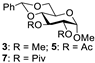
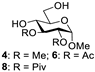
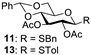
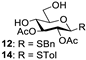
Abstract
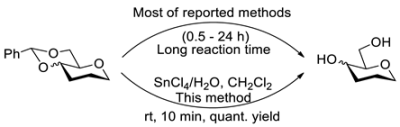
Acetalization and deacetalation are a pair of routine manipulations to protect and deprotect the 4- and 6-hydroxyl groups of glycosides in the synthesis of glycosyl building blocks. In this study, we found that treatment of SnCl4 with various carbohydrates containing acetal/ketal groups with the assistance of water in CH2Cl2 led to deacetalization/deketalization products in almost quantitative yields. In addition, for substrates containing both acetal/ketal and p-methoxylbenzyl groups, we also found that the p-methoxylbenzyl group was selectively cleaved by the use of a catalytic amount of SnCl4, while the acetal/ketal groups remained. Furthermore, based on this, 4,6-benzylidene glycosides can be conveniently converted to 4,6-OAc or 4-OH, 6-OAc glycosides.
1. Introduction
Orthogonally protected glycosyl building blocks play key roles in the synthesis of oligosaccharides, whose preparation usually requires multiple steps of selective protection and deprotection [1,2,3,4,5,6,7]. Acetalation is a routine manipulation for protecting the 4- and 6-hydroxyl groups of glycosides in the synthesis of glycosyl building blocks [8,9,10,11,12,13,14,15,16,17], and thus methods for removing 4,6-arylidene acetals were extensively reported [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Acidic hydrolysis of the 4,6-arylidene group is the most commonly used method, including the use of AcOH [18,19], CF3COOH [18,19], VO(OTf)2 [20], Er(OTf)3 [21], SnCl2 [22], NaHSO4 [23], I2 [24] and so on (Entries 1–7 in Table 1). Improved methods included the combined use of Lewis acids and dithiols (Entries 8–9) [25,26], and the use of silica gel supported acids (Entries 10–15) [27,28,29,30,31,32]. In addition, an improved method for deprotection of the 4,6-arylidene group under hydrogenation conditions was to use Et3SiH instead of H2 (Entry 16) [33]. These methods each have their own advantages and disadvantages, and the disadvantages usually include relatively harsh conditions, long reaction times, incompatibility with many functional groups and the formation of unwanted byproducts. SnCl4, as a Lewis acid, was used in the selective removal of benzyl groups in carbohydrate synthesis, but exhibited relatively low reactivity [34]. Considering the difference in the stability of benzyl and benzylidene under acidic conditions, we then attempted to use SnCl4 to achieve rapidly and highly selective removal of acetal and ketal groups. In this study, we found that treatment of carbohydrates containing acetal/ketal groups with SnCl4 ith the assistance of water in CH2Cl2 (DCM) led to deacetalization/deketalization products in almost quantitative yields. For substrates containing both acetal/ketal and PMB groups, we also found that the PMB group was selectively cleaved by the use of a catalytic amount of SnCl4 in DCM, while the acetal/ketal groups remained (Scheme 1a). Furthermore, based on SnCl4-promoted deacetalization, 4,6-benzylidene glycosides can be conveniently converted to 4,6-OAc glycosides (Scheme 1b) or 4-OH, 6-OAc glycosides (Scheme 1c).

Table 1.
Comparison of methods for removing 4,6-arylidene acetals.
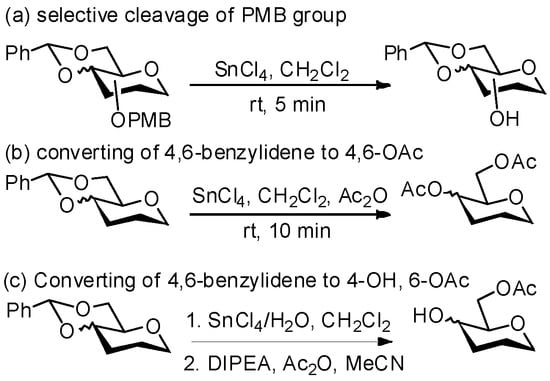
Scheme 1.
Application of SnCl4-promoted cleavage of acetal/PMB in this study.
2. Results
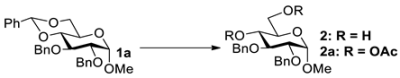
We first evaluated the potential of SnCl4 to remove the 4,6-O-benzylidene group of glycosides using methyl 2,3-di-O-benzyl-4,6-O-benzylidene-α-D-glucopyranoside 1a as a model compound. Thus, 1a was allowed to react with SnCl4 in DCM at rt (Table 2). Interestingly, as the used amount of SnCl4 was gradually increased from 0.2 equiv to 2.5 equiv, the yield of the deacetalation product 2 increased from 19% to a nearly quantitative yield (Entries 1–3). These results seem to support an equilibrium reaction. The reaction mechanism is proposed in Figure 1a, where the coordination of SnCl4 to the 4,6-oxygen atoms of 1a leads to the cleavage of the benzylidene group, and the formation of the intermediate M and dichlorotoluene. However, we failed when we tried to capture dichlorotoluene by an NMR experiment to support this mechanism (Figure S1 in SI). The fact that benzaldehyde was captured instead of dicholorotoluene supports the mechanism shown in Figure 1b, where trace amounts of water play an indispensable role in the cleavage of the benzylidene group. The mechanism also explained why it is not feasible to use a catalytic amount of SnCl4 in the reaction. When the solvent used in the reaction was changed from DCM to the more polar methanol and acetonitrile, the yields of 2 were greatly reduced (Entry 4). The reason must be due to the competitive coordination of SnCl4 with polar solvents.

Table 2.
Various conditions for deacetalation of 1 a.
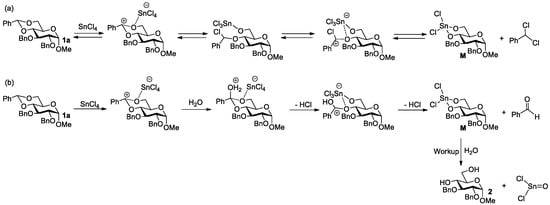
Figure 1.
Proposed mechanism for deacetalation of 1a by SnCl4. (a) No water involved; (b) Water involved.
As can be seen from the NMR spectrum (Figure S1 in SI), the reaction was terminated when the trace amounts of water in the d-choloroform was consumed. We then tried using water to assist this reaction (Entries 5–8). As can be seen, optimal conditions were to use 1.2–1.5 equiv of SnCl4 and 1.0–1.5 equiv of water; reaction at rt for 10 min under these conditions led to 2 in a nearly quantitative yield (Entry 8). Similar results for Entries 5 and 6 indicate that the simultaneous addition of SnCl4 and water had no adverse effect on the yield of 2. However, methanol used as a hydrogen source instead of water in the reaction proved to be ineffective (Entry 9). The use of HCl, SnCl2, FeCl3, CuCl2 and Cu(OTf )2 instead of SnCl4 in the reaction resulted in varying degrees of reduced yields of 2 (Entries 10–12). We also envisaged that the reaction of AcCl/Ac2O with M might produce selectively acetylated products. Therefore, AcCl/Ac2O instead of water was added to the reaction. However, the addition of AcCl led to the formation of a complex mixture (Entry 13), and the addition of Ac2O led to the formation of 4,6-OAc product 2a as the main product (Entry 14), indicating poor selectiveacetylations.
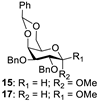
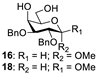
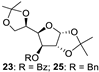
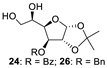
With the optimized conditions in hand, 4,6-O-arylidene glycosides 1b, 1c, 3, 5, 7, 11, 13, 15, 17 and 19 were further evaluated in the reaction (Entries 1–6 in Table 3). It can be seen that deacetalized products 2, 4, 6, 8, 10, 12, 14, 16, 18 and 20 were obtained in 89–98% yields after treating the substrates with 1.5 equiv of SnCl4 in the presence of 1.0 equiv of water in DCM for 10 min at rt. The results indicate that this method was compatible with various configurations of glycosides and functional groups. In particular, unlike the reported effect of SnCl4 on 1-STol/SBn glycosides [35], SnCl4 did not cause cleavage or configurational isomerization of the 1-STol/SBn group for 1-STol/SBn glycoside substrates (Entry 4). Encouraged by these results, we further tested removal of the isopropylidene ketal protecting group under these conditions. Similarly, the deketalized products 22, 24 and 26 were obtained in 94–97% yields from 21, 23 and 25 after only 10 min of reaction at rt (Entries 7 and 8). Especially, for the 1,2,5,6-diisopropylidene ketal-protected furanose substrates 23 and 25, the 5,6-isopropylidene ketal were preferentially removed to obtain the 1,2-isopropylidene ketal-protected furanose products 24 and 26 in excellent yields by this method (Entry 8).

Table 3.
Deprotection of Acetal, Ketal and PMB groups with SnCl4/H2O a.
We also noticed that the 4-methoxylbenzyl (PMB) protecting group could be deprotected in the presence of catalytic amounts of SnCl4 [36]. The catalytic mechanism involved the formation of the desired alcohol, the release of a PMB cation, and the subsequent formation of a lipophilic side product through the Friedel–Crafts alkylation of the PMB cation with another PMB ether [37,38]. Indeed, after treatment of methyl 2,4,6-tri-O-acetyl-3-O-PMB-galactoside/mannoside 27/29 with 0.2 equiv of SnCl4 in DCM at rt for 10 min, the PMB-removed products 28/30 were obtained in 92/98% yield (Entries 9 and 10). Since the reactivity for removing PMB is much higher than that for removing acetal/ketal by SnCl4, we guessed that PMB should be preferentially removed from substrates containing both PMB and acetal/ketal in the presence of catalytic amounts of SnCl4. Therefore, four substrates 31, 33, 35 and 37 containing both PMB and acetal/ketal were treated with 0.2–0.5 equiv of SnCl4 in DCM at rt for 5 min, leading to the selective PMB-removed products 32, 34, 36 and 38 in 80–85% yields (Scheme 2).
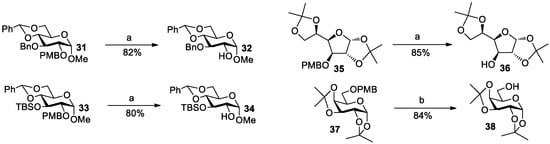
Scheme 2.
Selective cleavage of PMB group in the presence of SnCl4. Reagents and conditions: (a) SnCl4 (0.2 equiv), DCM, rt, 5 min; (b) SnCl4 (0.5 equiv), DCM, rt, 5 min.
In the control experiment shown for Entry 14 in Table 2, the 4,6-benzylidene acetal was conveniently converted to 4,6-OAc for 1a when acetic anhydride was used instead of H2O during the SnCl4-promoted deacetalation; in addition, a 78% yield of 2a was obtained when acetic anhydride was directly added dropwise to the reaction mixture. Further experiments indicate that the yield of 2a increased to 91% when a solution of acetic anhydride in dry acetonitrile was added dropwise to the reaction mixture (Scheme 3). Using this method, 4,6-OAc glycosides 41 (82%), 42 (85%) and 43 (88%) were obtained in high yields from 4,6-benzylidene glycosides 15, 39 and 40 (Scheme 3). As seen in Table 2, the SnCl4-mediated deacetalation method led to deprotected diol products in almost quantitative yields. We thus envisioned a directly selective acetylation of 6-OH after deprotection of 4,6-benzylidene glycosides without purification of the 4,6-OH glycoside products. After the SnCl4-promoted deacetalation of 4,6-O-benzylidene glycosides 1a, 15, 39 and 40 was completed, the reaction mixture was dissolved in dichloromethane and extracted using a saturated sodium bicarbonate solution and a saturated sodium potassium tartrate solution. The concentrated crude products were then dissolved in dry acetonitrile, followed by the addition of 1.1 equiv of Ac2O and 0.2 equiv of DIPEA [39]. The reaction proceeded at 40 °C for 12 h, resulting in 6-OAc products 44, 45, 46 and 47 in 70%, 64%, 69% and 65% yields, respectively (Scheme 3).
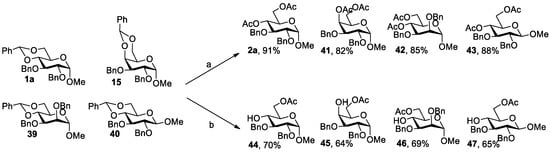
Scheme 3.
Converting of 4,6-benzylidene to 4,6-OAc and 4-OH, 6-OAc for glycosides. Reagents and conditions: (a) SnCl4 (1.5 equiv), Ac2O (2 equiv), DCM, rt, 10 min; (b) (i): SnCl4 (1.5 equiv), H2O (1 equiv), DCM, rt, 10 min; (ii): DIPEA (0.2 equiv), Ac2O (1.1 equiv), MeCN, 40 °C, 12 h.
3. Conclusions
In this study, it was found that acetal and ketal protective groups could be efficiently removed in the presence of SnCl4 for orthogonally protected carbohydrate substrates. The reaction can be completed in DCM within 10 min at room temperature, and a small amount of water has an obvious promoting effect on the reaction. It was also found that the PMB could be preferentially removed from the substrates containing both acetal/ketal and PMB by a catalytic amount of SnCl4. Based on SnCl4-promoted deacetalation, 4,6-benzylidene glycosides can be conveniently converted to 4,6-OAc glycosides and 4-OH, 6-OAc glycosides. These methods provide efficient approaches to synthesizing orthogonally protected carbohydrate building blocks.
4. Materials and Methods
General Methods. All chemicals were purchased as reagent grade and used without further purification. The solvents were purified before use and CH3CN was distilled from CaH2. Chemical reactions were monitored by thin-layer chromatography using precoated silica gel 60 (0.25 mm thickness) plates. Flash column chromatography was performed on silica gel 60 (SDS 0.040–0.063 mm). Spots were visualized by UV light (254 nm) then by charring with a solution of H2SO4 (5%) in ethanol. 1H NMR spectra were recorded by 400 MHz or 600 MHz (1H) and 100 MHz (13C) at 298 K in CDCl3 using the residual signals from CDCl3 (1H: δ = 7.26 ppm; 13C: δ = 77.16 ppm) or CD3OD (1H: δ = 3.31 ppm) as the internal standard. 1H peak assignments were made by first order analysis of the spectra, supported by standard 1H–1H correlation spectroscopy (COSY). High-resolution mass spectra (HRMS) were obtained by TOF detection. Optical rotations were measured on an SGW-1 automatic polarimeter with [α]D values reported in degrees; concentration (c) is in g/100 mL.
General procedure A for SnCl4-mediated deacetalization. SnCl4 (1.5 equiv) and H2O (1.0 equiv) were added to a solution of a carbohydrate substrate containing acetal/ketal in DCM (1 mL). The mixture was stirred at rt for 10 min and then poured onto a cold saturated NaHCO3 solution. The organic phase was separated and the aqueous phase was extracted with dichloromethane (3 × 10 mL). The combined organic phase was washed with saturated sodium potassium tartrate solution (1 × 15 mL), dried with anhydrous MgSO4, and concentrated in vacuo. The residue was purified by silica gel flash chromatography.
General procedure B for SnCl4-mediated removal of PMB. SnCl4 (0.2–0.5 equiv) was added to a solution of a carbohydrate substrate containing PMB in DCM (1 mL). The mixture was stirred at rt for 5 min and then poured onto a cold saturated NaHCO3 solution. The organic phase was separated and the aqueous phase was extracted with dichloromethane (3 × 10 mL). The combined organic phase was washed with the saturated NaHCO3 solution (1 × 15 mL), dried with anhydrous MgSO4, and concentrated in vacuo. The residue was purified by silica gel flash chromatography.
General procedure C for converting of 4,6-benzylidene to 4,6-OAc for glycosides. SnCl4 (1.5 equiv) and a solution of acetic anhydride (2.0 equiv), added dropwise in dry acetonitrile (0.5 mL), were added to a solution of a 4,6-benzylidene glycoside in DCM (1 mL). The mixture was stirred at rt for 10 min and then poured onto a cold saturated NaHCO3 solution. The organic phase was separated and the aqueous phase was extracted with dichloromethane (3 × 10 mL). The combined organic phase was washed with the saturated NaHCO3 solution (1 × 15 mL), dried with anhydrous MgSO4, and concentrated in vacuo. The residue was purified by silica gel flash chromatography.
General procedure D for converting of 4,6-benzylidene to 4-OH, 6-OAc for glycosides. SnCl4 (1.5 equiv) and H2O (1.0 equiv) were added to a solution of a carbohydrate substrate containing acetal/ketal in DCM (1 mL). The mixture was stirred at rt for 10 min and then poured onto cold saturated a NaHCO3 solution. The organic phase was separated and the aqueous phase was extracted with dichloromethane (3 × 10 mL). The combined organic phase was washed with saturated sodium potassium tartrate solution (1 × 15 mL), dried with anhydrous MgSO4, and concentrated in vacuo. The residue was allowed to react with acetic anhydride (1.1 equiv) in the presence of DIPEA (0.2 equiv) in dry acetonitrile (1 mL) at 40 °C for 12 h. After cooling and evaporation of the solvent, the reaction mixture was directly purified by flash column chromatography.
Methyl 2,3-di-O-benzyl-α-D-glucopyranoside (2) [26]. Following general process A, starting from 1a (100 mg, 0.22 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 2 as a white solid (77 mg, 95%). 1H NMR (600 MHz, CDCl3) δ 7.40–7.28 (m, 10H), 5.03 (d, J = 11.5 Hz, 1H), 4.77 (d, J = 12.0 Hz, 1H), 4.70 (d, J = 11.5 Hz, 1H), 4.66 (d, J = 12.0 Hz, 1H), 4.60 (d, J = 3.5 Hz, 1H), 3.83–3.76 (m, 2H), 3.74 (dd, J = 11.5, 4.6 Hz, 1H), 3.62 (dt, J = 8.4, 4.1 Hz, 1H), 3.55–3.47 (m, 2H), 3.38 (s, 3H).
Methyl 2,3-di-O-methyl-α-D-glucopyranoside (4) [40]. Following general process A, starting from 3 (72 mg, 0.23 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 4 as a white solid (46.7 mg, 91%). 1H NMR (400 MHz, CDCl3) δ 4.85 (d, J = 3.5 Hz, 1H), 3.92–3.74 (m, 2H), 3.64 (s, 4H), 3.53–3.46 (m, 5H), 3.44 (s, 3H), 3.28–3.18 (m, 1H).
Methyl 2,3-di-O-acetyl-α-D-glucopyranoside (6) [26]. Following general process A, starting from 5 (41 mg, 0.11 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 6 as a colorless oil (30.5 mg, 98%). 1H NMR (400 MHz, CDCl3) δ 5.34–5.25 (m, 1H), 4.91 (d, J = 3.6 Hz, 1H), 4.83 (dd, J = 10.1, 3.6 Hz, 1H), 3.95– 3.82 (m, 2H), 3.76–3.65 (m, 2H), 3.40 (s, 3H), 2.11 (s, 3H), 2.09 (s, 3H).
Methyl 2,3-di-O-pivaloyl-α-D-glucopyranoside (8) [41]. Following general process A, starting from 7 (28.2 mg, 0.063 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 8 as a white solid (22 mg, 97%). 1H NMR (400 MHz, CD3OD) δ 5.46–5.30 (m, 1H), 4.73 (dd, J = 10.1, 3.6 Hz, 1H), 3.85 (dd, J = 11.9, 2.2 Hz, 1H), 3.74 (dd, J = 11.9, 5.1 Hz, 1H), 3.66 (ddd, J = 10.1, 5.1, 2.2 Hz, 1H), 3.58 (t, J = 9.5 Hz, 1H), 3.42 (s, 3H), 3.36–3.30 (m, 1H), 1.21 (s, 9H), 1.18 (s, 9H).
Methyl 2,3-di-O-benzoyl-β-D-glucopyranoside (10) [26]. Following general process A, starting from 9 (64 mg, 0.13 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 10 as a colorless oil (49.8 mg, 95%). 1H NMR (400 MHz, CDCl3) δ 8.02–7.89 (m, 4H), 7.51 (t, J = 7.8 Hz, 2H), 7.37 (td, J = 7.8, 3.6 Hz, 4H), 5.49–5.28 (m, 2H), 4.74–4.54 (m, 1H), 4.04–3.90 (m, 3H), 3.60 (dt, J = 9.2, 4.0 Hz, 1H), 3.53 (s, 3H).
Benzyl 2,3-di-O-acetyl-1-thio-β-D-glucopyranoside (12). Following general process A, starting from 11 (25 mg, 0.055 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 12 as a colorless oil (18.8 mg, 93%). [α]D12 = −88 (c 0.2, CH2Cl2), 1H NMR (400 MHz, CDCl3) δ 7.42–7.23 (m, 5H), 5.07–4.91 (m, 2H), 4.39 (d, J = 8.0 Hz 1H), 3.95–3.87 (m, 3H), 3.80–3.64 (m, 2H), 3.39–3.17 (m, 2H), 2.34–2.18 (b, 1H), 2.08 (s, 3H), 2.03 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 171.58, 169.68, 137.45, 128.95, 128.61, 128.56, 127.38, 82.63, 79.65, 69.69, 69.28, 62.12, 34.40, 20.86, 20.74. HRMS (ESI-TOF) m/z [M + Na]+ calcd for [C17H22O7SNa]+: 393.0984; found: 393.0964.
4-Methylphenyl 2,3-di-O-acetyl-1-thio-D-glucopyranoside (14) [25]. Following general process A, starting from 13 (26 mg, 0.06 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 14 as a colorless oil (19.4 mg, 94%). 1H NMR (400 MHz, CDCl3) δ 7.41–7.35 (m, 2H), 7.15 (d, J = 7.9 Hz, 2H), 5.05 (t, J = 9.3 Hz, 1H), 4.92 (t, J = 9.6 Hz, 1H), 4.69 (d, J = 10.0 Hz, 1H), 4.02–3.89 (m, 1H), 3.83 (d, J = 12.2 Hz, 1H), 3.73 (t, J = 9.6 Hz, 1H), 3.53–3.42 (m, 1H), 2.36 (s, 3H), 2.12 (s, 3H), 2.10 (s, 3H).
Methyl 2,3-di-O-benzyl-α-D-galactopyranoside (16) [20]. Following general process A, starting from 15 (53 mg, 0.11 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 16 as a colorless oil (38 mg, 89%). 1H NMR (600 MHz, CDCl3) δ 7.42–7.26 (m, 10H), 4.81 (d, J = 11.8 Hz, 2H), 4.70 (dd, J = 7.4, 4.2 Hz, 2H), 4.67 (d, J = 12.1 Hz, 1H), 4.05 (dd, J = 2.9, 1.4 Hz, 1H), 3.93–3.83 (m, 3H), 3.79–3.73 (m, 2H), 3.38 (s, 3H).
Methyl 2,3-di-O-benzyl-β-D-galactopyranoside (18) [42]. Following general process A, starting from 17 (48 mg, 0.1 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 18 as a white solid (38.8 mg, 90%). 1H NMR (400 MHz, CDCl3) δ 7.42–7.27 (m, 10H), 4.90 (d, J = 11.0 Hz, 1H), 4.77–4.68 (m, 3H), 4.30 (d, J = 7.7 Hz, 1H), 4.04–3.93 (m, 2H), 3.83 (dd, J = 11.7, 4.5 Hz, 1H), 3.67–3.61 (m, 1H), 3.58 (s, 3H), 3.53–3.46 (m, 2H), 2.66 (s, 1H), 2.21 (t, J = 7.6 Hz, 1H).
Methyl 2,3-di-O-acetyl-α-D-mannopyranoside (20) [43]. Following general process A, starting from 19 (27 mg, 0.07 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 20 as a white solid (19.5 mg, 95%). 1H NMR (400 MHz, CDCl3) δ 5.22 (dd, J = 3.5, 1.7 Hz, 1H), 5.17 (dd, J = 9.9, 3.4 Hz, 1H), 4.67 (d, J = 1.7 Hz, 1H), 3.98 (t, J = 9.8 Hz, 1H), 3.89 (dd, J = 4.0, 2.1 Hz, 2H), 3.70 (dt, J = 9.8, 3.9 Hz, 1H), 3.39 (s, 3H), 2.69 (s, 1H), 2.12 (s, 3H), 2.07 (s, 3H), 1.79 (s, 1H).
Methyl 2,6-di-O-benzyl-α-D-galactopyranoside (22) [44]. Following general process A, starting from 21 (64 mg, 0.15 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 22 as a colorless oil (56 mg, 97%). 1H NMR (400 MHz, CDCl3) δ 7.43–7.22 (m, 10H), 4.70 (d, J = 3.7 Hz, 1H), 4.70–4.61 (m, 2H), 4.57 (d, J = 1.9 Hz, 2H), 4.04 (d, J = 3.4 Hz, 1H), 3.96 (dd, J = 9.8, 3.4 Hz, 1H), 3.94–3.86 (m, 1H), 3.76–3.68 (m, 3H), 3.35 (s, 3H).
3-O-benzoyl-1,2-O-isopropylidene-α-D-glucofuranose (24) [45]. Following general process A, starting from 23 (78.7 mg, 0.22 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 24 as a colorless oil (66.1 mg, 94%). 1H NMR (400 MHz, CDCl3) δ 8.14–7.98 (m, 2H), 7.67–7.57 (m, 1H), 7.47 (t, J = 7.7 Hz, 2H), 6.01 (d, J = 3.7 Hz, 1H), 5.53 (d, J = 2.6 Hz, 1H), 4.73 (d, J = 3.7 Hz, 1H), 4.30 (dd, J = 8.6, 2.6 Hz, 1H), 3.87 (d, J = 8.1 Hz, 1H), 3.75 (d, J = 8.3 Hz, 2H), 3.19 (s, 1H), 2.29–2.13 (m, 1H), 1.56 (s, 3H), 1.34 (s, 3H).
3-O-Benzyl-1,2-O-isopropylidene-α-D-glucofuranose (26) [26]. Following general process A, starting from 25 (139 mg, 0.4 mmol), purification by column chromatography (petroleum ether/ethyl acetate 1/1.5) afforded 26 as a colorless oil (112 mg, 91%). 1H NMR (400 MHz, CDCl3) δ 7.44–7.29 (m, 5H), 5.96 (d, J = 3.8 Hz, 1H), 4.75 (d, J = 11.8 Hz, 1H), 4.65 (d, J = 3.8 Hz, 1H), 4.58 (d, J = 11.7 Hz, 1H), 4.14–4.09 (m, 2H), 4.05–4.00 (m, 1H), 3.82 (dd, J = 11.5, 3.4 Hz, 1H), 3.71 (dd, J = 11.5, 5.4 Hz, 1H), 2.70 (s, 1H), 1.91 (s, 1H), 1.50 (s, 3H), 1.34 (s, 3H).
Methyl 2,4,6-tri-O-acetyl-α-D-galactopyranoside (28) [46]. Following general process B, starting from 27 (45 mg, 0.1 mmol), purification by column chromatography (petroleum ether/ethyl acetate 2/1) afforded 28 as a white solid (30 mg, 92%). 1H NMR (400 MHz, CDCl3) δ 5.35 (dd, J = 3.7, 1.2 Hz, 1H), 4.98 (dd, J = 10.1, 8.0 Hz, 1H), 4.38 (d, J = 7.9 Hz, 1H), 4.22–4.17 (m, 2H), 3.91–3.84 (m, 1H), 3.54 (s, 3H), 2.58 (d, J = 6.3 Hz, 1H), 2.20 (s, 3H), 2.16 (s, 3H), 2.09 (s, 3H).
Methyl 2,4,6-tri-O-acetyl-α-D-mannopyranoside (30) [43]. Following general process B, starting from 29 (88 mg, 0.2 mmol), purification by column chromatography (petroleum ether/ethyl acetate 2/1) afforded 30 as a colorless oil (53 mg, 98%). 1H NMR (400 MHz, CDCl3) δ 5.15–5.00 (m, 2H), 4.77 (d, J = 1.6 Hz, 1H), 4.31 (dd, J = 12.2, 5.4 Hz, 1H), 4.14 (dd, J = 12.1, 2.3 Hz, 1H), 4.10–4.03 (m, 1H), 3.94–3.89 (m, 1H), 3.39 (s, 3H), 2.33–2.26 (m, 1H), 2.17 (s, 3H), 2.13 (s, 3H), 2.11 (s, 3H).
Methyl 3-O-benzyl-4,6-di-O-benzylidene-α-D-glucopyranoside (32) [5]. Following general process B, starting from 31 (138 mg, 0.28 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 32 as a colorless oil (85.4 mg, 82%). 1H NMR (400 MHz, CDCl3) δ 7.57–7.43 (m, 2H), 7.44–7.23 (m, 9H), 5.58 (s, 1H), 4.97 (d, J = 11.6 Hz, 1H), 4.89–4.70 (m, 2H), 4.30 (dd, J = 9.8, 4.3 Hz, 1H), 3.91–3.67 (m, 4H), 3.65 (t, J = 9.1 Hz, 1H), 3.46 (s, 3H).
Methyl 3-O-(tert-butyl-dimethylsilyl)-4,6-di-O-benzylidene-α-D-glucopyranoside (34) [47]. Following general process B, starting from 33 (49 mg, 0.1 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 34 as a colorless oil (30 mg, 80%). 1H NMR (400 MHz, CDCl3) δ 7.55–7.44 (m, 2H), 7.36 (dd, J = 5.2, 2.0 Hz, 3H), 5.50 (s, 1H), 4.81 (d, J = 3.9 Hz, 1H), 4.27 (dd, J = 9.4, 4.1 Hz, 1H), 3.90 (t, J = 9.0 Hz, 1H), 3.81–3.71 (m, 3H), 3.59 (d, J = 3.9 Hz, 1H), 3.45 (s, 3H), 2.13 (d, J = 7.8 Hz, 1H), 0.87 (s, 9H), 0.10 (s, 3H), 0.02 (s, 3H).
1,2:5,6-Di-O-isopropylidene-α-D-glucofuranose (36) [48]. Following general process B, starting from 35 (179 mg, 0.47 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 36 as a white solid (104 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 5.95 (d, J = 3.6 Hz, 1H), 4.54 (d, J = 3.6 Hz, 1H), 4.37–4.33 (m, 2H), 4.17 (dd, J = 8.6, 6.2 Hz, 1H), 4.07 (dd, J = 7.6, 2.8 Hz, 1H), 3.99 (dd, J = 8.7, 5.4 Hz, 1H), 2.58 (d, J = 3.5 Hz, 1H), 1.50 (s, 3H), 1.45 (s, 3H), 1.37 (s, 3H), 1.32 (s, 3H).
1,2:3,4-Di-O-isopropylidene-α-D-galactopyranose (38) [49]. Following general process B, starting from 37 (65 mg, 0.17 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 38 as a colorless oil (37.1 mg, 84%). 1H NMR (400 MHz, CDCl3) δ 5.57 (d, J = 5.0 Hz, 1H), 4.62 (dd, J = 8.0, 2.4 Hz, 1H), 4.34 (dd, J = 5.1, 2.4 Hz, 1H), 4.32–4.23 (m, 1H), 3.87 (d, J = 9.4 Hz, 2H), 3.82–3.68 (m, 1H), 1.54 (s, 3H), 1.46 (s, 3H), 1.34 (s, 6H).
Methyl 2,3-di-O-benzyl-4,6-di-O-acetyl-α-D-glucopyranoside (2a) [29]. Following general process C, starting from 1a (100 mg, 0.22 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 2a as a white solid (90 mg, 91%). 1H NMR (400 MHz, CDCl3) δ 7.42–7.28 (m, 10H), 4.99 (dd, J = 10.3, 9.3 Hz, 1H), 4.89 (d, J = 11.6 Hz, 1H), 4.81 (d, J = 12.1 Hz, 1H), 4.65 (d, J = 12.1 Hz, 2H), 4.59 (d, J = 3.6 Hz, 1H), 4.21 (dd, J = 12.1, 4.9 Hz, 1H), 4.00 (dd, J = 12.1, 2.3 Hz, 1H), 3.92 (t, J = 9.4 Hz, 1H), 3.88–3.82 (m, 1H), 3.59 (dd, J = 9.6, 3.5 Hz, 1H), 3.39 (s, 3H), 2.06 (s, 3H), 1.91 (s, 3H).
Methyl 2,3-di-O-benzyl-4,6-di-O-acetyl-α-D-galactopyranoside (41) [29]. Following general process C, starting from 15 (80 mg, 0.17 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 41 as a colorless oil (65 mg, 82%). 1H NMR (400 MHz, CDCl3) δ 7.43–7.22 (m, 10H), 5.54 (d, J = 3.4 Hz, 1H), 4.85 (d, J = 12.1 Hz, 1H), 4.74 (d, J = 11.2 Hz, 1H), 4.69 (d, J = 3.6 Hz, 1H), 4.65 (d, J = 12.1 Hz, 1H), 4.57 (d, J = 11.1 Hz, 1H), 4.14–4.05 (m, 3H), 3.97 (dd, J = 10.0, 3.5 Hz, 1H), 3.78 (dd, J = 10.0, 3.7 Hz, 1H), 3.39 (s, 3H), 2.13 (s, 3H), 2.07 (s, 3H).
Methyl 2,3-di-O-benzyl-4,6-di-O-acetyl-α-D-mannopyranoside (42) [50]. Following general process C, starting from 39 (180 mg, 0.4 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 42 as a colorless oil (151 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 11H), 5.41 (dd, J = 10.0, 9.1 Hz, 1H), 4.81–4.74 (m, 2H), 4.69 (d, J = 12.4 Hz, 1H), 4.58 (d, J = 12.2 Hz, 1H), 4.45 (d, J = 12.2 Hz, 1H), 4.23 (dd, J = 12.1, 5.6 Hz, 1H), 4.13 (dd, J = 12.1, 2.5 Hz, 1H), 3.85–3.75 (m, 3H), 3.33 (s, 3H), 2.09 (s, 3H), 2.01 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 170.91, 169.75, 138.17, 138.12, 128.36, 128.32, 127.86, 127.65, 127.63, 127.41, 99.44, 73.96, 72.86, 71.86, 68.97, 68.08, 63.06, 55.00, 29.71, 20.92, 20.84.
Methyl 2,3-di-O-benzyl-4,6-di-O-acetyl-β-D-glucopyranoside (43) [50]. Following general process C, starting from 40 (90 mg, 0.19 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 43 as a colorless oil (78 mg, 88%). 1H NMR (400 MHz, CDCl3) δ 7.43–7.14 (m, 10H), 5.04 (dd, J = 10.0, 9.3 Hz, 1H), 4.91 (d, J = 10.9 Hz, 1H), 4.83 (d, J = 11.5 Hz, 1H), 4.70 (d, J = 11.0 Hz, 1H), 4.61 (d, J = 11.7 Hz, 1H), 4.34 (d, J = 7.7 Hz, 1H), 4.25 (dd, J = 12.2, 5.0 Hz, 1H), 4.08 (dd, J = 12.2, 2.4 Hz, 1H), 3.64– 3.52 (m, 5H), 3.49 (dd, J = 9.2, 7.7 Hz, 1H), 2.08 (s, 3H), 1.92 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 170.85, 169.58, 138.25, 138.23, 128.40, 128.38, 128.15, 127.83, 127.78, 127.69, 104.72, 81.96, 81.52, 75.15, 74.91, 71.81, 69.72, 62.40, 57.29, 20.80.
Methyl 2,3-di-O-benzyl-6-acetyl-α-D-glucopyranoside (44) [39]. Following general process D, starting from 1a (100 mg, 0.22 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 44 as a colorless oil (63.5 mg, 70%). 1H NMR (400 MHz, CDCl3) δ 7.40–7.27 (m, 10H), 5.00 (d, J = 11.3 Hz, 1H), 4.82–4.72 (m, 2H), 4.66 (d, J = 12.1 Hz, 1H), 4.62 (d, J = 3.5 Hz, 1H), 4.42 (dd, J = 12.1, 4.7 Hz, 1H), 4.21 (dd, J = 12.1, 2.2 Hz, 1H), 3.79 (t, J = 9.2 Hz, 1H), 3.74 (ddd, J = 10.0, 4.7, 2.2 Hz, 1H), 3.51 (dd, J = 9.6, 3.6 Hz, 1H), 3.42 (dd, J = 10.0, 8.9 Hz, 1H), 3.38 (s, 3H), 2.51 (s, 1H), 2.08 (s, 3H).
Methyl 2,3-di-O-benzyl-6-acetyl-α-D-galactopyranoside (45) [51]. Following general process D, starting from 15 (155 mg, 0.33 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 45 as a colorless oil (89 mg, 64%). 1H NMR (400 MHz, CDCl3) δ 7.41–7.22 (m, 10H), 4.81 (dd, J = 11.8, 2.2 Hz, 2H), 4.74–4.62 (m, 3H), 4.34–4.19 (m, 2H), 3.97 (t, J = 2.8 Hz, 1H), 3.96–3.82 (m, 3H), 3.37 (s, 3H), 2.55 (t, J = 1.5 Hz, 1H), 2.07 (s, 3H). 1H NMR (400 MHz, DMSO-d6) δ 7.43–7.11 (m, 10H), 4.98 (d, J = 5.0 Hz, 1H), 4.79 (d, J = 3.6 Hz, 1H), 4.73–4.55 (m, 4H), 4.20–4.06 (m, 2H), 4.06–4.00 (m, 1H), 3.77–3.74 (m, 2H), 3.67 (dd, J = 10.1, 3.1 Hz, 1H), 3.26 (s, 3H), 2.05 (s, 3H).
Methyl 2,3-di-O-benzyl-6-acetyl-α-D-mannopyranoside (46) [39]. Following general process D, starting from 39 (116 mg, 0.25 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 46 as a colorless oil (72 mg, 69%). 1H NMR (400 MHz, CDCl3) δ 7.42–7.23 (m, 10H), 4.78 (d, J = 1.7 Hz, 1H), 4.66 (q, J = 12.3 Hz, 2H), 4.52 (dd, J = 11.7, 36 Hz, 2H), 4.38 (qd, J = 12.0, 3.8 Hz, 2H), 3.95 (td, J = 9.7, 2.2 Hz, 1H), 3.79 (dd, J = 3.1, 1.8 Hz, 1H), 3.76–3.65 (m, 2H), 3.34 (s, 3H), 2.62 (d, J = 2.7 Hz, 1H), 2.09 (s, 3H).
Methyl 2,3-di-O-benzyl-6-acetyl-β-D-glucopyranoside (47) [51]. Following general process D, starting from 40 (190 mg, 0.41 mmol), purification by column chromatography (petroleum ether/ethyl acetate 3/1) afforded 47 as a white solid (111 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 7.52–7.13 (m, 10H), 4.93 (dd, J = 11.2, 6.6 Hz, 2H), 4.72 (dd, J = 11.2, 5.8 Hz, 2H), 4.41 (dd, J = 12.1, 4.4 Hz, 1H), 4.36–4.25 (m, 2H), 3.57 (s, 3H), 3.49–3.34 (m, 4H), 2.65 (d, J = 2.2 Hz, 1H), 2.09 (s, 3H).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238258/s1, Synthesis of substrates [26,49,52,53,54,55,56,57,58,59,60,61,62,63], 1H NMR and 13C NMR spectra. Figure S1. Treatment of methyl 2,3-di-O-pivaloyl-α-D-glucopyranoside 8 with 1 equiv of SnCl4 in dry d-CH3Cl.
Author Contributions
Conceptualization, T.L. and H.D.; methodology, T.L. and H.D.; validation, T.-T.X., Y.-F.G. and T.L.; formal analysis, T.L.; data curation, T.L.; writing—original draft preparation, T.L. and H.D.; writing—review and editing, T.L. and H.D.; supervision, H.D. project administration, H.D.; funding acquisition, H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Nature Science Foundation of China (No. 21772049).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Hai Dong thanks his good friend, Zhixin Zhang, for his personal financial support for the scientific research of Dong’s group. The authors are also grateful to the staff at the Analytical and Test Centre at HUST for support with the NMR instruments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, H.-Y.; Blaszczyk, S.A.; Xiao, G.-Z.; Tang, W.-P. Chiral reagents in glycosylation and modification of carbohydrates. Chem. Soc. Rev. 2018, 47, 681–701. [Google Scholar] [CrossRef] [PubMed]
- Dimakos, V.; Taylor, M.S. Site-Selective Functionalization of Hydroxyl Groups in Carbohydrate Derivatives. Chem. Rev. 2018, 118, 11457–11517. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, C.-Y.; Guo, Y.-F.; Feng, G.-J.; Dong, H. SnCl2-Catalyzed Acetalation/Selective Benzoylation Sequence for the Synthesis of Orthogonally Protected Glycosyl Acceptors. Eur. J. Org. Chem. 2022, 2022, e202101565. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Luo, T.; Feng, G.-J.; Liu, C.-Y.; Dong, H. Efficient Synthesis of 2-OH Thioglycosides from Glycals Based on the Reduction of Aryl Disulfides by NaBH4. Molecules 2022, 27, 5980. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yu, J.-C.; Feng, G.-J.; Luo, T.; Dong, H. Stannous chloride as a low toxicity and extremely cheap catalyst for regio-/site-selective acylation with unusually broad substrate scope. Green Chem. 2020, 22, 6936–6942. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, F.-L.; Luo, T.; Pei, Z.; Dong, H. Regio/Stereoselective Glycosylation of Diol and Polyol Acceptors in Efficient Synthesis of Neu5Ac-α-2,3-LacNPhth Trisaccharide. Chem. Asian J. 2019, 14, 223–234. [Google Scholar] [CrossRef]
- Ramadan, S.; Yang, W.-Z.; Zhang, Z.-R.; Huang, X.-F. Synthesis of Chondroitin Sulfate A Bearing Syndecan 1 Glycopeptide. Org. Lett. 2017, 19, 4838–4841. [Google Scholar] [CrossRef]
- Lv, J.; Zhu, J.-J.; Liu, Y.; Dong, H. Regioselective Sulfonylation/Acylation of Carbohydrates Catalyzed by FeCl3 Combined with Benzoyltrifluoroacetone and Its Mechanism Study. J. Org. Chem. 2020, 85, 3307–3319. [Google Scholar] [CrossRef]
- Lv, J.; Liu, Y.; Zhu, J.-J.; Zou, D.; Dong, H. Regio/site-selective alkylation of substrates containing a cis-, 1,2- or 1,3-diol with ferric chloride and dipivaloylmethane as the catalytic system. Green Chem. 2020, 22, 1139–1144. [Google Scholar] [CrossRef]
- Lv, J.; Ge, J.-T.; Luo, T.; Dong, H. An inexpensive catalyst, Fe(acac)3, for regio/siteselective acylation of diols and carbohydrates containing a 1,2-cis-diol. Green Chem. 2018, 20, 1987–1991. [Google Scholar] [CrossRef]
- Xu, H.-F.; Ren, B.; Zhao, W.; Xin, X.-T.; Lu, Y.-C.; Pei, Y.-X.; Dong, H.; Pei, Z.-C. Regioselective mono and multiple alkylation of diols and polyols catalyzed by organotin and its applications on the synthesis of value-added carbohydrate intermediates. Tetrahedron 2016, 72, 3490–3499. [Google Scholar] [CrossRef]
- Traboni, S.; Bedini, E.; Giordano, M.; Iadonisi, A. Three Solvent-Free Catalytic Approaches to the Acetal Functionalization of Carbohydrates and Their Applicability to One-Pot Generation of Orthogonally Protected Building Blocks. Adv. Synth. Catal. 2015, 357, 3562–3572. [Google Scholar] [CrossRef]
- Tran, A.T.; Jones, R.A.; Pastor, J.; Boisson, J.; Smith, N.; Galan, M.C. Copper(II) Triflate: A Versatile Catalyst for the One-Pot Preparation of Orthogonally Protected Glycosides. Adv. Synth. Catal. 2011, 353, 2593–2598. [Google Scholar] [CrossRef]
- Jones, R.A.; Davidson, R.; Tran, A.T.; Smith, N.; Galan, M.C. Iodine-catalyzed one-pot acetalation-esterification reaction for the preparation of orthogonally protected glycosides. Carbohydr. Res. 2010, 345, 1842–1845. [Google Scholar] [CrossRef]
- Vohra, Y.; Vasan, M.; Venot, A.; Boons, G.J. One-Pot Synthesis of Oligosaccharides by Combining Reductive Openings of Benzylidene Acetals and Glycosylations. Org. Lett. 2008, 10, 3247–3250. [Google Scholar] [CrossRef]
- Wang, C.-C.; Lee, J.-C.; Luo, S.-Y.; Kulkarni, S.S.; Huang, Y.-W.; Lee, C.-C.; Chang, K.-L.; Hung, S.-C. Regioselective one-pot protection of carbohydrates. Nature 2007, 446, 896–899. [Google Scholar] [CrossRef]
- Shie, C.-R.; Tzeng, Z.-H.; Kulkarni, S.S.; Uang, B.-J.; Hsu, C.-Y.; Hung, S.-C. Cu(OTf)2 as an Efficient and Dual-Purpose Catalyst in the Regioselective Reductive Ring Opening of Benzylidene Acetals. Angew. Chem. Int. Ed. 2005, 44, 1665–1668. [Google Scholar] [CrossRef]
- Garegg, P.J.; Kvarnstrom, I.; Niklasson, A.; Niklasson, G.; Svensson, S.C.T. Partial Substitution of Thioglycosides by Phase Transfer Catalyzed Benzoylation and Benzylation. J. Carbohydr. Chem. 1993, 12, 933–953. [Google Scholar] [CrossRef]
- Abronina, P.I.; Malysheva, N.N.; Litvinenko, V.V.; Zinin, A.I.; Kolotyrkina, N.G.; Kononov, L.O. A Ring Contraction of 2,3-Di-O-Silylated Thiopyranosides To Give Thiofuranosides under Mildly Acidic Conditions. Org. Lett. 2018, 20, 6051–6054. [Google Scholar] [CrossRef]
- Yan, M.-C.; Chen, Y.-N.; Wu, H.-T.; Lin, C.-C.; Chen, C.-T.; Lin, C.-C. Removal of Acid-Labile Protecting Groups on Carbohydrates Using Water-Tolerant and Recoverable Vanadyl Triflate Catalyst. J. Org. Chem. 2007, 72, 299–302. [Google Scholar] [CrossRef]
- Procopio, A.; Dalpozzo, R.; Nino, A.D.; Maiuolo, L.; Nardi, M.; Romeo, G. Mild and efficient method for the cleavage of benzylidene acetals by using erbium (III) triflate. Org. Biomol. Chem. 2005, 3, 4129–4133. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Hui, Y.-Z. A Convenient Method for Highly Selective Deprotection of Benzylidene Acetals from Sugars. Synth. Commun. 1996, 26, 881–886. [Google Scholar] [CrossRef]
- Michigami, K.; Terauchi, M.; Hayashi, M. Cleavage of 4,6-O-Benzylidene Acetal Using Sodium Hydrogen Sulfate Monohydrate. Synthesis 2013, 45, 1519–1523. [Google Scholar]
- Szarek, W.A.; Zamojski, A.; Tiwari, K.N.; Ison, E.R. A new, facile method for cleavage of acetals and dithioacetals in carbohydrate derivatives. Tetrahedron Lett. 1986, 27, 3827–3830. [Google Scholar] [CrossRef]
- Chen, C.-T.; Lin, Y.-D.; Liu, C.-Y. Catalytic carbon–sulfur bond formation by amphoteric vanadyl triflate: Exploring with thia-Michael addition, thioacetalization, and transthioacetalization reactions. Tetrahedron 2009, 65, 10470–10476. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, J.; Sun, J.-C.; Cai, L.; Zhao, Y.-Q.; Fang, J.; Hu, B.; Shu, P.-H.; Meng, L.-K.; Wan, Q. 1,4-Dithiothreitol mediated cleavage of the acetal and ketal type of diol protecting groups. Org. Chem. Front. 2018, 5, 2427–2431. [Google Scholar] [CrossRef]
- Kim, K.-S.; Song, Y.-H.; Lee, B.-H.; Hahn, C.-S. Efficient and Selective Cleavage of Acetals and Ketals Using Ferric Chloride Adsorbed on Silica Gel. J. Org. Chem. 1986, 51, 404–407. [Google Scholar] [CrossRef]
- Niu, Y.-H.; Wang, N.; Cao, X.-P.; Ye, X.-S. Efficient Formation and Cleavage of Benzylidene Acetals by Sodium Hydrogen Sulfate Supported on Silica Gel. Synlett 2007, 13, 2116–2120. [Google Scholar] [CrossRef]
- Agnihotri, G.; Misra, A.K. Mild and efficient method for the cleavage of benzylidene acetals using HClO4-SiO2 and direct conversion of acetals to acetates. Tetrahedron Lett. 2006, 47, 3653–3658. [Google Scholar] [CrossRef]
- Roy, B.; Verma, P.; Mukhopadhyay, B. H2SO4-silica-promoted ‘on-column’ removal of benzylidene, isopropylidene, trityl and tert-butyldimethylsilyl groups. Carbohydr. Res. 2009, 344, 145–148. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumar, G.D.K.; Baskaran, S. Truly Catalytic and Chemoselective Cleavage of Benzylidene Acetal with Phosphomolybdic Acid Supported on Silica Gel. Eur. J. Org. Chem. 2008, 2008, 6063–6067. [Google Scholar] [CrossRef]
- Couri, M.R.C.; Evangelista, E.A.; Alves, R.B.; Prado, M.A.F.; Gil, R.P.F.; De Almeida, M.V.; Raslan, D.S. Microwave-Assisted Rapid Deacetalation of Carbohydrates. Synth. Commun. 2005, 35, 2025–2031. [Google Scholar] [CrossRef]
- Santra, A.; Ghosh, T.; Misra, A.K. Removal of benzylidene acetal and benzyl ether in carbohydrate derivatives using triethylsilane and Pd/C. Beilstein J. Org. Chem. 2013, 9, 74–78. [Google Scholar] [CrossRef]
- Hori, H.; Nishida, Y.; Ohrui, H.; Meguro, H. Regioselective De-O-benzylation with Lewis Acids. J. Org. Chem. 1989, 54, 1346–1353. [Google Scholar] [CrossRef]
- Doyle, L.M.; O’Sullivan, S.; Di Salvo, C.; McKinney, M.; McArdle, P.; Murphy, P.V. Stereoselective Epimerizations of Glycosyl Thiols. Org. Lett. 2017, 19, 5802–5805. [Google Scholar] [CrossRef] [PubMed]
- Kartha, K.P.R.; Kiso, M.; Hasegawa, A.; Jennings, H.J. Novel Selectivity in Carbohydrate Reactions III. Selective Deprotection of p-Methoxybenzyl (PMBn) Ethers of Carbohydrates by Tin(IV) Chloride. J. Carbohydr. Chem. 1998, 17, 811–817. [Google Scholar] [CrossRef]
- Sawama, Y.; Masuda, M.; Asai, S.; Goto, R.; Nagata, S.; Nishimura, S.; Monguchi, Y.; Sajiki, H. FeCl3-Catalyzed Self-Cleaving Deprotection of Methoxyphenylmethyl-Protected Alcohols. Org. Lett. 2015, 17, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Kern, N.; Dombray, T.; Blanc, A.; Weibel, J.M.; Pale, P. Silver(I)-Catalyzed Deprotection of p-Methoxybenzyl Ethers: A Mild and Chemoselective Method. J. Org. Chem. 2012, 77, 9227–9235. [Google Scholar] [CrossRef]
- Ren, B.; Gan, L.; Zhang, L.; Yan, N.-N.; Dong, H. Diisopropylethylamine-triggered, highly efficient, self-catalyzed regioselective acylation of carbohydrates and diols. Org. Biomol. Chem. 2018, 16, 5591–5597. [Google Scholar] [CrossRef]
- Matwiejuk, M.; Thiem, J. Defining oxyanion reactivities in base-promoted glycosylations. Chem. Commun. 2011, 47, 8379–8381. [Google Scholar] [CrossRef]
- Rocheleau, S.; Pottel, J.; Huskić, I.; Moitessier, N. Highly Regioselective Monoacylation of Unprotected Glucopyranoside Using Transient Directing-Protecting Groups. Eur. J. Org. Chem. 2017, 2017, 646–656. [Google Scholar] [CrossRef]
- Medgyes, G.; Jerkovich, G.; Kuszmann, J.; Fügedi, P. Synthesis of sorbistin analogues. Carbohydr. Res. 1989, 186, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Moen, A.R.; Anthonsen, T. Screening of the regioselectivity of acetyl xylan esterase from Bacillus pumilus as a catalyst for the deacetylation of glycoside acetates. Biocatal. Biotransformation 2009, 27, 226–236. [Google Scholar] [CrossRef]
- Attouche, A.; Urban, D.; Beau, J.M. A Tin-Free Regioselective Radical De-O-benzylation by an Intramolecular Hydrogen Atom Transfer on Carbohydrate Templates. Angew. Chem. Int. Ed. 2013, 52, 9572–9575. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-Z.; Wang, K.-X.; Ma, K.-R.; Zhao, W.; Zhang, G.-Q. Preparation of rare L-idose derivatives from D-glucofuranose via neighboring acyl group assistance. Tetrahedron Lett. 2021, 73, 153135–153139. [Google Scholar] [CrossRef]
- Kajihara, Y.; Kodam, H.; Endo, T.; Hashimoto, H. Novel features of acceptor recognition by β-(1→4)-galactosyltransferase. Carbohydr. Res. 1998, 306, 361–378. [Google Scholar] [CrossRef]
- Fujiki, K.; Tanaka, K. Exploration of the Fluoride Reactivity of Aryltrifluoroborate on Selective Cleavage of Diphenylmethylsilyl Groups. Eur. J. Org. Chem. 2020, 29, 4616–4620. [Google Scholar] [CrossRef]
- Cavedon, C.; Sletten, E.T.; Madani, A.; Niemeyer, O.; Seeberger, P.H.; Pieber, B. Visible-Light-Mediated Oxidative Debenzylation Enables the Use of Benzyl Ethers as Temporary Protecting Groups. Org. Lett. 2021, 23, 514–518. [Google Scholar] [CrossRef]
- Johnsson, R.; Ohlin, M.; Ellervik, U. Reductive Openings of Benzylidene Acetals Revisited: A Mechanistic Scheme for Regio- and Stereoselectivity. J. Org. Chem. 2010, 75, 8003–8011. [Google Scholar] [CrossRef]
- Borén, H.B.; Garegg, P.J.; Pilotti, Å.; Swahn, C.-G. NMR Spectra of Some Glycoside Acetates in the Presence of Tris(dipivaloylmethanato)europium. Acta Chem. Scand. 1972, 26, 3261–3268. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.; Ramstrom, O.; Dong, H. Organosilicon-mediated regioselective acetylation of carbohydrates. Chem. Commun. 2012, 48, 5370–5372. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Senthilkumar, S.; Baskaran, S. Benzylidene acetal protecting group as a carboxylic acid surrogate: Synthesis of functionalized uronic acids and sugar amino acids. Chem. Eur. J. 2016, 22, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Dayoub, W.; Chen, G.-R.; Lemaire, M. TMDS as a Dual-Purpose Reductant in the Regioselective Ring Cleavage of Hexopyranosyl Acetals to Ethers. Eur. J. Org. Chem. 2012, 2012, 1960–1966. [Google Scholar] [CrossRef]
- Seitz, A.; Wende, R.C.; Roesner, E.; Niedek, D.; Topp, C.; Colgan, A.C. McGarrigle, E.M.; Schreiner, P.R. Site-Selective Acylation of Pyranosides with Oligopeptide Catalysts. J. Org. Chem. 2021, 86, 3907–3922. [Google Scholar] [CrossRef] [PubMed]
- van der Vorm, S.; Hansen, T.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. The Influence of Acceptor Nucleophilicity on the Glycosylation Reaction Mechanism. Chem. Sci. 2017, 8, 1867–1875. [Google Scholar] [CrossRef]
- Maki, Y.; Nomura, K.; Okamoto, R.; Izumi, M.; Mizutani, Y.; Kajihara, Y. Acceleration and Deceleration Factors on the Hydrolysis Reaction of 4,6-O-Benzylidene Acetal Group. J. Org. Chem. 2020, 85, 15849–15856. [Google Scholar] [CrossRef]
- Emmadi, M.; Kulkarni, S.S. Synthesis of Rare Deoxy Amino Sugar Building Blocks Enabled the Total Synthesis of a Polysaccharide Repeating Unit Analogue from the LPS of Psychrobacter cryohalolentis K5T. J. Org. Chem. 2018, 83, 14323–14337. [Google Scholar] [CrossRef]
- Ye, D.-F.; Liu, Z.-Y.; Chen, H.; Sessler, J.L.; Lei, C.-H. Cesium Carbonate Catalyzed Esterification of N-Benzyl-N-Bocamides under Ambient Conditions. Org. Lett. 2019, 21, 6888–6892. [Google Scholar] [CrossRef]
- Bauder, C. A Convenient synthesis of orthogonally protected 2-deoxystreptamine (2-DOS) as an aminocyclitol scaffold for the development of novel aminoglycoside antibiotic derivatives against bacterial resistance. Org. Biomol. Chem. 2008, 6, 2952–2960. [Google Scholar] [CrossRef]
- Babu, R.B.R.; Sørensen, M.D. Parmar, V.S.; Harrit, N.H.; Wengel, J. Oligodeoxynucleotides containing α-L-ribo confifigured LNA-type C-aryl nucleotides. Org. Biomol. Chem. 2004, 2, 80–89. [Google Scholar]
- Viuffa, A.H.; Heuckendorfa, M.; Jensen, H.H. p-Chlorobenzyl Ether: A p-Methoxybenzyl Ether in Disguise. Org. Lett. 2016, 18, 5773–5775. [Google Scholar] [CrossRef] [PubMed]
- Crich, D.; Banerjee, A.; Yao, Q.-J. Direct Chemical Synthesis of the β-d-Mannans: The β-(1→2) and β-(1→4) Series. J. Am. Chem. Soc. 2004, 126, 14930–14934. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Y.; Kawada, T.; Rosenau, T.; Kosma, P. Synthesis of methyl 4′-O-methyl-13C12-β-D-cellobioside from 13C6-D-glucose. Part 1: Reaction optimization and synthesis. Carbohydr. Res. 2005, 340, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).