development, inventions, and patent trends on Mobocertinib succinate: The first-in-class oral treatment for NSCLC with EGFR Exon 20 insertions. Biomedicines 2021, 9, 1938. [Google Scholar] [CrossRef]
- Ichim, T.E.; Dixon, T.G.; Veltmeyer, J. Nutraceuticals for Suppressing Indolamine 2,3 Deoxygenase. U.S. Patent Number US11229674B1, 25 January 2022. [Google Scholar]
- Ichim, T.E.; Dixon, T.G. Nutraceuticals for the Prevention, Inhibition, and Treatment of SARS-Cov-2 and Associated COVID-19. U.S. Patent Application Publication Number US20210338763A1, 4 November 2021. [Google Scholar]
- Haddadi, M.; Taghdisi, H.P.M. Herbal Medicine for the Treatment of COVID-19. PCT International Patent Application Publication Number WO2022009236A1, 13 January 2022. [Google Scholar]
- Al Jasim, N.A.N.; Ahmed, N.Z. Immunomodulatory Composition to Treat and/or Prevent COVID-19 Illness. U.S. Patent Application Publication Number US20220000958A1, 6 January 2022. [Google Scholar]
- Omar, A. Herbal Anti-Viral Drug Containing Black Seed Oil for Treating Coronavirus Disease (COVID-19). Turkish Patent Application Publication Number TR2020004046A2, 21 April 2020. [Google Scholar]
- Muhammad, T. Compositions and Prevention and Intervention Methods for COVID-19 with Divine Ayats’ Fitra30 COVID-19 Protocol. PCT International Patent Application Publication Number WO202120, 14 October 2021. [Google Scholar]
- Ichim, T.E.; Veltmeyer, J.; Dixon, T.G. Additive and/or Synergistic Combinations of Metformin with Nutraceuticals for the Prevention, Inhibition and Treatment of Sars-Cov-2 and Associated COVID-19. U.S. Patent Application Publication Number US2022023237A1, 27 January 2022. [Google Scholar]
- Ichim, T.E.; Dixon, T.G.; Veltmeyer, J. Neuroprotection and Neuroregeneration by Pterostilbene and Compositions Thereof. U.S. Patent Application Publication Number US2022031793A1, 3 February 2022. [Google Scholar]
- Ichim, T.E.; Ramos, F.; Veltmeyer, J.; Dixon, T.G. Prevention of Neuroinflammation Associated Memory Loss Using Nutraceutical Compositions. U.S. Patent Application Publication Number US2022040248A1, 10 February 2022. [Google Scholar]
- Alkalay, R. Compositions and Methods for Treating and Preventing a Coronavirus Infection. PCT International Patent Application Publication Number WO2021186453A1, 23 September 2021. [Google Scholar]
- Alkalay, R. Compositions and Methods for Treating and Preventing Non-Malignant Respiratory Disease. PCT International Patent Application Publication Number WO2021186454A1, 23 September 2021. [Google Scholar]
- Alkalay, R. Compositions and Methods for Treating or Preventing Inflammatory Diseases Including Diabetes Mellitus 10 Type I and Type II and Thyroid Diseases. PCT International Patent Application Publication Number WO2021186455A1, 23 September 2021. [Google Scholar]
- Alkalay, R. Compositions and Methods for Treating Solid and Soft Tumors and Proliferative Diseases. PCT International Patent Application Publication Number WO2021186456A1, 23 September 2021. [Google Scholar]
- Popov, T.; Josling, P.D. Compositions and Applications Thereof. PCT International Patent Application Publication Number WO2021160982A1, 19 August 2021. [Google Scholar]
- Hoag, G.E.; Salerno, J. Method for Treating Viral and Bacterial Infection through Inhalation Therapy. PCT International Patent Application Publication Number WO2021216749A1, 28 October 2021. [Google Scholar]
- Mehmood, A.; Khan, S.; Khan, S.; Ahmed, S.; Ali, A.; Xue, M.; Ali, L.; Hamza, M.; Munir, A.; Ur Rehman, S.; et al. In silico analysis of quranic and prophetic medicinals plants for the treatment of infectious viral diseases including corona virus. Saudi J. Biol. Sci. 2021, 28, 3137–3151. [Google Scholar] [CrossRef]
- Holgersen, E.M.; Gandhi, S.; Zhou, Y.; Kim, J.; Vaz, B.; Bogojeski, J.; Bugno, M.; Shalev, Z.; Cheung-Ong, K.; Gonçalves, J.; et al. Transcriptome-Wide off-target effects of steric-blocking oligonucleotides. Nucleic Acid Ther. 2021, 31, 392–403. [Google Scholar] [CrossRef]
- From the American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR); Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS); European Society of Minimally Invasive Neurological Therapy (ESMINT); European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI); Society of Interventional Radiology (SIR); et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Zarei, A.; Soleymani, S.; Jamalimoghadamsiahkali, S.; Asadi, A.; Shati, M.; Jafari, M.; Rezadoost, H.; Kordafshar, G.; Naghizadeh, A.; et al. Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial. Phytother. Res. 2021, 35, 6295–6309. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Kulyar, M.F.; Li, R.; Mehmood, K.; Waqas, M.; Li, K.; Li, J. Potential influence of Nigella sativa (Black cumin) in reinforcing immune system: A hope to decelerate the COVID-19 pandemic. Phytomedicine 2021, 85, 153277. [Google Scholar] [CrossRef] [PubMed]
- Kadil, Y.; Mouhcine, M.; Filali, H. In Silico Investigation of the SARS CoV2 protease with thymoquinone, the major constituent of Nigella Sativa. Curr. Drug Discov. Technol. 2021, 18, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Younus, H. Potential implications of black seed and its principal constituent thymoquinone in the treatment of COVID-19 patients. Curr. Pharm. Biotechnol. 2021, 22, 1315–1324. [Google Scholar] [CrossRef]
- Awad, E.M.; Binder, B.R. In vitro induction of endothelial cell fibrinolytic alterations by Nigella Sativa. Phytomedicine 2005, 12, 194–202. [Google Scholar] [CrossRef]
- Imran, M.; Alshrari, A.S.; Asdaq, S.M.B.; Abida. Trends in the development of remdesivir based inventions against COVID-19 and other disorders: A patent review. J. Infect. Public Health 2021, 14, 1075–1086. [Google Scholar] [CrossRef]
- Imran, M.; Kumar, A.M.; Asdaq, S.M.B.; Khan, S.A.; Alaqel, S.I.; Alshammari, M.K.; Alshehri, M.M.; Alshrari, A.S.; Mateq, A.A.; Al-Shammeri, A.M.; et al. Discovery, development, and patent trends on molnupiravir: A prospective oral treatment for COVID-19. Molecules 2021, 26, 5795. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of Black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Mahdian, V.; Razavi, B.M.; Hosseinzadeh, H. Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone. J. Pharmacopunct. 2017, 20, 179–193. [Google Scholar]
- Webber, P.M. A guide to drug discovery. Protecting your inventions: The patent system. Nat. Rev. Drug Discov. 2003, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Jalal, Z.; Bakour, M.; Lyoussi, B. Medicinal Plants and zinc: Impact on COVID-19 pandemic. Sci. World J. 2021, 2021, 9632034. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).