Abstract
The noncovalent interactions of (5,10,15,20-tetra(4-methylphenyl)porphinato)cobalt(II) (CoTTP) with C60 and 1-N-methyl-2-(pyridin-4-yl)-3,4-fullero[60]pyrrolidine (PyC60) were studied in toluene using absorption and fluorescence titration methods. The self-assembly in the 2:1 complexes (the triads) (C60)2CoTTP and (PyC60)2CoTTP was established. The bonding constants for (C60)2CoTTP and (PyC60)2CoTTP are defined to be (3.47 ± 0.69) × 109 and (1.47 ± 0.28) × 1010 M−2, respectively. 1H NMR, IR spectroscopy, thermogravimetric analysis and cyclic voltammetry data have provided very good support in favor of efficient complex formation in the ground state between fullerenes and CoTTP. PyC60/C60 fluorescence quenching in the PyC60/C60–CoTTP systems was studied and the fluorescence lifetime with various CoTTP additions was determined. The singlet oxygen quantum yield was determined for PyC60 and the intensity decrease in the 1O2 phosphorescence for C60 and PyC60 with the CoTTP addition leading to the low efficiency of intercombination conversion for the formation of the 3C60* triplet excited state was found. Using femtosecond transient absorption measurements in toluene, the photoinduced electron transfer from the CoTTP in the excited singlet state to fullerene moiety was established. Quantum chemical calculations were used for the determination of molecular structure, stability and the HOMO/LUMO energy levels of the triads as well as to predict the localization of frontier orbitals in the triads.
1. Introduction
Among the wide variety of fullerenes, C60 is one of the most common and actively studied fullerenes due to its pronounced electron-withdrawing property and great potential for application in many areas of applied science [,,,,,]. C60 fullerene is known to be a strong singlet oxygen generator, and it has only limited use in photodynamic therapy due to its extremely low solubility in water []. However, there are investigations devoted to the study of the effect of aqueous suspensions of C60 fullerene on microorganisms [,]. The modification of the fullerene core by attaching various groups or introducing a metal atom into the carbon cage provides the possibility of fine-tuning the physical and chemical properties of these compounds. The fullerene derivatization increases its solubility in an aqueous solvent and reduces aggregation to possible application in medicinal chemistry as antibacterial/antifungal agents [,,,]. The transition to trifluoromethyl derivatives of C60 leads to the increase in the electron affinity compared to initial fullerene and in the ability to form long-lived anion-radical particles [,]. The dimerization of fullerenes, namely the formation of pyrrolizidine/cyclobutane-bridged double-caged fullerene derivatives, promotes the charge delocalization over both carbon cages, upshifts energies of frontier molecular orbitals, a narrowed bandgap and a reduced electron-transfer reorganization energy relative to molecular C60 [,]. The endohedral C60 derivatives are interesting because a metal atom encapsulated into C60 is able to transfer a part of its spin density to the carbon cage, which ensures long spin relaxation times []. The modification of C60 by pyridyl groups, when the pyridyl nitrogen atom donates its lone pair of electrons to the electron-deficient π-system of fullerene, is used in the control of electron-withdrawing properties (LUMO energy) of fullerene derivatives. Such modification also allows for the design of various donor–acceptor systems, providing the high efficiency of photoinduced separation of charges when irradiated with the visible light [,,,,].
One of the strategies for improving the charge-separation process is the formation of covalent and noncovalent supramolecular systems based on fullerenes and electro/photoactive compounds as acceptors and donors, respectively []. Porphyrins and their complexes are promising among such compounds due to their structural diversity, photophysical/photochemical properties, broadband spectrum, high extinction coefficients, stability at relatively high temperatures and the presence of a π-conjugated macrocycle [,]. These systems offer new opportunities in the preparation of materials that may produce long-lived charge-separated states in high quantum yields.
The supramolecular interactions of the fullerenes with the porphyrins have been extensively studied in recent decades [,]. However, there is little data on the interaction of the non-functionalized fullerene C60 with metalloporphyrins (MPs). The C60 complexes with Zn(II), Co(II) and Fe(III) octaethylporphyrins (OEPs) bonding due to the van der Waals interactions were obtained by M.M. Olmstead et al. in 1999 []. Their structures, established by X-ray diffraction, contained one C60 molecule surrounded by two metalloporphyrin molecules in the case of ZnOEP and CoOEP, but one metalloporphyrin molecule in the case of ClFeOEP []. In the work of [], molecular complexes of fullerene C60 with Mn(II), Co(II), Cu(II), Zn(II) and Fe(III) tetraphenylporphyrins (TPPs) were synthesized and studied by ESR, IR, UV–vis and X-ray photoelectron spectroscopy. The formation of molecular complexes with fullerenes affects the ESR spectra of MPs lowering the spin state in the case of MnTPP or changing the constant of hyperfine interaction in the case of CoTPP and CuTPP []. Additionally, C60-containing complexes based on jaw-like Pd, Zn, Cu, Co, Fe and Mn bis(metalloporphyrins) were described in the work of []. In 2007, Aida et al. reported that iridium(III) porphyrin cyclic dimer bonds C60 to form the 1:1 complex []. In 2017, Yamada et al. have synthesized iridium porphyrin bearing peripheral alkyl chains, which forms not only the 1:1 complex but also the 2:1 one in toluene []. The electronic interactions between zinc(II) porphyrin containing the reactive amino groups and C60 were established by steady state fluorescence, time correlated single photon counting measurements and DFT calculations []. These new data point to the consideration of metalloporphyrin–C60 systems as the basis for numerous photovoltaic devices.
The self-assembly of the cobalt(II) porphyrin (CoP) coordination triads (where the triad is the molecule with various combinations of electron donor and acceptor) with fullero[60]pyrrolidines have been reported in our early works [,,,], while the one with non-functionalized fullerene C60 have not been explored to date. We report here about the ability of cobalt(II) porphyrin bearing peripheral 4-methylphenyl groups to bond both C60 and PyC60, thus forming the 2:1 coordination complexes. The self-assembly in cobalt(II) porphyrin–C60/PyC60 systems in toluene was studied by the absorption and fluorescence titration methods. The spectral properties of triads in the ground and excited states are observed and discussed in this article.
2. Results and Discussion
The UV–vis and fluorescence spectroscopy are suitable methods to study the supramolecular interaction of metalloporphyrins upon bonding fullerenes [,,]. Both methods allow one to easily determine the stability constants of the CoTTP–C60/PyC60 triads. The spectrophotometric methods are attractive due to the large changes in the CoPs UV–vis spectra when a non-covalent bond is formed along the axial axis causes.
Figure 1 shows the scheme of the CoTTP reaction with PyC60 and the corresponding UV–vis spectrum transformations during the titration of the CoTTP solution in toluene by PyC60. The time-dependent titration at τ = 0 and τ = ∞ [] allowed us to describe both rapid/slow equilibriums and the rate of the second-stage reaction. The fast reversible (PyC60)CoTTP formation (at τ = 0) is characterized by the gradual decrease in the intensity at 414 nm in the CoTTP spectrum. The (PyC60)2CoTTP formation occurs over time (within 100–1100 s depending on the PyC60 concentration, see Figure S1 in Supplementary Materials). It is accompanied by the gradual decrease in the absorption at 414 nm together with the appearance of the new band at 437 nm. The analysis of the logI-logCPyC60 dependence slope has confirmed one PyC60 molecule bonding at the first and second stage (Figure S2, Supplementary Materials). The step constants, K1 and K2, were calculated by the equation for the three-component equilibrium system (see Equation (S1) in Supplementary Materials) and were found to be (2.3 ± 0.3) × 104 and (6.4 ± 0.9) × 105 M−1, respectively. The stability constant of the (PyC60)2CoTTP triad, β = K1 × K2 is (1.47 ± 0.28) ×1010 M−2.
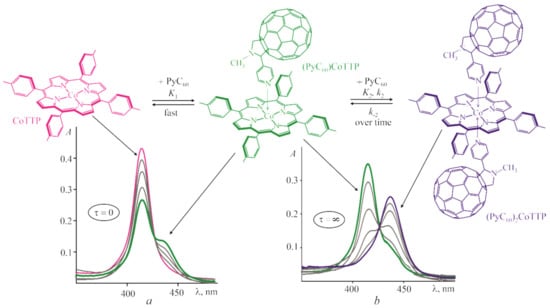
Figure 1.
The scheme of the (PyC60)2CoTTP formation and UV–vis spectrum transformations during the titration of CoTTP with PyC60 (CPyC60 = 0 ÷ 7.7 × 10−5 M) in toluene immediately after mixing the reactants (a) and at the end of the reactions (b).
The rate of forward reactions (k2obs) in the slow equilibrium was measured (Table S1, Supplementary Materials). Using the data in Table S1 and Figure S3, the first order with respect to CoTTP and PyC60, respectively, are determined (Figure S3, Supplementary Materials). The rate constant k2 found from Figure S3 data is (62.3 ± 5.1) M s−1. The rate constant k−2 equal k2/K2 is 9.7 × 10−5 M2 s−1.
UV–vis spectral changes observed during CoTTP bonding with C60 are similar to the ones for the CoTTP–PyC60 system. The formation of a supramolecular complex between CoTTP and C60 is accompanied by the Soret band shift from 414 nm to 432 nm. Job’s plot points to the CoTTP–C60 bonding stoichiometry of 1:2 (Figure 2). The use the time-dependent titration method for the CoTTP–C60 system allowed us to also identify two stages (the fast equilibrium and slow two-way reaction of C60 coordination, Figure S4, Supplementary Materials) and to determine the thermodynamic stability constants, which were found to be K1 = (5.02 ± 0.94) × 104 M−1, K2 = (6.63 ± 0.98) × 104 M−1 and β = (3.47 ± 0.69) × 109 M−2.
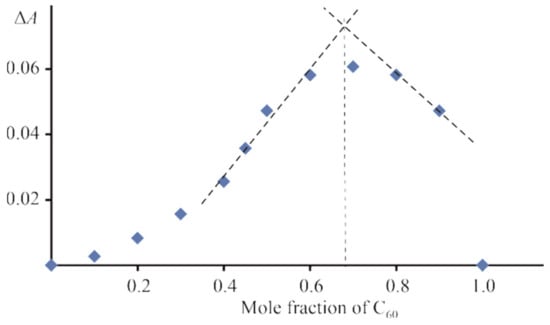
Figure 2.
Job’s plot for CoTTP–C60 system in toluene; in Job’s experiment, the concentration of CoTTP and C60 are continuously varied in the concentration range from 1.06 × 10−6 M to 1.06 × 10−5 M.
The stability constants (the formation ones) of (PyC60)2CoTTP and (C60)2CoTTP were also determined with the help of steady state fluorescence measurements in toluene. Since cobalt(II) porphyrins were non-fluorescent [], and C60 [] as well as their derivatives [] were fluorescent with a low fluorescence quantum yield (3.2 × 10−4 for C60), we studied the fluorescence of C60/PyC60 with various additions of CoTTP at thermostating solutions for 20 min (τ = ∞). The fluorescence quantum yield of PyC60 determined in the toluene is 5.3 × 10−4. It was established that the C60/PyC60 fluorescence upon excitation at 450 nm is quenched following the gradual addition of CoTTP. The steady state fluorescence titration experiments for the PyC60–CoTTP and C60–CoTTP systems recorded in toluene are demonstrated in Figure 3a,b, respectively. The process of C60/PyC60 fluorescence quenching with CoTTP was investigated using the Stern–Volmer equation. The results are given in Figure 3c,d. The Stern–Volmer plot is linear in both cases. The fluorescence-quenching constants (KSV) were found to be (5.47 ± 1.34) × 104 and (5.66 ± 0.45) × 103 M−1 for (PyC60)2CoTTP and (C60)2CoTTP, respectively. The stability constants (KBH) for the triads were determined by the Benesi–Hildebrand equation plotting I0/(I0 − I) vs. 1/[CCoTTP] (Figure S5, Supplementary Materials), the straight line was obtained and the value of KBH was determined as (1.2 ± 0.3) × 1010 and (1.3 ± 0.3) × 109 M−1 for (PyC60)2CoTTP and (C60)2CoTTP, respectively. These values are in agreement with the values from UV–vis spectrophotometric titration, although the standard deviation in the latter case is approximately 1.3 times less.
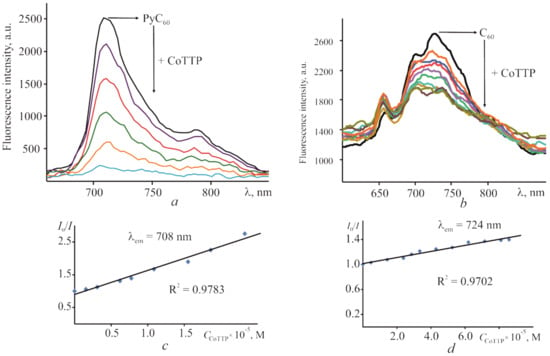
Figure 3.
The changes in the PyC60 stationary fluorescence spectrum (CPyC60 = 4.39 × 10−6 M) in the presence of CoTTP (CCoTTP = 1.55 × 10−6–2.33 × 10−5 M) (a) and the ones for C60 (CC60 = 9.08 × 10−5 M) in the presence of CoTTP (CCoTTP = 4.77 × 10−6–8.58 × 10−5 M) (b) in toluene; (c) Stern–Volmer plot for the PyC60–CoTTP (c)/C60–CoTTP (d) system in toluene. λexc = 450 nm.
According to the literature data [], the fluorescence lifetime of C60 varies from 1.17 to 1.3 ns. As the results of our measurements, the fluorescence lifetime of C60 in toluene was determined to be 1.38 ns. C60 exhibits bi-exponential decay which is in agreement with the works of [,]. PyC60 also exhibits bi-exponential decay with the fluorescence lifetime of 1.61 ns.
The time-resolved fluorescence spectroscopy results for the C60, PyC60, C60–CoTTP and PyC60–CoTTP solutions in toluene are shown in Table S2, Supplementary Materials. The different effect in the C60 and PyC60 fluorescence lifetime from the CoTTP addition points to the different nature of fullerene quenching. The C60 fluorescence lifetime decreases to 1 ns with the addition of 2.4 × 10−5 M CoTTP. The PyC60 fullerene fluorescence lifetime remains unchanged in the PyC60–CoTTP mixtures. This behavior is associated with the static and dynamic mechanism of fluorescence quenching upon bonding CoTTP with PyC60 and C60, respectively.
Another feature of fullerenes is their ability to generate singlet molecular oxygen (1O2) with high quantum yields [], which we have used to confirm the formation of supramolecular systems with the charge-separation state []. The 1O2 quantum yield for C60 is often equated to 1 [,]. Given these data, the quantum yield of singlet oxygen of PyC60 was determined and found to be 0.7 (see Section 3.4). This value is consistent with the higher triplet energies and the lower quantum yields of triplet and singlet oxygen for functionalized C60 []. The intensity of the 1O2 phosphorescence decreases when the addition of CoTTP is increased. Emission spectra of the 1O2 obtained by the photoirradiation (λ = 453 nm) of the C60, PyC60, C60–CoTTP and PyC60–CoTTP solutions are shown in Figure 4. The phosphorescence decay of singlet oxygen formed by the sensitization with PyC60 and PyC60–CoTTP is represented in Table S3, Supplementary Materials. The intensity of the 1O2 phosphorescence for C60 and PyC60 decreases with the maximum CoTTP addition by 3.5 and almost 6 times, respectively. This fact additionally confirms the realization of photoinduced electron transfer (PET) in (PyC60)2CoTTP/(C60)2CoTTP from the donor CoTTP fragment to the acceptor fullerene-containing fragment, since this process possibly leads to the low efficiency of intercombination conversion for the formation of the 3C60* triplet excited state.
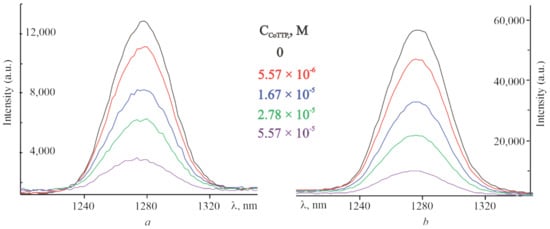
Figure 4.
The emission spectra of singlet oxygen formed by the sensitization with C60 (a), PyC60 (b), C60–CoTTP (a) and PyC60–CoTTP (b).
The interactions between CoTTP and C60/PyC60 in the ground states are confirmed by spectral methods such as mass spectrometry and IR/1H NMR spectroscopy. MALDI mass spectrometry is a rapid qualitative test for fullerene bonding by porphyrin []. Using the dithronol matrix, (PyC60)2CoTTP gives the exact mass peaks corresponding to both the supramolecular complex (2436.88 [M]+) and its constituents (728.61 [CoTTP]+ and 855.66 [PyC60]+) (Figure 5). No molecular ion peak of (C60)2CoTTP was observed due to the dissociation of the noncovalent complex in the conditions of the mass spectral experiments.
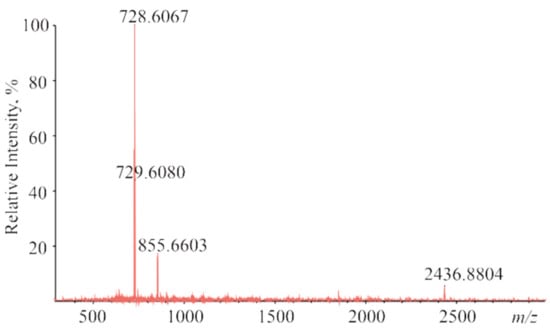
Figure 5.
The MALDI-TOF mass spectrum of (PyC60)2CoTTP.
According to IR spectral studies, the position and intensity of the pyrrole ring stretching vibrations of CoTTP remain practically unchanged in the IR spectra of (PyC60)2CoTTP and (C60)2CoTTP, pointing to the Co atom location in the macrocycle plane [,]. This is related to the signals at 1684 cm−1, ν (C=N), 1452 cm−1, 1404 cm−1, 1377 cm−1 ν(C-H)pyr in the CoTTP spectrum that is listed in Experimental Section. The planar coordination center was also confirmed by both theoretical calculations (see next section) and the data from the work of [] on the example of (2,3,7,8,12,18-hexamethyl,13,17-diethyl,5-(2-pyridyl)porphinato)cobalt(II) and its triads with PyC60. Along with the signals of the porphyrin macrocycle, there are signals of coordinated PyC60 in the IR spectrum of (PyC60)2CoTTP (Table S4, Supplementary Materials). The vibration signals of the C60 skeleton at 527 cm−1, 574 cm−1 and 1428 cm−1 as well as signals of pyridyl/pyrrolidinyl fragments of the fullerene core at 1245 cm−1, 1281 cm−1 and in the 400–710 cm−1 range (Figure 6, Table S4, Supplementary Materials) are observed. These signals are shifted by 1–14 cm−1 relative to uncoordinated PyC60 [,]. The signal at 456 cm−1 related to ν(Co-N) is considerably broadened; the ν(Co-NPyC60) signal appears at 463 cm−1 as the shoulder (Figure 6). The IR (C60)2CoTTP spectrum is the sum of the C60 [] and CoTTP spectra, suggesting the weak interaction between C60 and CoTTP in the ground state.
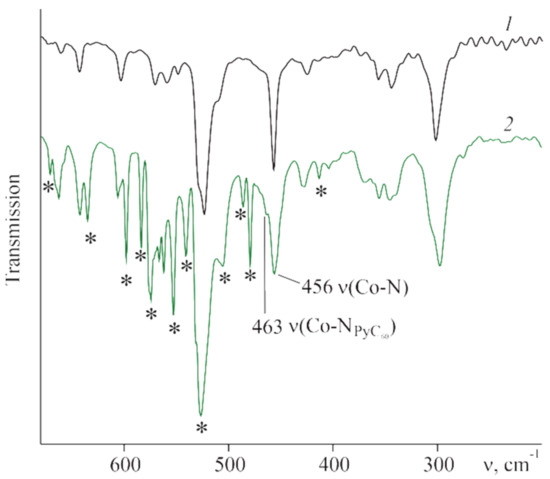
Figure 6.
The IR spectra of CoTTP (1) and (PyC60)2CoTTP (2) in CsBr. The peaks corresponding to vibrations of PyC60 are denoted with asterisks.
The CoTTP 1H NMR spectrum (Figure S6, Supplementary Materials), typical of other cobalt(II) porphyrins [,,], is represented by the signals of β-pyrrole, ortho-phenyl and meta-phenyl protons as the broadened singlets at 15.98 ppm, 13.07 ppm and 9.75 ppm, respectively. The upfield shifts of these proton signals in the 1H NMR spectrum of the (PyC60)2CoTTP (Figure 7) due to the ring current effect of the fullerene on the porphyrin indicate the fullerene coordination state.
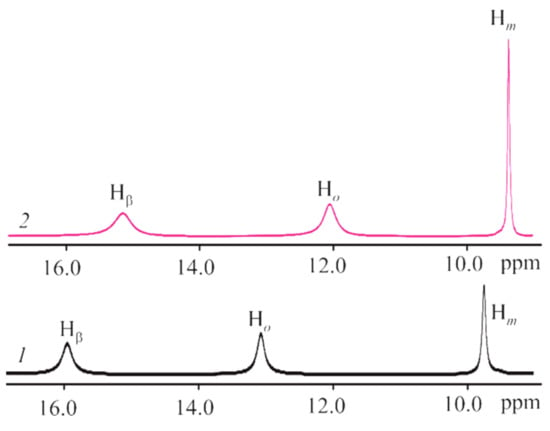
Figure 7.
The 1H NMR spectra of CoTTP (1) and its triad, (PyC60)2CoTTP (2) in CDCl3.
The thermal stability of (PyC60)2CoTTP/(C60)2CoTTP studied in comparison with the its precursors by the TGA method demonstrates the high parameters of most porphyrins [,]. Figure S7a, Supplementary Materials, shows the TG curve and its first derivative (DTG) for CoTTP. The weight loss of 0.85% between 25 °C and 479 °C is connected with the desorption of solvent molecules rather than the metalloporphyrin degradation. The elimination of the tolyl groups and the degradation of macrocycle ring manifest itself in the form of the weight loss of 12% in the temperature range of 479–509 °C with the major weight loss at 504 °C and of 19.65% between 719 °C and 750 °C with the maximum value at 738 °C, respectively. C60 has also displayed the high thermal stability, exhibiting the decomposition process in the range of 827–937 °C (Figure S7b, Supplementary Materials). The TGA results for PyC60 (Figure S7c, Supplementary Materials) show the start of the compound decomposition from around 374 °C to 454 °C, i.e., at a considerably lower temperature than the one for pure C60. The main drop in the PyC60 mass corresponding to the fullerene cage decomposition occurs between 807 °C and 904 °C; the peak of the derivative on the weight loss is observed at 883 °C.
The decomposition of triads shown in Figure 8 is the multi-stage process which includes the degradation of both the porphyrin macrocycle and the fullerene core. In the case of (C60)2CoTTP, the peaks of the derivative on the weight loss at 494 °C/738 °C and 823 °C correspond to the thermal decomposition of CoTTP and C60, respectively (Figure 8a). The thermal behavior of (PyC60)2CoTTP (Figure 8b) was similar. The peaks of the derivatives on the weight loss for both triads are observed at temperatures lower than that of their individual components. Thus, the results of the TGA experiment suggest the direct bonding between CoTTP and C60/PyC60 in the high stable triads.
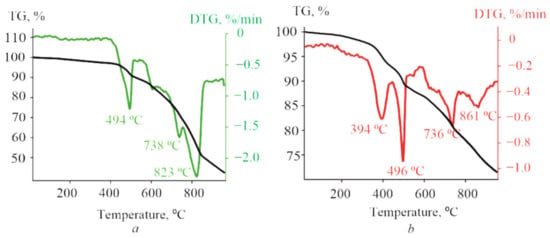
Figure 8.
The TG and DTG curves for the (C60)2CoTTP (a) and (PyC60)2CoTTP (b) powder from 25 °C to 920 °C. The heating rate is 10 °C/min.
The cyclic voltammograms (CVs) of the triads and their individual components are represented in Figure 9. There are two reversible peaks in the positive potential range for CoTTP. The first and the second oxidation/reduction correspond to the central metal atom and the macrocycle, respectively []. The cyclic voltammograms of C60 and PyC60 in CH2Cl2 are characterized by three pairs of peaks, which is typical of fulleropyrrolidines [,]. The redox potentials for all these compounds are given in Table S5, Supplementary Materials. The CV of (C60)2CoTTP and (PyC60)2CoTTP are essentially the sum of the CoTTP and C60/PyC60 peaks. Three well-pronounced reversible redox waves corresponding to the sequential addition of electrons to the fullerene unit are observed in the negative potential window. Two reversible redox waves in the positive potential window correspond to the central metal atom and the macrocycle of CoTTP. However, these peak potentials are shifted relative to ones for the individual components (Table S5, Supplementary Materials), which indicates the interaction between the π system of CoTTP and C60/PyC60 and the redistribution of the electron density within the triad.
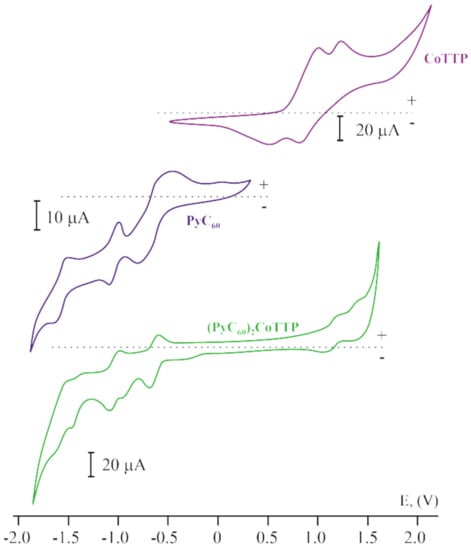
Figure 9.
The cyclic voltammograms for CoTTP, PyC60 and (PyC60)2CoTTP in CH2Cl2 containing the 0.1 M (n-Bu)4NClO4 supporting electrolyte. The scan rate is 100 mV s−1.
The DFT calculations have demonstrated the higher stability of (PyC60)2CoTTP compared with (C60)2CoTTP. The bonding energy in (PyC60)2CoTTP and (C60)2CoTTP calculated as the difference between the total energy of triad minus the sum of the energies of CoTTP and C60/PyC60 is 253 kJ·mol−1 and 293 kJ·mol−1, respectively. The coordination of CoTTP is accompanied by a distortion of the macrocycle and its deviation from planarity by 7.31° (Figure 10). The coordination center has an octahedral geometry with average values of Co–Nmacrocycle and Co–NPyC60 bond lengths of 2.002 Å and 2.382 Å, respectively (Figure S8a, Supplementary Materials). The additional (PyC60)2CoTTP stabilization is facilitated by both π-π CoTTP–PyC60 stacking and the peripheral tolyl CH3–PyC60·π interactions.
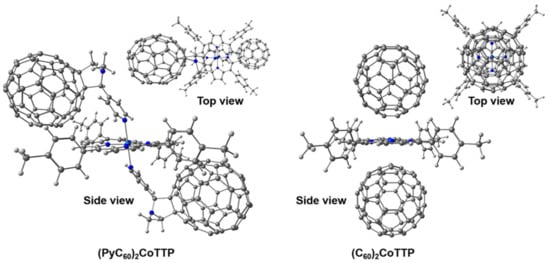
Figure 10.
The optimized structures of (PyC60)2CoTTP (left) and (C60)2CoTTP (right).
In contrast to donor–acceptor bonding, the effect of CoTTP–C60 π-π stacking in the macrocycle geometry, with a slight distortion of 1.47°, is softer. The average length of the Co–Nmacrocycle bonds is 2.004 Å (Figure S8b, Supplementary Materials); the distance between the centers of the CoTTP and C60 closest contacts is 2.686 Å. In addition, one can note the presence of the peripheral tolyl CH3—C60·π contacts. The characteristic feature of the considered triads is the charge transfer from the porphyrin macrocycle to the acceptor fullerene moieties. This is clearly seen as the decrease in negative charge of the CoTTP nitrogen atoms in (PyC60)2CoTTP and (C60)2CoTTP (Figure S9, Supplementary Materials). At the same time, the pyrrolidine moiety in (PyC60)2CoTTP and the fullerene fragment in (C60)2CoTTP acts as the donors (the latter due to the interactions of the carbon atoms of the electron-saturated [6,6] double bonds with the complexing agent atom), transferring the electron density to the metal atom. This is confirmed by the CoTTP central atom positive charge decrease in (PyC60)2CoTTP and (C60)2CoTTP (Figure S9, Supplementary Materials). The axial coordination does not lead to the cobalt atom displacement from the macrocycle plane.
The analysis of the frontier molecular orbitals (FMOs) of (PyC60)2CoTTP and (C60)2CoTTP has shown that HOMO and LUMO in (PyC60)2CoTTP are localized on CoTTP/the pyridine moiety nitrogen atom and on both PyC60 fullerene moieties, respectively (Figure 11). In (C60)2CoTTP, HOMO is localized on both CoTTP and the carbon atoms of the electron-saturated [6,6] double bonds while LUMO is localized on both C60 moieties (Figure 11). Such localization of FMOs in the triads may indicate the mechanism of the implementation PET from the donor CoTTP moiety to the acceptor fullerene-containing moiety. The triad HOMO–LUMO gaps obtained from the DFT calculations (Figure 11) are consistent with the ones estimated from the oxidation and reduction potentials as difference between the first CoTTP oxidation and the first fullerene reduction potentials []. The HOMO–LUMO gap is 1.19 V and 1.45 V for (C60)2CoTTP and (PyC60)2CoTTP, respectively.
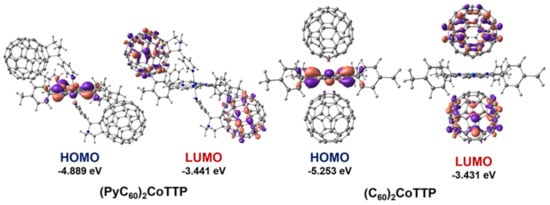
Figure 11.
The FMOs in (PyC60)2CoTTP (left) and (C60)2CoTTP (right).
The information about the excited state of CoTTP and its linear spectra dynamic obtained by the transient absorption measurements using a femtosecond pump–supercontinuum probe setup is as follows: as shown in Section 3.2, the CoTTP Soret band at 414 nm displays an almost 15 times higher molar extinction coefficient than the Q band at 529 nm. The negative signal at 416 nm related to ground-state bleaching and dominating the spectral range of transient absorption spectra was excluded from the studied spectral window for greater visibility and greater significance of the positive signals of in femtosecond transient absorption (FTA) spectra. The CoTTP FTA spectra recorded after the 435 nm laser pulse excitation were collected between 430 nm and 780 nm in different time windows (Figure S10, Supplementary Materials). The CoTTP FTA spectrum displays the characteristic spectral shape of porphyrin singlet excited states []. There is the broad intense absorption band with the maximum at 443 nm as well as the Q-band at 527 nm in the CoTTP singlet excited state spectrum, which bleaches at the respective ground-state absorption band (Figure S10, Supplementary Materials). The excitation wavelength 435 nm used in the present work corresponds to the transition from S0 to the vibrationally excited level of the S2 state of porphyrins []. The analysis of transient absorbance kinetics at 443 nm at short delays has revealed the fast rise component with a time constant of only 0.11 ps, which can be presumably attributed to the S2→S1 internal conversion (Figure S11, Supplementary Materials). Thus, this time constant can be interpreted as the lifetime of the S2 excited state (τ1). This is in agreement with the data reported earlier for other Co(II) porphyrins [,]. The τ1 values of 0.08 ps, 0.17 ps and 0.099 ps were found for the toluene solutions of (5,10,15,20-tetraphenylporphinato)cobalt(II), (5,10,15,20-tetra(4-trifluoromethylphenyl)porphinato)cobalt(II) [] and meso-carbazole substituted cobalt(II)porphyrins, respectively []. The singlet excited state (S1) lifetime (τ2) of CoTTP was calculated from the monoexponential fit to the decay profile at 443 nm and was found to be 5.95 ps (Figure S11, Supplementary Materials).
The (PyC60)2CoTTP and (C60)2CoTTP FTA spectra (Figure 12) differ from these for CoTTP. The detection of both the CoTTP radical cation, CoTTP•+ and the PyC60/C60 radical anion, PyC60•−/C60•−, would confirm PET from porphyrin to fullerene. The PyC60•−/C60•− band is known to appear as broad near-IR peak in the 950 nm to 1020 range []. For the spectral range of our instrument setup from 400 nm to 850 nm, it is not possible to detect a similar peak. That is why the charge-separated states formed in the triads are confirmed in our work by the changing CoTTP•+ bands and S2 and S1 excited state lifetimes.
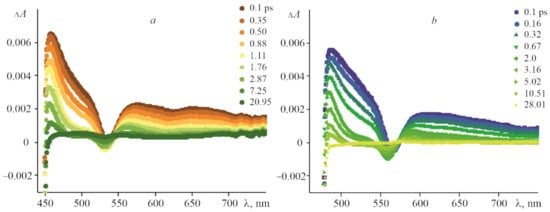
Figure 12.
The FTA spectra at various time delays for (PyC60)2CoTTP (a) and (C60)2CoTTP (b) in toluene following the 435 nm laser excitation.
As shown in Figure 12, the positive signal at 456 nm shifted by 13 nm compared to that of CoTTP is observed after the excitation of the (PyC60)2CoTTP. This signal, displayed at 486 nm in the case of (C60)2CoTTP, shifts much more (Figure 12). The time profiles of the CoTTP•+ bands are represented in Figure 13. The difference within the same time observation window (100 ps) can be seen between the two triads studied. The time constants of the (PyC60)2CoTTP and (C60)2CoTTP singlet excited state, τ2, extracted from the CoTTP•+ decay are 7.28 ps and 14.53 ps, respectively. The analysis of the τ2 values shows a not too long lifetime for the radical ion-pairs of the triads, CoTTP•+:PyC60•−/C60•−, in toluene; however, these results indicate PET from CoTTP to bonded PyC60/C60. The excited state lifetime can be changed by the variation of solvent properties. The charge-separated state lifetime of triad based on CoTTP and phenyl imidazole functionalized fullero[60]pyrrolidine was found at about 1.61 ns in o-dichlorobenzene [].
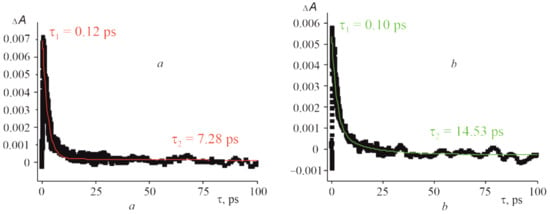
Figure 13.
The time profile of CoTTP•+ band at 456 nm for CoTTP•+:PyC60•− (a) and at 486 nm for CoTTP•+:C60•− (b) (λexc = 435 nm) in toluene. (Red and green monoexponential fit the decay profile).
3. Materials and Methods
3.1. General Information
Organic solvents, chromatographic materials and fullerene (C60) were obtained from Aldrich Chemicals and Acros Organics and used as received without further purification. Meso-tetra(4-tolylphenyl)porphyrin was obtained from PorphyChem.
The UV–vis spectra were measured on an Agilent 8454 UV–visible spectrophotometer in toluene. The stationary fluorescence, emission spectra of singlet molecular oxygen and time-resolved fluorescence measurements were carried out by means of a high-performance fluorescence lifetime and steady-state spectrometer FluoTime 300 with a laser 450 nm, LDH-P-C-450, (PicoQuant, Berlin, Germany) as an excitation source. 1H NMR spectra were recorded on a Bruker Avance III- 500 NMR spectrometer in CDCl3 at room temperature. Chemical shifts (δ) were referenced to the residual solvent peak (CDCl3, 7.26 ppm). IR spectra were obtained using a VERTEX 80v spectrometer in KBr and CsBr pellets. The solid samples of the triads for the IR measurements were prepared by the vacuum removal of the solvent. Mass spectra were performed on an Axima Confidence (Shimadzu Biotech, a 337 nm nitrogen laser) spectrometer. Mass spectra were accumulated using reflectron positive ion mode for MALDI-TOF MS.
Thermal analysis (TG/DTG) of the complexes was carried out in argon using a Netzsch TG 209 F1 microthermal balance. The rate of sample heating, the sample weights and the temperature range were 10 °C/min, 3.5–4.7 mg and 25–920 °C, respectively. The resolution of microthermal balances is 1 × 10−4 mg.
The experiments of cyclic voltammetry (dichloromethane, 0.1 M (n-Bu)4NClO4) were performed on a Solartron SI 1260 using the three-electrode system (a platinum button, a platinum wire and a saturated calomel electrode (SCE) as the working, counter and reference electrode, respectively). Cyclic voltammograms were measured at the potential sweep rate of 1000 mV·s−1. All solutions were purged from the dioxygen prior to electrochemical measurements using an argon gas.
3.2. Synthesis and Analysis of Compounds
(5,10,15,20-tetra(4-methylphenyl)porphinato)cobalt(II), CoTTP, was synthesized by reaction of 5,10,15,20-tetra(4-methylphenyl)porphin (30 mg, 0.041 mmol) with Co(AcO)2·4H2O (51 mg, 0.21 mmol) in boiling dimethylformamide (DMF) for 40 min. The contents of the reaction flask were cooled and diluted with water. The products were extracted into chloroform. The solution in CHCl3 was repeatedly washed with distilled water to remove DMF; then, CHCl3 was partially distilled. The residual solution was purified by chromatography on the Al2O3 column (the grade II activity according to Brockman) using chloroform. The yield is 82%. UV–vis (toluene) λmax/nm (logε): 414 (5.28), 529 (3.86). IR (KBr, cm−1) νmax: 3021 ν(C-H)Ph; 2919, 2856 ν(CH3); 1684 ν (C=N)Pyr; 1612, 1549, 1508 ν(C=C); 1452, 1404, 1377 ν(C-H)Pyr; 1351, 1310, 1238 ν(C–N); 1209, 1182, 1108, 1074, 1036, 1002, 967, 849, 841 δ(C-H)Ph/Pyr; 799, 717 γ(C-H)Ph/Pyr. IR (CsBr, cm−1) νmax: 661, 642, 603, 570, 558, 523, 456 ν(Co-N), 424, 356, 344, 301. 1H NMR (CDCl3), δ (ppm): 15.95 (s, 8Hβ), 13.07 (br. s, 8Ho), 9.75 (br. s, 8Hm), 4.16 (s, 12Htolyl). MS (MALDI-TOF): m/z calcd for C48H36N4Co [M]+ 727.78; found: 728.32 [M]+ (Figure S12, Supplementary Materials).
1-Methyl-2-(pyridin-4′-yl)-3,4-fullero[60]pyrrolidine, PyC60. The synthesis and spectral characteristics of PyC60 are represented in the work of [].
3.3. Thermodynamics and Kinetics
The two-way reaction of CoTTP with C60 and PyC60 in toluene was spectrophotometrically studied at 298 K using the time-dependent titration method in which the thermodynamic and kinetic reaction parameters are obtained in one experimental series (see Supplementary Materials for details).
3.4. Fluorescence Spectroscopy
The fluorescence spectra of C60/PyC60 and its complexes with CoTTP (after excitation 450 nm (LDH-P-C-450)) were measured in toluene in quartz cuvettes (10 mm × 10 mm) on FluoTime 300 PicoQuant spectrofluorimeter. The self-assembly in the systems CoTTP–C60/PyC60 were studied using the method of the molar ratios. The molar series of the solutions with constant concentrations of C60/PyC60 and varying concentrations of CoTTP were prepared by adding CoTTP solution to the C60 (6.51 × 10−5 M) and PyC60 (6.55 × 10−5 M) solutions in toluene. To evaluate the efficiency of the C60/PyC60 fluorescence quenching, the Stern–Volmer constants (KSV) were determined using the equation:
where I0 and I are the fluorescence intensity of C60/PyC60 in the absence and presence of CoTTP, respectively.
The CoTTP–C60/PyC60 bonding constants (KBH) were estimated using modified Benesi–Hildebrand equation:
where I0, Ix and Imax are the fluorescence intensities of C60/PyC60 in the absence of CoTTP, at an intermediate C60/PyC60 concentration and at the concentration of complete interaction, respectively.
The PyC60 fluorescence quantum yield was determined in the toluene at room temperature using C60 in toluene as a fluorescence standard (Φst, = 3.2×10−4) []. The value of ΦF was calculated using the equation:
where ΦF is the fluorescence quantum yield, Φstd—the fluorescence quantum yield of the standard, S—the integrated fluorescence intensity (the area under spectrum), A—the absorbance at the excitation wavelength and n—the refractive index of the solvents used.
The fluorescence decay curves were measured and the fluorescence lifetimes were obtained by the reconvolution of the decay curves using the EasyTau 2 software package (PicoQuant). The biexponential fluorescence decay model was applied for C60, PyC60 and C60–CoTTP/PyC60–CoTTP systems in toluene. The instrument response function (IRF) of the system was measured with the stray light signal of the dilute colloidal silica suspension (LUDOX®).
Singlet molecular oxygen was detected directly during 1O2 luminescence spectrum recording in the range from 1200 nm to 1350 nm for C60, PyC60 and C60–CoTTP/PyC60–CoTTP systems in toluene. The PyC60 luminescence signal of singlet oxygen was measured and compared with the similar C60 signal for which value in toluene is known []. The PyC60 value was calculated using the equation:
where S is the area of singlet oxygen emitted spectrum, A is the absorbance of the solution at the excitation wavelength and τ is the singlet oxygen lifetime of PyC60 or C60. The symbol reported as subscript (std) refers to the C60 standard sample.
3.5. Femtosecond Laser Photolysis Setup
Femtosecond transient absorption spectra were measured at 20 °C on a pump and light supercontinuum probe setup with a 3.3 fs to 1 ps delay step and a 1 nm wavelength step. The spectra were subjected to correction procedure [,,]. The details of the transient spectra measurement were described in Supplementary Materials. The analysis of the characteristic times (τ) of the transient absorption spectra was carried out using the kinetic simulation based on the singular value decomposition (SVD) of the obtained data matrix. This approach involves the consideration of the entire array of the experimental values ΔA(λ, t). Exponential processing of the obtained data at the wavelength of the excited state is used to determine the lifetime of the excited state.
3.6. DFT Calculations
The DFT calculations were carried out for (PyC60)2CoTTP and (C60)2CoTTP using the Gaussian 16, Revision C.01 program package []. The B3LYP functional [] and the def2-SVP [] basis set on all atoms (the def2-ECP is automatically assigned to cobalt atoms) were used for the geometric optimization of the triads. Influence of intramolecular dispersion interactions on molecular structure was explored using the dispersion corrections introduced by S. Grimme (D3) []. The geometric optimization of the triads was carried out without limitation in symmetry. Harmonic vibrational frequencies were calculated at the same theory level in order to characterize the stationary points as true minima, representing the equilibrium structures on the potential energy surfaces. ChemCraft 1.8 [] (the graphical program for visualization of quantum chemistry computations, https://chemcraftprog.com) was used for analyses of results and molecular graphics.
The bonding energy (Ebonding) was calculated as follows: Ebonding = |Etriad − (ECoTTP + EC60/PyC60)|.
4. Conclusions
(5,10,15,20-tetra(4-methylphenyl)porphinato)cobalt(II) (CoTTP) in toluene bonds fullerene (C60) and 1-N-methyl-2-(pyridin-4-yl)-3,4-fullero[60]pyrrolidine (PyC60) in the two-step two-ways processes end in the noncovalent 1: 2 π-π complex and coordination complex, respectively. The bonding constants are (3.47 ± 0.69) ×109 M−2 and (1.47 ± 0.28) ×1010 M−2, respectively, according to the methods of electron absorption spectroscopy. The fluorescence titration gives the close values of the constants with an approximately 1.3 times higher standard deviation. 1H NMR, IR, thermogravimetric analysis and cyclic voltammetry studies of CoTTP, fullerenes and their complexes provide very good support in favor of the effective ground-state complexation between fullerenes and CoTTP. The quenching of the PyC60/C60 fluorescence and the decreasing intensity of the singlet molecular oxygen phosphorescence parallel to the gradual increase in the CoTTP additions during the steady-state emission experiments have confirmed the photoinduced electron transfer (PET) in the triads, (PyC60)2CoTTP/(C60)2CoTTP. The PyC60/C60 fluorescence lifetime and the exited-state characteristics were obtained using fluorescence and femtosecond transient absorption. DFT calculations used to predict the localization of the frontier orbitals and to determine the HOMO/LUMO energy levels have also confirmed the PET from the singlet excited CoTTP to the fullerene moiety in the triads. These results are of particular importance in the development of the porphyrin-fullerene systems, having potential for energy-harvesting applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27248900/s1, Figure S1: The transformation of UV–vis spectrum of the CoTTP–5.76 × 10−5 M PyC60 mixture in toluene at 298 K during 500 s; Figure S2: The plots of logI vs. logCPyC60 at the first and the second stage for the reaction of CoTTP with PyC60; Table S1: The rate constants of the (PyC60)CoTTP reaction with PyC60 in toluene at 298 K; Figure S3: The plot of logkobs vs. logCPyC60 for the reaction of (PyC60)CoTTP with PyC60 in toluene at 298 K; Figure S4: The UV–vis spectrum transformations during the titration of CoTTP with C60 (CC60 = 0 ÷ 9.0 × 10−5 M) in toluene at τ = 0 (a) and τ = ∞ (b). Insets: the plots of logI vs. logCC60; Figure S5: The Benesi–Hildebrand plots for (PyC60)2CoTTP (a) and (C60)2CoTTP (b); Table S2: The time-resolved fluorescence spectroscopy results for C60, PyC60, C60–CoTTP and PyC60–CoTTP systems; Table S3: The phosphorescence decay of singlet oxygen formed by sensitization with PyC60 and the PyC60–CoTTP system; Table S4: The IR bands of fullerenes in the (PyC60)2CoTTP and (C60)2CoTTP triads; Figure S6: The 1H NMR spectra of CoTTP in CDCl3; Figure S7: The TG and DTG curves for the CoTTP (a), C60 (b) and PyC60 (c) powder from 25 °C to 920 °C, with the heating rate of 10 °C/min; Table S5: The peak potentials for CoTTP, C60/PyC60 and triad based on them in CH2Cl2 containing 0.1 M (n-Bu)4NClO4; Figure S8: The values of the (PyC60)2CoTTP (a) and (C60)2CoTTP (b) coordination center bond lengths; Figure S9: The Mulliken charge values of the atoms forming the coordination center of CoTTP (a), (PyC60)2CoTTP (b) and (C60)2CoTTP (c); Figure S10: The femtosecond transient absorption spectra registered at various time delays for CoTTP in toluene following the 435 nm laser excitation; Figure S11: The transient absorption decays at 443 nm at early time (0–1 ps) (a) and (1–500 ps) (b) in toluene recorded for CoTTP (τexc = 435 nm), the green line is monoexponential fit to the decay profile; Figure S12: The MALDI-TOF spectrum of CoTTP; thermodynamics and kinetics; femtosecond laser photolysis setup.
Author Contributions
Conceptualization, N.G.B.; methodology, V.A.M., N.O.K., I.V.S., A.A.K. and F.E.G.; validation, N.G.B., E.N.O. and T.N.L.; formal analysis, E.N.O. and N.G.B.; investigation, N.G.B., E.N.O., I.V.S., A.A.K., V.A.M., N.O.K. and F.E.G.; writing—original draft preparation, E.N.O. and A.A.K.; writing—review and editing, N.G.B., E.N.O. and T.N.L.; visualization, N.G.B., E.N.O. and A.A.K.; supervision, N.G.B.; funding acquisition, N.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (Project number no. 21-73-20090) (as part of the femtosecond transient absorption studies), the Ministry of Science and Higher Education of the Russian Federation (No. 075-15-2021-579) (G.A. Krestov Institute of Solution Chemistry of the Russian Academy of Sciences) (the investigation of the ability to generate singlet oxygen, DFT calculations).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the center of the scientific equipment collective use «The upper Volga region center of physicochemical research». Optical measurements were performed using core research facilities of FRCCP RAS (no. 1440743, 506694).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tanzi, L.; Terreni, M.; Zhang, Y. Synthesis and biological application of glyco- and peptide derivatives of fullerene C60. Eur. J. Med. Chem. 2022, 230, 114104. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; He, Q.; Yuan, X.; Huang, D.; et al. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B Environ. 2020, 265, 118579. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Durantini, J.E.; Durantini, E.N. Fullerene C60 derivatives as antimicrobial photodynamic agents. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100471. [Google Scholar] [CrossRef]
- Yan, W.; Seifermann, S.M.; Pierrat, P.; Bräse, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Li, B.; Fu, L.; Yin, L.-W. One-step-spin-coating route for homogeneous perovskite/pyrrole-C60 fullerene bulk heterojunction for high performance solar cells. J. Power Sources 2019, 419, 27–34. [Google Scholar] [CrossRef]
- Porcu, P.; Estrada-Montaño, A.S.; Vonlanthen, M.; Cuétara-Guadarrama, F.; González-Méndez, I.; Sorroza-Martínez, K.; Zaragoza-Galán, G.; Rivera, E. Azobenzene dyads containing fullerene, porphyrin and pyrene chromophores: Molecular design and optical properties. Dye. Pigment. 2022, 197, 109858. [Google Scholar] [CrossRef]
- Stasheuski, A.S.; Galievsky, V.A.; Stupak, A.P.; Dzhagarov, B.M.; Choi, M.J.; Chung, B.H.; Jeong, J.Y. Photophysical Properties and Singlet Oxygen Generation Efficiencies of Water-Soluble Fullerene Nanoparticles. Photochem. Photobiol. 2014, 90, 997–1003. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial Cytotoxicity of Carbon-Based Nanomaterials: Implications for River Water and Wastewater Effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Brunet, L.; Hinkal, G.W.; Wiesner, M.R.; Alvarez, P.J.J. Antibacterial Activity of Fullerene Water Suspensions (nC60) Is Not Due to ROS-Mediated Damage. Nano Lett. 2008, 8, 1539–1543. [Google Scholar] [CrossRef]
- Kubatova, H.; Zemanova, E.; Klouda, K.; Bilek, K.; Kadukova, J. Effects of C60 Fullerene and its Derivatives on Selected Microorganisms. J. Microbiol. Res. 2013, 3, 152–162. [Google Scholar] [CrossRef]
- Fedorova, N.E.; Klimova, R.R.; Tulenev, Y.A.; Chichev, E.V.; Kornev, A.B.; Troshin, P.A.; Kushch, A.A. Carboxylic Fullerene C60 Derivatives: Efficient Microbicides Against Herpes Simplex Virus And Cytomegalovirus Infections In Vitro. Mendeleev Commun. 2012, 22, 254–256. [Google Scholar] [CrossRef]
- Wang, M.; Maragani, S.; Huang, L.; Jeon, S.; Canteenwala, T.; Hamblin, M.R.; Chiang, L.Y. Synthesis of decacationic [60]fullerene decaiodides giving photoinduced production of superoxide radicals and effective PDT-mediation on antimicrobial photoinactivation. Eur. J. Med. Chem. 2013, 63, 170–184. [Google Scholar] [CrossRef]
- Siddiquie, R.Y.; Agrawal, A.; Joshi, S.S. Surface Alterations to Impart Antiviral Properties to Combat COVID-19 Transmission. Trans. Indian Natl. Acad. Eng. 2020, 5, 343–347. [Google Scholar] [CrossRef]
- Vorobiev, A.K.; Markov, V.Y.; Samokhvalova, N.A.; Samokhvalov, P.S.; Troyanov, S.I.; Sidorov, L.N. Stable trifluoromethylated fullerene radicals C60(CF3)15 and C60(CF3)17. Mendeleev Commun. 2010, 20, 7–9. [Google Scholar] [CrossRef]
- Samokhvalova, N.A.; Khavrel’, P.A.; Goryunkov, A.A.; Ioffe, I.N.; Karnatsevich, V.L.; Sidorov, L.N.; Kemnitz, E.; Troyanova, S.I. New isomers of trifluoromethylated fullerene: C60(CF3)12 and C60(CF3)14. Russ. Chem. Bull. 2008, 57, 2526–2534. [Google Scholar] [CrossRef]
- Brotsman, V.A.; Ioutsi, V.A.; Rybalchenko, A.V.; Markov, V.Y.; Belov, N.M.; Lukonina, N.S.; Troyanov, S.I.; Ioffe, I.N.; Trukhanov, V.A.; Galimova, G.K.; et al. Tightly Bound Double-Caged [60]Fullerene Derivatives with Enhanced Solubility: Structural Features and Application in Solar Cells. Chem. Asian J. 2017, 12, 1075–1086. [Google Scholar] [CrossRef]
- Brotsman, V.A.; Rybalchenko, A.V.; Zubov, D.N.; Paraschuk, D.Y.; Goryunkov, A.A. Double-caged fullerene acceptors: Effect of alkyl chain length on photovoltaic performance. J. Mater. Chem. C 2019, 7, 3278–3285. [Google Scholar] [CrossRef]
- Harneit, W.; Meyer, C.; Weidinger, A.; Suter, D.; Twamley, J. Architectures for a Spin Quantum Computer Based on Endohedral Fullerenes. Phys. Status Solidi (b) 2002, 233, 453–461. [Google Scholar] [CrossRef]
- Troshin, P.A.; Peregudov, A.S.; Troyanov, S.I.; Lyubovskaya, R.N. New pyrrolidine and pyrroline derivatives of fullerenes: From the synthesis to the use in light-converting systems. Russ. Chem. Bull. 2008, 57, 887–912. [Google Scholar] [CrossRef]
- Kc, C.B.; D’Souza, F. Design and photochemical study of supramolecular donor–acceptor systems assembled via metal–ligand axial coordination. Coord. Chem. Rev. 2016, 322, 104–141. [Google Scholar] [CrossRef]
- Borges-Martínez, M.; Montenegro-Pohlhammer, N.; Zhang, X.; Galvez-Aranda, D.E.; Ponce, V.; Seminario, J.M.; Cárdenas-Jirón, G. Fullerene binding effects in Al(III)/Zn(II) Porphyrin/Phthalocyanine photophysical properties and charge transport. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 269, 120740. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, B.; Waszkowska, K.; Busseau, A.; Villegas, C.; Hudhomme, P.; Dabos-Seignon, S.; Zawadzka, A.; Legoupy, S.; Sahraoui, B. Penta(zinc porphyrin)[60]fullerenes: Strong reverse saturable absorption for optical limiting applications. Appl. Surf. Sci. 2020, 533, 147468. [Google Scholar] [CrossRef]
- Costa, J.I.T.; Farinha, A.S.F.; Neves, M.; Tome, A.C. An easy access to porphyrin triads and their supramolecular interaction with a pyridyl 60 fulleropyrrolidine. Dye. Pigment. 2016, 135, 163–168. [Google Scholar] [CrossRef]
- Guldi, D.M. Fullerenes: Three dimensional electron acceptor materials. Chem. Commun. 2000, 321–327. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Bichan, N.G.; Gruzdev, M.S.; Ksenofontov, A.A.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Lomova, T.N. Carbazole-functionalized cobalt(ii) porphyrin axially bonded with C60/C70 derivatives: Synthesis and characterization. New J. Chem. 2021, 45, 9053–9065. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Ksenofontov, A.A.; Mozgova, V.A.; Gruzdev, M.S.; Chervonova, U.V.; Shelaev, I.V.; Lomova, T.N. Meso-carbazole substituted porphyrin complexes: Synthesis and spectral properties according to experiment, DFT calculations and the prediction by machine learning methods. Dye. Pigment. 2022, 204, 110470. [Google Scholar] [CrossRef]
- Morisue, M.; Saito, G.; Sasada, D.; Umeyama, T.; Imahori, H.; Mitamura, K.; Masunaga, H.; Hoshino, T.; Sakurai, S.; Sasaki, S. Glassy Porphyrin/C60 Composites: Morphological Engineering of C60 Fullerene with Liquefied Porphyrins. Langmuir 2020, 36, 13583–13590. [Google Scholar] [CrossRef]
- Pratik, S.M.; Gagliardi, L.; Cramer, C.J. Boosting Photoelectric Conductivity in Porphyrin-Based MOFs Incorporating C60. J. Phys. Chem. C 2020, 124, 1878–1887. [Google Scholar] [CrossRef]
- Olmstead, M.M.; Costa, D.A.; Maitra, K.; Noll, B.C.; Phillips, S.L.; Van Calcar, P.M.; Balch, A.L. Interaction of Curved and Flat Molecular Surfaces. The Structures of Crystalline Compounds Composed of Fullerene (C60, C60O, C70, and C120O) and Metal Octaethylporphyrin Units. J. Am. Chem. Soc. 1999, 121, 7090–7097. [Google Scholar] [CrossRef]
- Konarev, D.V.; Neretin, I.S.; Slovokhotov, Y.L.; Yudanova, E.I.; Drichko, N.V.; Shul’ga, Y.M.; Tarasov, B.P.; Gumanov, L.L.; Batsanov, A.S.; Howard, J.A.K.; et al. New molecular complexes of fullerenes C60 and C70 with tetraphenylporphyrins [M(tpp)], in which M = H2, Mn, Co, Cu, Zn, and FeCl. Chem. Eur. J. 2001, 7, 2605–2616. [Google Scholar] [CrossRef]
- Sun, D.; Tham, F.S.; Reed, C.A.; Chaker, L.; Boyd, P.D.W. Supramolecular Fullerene-Porphyrin Chemistry. Fullerene Complexation by Metalated “Jaws Porphyrin” Hosts. J. Am. Chem. Soc. 2002, 124, 6604–6612. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Tashiro, K.; Yamasaki, M.; Aida, T. Hosting Fullerenes by Dynamic Bond Formation with an Iridium Porphyrin Cyclic Dimer: A “Chemical Friction” for Rotary Guest Motions. J. Am. Chem. Soc. 2007, 129, 11912–11913. [Google Scholar] [CrossRef]
- Yamada, M.; Murakami, G.; Kobayashi, S.; Maeda, Y. Enhancing solution-phase supramolecular interactions between monomeric porphyrins and [60]fullerene by simple chemical modification. Tetrahedron Lett. 2017, 58, 4514–4518. [Google Scholar] [CrossRef]
- Nayak, S.; Ray, A.; Bhattacharya, S. Size selective supramolecular interaction upon molecular complexation of a designed porphyrin with C60 and C70 in solution. J. Mol. Liq. 2021, 321, 114367. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Mozgova, V.A.; Kudryakova, N.O.; Lomova, T.N. Formation Reaction, Spectroscopy, and Photoelectrochemistry of the Donor–Acceptor Complex (5,10,15,20-Tetraphenyl-21,23H-porphinato)cobalt(II) with Pyridyl-Substituted Fullero[60]pyrrolidine. Russ. J. Inorg. Chem. 2019, 64, 605–614. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Gruzdev, M.S.; Lomova, T.N. Formation Reaction and Chemical Structure of a Novel Supramolecular Triad Based on Cobalt(II) 5,10,15,20-(Tetra-4-Tert-Butylphenyl)-21H,23H-Porphyrin and 1-Methyl-2-(Pyridin-4′-yl)-3,4-Fullero 60 Pyrrolidine. J. Struct. Chem. 2018, 59, 711–719. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Mozgova, V.A.; Kudryakova, N.O.; Gruzdev, M.S.; Lomova, T.N. Mechanism of the Self-Assembly of Donor–Acceptor Triads Based on Cobalt(II) Porphyrin Complex and Fullero[60]pyrrolidine, According to Data Obtained by Spectroscopic and Electrochemical Means. Russ. J. Phys. Chem. A 2020, 94, 1159–1166. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Mozgova, V.A.; Kudryakova, N.O.; Lomova, T.N. Three cobalt(II) porphyrins ligated with pyridyl-containing nanocarbon/gold(III) porphyrin for solar cells: Synthesis and characterization. Polyhedron 2021, 203, 115223. [Google Scholar] [CrossRef]
- Gacka, E.; Burdzinski, G.; Marciniak, B.; Kubas, A.; Lewandowska-Andralojc, A. Interaction of light with a non-covalent zinc porphyrin–graphene oxide nanohybrid. Phys. Chem. Chem. Phys. 2020, 22, 13456–13466. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Bichan, N.G.; Ksenofontov, A.A.; Lomova, T.N. New dyads based on trifluoromethylated phthalocyanine derivatives and substituted fullerene with possible application photoinduced electron transfer. J. Fluor. Chem. 2019, 224, 113–120. [Google Scholar] [CrossRef]
- Poddutoori, P.K.; Lim, G.N.; Vassiliev, S.; D’Souza, F. Ultrafast charge separation and charge stabilization in axially linked ‘tetrathiafulvalene–aluminum(iii) porphyrin–gold(iii) porphyrin’ reaction center mimics. Phys. Chem. Chem. Phys. 2015, 17, 26346–26358. [Google Scholar] [CrossRef] [PubMed]
- Subedi, D.R.; Jang, Y.; Ganesan, A.; Schoellhorn, S.; Reid, R.; Verbeck, G.F.; D’Souza, F. Donor-acceptor conjugates derived from cobalt porphyrin and fullerene via metal-ligand axial coordination: Formation and excited state charge separation. J. Porphyr. Phthalocyanines 2021, 25, 533–546. [Google Scholar] [CrossRef]
- Ma, B.; Sun, Y.-P. Fluorescence spectra and quantum yields of [60]fullerene and [70]fullerene under different solvent conditions. A quantitative examination using a near-infrared-sensitive emission spectrometer. J. Chem. Soc. Perkin Trans. 1996, 2, 2157–2162. [Google Scholar] [CrossRef]
- Brites, M.J.; Santos, C.; Nascimento, S.; Gigante, B.; Luftmann, H.; Fedorov, A.; Berberan-Santos, M.N. Synthesis and fluorescence properties of [60] and [70]fullerene–coumarin dyads: Efficient dipole–dipole resonance energy transfer from coumarin to fullerene. New J. Chem. 2006, 30, 1036–1045. [Google Scholar] [CrossRef]
- Catalan, J.; Elguero, J. Fluorescence of fullerenes (C60 and C70). J. Am. Chem. Soc. 1993, 115, 9249–9252. [Google Scholar] [CrossRef]
- Song, J.; Li, F.-m.; Qian, S.-x.; Li, Y.-f.; Peng, W.-j.; Zhou, J.-y.; Yu, Z.-x. Time decay behavior of fullerene-C60 studied by time-resolved photoluminescence. Acta Phys. Sin. (Overseas Ed.) 1995, 4, 175. [Google Scholar] [CrossRef]
- Shihong, M.; Xingze, L.; Jianhua, X.; Zhigang, C.; Jianying, Z.; Wencheng, W.; Zhiming, Z. Photoluminescence of fullerene[60] in ultrathin ordered multilayers: The dissociation of molecular aggregation. Chin. Sci. Bull. 1998, 43, 1004–1008. [Google Scholar] [CrossRef]
- Prat, F.; Martí, C.; Nonell, S.; Zhang, X.; Foote, C.S.; Moreno, R.G.; Bourdelande, J.L.; Font, J. C60 Fullerene-based materials as singlet oxygen O2(1Δg) photosensitizers: A time-resolved near-IR luminescence and optoacoustic study. Phys. Chem. Chem. Phys. 2001, 3, 1638–1643. [Google Scholar] [CrossRef]
- Ooyama, Y.; Enoki, T.; Ohshita, J.; Kamimura, T.; Ozako, S.; Koide, T.; Tani, F. Singlet oxygen generation properties of an inclusion complex of cyclic free-base porphyrin dimer and fullerene C60. RSC Adv. 2017, 7, 18690–18695. [Google Scholar] [CrossRef]
- Hung, R.R.; Grabowski, J.J. A precise determination of the triplet energy of carbon (C60) by photoacoustic calorimetry. J. Phys. Chem. 1991, 95, 6073–6075. [Google Scholar] [CrossRef]
- Hamano, T.; Okuda, K.; Mashino, T.; Hirobe, M.; Arakane, K.; Ryu, A.; Mashiko, S.; Nagano, T. Singlet oxygen production from fullerene derivatives: Effect of sequential functionalization of the fullerene core. Chem. Commun. 1997, 21–22. [Google Scholar] [CrossRef]
- Prat, F.; Stackow, R.; Bernstein, R.; Qian, W.; Rubin, Y.; Foote, C.S. Triplet-State Properties and Singlet Oxygen Generation in a Homologous Series of Functionalized Fullerene Derivatives. J. Phys. Chem. A 1999, 103, 7230–7235. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Klyueva, M.E.; Lomova, T.N. Pyridine coordination to manganese(III) porphyrins: The effect of multiple functional substitution in porphyrin. Russ. J. Inorg. Chem. 2017, 62, 1483–1487. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Bichan, N.G.; Lomova, T.N. Synthesis and properties of a new (octaethylporphyrinato)-manganese(III)-pyridinyl-substituted pyrrolidinofullerene dyad. Russ. J. Org. Chem. 2016, 52, 1503–1508. [Google Scholar] [CrossRef]
- Bichan, N.G.; Ovchenkova, E.N.; Kudryakova, N.O.; Ksenofontov, A.A.; Gruzdev, M.S.; Lomova, T.N. Self-assembled cobalt(ii)porphyrin–fulleropyrrolidine triads via axial coordination with photoinduced electron transfer. New J. Chem. 2018, 42, 12449–12456. [Google Scholar] [CrossRef]
- Qu, Y.; Yu, W.; Liang, S.; Li, S.; Zhao, J.; Piao, G. Structure and Morphology Characteristics of Fullerene C60 Nanotubes Fabricated with N-Methyl-2-pyrrolidone as a Good Solvent. J. Nanomater. 2011, 2011, 706293. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Yang, W.-J.; Zhou, Y.; Pan, W.-H.; Wei, C.-X.; Yuen, A.C.Y.; Chen, T.B.Y.; Yeoh, G.H.; Lu, H.-D.; Yang, W. Synthesis of zinc porphyrin complex for improving mechanical, UV-resistance, thermal stability and fire safety properties of polystyrene. Chem. Eng. J. 2022, 442, 136367. [Google Scholar] [CrossRef]
- Jin, J.; Dong, Z.; He, J.; Li, R.; Ma, J. Synthesis of Novel Porphyrin and its Complexes Covalently Linked to Multi-Walled Carbon Nanotubes and Study of their Spectroscopy. Nanoscale Res. Lett. 2009, 4, 578. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.; Ozoemena, K.I.; Maree, D.M.; Nyokong, T. Synthesis and electrochemical studies of a covalently linked cobalt(ii) phthalocyanine–cobalt(ii) porphyrin conjugate. Dalton Trans. 2005, 1241–1248. [Google Scholar] [CrossRef]
- D’Souza, F.; Deviprasad, G.R.; Zandler, M.E.; Hoang, V.T.; Klykov, A.; VanStipdonk, M.; Perera, A.; El-Khouly, M.E.; Fujitsuka, M.; Ito, O. Spectroscopic, Electrochemical, and Photochemical Studies of Self-Assembled via Axial Coordination Zinc Porphyrin−Fulleropyrrolidine Dyads. J. Phys. Chem. A 2002, 106, 3243–3252. [Google Scholar] [CrossRef]
- Troshin, P.A.; Peregudov, A.S.; Mühlbacher, D.; Lyubovskaya, R.N. An Efficient [2+3] Cycloaddition Approach to the Synthesis of Pyridyl-Appended Fullerene Ligands. Eur. J. Org. Chem. 2005, 2005, 3064–3074. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Motorina, E.V.; Bichan, N.G.; Gostev, F.E.; Lomova, T.N. Self-assembling cobalt(II) porphyrin—Fullero [60]pyrrolidine triads. Synthesis and spectral properties in the ground and excited state. J. Organomet. Chem. 2022, 977, 122458. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Tsaturyan, A.A.; Bichan, N.G.; Lomova, T.N. N Basicity of Substituted Fullero[60]/[70]pyrrolidines According to DFT/TD-DFT Calculations and Chemical Thermodynamics. J. Phys. Chem. A 2021, 125, 5365–5374. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, S.A.; Dobryakov, A.L.; Ruthmann, J.; Ernsting, N.P. Femtosecond spectroscopy of condensed phases with chirped supercontinuum probing. Phys. Rev. A 1999, 59, 2369–2384. [Google Scholar] [CrossRef]
- Shelaev, I.V.; Gostev, F.E.; Vishnev, M.I.; Shkuropatov, A.Y.; Ptushenko, V.V.; Mamedov, M.D.; Sarkisov, O.M.; Nadtochenko, V.A.; Semenov, A.Y.; Shuvalov, V.A. P680 (PD1PD2) and ChlD1 as alternative electron donors in photosystem II core complexes and isolated reaction centers. J. Photochem. Photobiol. B Biol. 2011, 104, 44–50. [Google Scholar] [CrossRef]
- Dobryakov, A.L.; Pérez Lustres, J.L.; Kovalenko, S.A.; Ernsting, N.P. Femtosecond transient absorption with chirped pump and supercontinuum probe: Perturbative calculation of transient spectra with general lineshape functions, and simplifications. Chem. Phys. 2008, 347, 127–138. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2019.
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Zhurko, G.A. Chemcraft- Graphical Program for Visualization of Quantum Chemistry Computations, Ver. 1.8. Available online: http://www.chemcraftprog.com (accessed on 19 November 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).