A Novel PIFA/KOH Promoted Approach to Synthesize C2-arylacylated Benzothiazoles as Potential Drug Scaffolds
Abstract
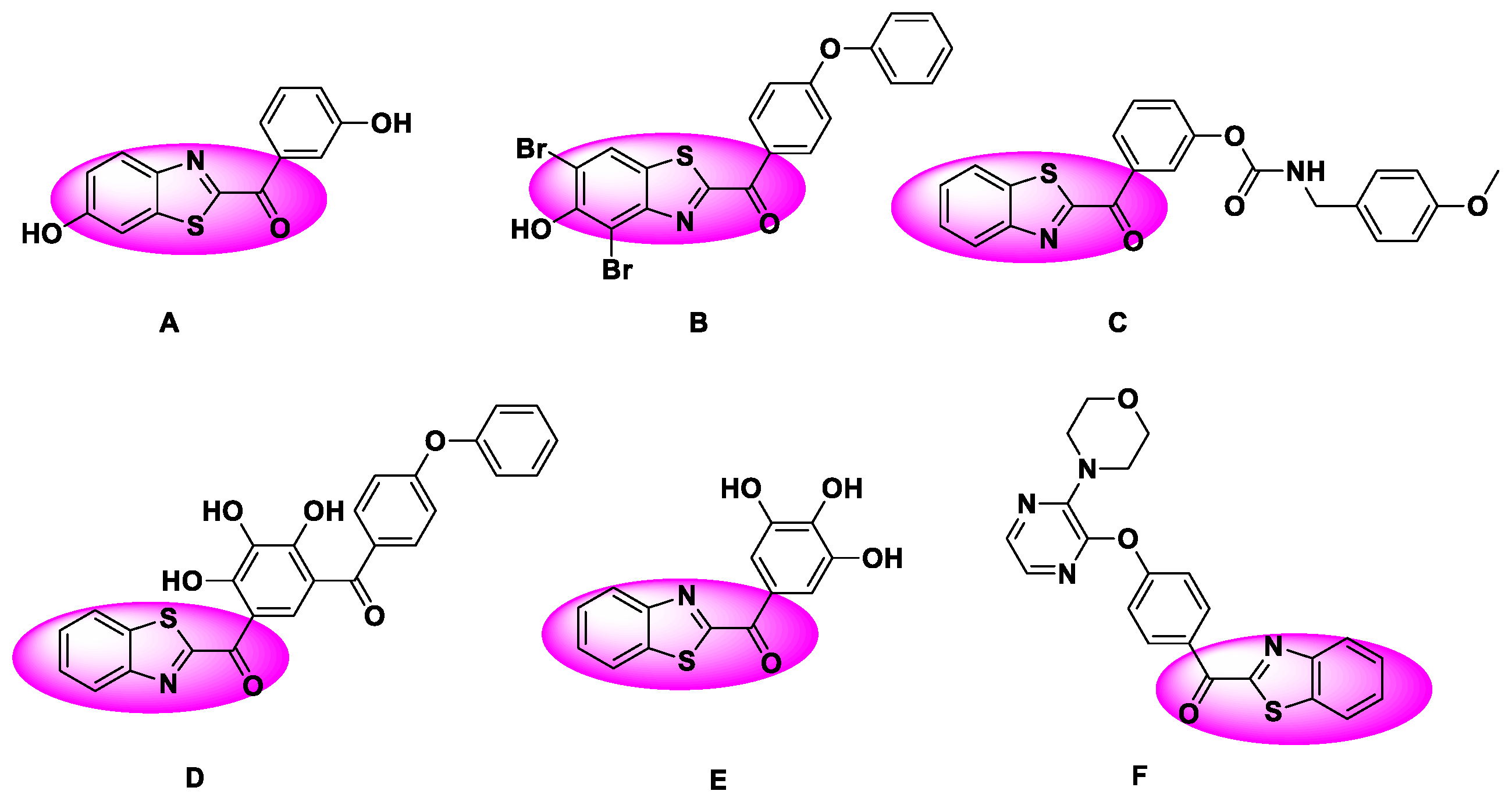
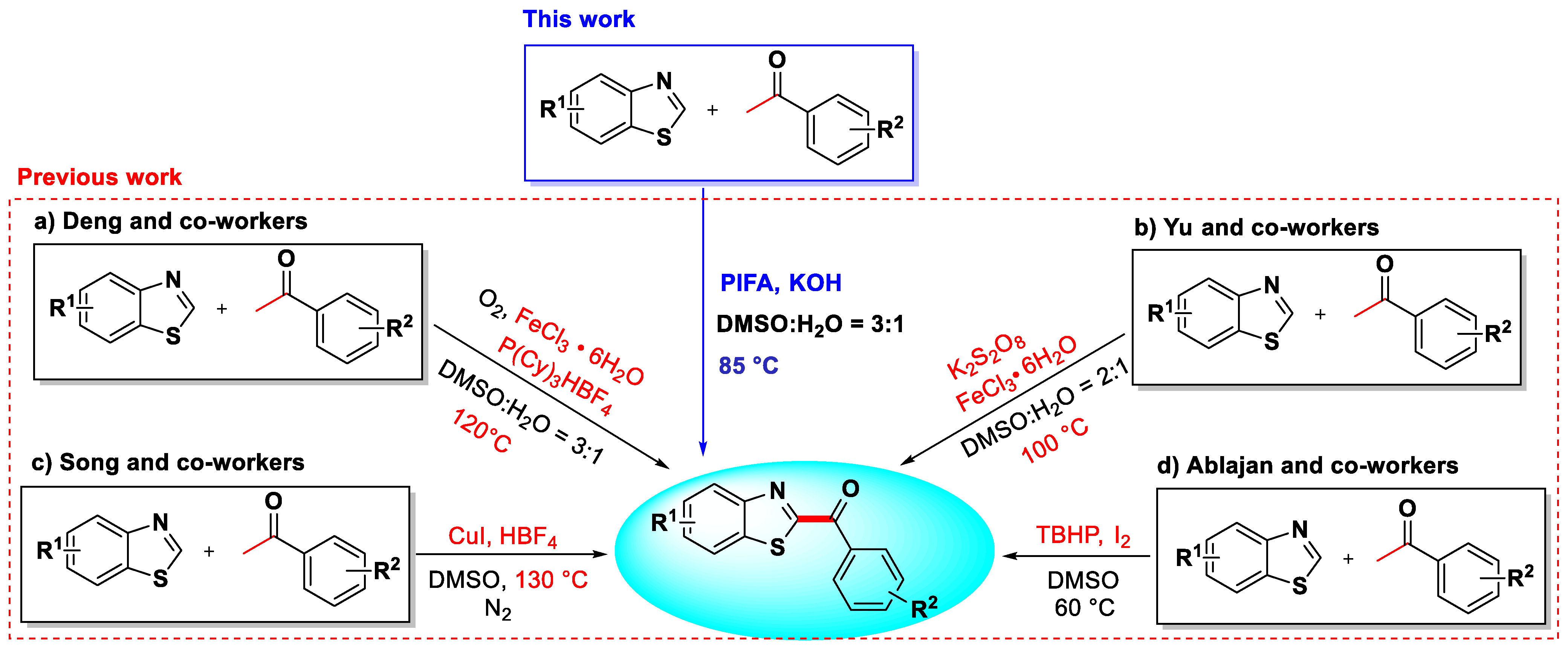
1. Introduction
2. Results and Discussion
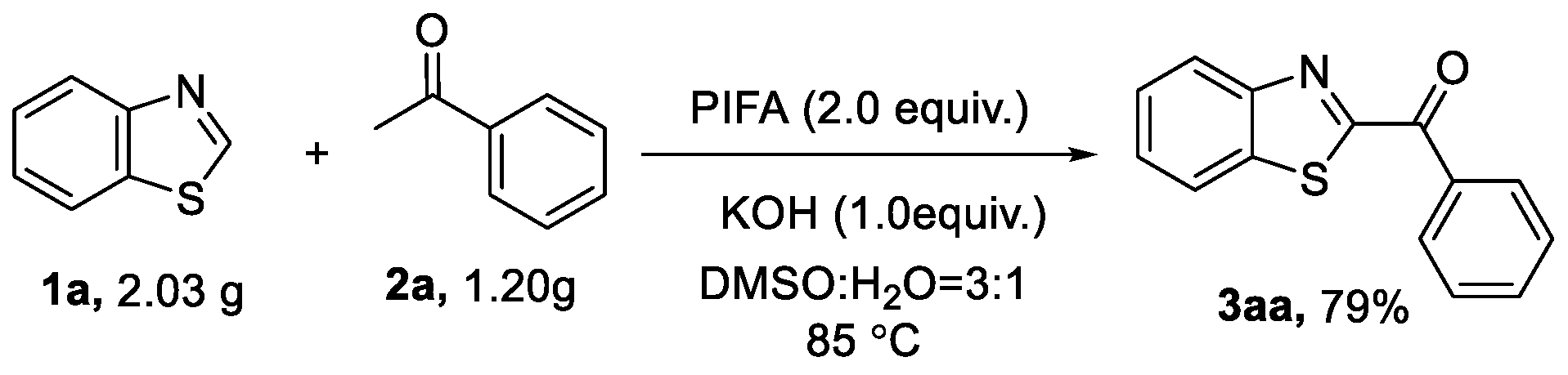
2.1. Optimization of Reaction Conditions
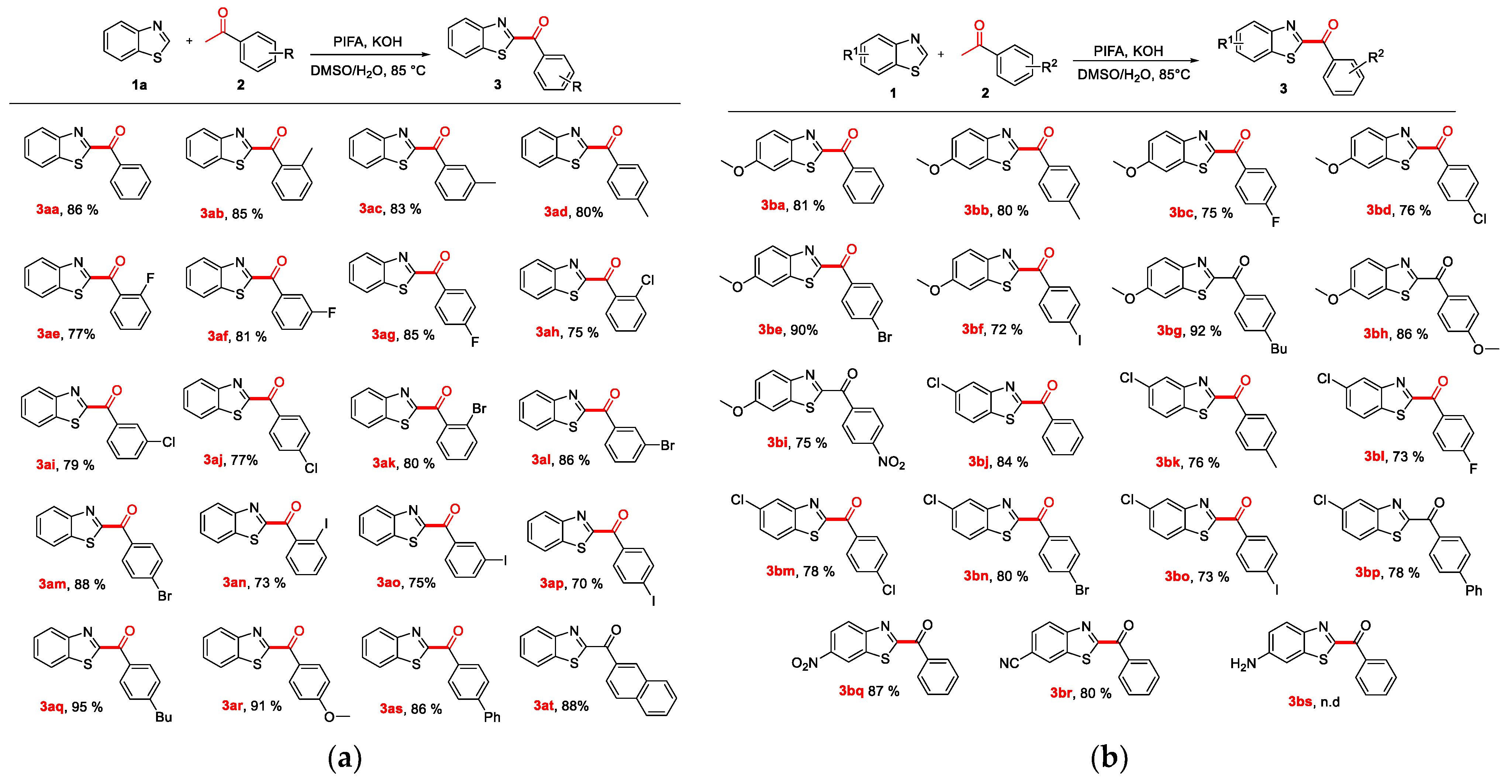
2.2. Expansion of Substrate Scope
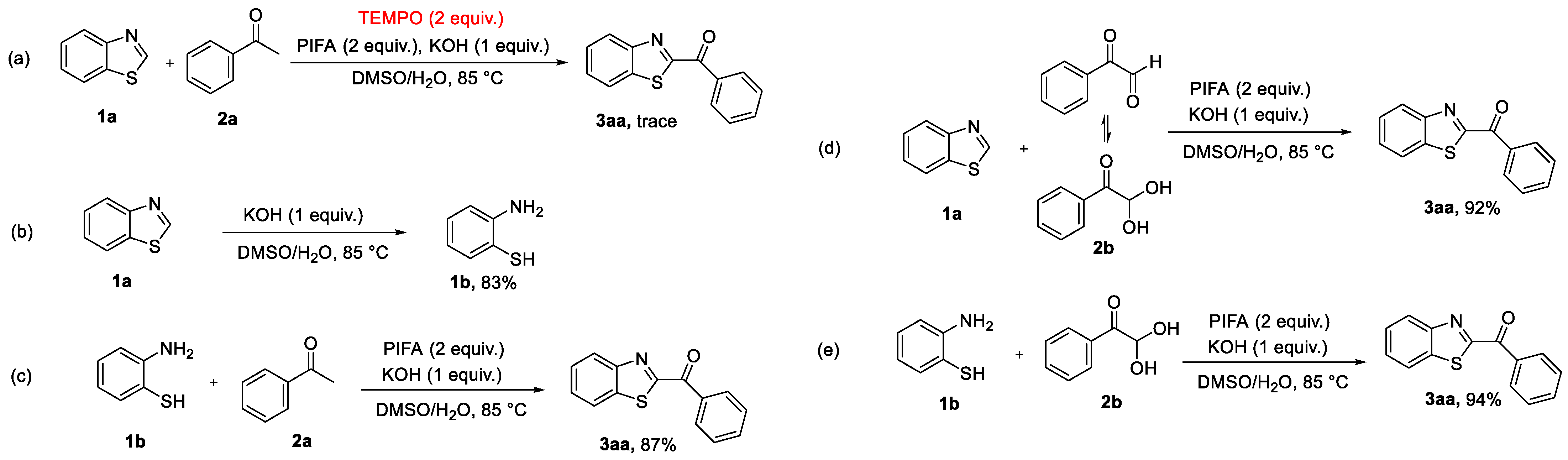
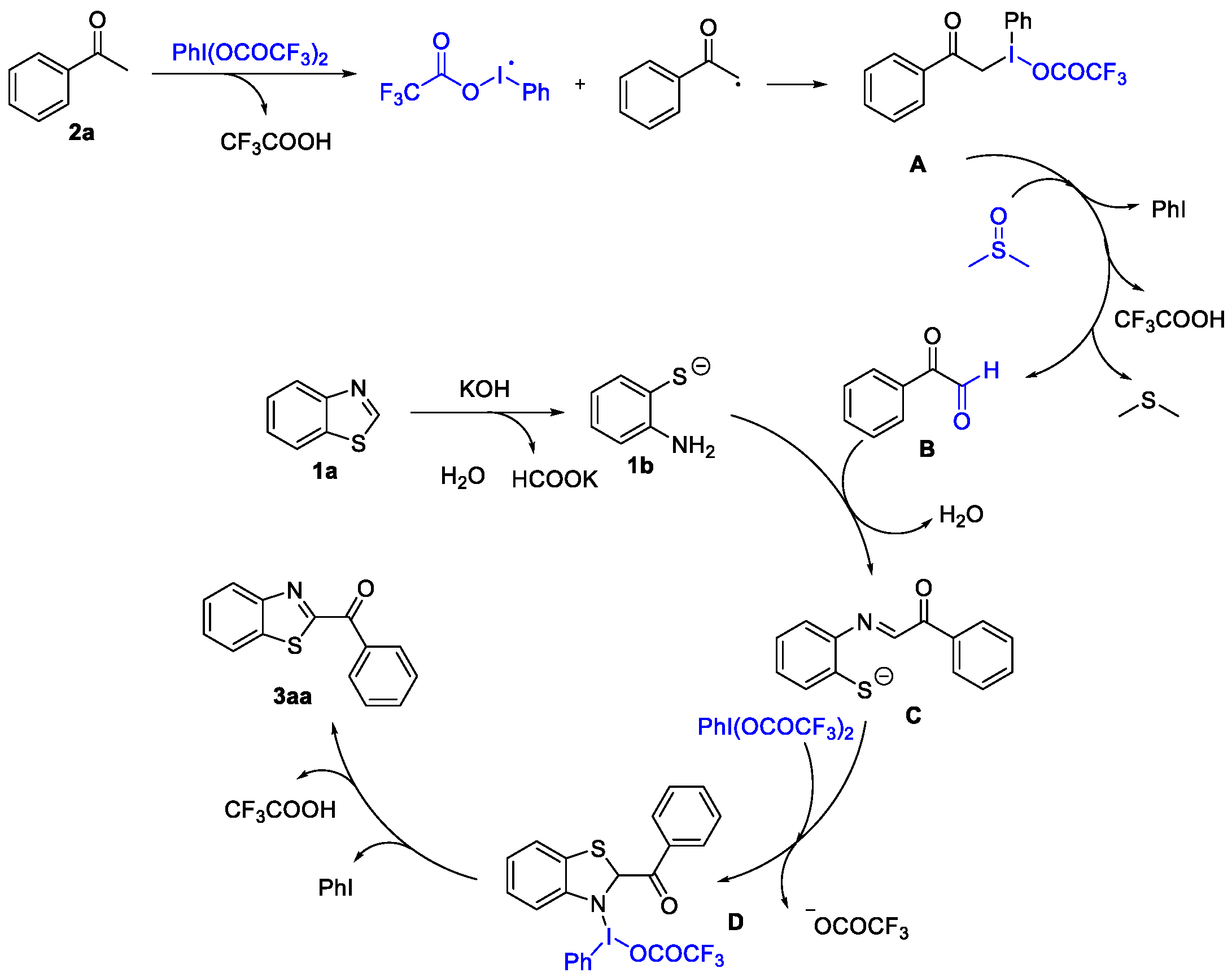
2.3. Mechanism Study
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Synthesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Blunt, C.E.; Nawrat, C.C.; LeBozec, L.; Liutkus, M.; Liu, Y.; Lewis, W.; Moody, C.J. Oxidative routes to the heterocyclic cores of benzothiazole natural products. Synlett 2016, 27, 37–40. [Google Scholar]
- Sumit; Kumar, A.; Mishra, A.K. Advancement in pharmacological activities of benzothiazole and its derivatives: An up to date review. Mini-Rev. Med. Chem. 2021, 21, 314–335. [Google Scholar]
- Ballari, M.S.; Cano, N.H.; Wunderlin, D.A.; Feresin, G.E.; Santiago, A.N. One-pot sequential synthesis and antifungal activity of 2-(benzylsulfonyl)benzothiazole derivatives. RSC Adv. 2019, 9, 29405–29413. [Google Scholar] [CrossRef]
- Bhat, M.; Belagali, S.L. Structural activity relationship and importance of benzothiazole derivatives in medicinal chemistry: A comprehensive review. Mini-Rev. Org. Chem. 2020, 17, 323–350. [Google Scholar] [CrossRef]
- Dhumal, S.T.; Deshmukh, A.R.; Kharat, K.R.; Sathe, B.R.; Chavan, S.S.; Mane, R.A. Copper fluorapatite assisted synthesis of new 1,2,3-triazoles bearing a benzothiazolyl moiety and their antibacterial and anticancer activities. New J. Chem. 2019, 43, 7663–7673. [Google Scholar] [CrossRef]
- El-Mekabaty, A.; Sofan, M.A.; Hasel, A.M.; Said, S.B. Concise synthesis of some new benzothiazole-based heterocycles as probable anticancer and antioxidant agents. ChemistrySelect 2021, 6, 2569–2575. [Google Scholar] [CrossRef]
- Hori, N.; Tsukamoto, G.; Imamura, A.; Ohashi, M.; Saito, T.; Yoshino, K. Novel disease-modifying antirheumatic drugs.1. synthesis and antiarthritic activity of 2-(4-methylphenyl)benzothiazoles. Chem. Pharm. Bull. 1992, 40, 2387–2390. [Google Scholar] [CrossRef][Green Version]
- Haroun, M.; Tratrat, C.; Petrou, A.; Geronikaki, A.; Ivanov, M.; Ciric, A.; Sokovic, M. 2-Aryl-3-(6-trifluoromethoxy)benzo[d]thiazole-based thiazolidinone hybrids as potential anti-infective agents: Synthesis, biological evaluation and molecular docking studies. Bioorg. Med. Chem. Lett. 2021, 32, 127718. [Google Scholar] [CrossRef]
- Pal, N.; Arya, A.K. An efficient and facile synthesis of Zn(II) complexes with 2-substituted benzothiazoles and glycine- and alanine-based ligands having antifungal and antibacterial activities. Res. Chem. Intermed. 2013, 39, 553–560. [Google Scholar] [CrossRef]
- Gilani, S.J.; Hassan, M.Z.; Imam, S.S.; Kala, C.; Dixit, S.P. Novel benzothiazole hydrazine carboxamide hybrid scaffolds as potential in vitro GABA AT enzyme inhibitors: Synthesis, molecular docking and antiepileptic evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 1825–1830. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.B.; Zhong, X.M.; Wang, X.B.; Chen, L.J.; He, M.; Xue, W. Synthesis and biological activities of benzothiazole derivatives bearing a 1,3,4-thiadiazole moiety. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 241–248. [Google Scholar] [CrossRef]
- Miralinaghi, P.; Schmitt, C.; Hartmann, R.W.; Frotscher, M.; Engel, M. 6-Hydroxybenzothiophene ketones: Potent inhibitors of 17 beta-hydroxysteroid dehydrogenase type 1 (17 beta-HSD1) owing to favorable molecule geometry and conformational preorganization. ChemMedChem 2014, 9, 2294–2308. [Google Scholar] [CrossRef]
- Spadaro, A.; Negri, M.; Marchais-Oberwinkler, S.; Bey, E.; Frotscher, M. Hydroxybenzothiazoles as new nonsteroidal inhibitors of 17 beta-hydroxysteroid dehydrogenase Type 1 (17 beta-HSD1). PLoS ONE 2012, 7, e292522012. [Google Scholar] [CrossRef]
- Spadaro, A.; Frotscher, M.; Hartmann, R.W. Optimization of hydroxybenzothiazoles as novel potent and selective inhibitors of 17 beta-HSD1. J. Med. Chem. 2012, 55, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Komiya, M.; Asano, S.; Koike, N.; Koga, E.; Igarashi, J.; Nakatani, S.; Isobe, Y. Synthesis of novel benzo-fused heteroaryl derivatives as Ca2+/Calmodulin-dependent protein kinase II inhibitors. Chem. Pharm. Bull. 2013, 61, 1094–1097. [Google Scholar] [CrossRef]
- Myllymaki, M.J.; Saario, S.M.; Kataja, A.O.; Castillo-Melendez, J.A.; Nevalainen, T.; Juvonen, R.O.; Jarvinen, T.; Koskinen, A.M.P. Design, synthesis, and in vitro evaluation of carbamate derivatives of 2-benzoxazolyl- and 2-benzothiazolyl-(3-hydroxyphenyl)-methanones as novel fatty acid amide hydrolase inhibitors. J. Med. Chem. 2007, 50, 4236–4242. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Z.; Nikolovska-Coleska, Z.; Qiu, S.; Yang, C.Y.; Guo, J.; Wang, S.M. Acylpyrogallols as inhibitors of antiapoptotic Bcl-2 proteins. J. Med. Chem. 2008, 51, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Li, C.M.; Wang, J.; Ahn, S.; Wang, Z.; Lu, Y.; Dalton, J.T.; Miller, D.D.; Li, W. Synthesis and antiproliferative activity of novel 2-aryl-4-benzoyl-imidazole derivatives targeting tubulin polymerization. Bioorg. Med. Chem. 2011, 19, 4782–4795. [Google Scholar] [CrossRef]
- Hu, E.; Kunz, R.K.; Chen, N.; Rumfelt, S.; Siegmund, A.; Andrews, K.; Chmait, S.; Zhao, S.; Davis, C.; Chen, H.; et al. Design, optimization, and biological evaluation of novel keto-benzimidazoles as potent and selective inhibitors of phosphodiesterase 10A (PDE10A). J. Med. Chem. 2013, 56, 8781–8792. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Jia, F.C.; Liu, M.C.; Wu, A.X.A. Multipathway coupled domino strategy: Metal-free oxidative cyclization for one-pot synthesis of 2-acylbenzothiazoles from multiform substrates. Org. Lett. 2012, 14, 4414–4417. [Google Scholar] [CrossRef]
- Fan, X.S.; He, Y.; Zhang, X.Y.; Guo, S.G.; Wang, Y.Y. Synthesis of heteroaryl ketones via tandem reaction of 1,1-dibromoethenes. Tetrahedron 2011, 67, 6369–6374. [Google Scholar] [CrossRef]
- Meng, X.; Bi, X.R.; Yu, C.Y.; Chen, G.X.; Chen, B.H.; Jing, Z.Q.; Zhao, P.Q. Ball-milling synthesized hydrotalcite supported Cu-Mn mixed oxide under solvent-free conditions: An active catalyst for aerobic oxidative synthesis of 2-acylbenzothiazoles and quinoxalines. Green Chem. 2018, 20, 4638–4644. [Google Scholar] [CrossRef]
- Huynh, T.V.; Doan, K.V.; Luong, N.T.K.; Nguyen, D.T.P.; Doan, S.H.; Nguyen, T.T.; Phan, N.T.S. New synthesis of 2-aroylbenzothiazoles via metal-free domino transformations of anilines, acetophenones, and elemental sulfur. RSC Adv. 2020, 10, 18423–18433. [Google Scholar] [CrossRef]
- Liu, S.W.; Chen, R.; Chen, H.; Deng, G.J. Iron-catalyzed 2-acylbenzothiazole formation from aryl ketones and benzothiazoles using oxygen as oxidant. Tetrahedron Lett. 2013, 54, 3838–3841. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.Z.; Chen, S.Y.; Yu, X.Q. Iron-catalyzed arylation or aroylation of benzothiazoles with benzylic alcohols and aryl ketones. Tetrahedron 2014, 70, 245–250. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Q.L. Chemoselective copper-catalyzed acylation of benzothiazoles with aryl methyl ketones. Adv. Synth. Catal. 2014, 356, 2445–2452. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.W.; Guo, Z.Q.; Ablajan, K. Iodine- and TBHP-promoted acylation of benzothiazoles under metal-free conditions. Synthesis 2020, 52, 3058–3064. [Google Scholar]
- Hua, M.; Wang, C.Q.; Liu, Q.X.; Chen, D.Y.; Fu, H.; Zhou, H.F. Silver-mediated 2-arylation/alkylation/acylation of benzothiazoles with aldehydes in water. Heterocycles 2018, 96, 1226–1237. [Google Scholar]
- Yang, K.; Zhang, C.; Wang, P.; Zhang, Y.; Ge, H.B. Nickel-catalyzed decarboxylative acylation of heteroarenes by sp2 C-H functionalization. Chem.-Eur. J. 2014, 20, 7241–7244. [Google Scholar] [CrossRef]
- Yang, K.; Chen, X.Y.; Wang, Y.Q.; Li, W.Q.; Kadi, A.A.; Fun, H.K.; Sun, H.; Zhang, Y.; Li, G.G.; Lu, H.J. Cobalt-catalyzed decarboxylative 2-benzoylation of oxazoles and thiazoles with alpha-oxocarboxylic acids. J. Org. Chem. 2015, 80, 11065–11072. [Google Scholar] [CrossRef]
- Huang, T.H.; Wu, X.; Yu, Y.B.; An, L.; Yin, X.X. A convenient synthesis of 2-acyl benzothiazoles/thiazoles from benzothiazole/thiazole and N,N′-carbonyldiimidazole activated carboxylic acids. Tetrahedron Lett. 2019, 60, 1667–1670. [Google Scholar] [CrossRef]
- Lassalas, P.; Marsais, F.; Hoarau, C. DMAP-catalyzed regel-type direct C-2 (hetero)aroylation of oxazoles and thiazoles derivatives with acid chlorides. Synlett 2013, 24, 2233–2240. [Google Scholar] [CrossRef]
- Wirth, T. Hypervalent iodine chemistry in synthesis: Scope and new directions. Angew. Chem. Int. Edit. 2005, 44, 3656–3665. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.D.; Wirth, T. Hypervalent iodine goes catalytic. Angew. Chem. Int. Edit. 2006, 45, 4402–4404. [Google Scholar] [CrossRef]
- Merritt, E.A.; Olofsson, B. Alpha-functionalization of carbonyl compounds using hypervalent iodine reagents. Synthesis 2011, 517–538. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, X.T.; Kong, Y.L.; Liu, J.C.; Chen, B.; Weng, J.Q. C2-arylacylation of 2H-benzothiazoles with methyl arenes via selectfluor oxidation. Tetrahedron Lett. 2021, 75, 153184. [Google Scholar] [CrossRef]
- Weng, J.Q.; Xu, W.X.; Dai, X.Q.; Zhang, J.H.; Liu, X.H. Alkylation reactions of benzothiazoles with N,N-dimethylamides catalyzed by the two-component system under visible light. Tetrahedron Lett. 2019, 60, 390–396. [Google Scholar] [CrossRef]
- Xu, W.X.; Dai, X.Q.; Weng, J.Q. K2S2O8-mediated hydroxyalkylation of benzothiazoles with alcohols in aqueous solution. ACS Omega 2019, 4, 11285–11292. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.L.; Xu, W.X.; Liu, X.H.; Weng, J.Q. Visible light-induced hydroxyalkylation of 2H-benzothiazoles with alcohols via selectfluor oxidation. Chin. Chem. Lett. 2020, 31, 3245–3249. [Google Scholar] [CrossRef]
- Xu, W.X.; Ye, F.X.; Liu, X.H.; Weng, J.Q. NCS/TBHP promoted C2 arylation of benzothiazoles with aldehydes in DMSO. Tetrahedron Lett. 2020, 61, 151807. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Liu, M.C.; Jia, F.C.; Yuan, J.J.; Gao, Q.H.; Lian, M.; Wu, A.X. Metal-free sp3 C-H bond dual-(het)arylation: I2-promoted domino process to construct 2,2-bisindolyl-1-arylethanones. Org. Lett. 2012, 14, 3392–3395. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Taherinia, Z. New revolution in biaryl synthesis: Transition metal-free C-C bond formation promoted by the mixture of 2-mercaptoethanol/KOH/DMSO. ChemistrySelect 2019, 4, 4735–4738. [Google Scholar] [CrossRef]
- Matcha, K.; Antonchick, A.P. Metal-free cross-dehydrogenative coupling of heterocycles with aldehydes. Angew. Chem. Int. Edit. 2013, 52, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.M.; Han, X.L.; Zhao, J.Q.; Zhang, H.Y.; Zhang, Y.C. Direct C3 Alkoxylation of Quinoxalin-2(1H)-ones with Alcohols via Cross-Dehydrogenative Coupling under Catalyst-Free Conditions. J. Org. Chem. 2019, 84, 11417–11424. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.Y.; Guo, S.X.; Wang, W.; Zhang, Y.Q. PIFA-mediated cross-dehydrogenative coupling of N-heteroarenes with cyclic ethers: Ethanol as an efficient promoter. Eur. J. Org. Chem. 2021, 2021, 411–421. [Google Scholar] [CrossRef]
- Zhang, S.S.; Wang, S.W.; Leng, Y.T.; Wu, Y.J. One-pot synthesis of 2-acylbenzothiazoles from 2-aminobenzenethiols and arylacetonitriles via cyclization and sequential oxidation. Tetrahedron Lett. 2021, 79, 153300. [Google Scholar] [CrossRef]
- Fukuda, K.; Hasegawa, T.; Kotani, T.; Muramoto, H.; Okamoto, K. Benzothiazole Derivatives. U.S. Patent 5,900,426, 4 May 1999. [Google Scholar]
- Ma, R.C.; Ding, Y.X.; Chen, R.; Wang, Z.M.; Wang, L.; Ma, Y.M. Oxidant/solvent-controlled I2-catalyzed domino annulation for selective synthesis of 2-aroylbenzothiazoles and 2-arylbenzothiazoles under metal-free conditions. J. Org. Chem. 2021, 86, 310–321. [Google Scholar] [CrossRef]
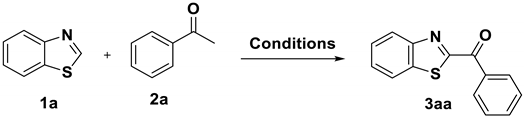 | ||||
| Entry | Oxidant (eq.) | Base (eq.) | Solvent (mL) | Yield (%) b |
| 1 | PIFA (2) | NaOH (1) | DMSO (2) | 7 |
| 2 | PIFA (2) | NaOH (1) | MeCN (2) | N.D. |
| 3 | PIFA (2) | NaOH (1) | DMF (2) | N.D. |
| 4 | PIFA (2) | NaOH (1) | H2O (2) | N.D. |
| 5 | PIFA (2) | NaOH (1) | DMSO/H2O 1:1 = (2) | 36 |
| 6 | PIFA (2) | NaOH (1) | DMSO/H2O 2:1 = (2) | 48 |
| 7 | PIFA (2) | NaOH (1) | DMSO/H2O 3:1 = (2) | 60 |
| 8 | PIFA (2) | NaOH (1) | DMSO/H2O 4:1 = (2) | 53 |
| 9 | PIFA (2) | K2CO3 (1) | DMSO/H2O 3:1 = (2) | 35 |
| 10 | PIFA (2) | Na2CO3 (1) | DMSO/H2O 3:1 = (2) | 32 |
| 11 | PIFA (2) | KOH (1) | DMSO/H2O 3:1 = (2) | 75 |
| 12 | PIFA (2) | none | DMSO/H2O 3:1 = (2) | N.D. |
| 13 | PIFA (2) | KOH (0.5) | DMSO/H2O 3:1 = (2) | 48 |
| 14 | PIFA (2) | KOH (1.5) | DMSO/H2O 3:1 = (2) | 53 |
| 15 | none | KOH (1) | DMSO/H2O 3:1 = (2) | N.D. |
| 16 | PIFA (0.5) | KOH (1) | DMSO/H2O 3:1 = (2) | 26 |
| 17 | PIFA (1.5) | KOH (1) | DMSO/H2O 3:1 = (2) | 38 |
| 18 | PIFA (2.5) | KOH (1) | DMSO/H2O 3:1 = (2) | 51 |
| 19 c | PIFA (2) | KOH (1) | DMSO/H2O 3:1 = (2) | 58 |
| 20 d | PIFA (2) | KOH (1) | DMSO/H2O 3:1 = (2) | 65 |
| 21 e | PIFA (2) | KOH (1) | DMSO/H2O 3:1 = (2) | 86 |
| 22 f | PIFA (2) | KOH (1) | DMSO/H2O 3:1 = (2) | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.-T.; Hu, Z.-G.; Huang, Z.; Zhou, L.-L.; Weng, J.-Q. A Novel PIFA/KOH Promoted Approach to Synthesize C2-arylacylated Benzothiazoles as Potential Drug Scaffolds. Molecules 2022, 27, 726. https://doi.org/10.3390/molecules27030726
Sun X-T, Hu Z-G, Huang Z, Zhou L-L, Weng J-Q. A Novel PIFA/KOH Promoted Approach to Synthesize C2-arylacylated Benzothiazoles as Potential Drug Scaffolds. Molecules. 2022; 27(3):726. https://doi.org/10.3390/molecules27030726
Chicago/Turabian StyleSun, Xiao-Tong, Zhi-Gang Hu, Zhen Huang, Ling-Li Zhou, and Jian-Quan Weng. 2022. "A Novel PIFA/KOH Promoted Approach to Synthesize C2-arylacylated Benzothiazoles as Potential Drug Scaffolds" Molecules 27, no. 3: 726. https://doi.org/10.3390/molecules27030726
APA StyleSun, X.-T., Hu, Z.-G., Huang, Z., Zhou, L.-L., & Weng, J.-Q. (2022). A Novel PIFA/KOH Promoted Approach to Synthesize C2-arylacylated Benzothiazoles as Potential Drug Scaffolds. Molecules, 27(3), 726. https://doi.org/10.3390/molecules27030726






