Precious Gene: The Application of RET-Altered Inhibitors
Abstract
1. Introduction
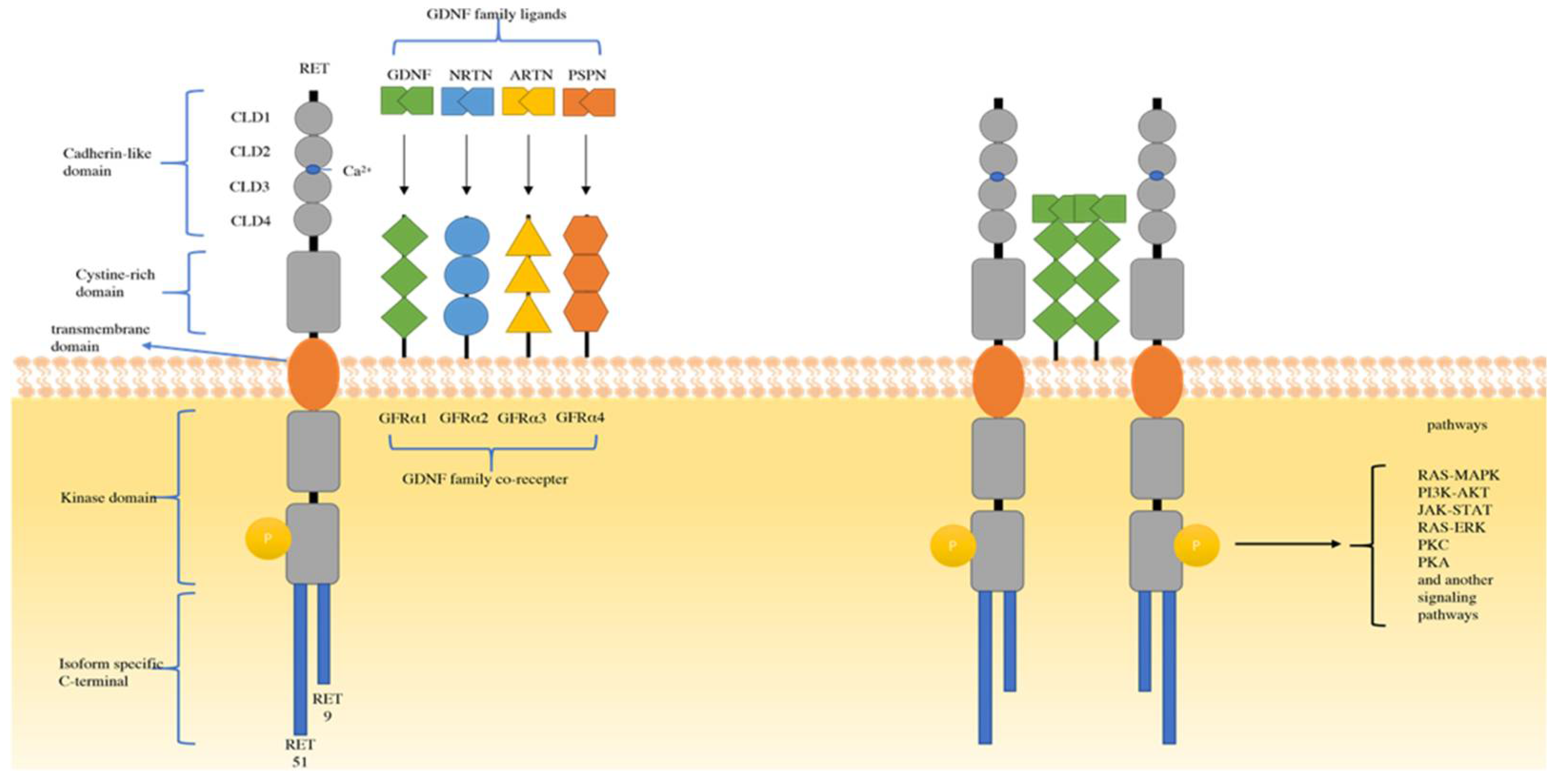
2. Mechanism and Biology of RET
3. RET-Targeted Therapies
3.1. Multikinase Inhibitors (MKIs)
3.1.1. MKIs in Lung Cancer
3.1.2. MKIs in Thyroid Cancer
3.1.3. MKI in Other Solid Tumors
3.1.4. Disadvantages of MKIs
3.2. Selective RET Inhibitors
3.2.1. Selpercatinib (LOXO-292)
3.2.2. Pralsetinib (BLU-667)
3.2.3. TPX-0046
3.2.4. Zeteletinib (BOS-172738; DS-5010)
4. Future Expectations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Goldman, J.M.; Melo, J.V. Targeting the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001, 344, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.-W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.-J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.Y.; Haaland, B.A.; Lopes, G.D.L. Gefitinib vs. chemotherapy as first-line therapy in advanced non-small cell lung cancer: Meta-analysis of phase III trials. Lung Cancer 2011, 74, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Lin, J.J.; Shaw, A.T. Resisting Resistance: Targeted Therapies in Lung Cancer. Trends Cancer 2016, 2, 350–364. [Google Scholar] [CrossRef]
- Gainor, J.F.; Shaw, A.T. Emerging Paradigms in the Development of Resistance to Tyrosine Kinase Inhibitors in Lung Cancer. J. Clin. Oncol. 2013, 31, 3987–3996. [Google Scholar] [CrossRef]
- Chi, X.; Michos, O.; Shakya, R.; Riccio, P.; Enomoto, H.; Licht, J.D.; Asai, N.; Takahashi, M.; Ohgami, N.; Kato, M.; et al. Ret-Dependent Cell Rearrangements in the Wolffian Duct Epithelium Initiate Ureteric Bud Morphogenesis. Dev. Cell 2009, 17, 199–209. [Google Scholar] [CrossRef]
- de Graaff, E.; Srinivas, S.; Kilkenny, C.; D’Agati, V.; Mankoo, B.S.; Costantini, F.; Pachnis, V. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001, 15, 2433–2444. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Takahashi, M.; Asai, N.; Iwashita, T.; Matsuyama, M.; Asai, J. Spatial and temporal expression of the ret proto-oncogene product in embryonic, infant and adult rat tissues. Oncogene 1995, 10, 191–198. [Google Scholar]
- Kohno, T.; Tabata, J.; Nakaoku, T. REToma: A cancer subtype with a shared driver oncogene. Carcinogenesis 2020, 41, 123–129. [Google Scholar] [CrossRef]
- Gainor, J.F.; Shaw, A.T. The New Kid on the Block: RET in Lung Cancer. Cancer Discov. 2013, 3, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Santoro, M.; Schlumberger, M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat. Rev. Endocrinol. 2021, 17, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Romei, C.; Ciampi, R.; Elisei, R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat. Rev. Endocrinol. 2016, 12, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, L.M. RET revisited: Expanding the oncogenic portfolio. Nat. Rev. Cancer 2014, 14, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Donis-Keller, H.; Dou, S.; Chi, D.; Carlson, K.M.; Toshima, K.; Lairmore, T.C.; Howe, J.; Moley, J.F.; Goodfellow, P.; Wells, J.S.A. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum. Mol. Genet. 1993, 2, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Ichikawa, H.; Totoki, Y.; Yasuda, K.; Hiramoto, M.; Nammo, T.; Sakamoto, H.; Tsuta, K.; Furuta, K.; Shimada, Y.; et al. KIF5B-RET fusions in lung adenocarcinoma. Nat. Med. 2012, 18, 375–377. [Google Scholar] [CrossRef]
- Drilon, A.; Rekhtman, N.; Arcila, M.; Wang, L.; Ni, A.; Albano, M.; Van Voorthuysen, M.; Somwar, R.; Smith, R.S.; Montecalvo, J.; et al. Cabozantinib in patients with advanced RET -rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016, 17, 1653–1660. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, J.-K.; Ahn, M.-J.; Kim, D.-W.; Sun, J.-M.; Keam, B.; Kim, T.; Heo, D.; Ahn, J.; Choi, Y.-L.; et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: A phase II clinical trial. Ann. Oncol. 2017, 28, 292–297. [Google Scholar] [CrossRef]
- Thein, K.Z.; Velcheti, V.; Mooers, B.H.; Wu, J.; Subbiah, V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer 2021, 7, 1074–1088. [Google Scholar] [CrossRef]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; A Cassier, P.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.; Loong, H.H.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Selpercatinib: First approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Pralsetinib: First approval. Drugs 2020, 80, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K. Discovery stories of ret fusions in lung cancer: A mini-review. Front. Physiol. 2019, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ritz, J.; Cooper, G.M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985, 42, 581–588. [Google Scholar] [CrossRef]
- Ishizaka, Y.; Itoh, F.; Tahira, T.; Ikeda, I.; Sugimura, T.; Tucker, J.; Fertitta, A.; Carrano, A.V.; Nagao, M. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 1989, 4, 1519–1521. [Google Scholar]
- Takeuchi, K.; Soda, M.; Togashi, Y.; Suzuki, R.; Sakata, S.; Hatano, S.; Asaka, R.; Hamanaka, W.; Ninomiya, H.; Uehara, H.; et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 2012, 18, 378–381. [Google Scholar] [CrossRef]
- Wang, X. Structural studies of GDNF family ligands with their receptors—Insights into ligand recognition and activation of receptor tyrosine kinase RET. Biochim. Biophys. Acta BBA Proteins Proteom. 2013, 1834, 2205–2212. [Google Scholar] [CrossRef]
- Goodman, K.M.; Kjær, S.; Beuron, F.; Knowles, P.P.; Nawrotek, A.; Burns, E.M.; Purkiss, A.G.; George, R.; Santoro, M.; Morris, E.P.; et al. RET Recognition of GDNF-GFRα1 Ligand by a Composite Binding Site Promotes Membrane-Proximal Self-Association. Cell Rep. 2014, 8, 1894–1904. [Google Scholar] [CrossRef]
- O’Leary, C.; Xu, W.; Pavlakis, N.; Richard, D.; O’Byrne, K. Rearranged During Transfection Fusions in Non-Small Cell Lung Cancer. Cancers 2019, 11, 620. [Google Scholar] [CrossRef]
- Ibáñez, C.F. Structure and Physiology of the RET Receptor Tyrosine Kinase. Cold Spring Harb. Perspect. Biol. 2013, 5, a009134. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.S.; Lee, W.-C.; Shin, J.-Y.; Lee, S.; Bleazard, T.; Won, J.-K.; Kim, Y.T.; Kim, J.-I.; Kang, J.-H.; Seo, J.-S. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012, 22, 436–445. [Google Scholar] [CrossRef] [PubMed]
- American Thyroid Association Guidelines Taskforce on Thyroid; Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; et al. Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Romei, C.; Vorontsova, T.; Cosci, B.; Veremeychik, V.; Kuchinskaya, E.; Basolo, F.; Demidchik, E.P.; Miccoli, P.; Pinchera, A.; et al. RET/PTC Rearrangements in Thyroid Nodules: Studies in Irradiated and Not Irradiated, Malignant and Benign Thyroid Lesions in Children and Adults1. J. Clin. Endocrinol. Metab. 2001, 86, 3211–3216. [Google Scholar] [CrossRef]
- Cheung, C.C.; Carydis, B.; Ezzat, S.; Bedard, Y.C.; Asa, S.L. Analysis of ret/PTC Gene Rearrangements Refines the Fine Needle Aspiration Diagnosis of Thyroid Cancer. J. Clin. Endocrinol. Metab. 2001, 86, 2187–2190. [Google Scholar] [CrossRef]
- Nikiforov, Y.E. RET/PTC Rearrangement in Thyroid Tumors. Endocr. Pathol. 2002, 13, 3–16. [Google Scholar] [CrossRef]
- Romei, C.; Elisei, R. RET/PTC Translocations and Clinico-Pathological Features in Human Papillary Thyroid Carcinoma. Front. Endocrinol. 2012, 3, 54. [Google Scholar] [CrossRef]
- Hamatani, K.; Eguchi, H.; Ito, R.; Mukai, M.; Takahashi, K.; Taga, M.; Imai, K.; Cologne, J.; Soda, M.; Arihiro, K.; et al. RET/PTC Rearrangements Preferentially Occurred in Papillary Thyroid Cancer among Atomic Bomb Survivors Exposed to High Radiation Dose. Cancer Res. 2008, 68, 7176–7182. [Google Scholar] [CrossRef]
- Hamatani, K.; Eguchi, H.; Koyama, K.; Mukai, M.; Nakachi, K.; Kusunoki, Y. A novel RET rearrangement (ACBD5/RET) by pericentric inversion, inv(10)(p12.1;q11.2), in papillary thyroid cancer from an atomic bomb survivor exposed to high-dose radiation. Oncol. Rep. 2014, 32, 1809–1814. [Google Scholar] [CrossRef][Green Version]
- Leeman-Neill, R.J.; Brenner, A.V.; Ma, M.P.L.; Bogdanova, T.I.; Hatch, M.; Zurnadzy, L.Y.; Mabuchi, K.; Tronko, M.D.; Nikiforov, Y.E. RET/PTC and PAX8/PPARγ chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer 2013, 119, 1792–1799. [Google Scholar] [CrossRef]
- Lipson, D.; Capelletti, M.; Yelensky, R.; Otto, G.; Parker, A.; Jarosz, M.; A Curran, J.; Balasubramanian, S.; Bloom, T.; Brennan, K.W.; et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat. Med. 2012, 18, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Yang, D.; Velcheti, V.; Drilon, A.; Meric-Bernstam, F. State-of-the-Art Strategies for Targeting RET-Dependent Cancers. J. Clin. Oncol. 2020, 38, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Klempner, S.; Bazhenova, L.A.; Braiteh, F.S.; Nikolinakos, P.G.; Gowen, K.; Cervantes, C.M.; Chmielecki, J.; Greenbowe, J.R.; Ross, J.S.; Stephens, P.J.; et al. Emergence of RET rearrangement co-existing with activated EGFR mutation in EGFR-mutated NSCLC patients who had progressed on first- or second-generation EGFR TKI. Lung Cancer 2015, 89, 357–359. [Google Scholar] [CrossRef]
- Le Rolle, A.-F.; Klempner, S.J.; Garrett, C.R.; Seery, T.; Sanford, E.M.; Balasubramanian, S.; Ross, J.S.; Stephens, P.J.; Miller, V.A.; Ali, S.M.; et al. Identification and characterization of RET fusions in advanced colorectal cancer. Oncotarget 2015, 6, 28929–28937. [Google Scholar] [CrossRef]
- Kim, S.Y.; Oh, S.O.; Kim, K.; Lee, J.; Kang, S.; Kim, K.-M.; Lee, W.; Kim, S.T.; Nam, D.N. NCOA4-RET fusion in colorectal cancer: Therapeutic challenge using patient-derived tumor cell lines. J. Cancer 2018, 9, 3032–3037. [Google Scholar] [CrossRef]
- Romei, C.; Cosci, B.; Renzini, G.; Bottici, V.; Molinaro, E.; Agate, L.; Passannanti, P.; Viola, D.; Biagini, A.; Basolo, F.; et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC). Clin. Endocrinol. 2011, 74, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Raue, F.; Frank-Raue, K. Update on Multiple Endocrine Neoplasia Type 2: Focus on Medullary Thyroid Carcinoma. J. Endocr. Soc. 2018, 2, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Jiao, Y.; Sausen, M.; Leary, R.; Bettegowda, C.; Roberts, N.; Bhan, S.; Ho, A.S.; Khan, Z.; Bishop, J.; et al. Exomic Sequencing of Medullary Thyroid Cancer Reveals Dominant and Mutually Exclusive Oncogenic Mutations in RET and RAS. J. Clin. Endocrinol. Metab. 2013, 98, E364–E369. [Google Scholar] [CrossRef]
- Gautschi, O.; Milia, J.; Filleron, T.; Wolf, J.; Carbone, D.P.; Owen, D.H.; Camidge, R.; Narayanan, V.; Doebele, R.C.; Besse, B.; et al. Targeting RET in Patients With RET-Rearranged Lung Cancers: Results from the Global, Multicenter RET Registry. J. Clin. Oncol. 2017, 35, 1403–1410. [Google Scholar] [CrossRef]
- Yoh, K.; Seto, T.; Satouchi, M.; Nishio, M.; Yamamoto, N.; Murakami, H.; Nogami, N.; Matsumoto, S.; Kohno, T.; Tsuta, K.; et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): An open-label, multicentre phase 2 trial. Lancet Respir. Med. 2017, 5, 42–50. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarząb, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in Progressive Medullary Thyroid Cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Fu, S.; Patel, M.R.; Fakih, M.; Wang, D.; Olszanski, A.J.; Morgensztern, D.; Liu, S.V.; Cho, B.C.; Bazhenova, L.; et al. A Phase I/Ib Trial of the VEGFR-Sparing Multikinase RET Inhibitor RXDX-105. Cancer Discov. 2019, 9, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A., Jr.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase III Trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Koehler, V.F.; Adam, P.; Frank-Raue, K.; Raue, F.; Berg, E.; Hoster, E.; Allelein, S.; Schott, M.; Kroiss, M.; Spitzweg, C.; et al. Real-World Efficacy and Safety of Cabozantinib and Vandetanib in Advanced Medullary Thyroid Cancer. Thyroid 2021, 31, 459–469. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef]
- Morandi, A.; Plaza-Menacho, I.; Isacke, C.M. RET in breast cancer: Functional and therapeutic implications. Trends Mol. Med. 2011, 17, 149–157. [Google Scholar] [CrossRef]
- Drilon, A.; Hu, Z.I.; Lai, G.G.Y.; Tan, D.S.W. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat. Rev. Clin. Oncol. 2018, 15, 151–167. [Google Scholar] [CrossRef]
- Das, T.K.; Cagan, R.L. KIF5B-RET Oncoprotein Signals through a Multi-kinase Signaling Hub. Cell Rep. 2017, 20, 2368–2383. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non–Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Subbiah, V.; Velcheti, V.; Tuch, B.; Ebata, K.; Busaidy, N.; Cabanillas, M.; Wirth, L.; Stock, S.; Smith, S.; Lauriault, V.; et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann. Oncol. 2018, 29, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Illini, O.; Hochmair, M.J.; Fabikan, H.; Weinlinger, C.; Tufman, A.; Swalduz, A.; Lamberg, K.; Hashemi, S.M.S.; Huemer, F.; Vikström, A.; et al. Selpercatinib in RET fusion-positive non-small-cell lung cancer (SIREN): A retrospective analysis of patients treated through an access program. Ther. Adv. Med. Oncol. 2021, 13, 17588359211019675. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Hu, X.; Liu, X.; Subbiah, V.; Mooers, B.H.M.; Wu, J. The L730V/I RET roof mutations display different activities toward pralsetinib and selpercatinib. npj Precis. Oncol. 2021, 5, 48. [Google Scholar] [CrossRef]
- Li, A.Y.; McCusker, M.G.; Russo, A.; Scilla, K.A.; Gittens, A.; Arensmeyer, K.; Mehra, R.; Adamo, V.; Rolfo, C. RET fusions in solid tumors. Cancer Treat. Rev. 2019, 81, 101911. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Subbiah, V.; Gautschi, O.; Tomasini, P.; de Braud, F.; Solomon, B.J.; Tan, D.S.-W.; Alonso, G.; Wolf, J.; Park, K.; et al. Selpercatinib in Patients with RET Fusion–Positive Non–Small-Cell Lung Cancer: Updated Safety and Efficacy from the Registrational LIBRETTO-001 Phase I/II Trial. J. Clin. Oncol. 2022, JCO.22.00393. Available online: https://ascopubs.org/doi/abs/10.1200/JCO.22.00393 (accessed on 19 September 2022). [CrossRef] [PubMed]
- Subbiah, V.; Gainor, J.F.; Oxnard, G.R.; Tan, D.S.; Owen, D.H.; Cho, B.C.; Loong, H.H.; McCoach, C.E.; Weiss, J.; Kim, Y.J.; et al. Intracranial Efficacy of Selpercatinib in RET Fusion-Positive Non–Small Cell Lung Cancers on the LIBRETTO-001 Trial. Clin. Cancer Res. 2021, 27, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.V.; Gerdemann, U.; Raju, S.G.; Henry, D.; Smith, S.; Rothenberg, S.M.; Cox, M.C.; Proust, S.; Bender, J.G.; Frazier, A.L.; et al. Activity of the Highly Specific RET Inhibitor Selpercatinib (LOXO-292) in Pediatric Patients with Tumors Harboring RET Gene Alterations. JCO Precis. Oncol. 2020, 4, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Gerdemann, U.; Lee, Y.A.; Henry, D.; Smith, S.; Ortiz, M.V.; Rothenberg, S.M.; Raju, S.G.; Cox, M.C.; Bender, J.L.G.; Pappo, A.S.; et al. First experience of LOXO-292 in the management of pediatric patients with RET-altered cancers. J. Clin. Oncol. 2019, 37, 10045. [Google Scholar] [CrossRef]
- Touyz, R.M.; Herrmann, J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. npj Precis. Oncol. 2018, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Ryan, K.R.; Lakkaniga, N.R.; Acharya, B.; Garcia, N.G.; Smith, E.L.; Frett, B. Targeting Rearranged during Transfection in Cancer: A Perspective on Small-Molecule Inhibitors and Their Clinical Development. J. Med. Chem. 2021, 64, 11747–11773. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Curigliano, G.; Thomas, M.; Subbiah, V.; Baik, C.; Tan, D.; Lee, D.; Misch, D.; Garralda, E.; Kim, D.-W.; et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: Update from the ARROW trial. Ann. Oncol. 2022, 33, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; I Hu, M.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Curigliano, G.; Brose, M.S.; Zhu, V.W.; Leboulleux, S.; Bowles, D.W.; et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): A multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021, 9, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion–positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Tan, L.; Lin, J.J.; Wong, S.Q.; Hollizeck, S.; Ebata, K.; Tuch, B.B.; Yoda, S.; Gainor, J.F.; Sequist, L.V.; et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J. Thorac. Oncol. 2020, 15, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.E.; Zhai, D.; Rogers, E.; Deng, W.; Zhang, X.; Ung, J.; Lee, D.; Rodon, L.; Graber, A.; Zimmerman, Z.F.; et al. The next-generation RET inhibitor TPX-0046 is active in drug-resistant and naïve RET-driven cancer models. J. Clin. Oncol. 2020, 38, 3616. [Google Scholar] [CrossRef]
- Drilon, A.; Rogers, E.; Zhai, D.; Deng, W.; Zhang, X.; Lee, D.; Ung, J.; Whitten, J.; Zhang, H.; Liu, J.; et al. TPX-0046 is a novel and potent RET/SRC inhibitor for RET-driven cancers. Ann. Oncol. 2019, 30, v190–v191. Available online: https://www.sciencedirect.com/science/article/pii/S0923753419587282 (accessed on 1 October 2019). [CrossRef]
- Turning Point Therapeutics Announces Initial Clinical Data from Phase 1/2 Sword-1 Study of Ret Inhibitor Tpx-0046. 2021. Available online: https://firstwordpharma.com/story/5266393 (accessed on 6 April 2021).
- Schoffski, P.; Cho, B.C.; Italiano, A.; Loong, H.H.F.; Massard, C.; Rodriguez, L.M.; Shih, J.-Y.; Subbiah, V.; Verlingue, L.; Andreas, K.; et al. BOS172738, a highly potent and selective RET inhibitor, for the treatment of RET-altered tumors including RET-fusion+ NSCLC and RET-mutant MTC: Phase 1 study results. J. Clin. Oncol. 2021, 39, 3008. [Google Scholar] [CrossRef]
- Schoffski, P.; Aftimos, P.G.; Massard, C.; Italiano, A.; Jungels, C.; Andreas, K.; Keegan, M.; Ho, P.T. A phase I study of BOS172738 in patients with advanced solid tumors with RET gene alterations including non-small cell lung cancer and medullary thyroid cancer. J. Clin. Oncol. 2019, 37, TPS3162. [Google Scholar] [CrossRef]
- Kaneta, Y.; Komatsu, T.; Miyamoto, M.; Goto, M.; Namiki, H.; Shibata, Y.; Kageji, H.; Inagaki, H.; Nakayama, K.; Tominaga, Y. Abstract b173: Preclinical characterization and antitumor efficacy of ds-5010, a highly potent and selective ret inhibitor. Mol. Cancer Ther. 2018, 17, B173. [Google Scholar] [CrossRef]
- Golding, B.; Luu, A.; Jones, R.; Viloria-Petit, A.M. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol. Cancer 2018, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, S.; McCoach, C.; Zhu, V.; Tan, A.; Yoda, S.; Peterson, J.; Do, A.; Prutisto-Chang, K.; Dagogo-Jack, I.; et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann. Oncol. 2020, 31, 1725–1733. [Google Scholar] [CrossRef]
- Jiang, Y.; Peng, X.; Ji, Y.; Dai, Y.; Fang, Y.; Xiong, B.; Ren, W.; Hu, Y.; Chen, Y.; Ai, J. The Novel RET Inhibitor SYHA1815 Inhibits RET-Driven Cancers and Overcomes Gatekeeper Mutations by Inducing G1 Cell-Cycle Arrest through c-Myc Downregulation. Mol. Cancer Ther. 2021, 20, 2198–2206. [Google Scholar] [CrossRef]
- Kolakowski, G.R.; Anderson, E.D.; Ballard, J.A.; Brandhuber, B.J.; Condroski, K.R.; Gomez, E.B.; Irvin, T.C.; Kumar, M.; Patel, N.A.; Watson, F.D.; et al. Abstract 1464: Pre-clinical characterization of potent and selective next-generation RET inhibitors. Cancer Res. 2021, 81, 1464. [Google Scholar] [CrossRef]

| Drug | Cancer | Phase | Location | Statue | NCT |
|---|---|---|---|---|---|
| Selpercatinib | |||||
| MTC, Solid tumor | II | China | active | NCT04280081(LIBRETTO-321) | |
| RET-altered advanced solid tumors, lymphomas, and histiocytic disorders in pediatric patients | II | US | recruiting | NCT04320888 | |
| NSCLC | III | global | recruiting | NCT04819100(LIBRETTO-432) | |
| NSCLC, MTC, colon cancer, advanced solid tumor | I/II | global | recruiting | NCT03157128 (LIBRETTO-001) | |
| MTC | III | global | recruiting | NCT04211337 (LIBRETTO-531) | |
| thyroid cancer | II | US | recruiting | NCT04759911 | |
| Advanced or Metastatic RET Fusion-Positive NSCLC | III | global | recruiting | NCT04194944 (LIBRETTO-431) | |
| NSCLC | II | US | recruiting | NCT05364645 | |
| NSCLC | II | US | active | NCT04268550 | |
| NSCLC, MTC, colon cancer, another solid tumor | N/A | global | available | NCT03906331 | |
| Advanced solid tumor and Primary CNS tumors in pediatric patients | I/II | global | recruiting | NCT03899792 (LIBRETTO-121) | |
| NSCLC | II | global | recruiting | NCT03944772 (ORCHARD) | |
| Advanced cancer, solid tumor | II | Finland | recruiting | NCT05159245 (FINPROVE) | |
| Advanced solid tumor, lymphomas, histiocytic disorders in pediatric patients | II | US | recruiting | NCT03155620 | |
| Pralsetinib | |||||
| MTC | III | Spain | Not yet recruiting | NCT04760288(AcceleRET-MTC) | |
| NSCLC, MTC | N/A | N | approved | NCT04204928 | |
| NSCLC, MTC, another solid tumor | I/II | global | active | NCT03037385 (ARROW) | |
| NSCLC | III | global | recruiting | NCT04222972 (AcceleRET-Lung) | |
| NSCLC | N/A | US, France, Switzerland | enrolling | NCT04697446 | |
| NSCLC | III | global | recruiting | NCT05170204 | |
| NSCLC | II | US | recruiting | NCT04302025 | |
| Advanced cancer, solid tumor | II | Finland | recruiting | NCT05159245(FINPROVE) | |
| Advanced Unresectable or Metastatic Solid Malignancy | II | US | recruiting | NCT04632992 (MyTACTIC) | |
| Solid tumor | II | global | recruiting | NCT04589845 | |
| TPX-0046 | |||||
| Advanced solid tumors | I/II | US, South Korea | recruiting | NCT04161391 | |
| TAS0953/HM06 | |||||
| Advanced solid tumors | I/II | US, Japan | recruiting | NCT04683250 | |
| SYHA1815 | |||||
| Advanced or metastatic solid tumors | N/A | China | recruiting | NCT05105464 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, Q.; Gan, X.; Li, L.; Gou, Q.; Zhang, T. Precious Gene: The Application of RET-Altered Inhibitors. Molecules 2022, 27, 8839. https://doi.org/10.3390/molecules27248839
Gou Q, Gan X, Li L, Gou Q, Zhang T. Precious Gene: The Application of RET-Altered Inhibitors. Molecules. 2022; 27(24):8839. https://doi.org/10.3390/molecules27248839
Chicago/Turabian StyleGou, Qitao, Xiaochuan Gan, Longhao Li, Qiheng Gou, and Tao Zhang. 2022. "Precious Gene: The Application of RET-Altered Inhibitors" Molecules 27, no. 24: 8839. https://doi.org/10.3390/molecules27248839
APA StyleGou, Q., Gan, X., Li, L., Gou, Q., & Zhang, T. (2022). Precious Gene: The Application of RET-Altered Inhibitors. Molecules, 27(24), 8839. https://doi.org/10.3390/molecules27248839





