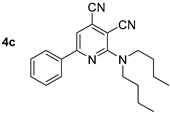
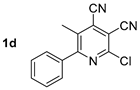
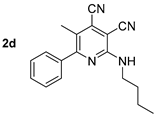
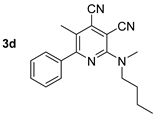
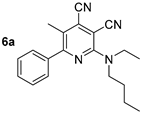
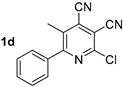
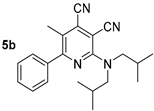
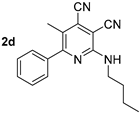
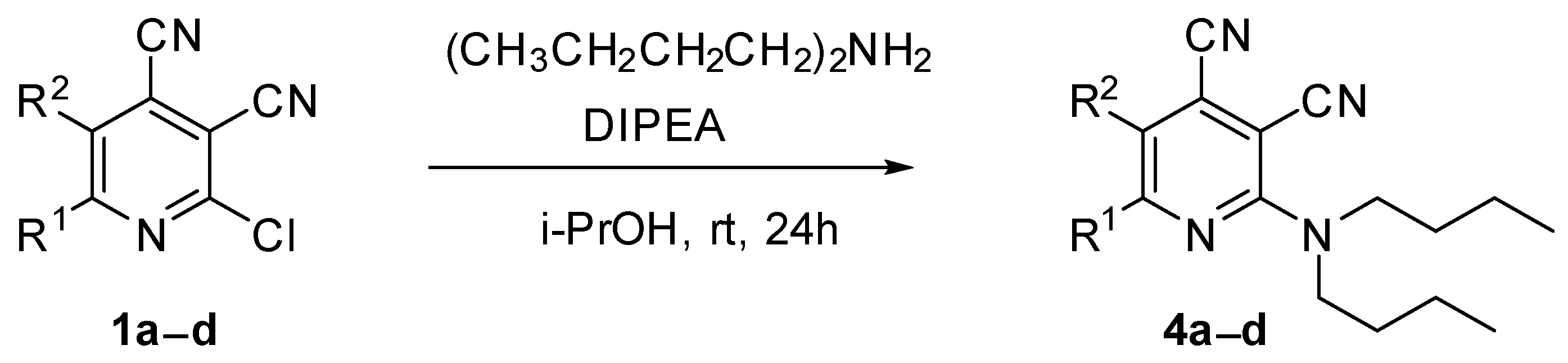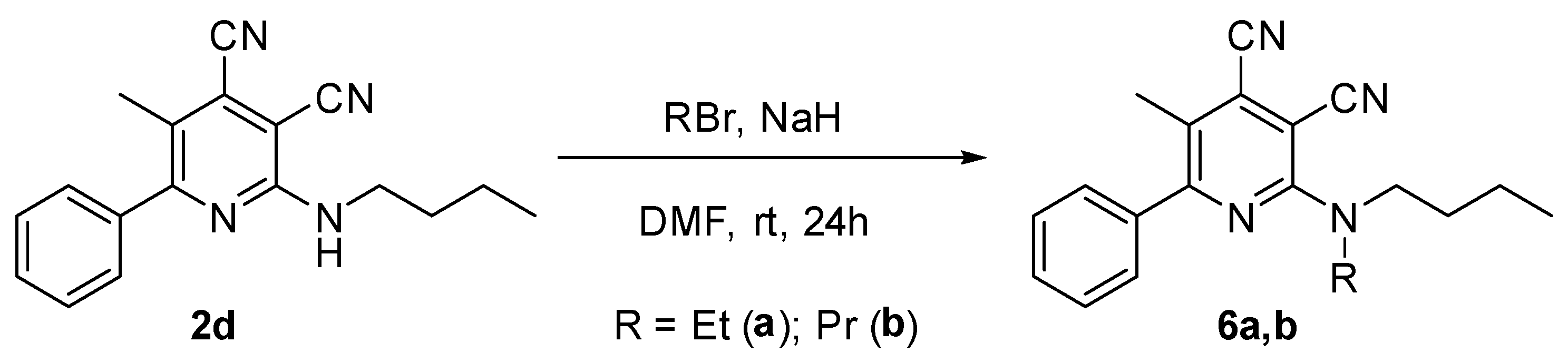Abstract
New representatives of 2-(butylamino)cinchomeronic dinitrile derivatives were synthesized as promising fluorophores showing dual-state emission. To characterize the influence of the length (from methyl to butyl) and the structure (both linear and branched) of the alkyl substituent at the amino nitrogen atom, the spectral fluorescence properties of all synthesized compounds were carefully studied both in solution and in solid state. The highest photoluminescence quantum yield values of 63% were noted for solutions of 2-(butylamino)-6-phenylpyridine-3,4-dicarbonitrile in DCM and 2-(butylamino)-5-methyl-6-phenylpyridine-3,4-dicarbonitrile in toluene.
1. Introduction
The development of new luminescent organic compounds has received continued attention due to their possible applications in many fields. The most promising fluorophores are compounds efficiently emitting visible light upon photoexcitation both in solution and in the solid state. Such a phenomenon is called dual-state emission (DSE) [1,2,3,4]. Usually, most fluorescent materials are emissive in one state only (in solution or in crystals). In many cases, luminogens exhibit an intense photoluminescence in dilute solutions and show no or weak emission in the solid state [5,6]. This is due to the fact that aggregated molecules are often affected by strong π-π stacking leading to energy exchange and aggregation-caused quenching (ACQ). In contrast to such fluorophores, some organic molecules are non- or weakly emissive in solution but demonstrate a so-called aggregation-induced emission (AIE)—strong fluorescence in the solid state [7,8,9]. The mentioned aggregation state restrictions significantly reduce the areas of possible use for most fluorescent molecules. Thus, the possibility to achieve strong emissions both in solution and in solid state simultaneously for the single molecule is a challenging task since the absorbed excitation energy can be released through various competing channels.
Over the past decade, there has been an exponential increase in the number of publications about the synthetic approaches to compounds showing dual-state emission (DSE) [1,2,3,4,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. This is due to the fact that DSE molecules are more versatile and are therefore intended for a wider range of applications. For example, they are successfully used in sensing (as fluorescence sensors and pH or ion indicators) [20,21,22,23,24,25], bioimaging [26,27,28], ultrahigh-density data recording and storage [29], super-resolution fluorescence microscopy [30], lasing [31], organic OLED devices [32,33,34,35], organic semiconductors [36], and NLO materials [37,38].
Previously, we reported that compounds with cinchomeronic dinitrile fragments exhibited fluorescence both in solutions and in solid state [39,40,41,42,43]. For example, sulfur-containing cinchomeronic dinitrile derivatives (ethyl 2-((3,4-dicyanopyridine-2-yl)thio)acetates) showed blue fluorescence in organic solvents and a green emission in powders [39]. Compounds such as 2-diethylaminocinchomeronic dinitriles were strongly emissive in the solid state and in nonpolar solvents, with fluorescence quantum yields up to 59% [40]. Compounds such as 2-(pyrrolidin-1-yl)-, 2-(piperidin-1-yl)-, and 2-(azepan-1-yl)-pyridine-3,4-dicarbonitrile derivatives showed even higher emission efficiencies both in solvents and in crystals up to 80% [41]. In addition, 2-(dicyanomethylene)-1,2-dihydropyridine-3,4-dicarbonitrile derivatives (4-CN-TCPy) possessed solid-state emissions in the red and NIR regions, while their solutions were characterized with strong solvatochromic effects and blue fluorescence [42]. It was also shown that the fluorescence of 4-CN-TCPy can be used for determination of the degree of substitution of the amino nitrogen atom with ethyl groups using gaseous ethylamine, diethylamine, and triethylamine [43]. Such above-mentioned promising fluorescence properties prompted us to continue our studies. Herein, we report our novel findings in the field of cinchomeronic dinitrile derivatives showing dual-state emissions (DSE): synthesis and characterization of new compounds along with investigation of their photophysical properties.
It is known that alkyl chains of a certain length and other terminal bulky groups are usually introduced not only to improve the solubility of conjugated fluorescent molecules, but also to prevent their intermolecular interactions and to provide “self-isolation” of fluorophores, increasing the emission intensity in both states [1,12,44]. According to this, we decided to synthesize previously undescribed N-butylamino-substituted derivatives of cinchomeronic dinitrile bearing both linear and branched alkyl chains and to study their photophysical properties.
2. Results
2.1. Synthesis and Photophysical Properties of 2-(Butylamino)Pyridine-3,4-Dicarbonitriles 2
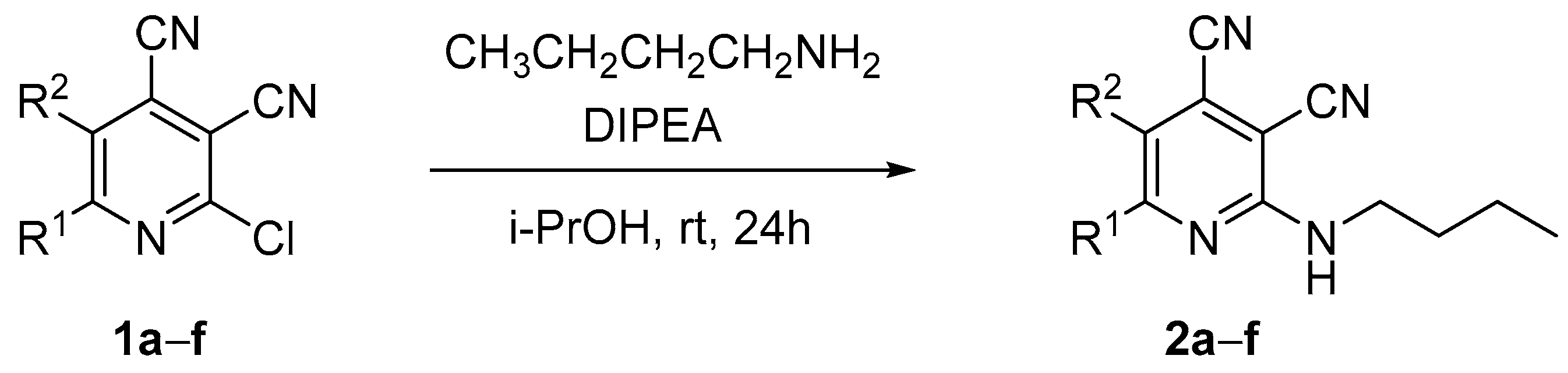
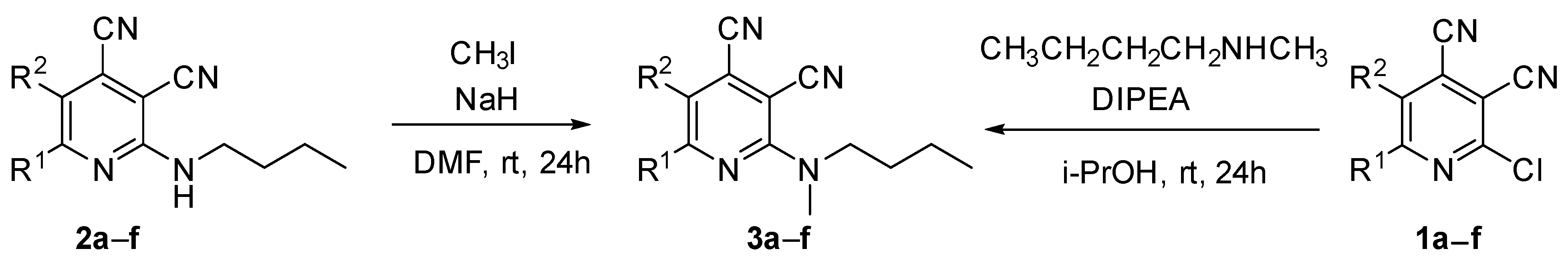
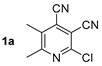
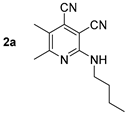
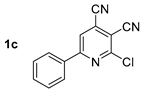
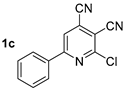
At first, we developed the method for the preparation of 2-butylaminopyridine-3,4-dicarbonitriles 2a–f (Scheme 1, Table 1) based on the reaction of available 2-chloropyridine-3,4-dicarbonitriles 1 [45] with N-butylamine in propan-2-ol in the presence of N,N-diisopropylethylamine (DIPEA). The reaction yield was 53–81%.
Scheme 1.
Synthesis of 2-(butylamino)pyridine-3,4-dicarbonitriles 2.

Table 1.
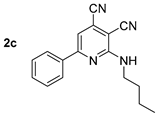
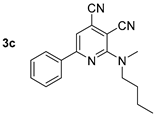
Structure of starting compounds 1 and isolated products 2–4.
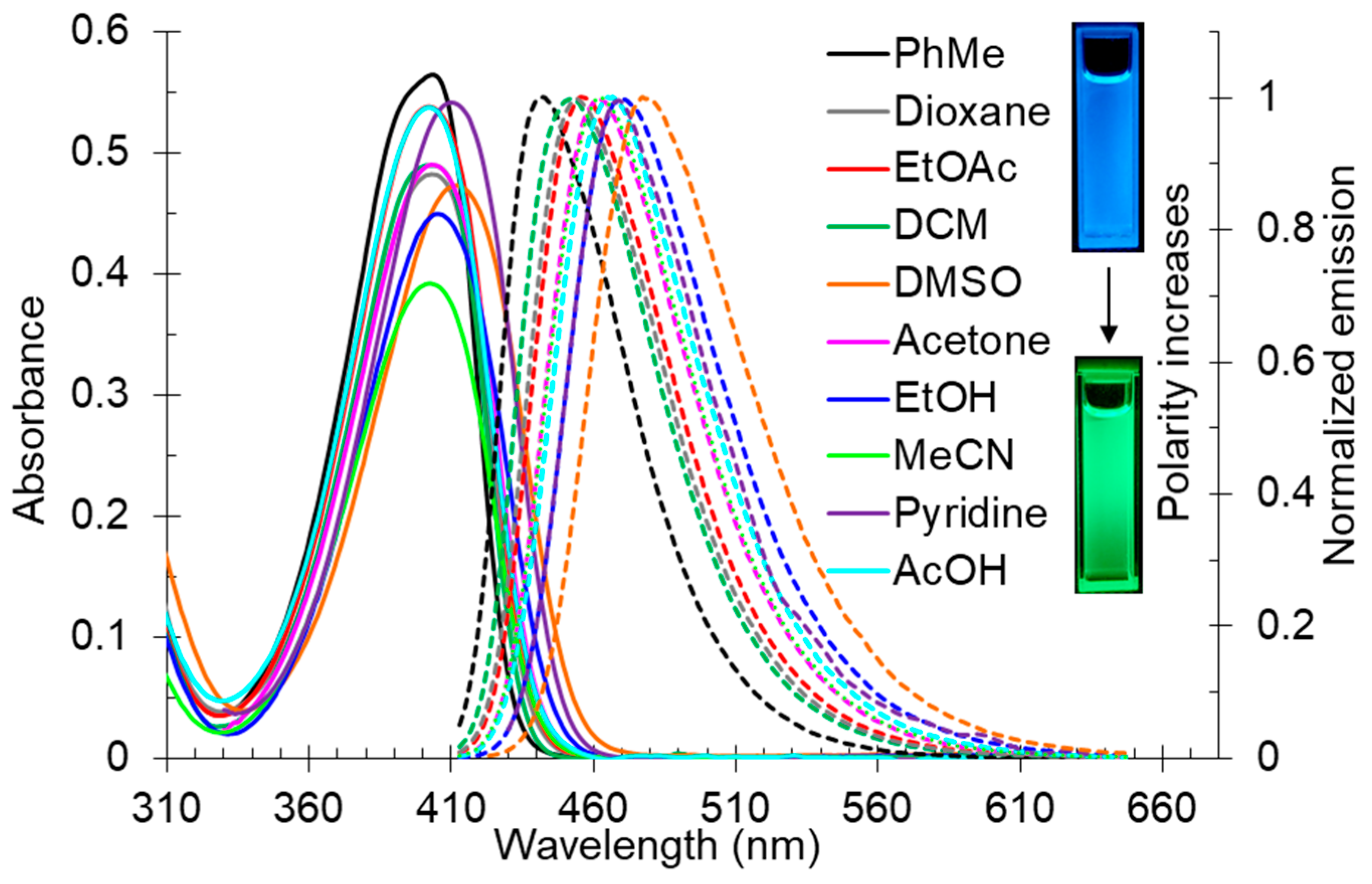
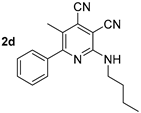
The solvatochromic behavior of 2-butylamino-substituted compounds 2 was studied using compound 2d (Table 2, Figure 1). It was found that the long-wavelength absorption band was slightly red-shifted upon increasing of the solvent polarity, and its maximum lay in the range of 402–410 nm. The fluorescence band was more affected by the polarity changes, and its maximum was in the range of 442–477 nm corresponding to the blue and blue-green region of the spectrum.

Table 2.
Solvatochromic properties of the compound 2d.
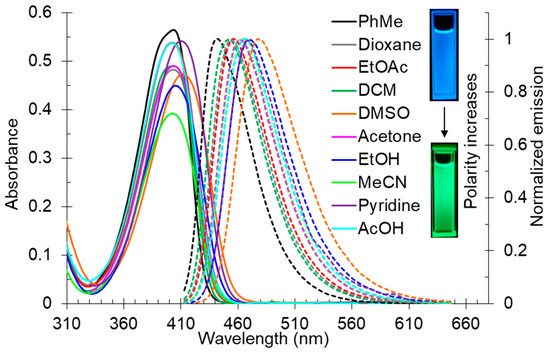
Figure 1.
UV-vis absorption (5 × 10−5 M, solid lines) and emission (5 × 10−5 M, dashed lines) spectra of compound 2d in various solvents. Photos of solution 2d in toluene and DMSO were taken under 365 nm of irradiation.
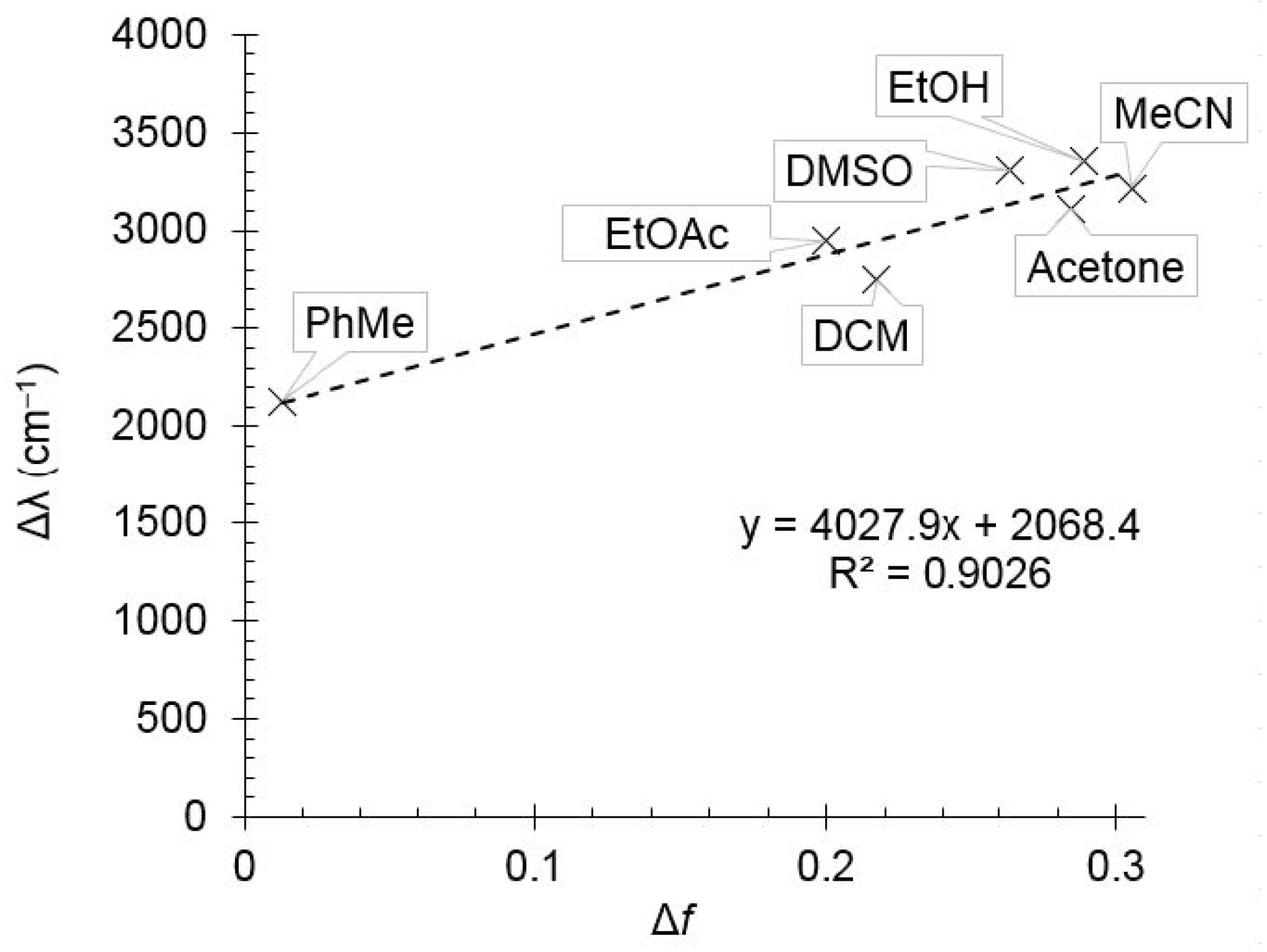
It was found that increasing of the solvent polarity caused a bathochromic shift in the emission maximum. It means that the excited state of compound 2d is more polar than the ground one, and therefore it should be better stabilized by polar solvents. This observation was supported by the Lippert–Mataga plot showing good linearity (Figure 2). The compound 1,4-dioxane was excluded from the plot due to its “effective” dielectric constant which should be considered much higher as the molecule is able to adopt the boat conformation around dipolar species. Pyridine and acetic acid were also excluded because of the pronounced acid–base properties.
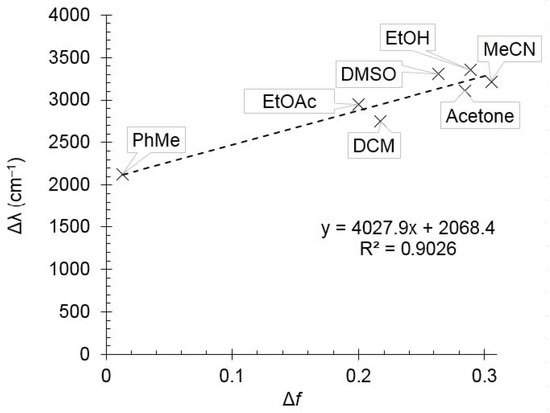
Figure 2.
The Lippert–Mataga plot for compound 2d.
Table 1 describes the relationship between the nonradiative excitation energy loss (Δλ) and the polarity function of a solvent (Δf).
where Δλ is Stokes shift value in cm−1, Δμ is the change in dipole moment upon photoexcitation, h is Planck’s constant, c is the speed of light in a vacuum, a is the cavity radius of fluorophore, and Δf is the orientation polarizability of the solvent which can be found from:
where ε is the dielectric constant and n is the refractive index of the solvent.
Δλ = (2 Δμ2/hca3) Δf,
Δf = [(ε − 1)/(2ε + 1)] − [(n2 − 1)/(2n2 + 1)],
The slope of the Lippert–Mataga plot was used to estimate the change in dipole moment of a molecule upon photoexcitation (Δμ), and for compound 2d it was found to be about 5.1 D, which can be attributed to the prevailing locally excited (LE) state.
Due to the higher dipole moment in the excited state, the photoluminescence quantum yield of compound 2d was also expected to be decreased in polar media. Thus, in nonpolar toluene and dichloromethane, it reached 60% and 63%, respectively, while in DMSO, it decreased to 26%. Basic pyridine led to almost complete fluorescence quenching, apparently due to deprotonation of the N–H fragment.
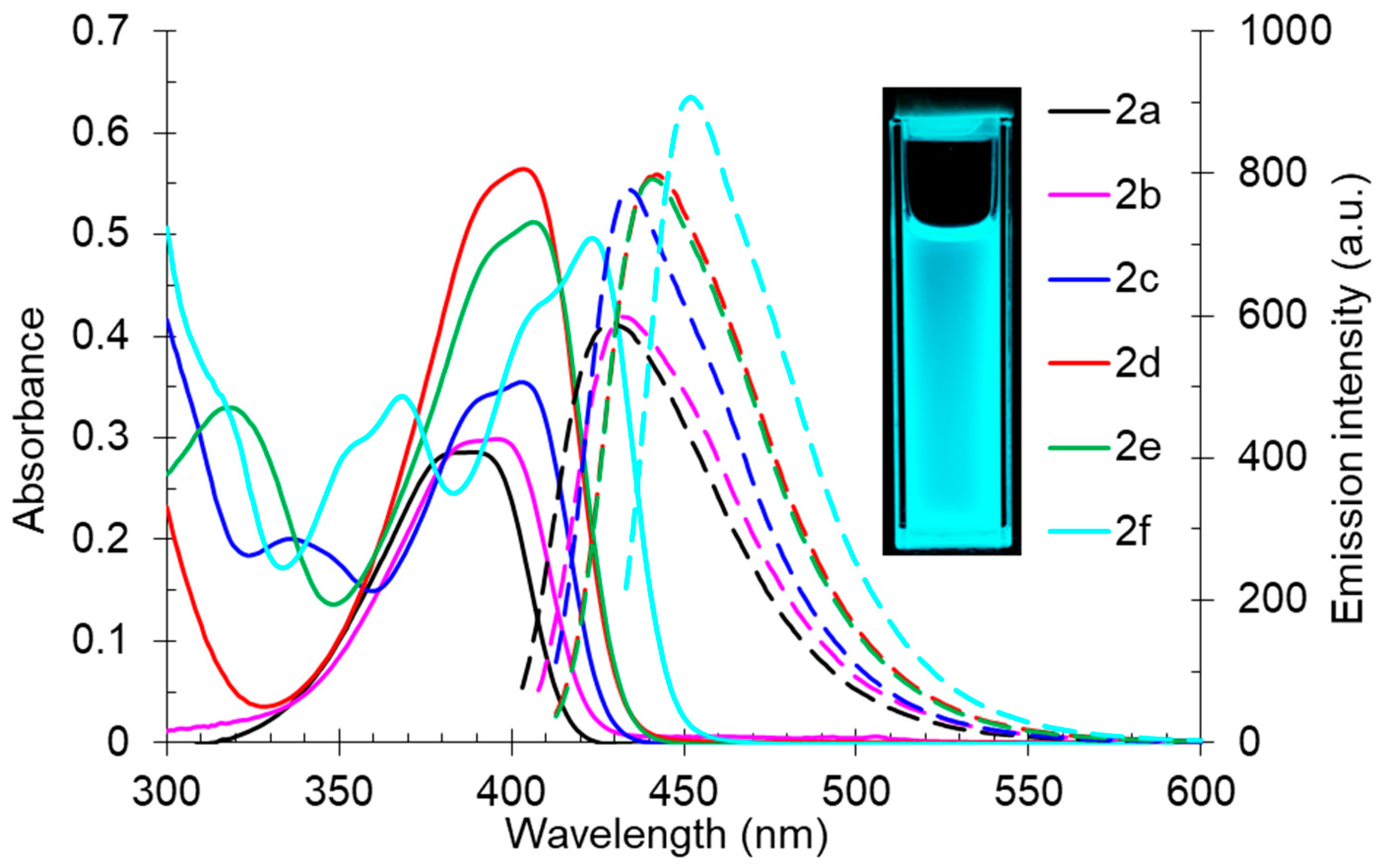
The study of the influence of compound 2’s structure on the photophysical properties showed that derivatives 2a and 2b, bearing aliphatic fragments at the pyridine ring, had shorter-wavelength absorption maxima at 389 nm and 396 nm, respectively, while for aryl-substituted structures 2c–f, these band maxima were in the range of 403–423 nm. All the studied compound 2s demonstrated good photoluminescence properties. Their emission maxima lay in the blue region of the spectrum between 429–452 nm. It should also be noted that the fluorescence quantum yield (Φs) was not decreased, even for aliphatic derivatives 2a,b. It indicates that the 2-butylamino-substituted cinchomeronic dinitrile moiety is essential for the radiative relaxation from the excited state. The maximum photoluminescence efficiency (Φs) was noted for compound 2c of about 63% (Table 3, Figure 3).

Table 3.
Photophysical parameters of the synthesized 2-(butylamino)cinchomeronic dinitrile derivatives 2–6.
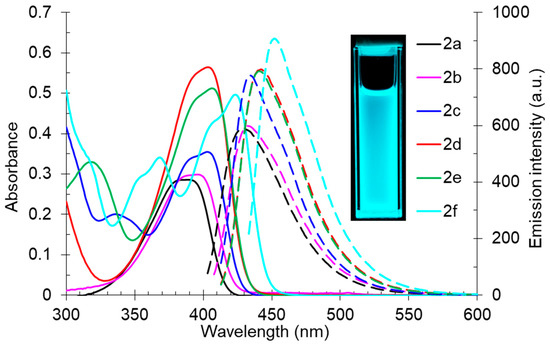
Figure 3.
UV-vis absorption (5 × 10−5 M, solid lines) and emission (5 × 10−5 M, dashed lines) spectra of compound 2 in toluene. A photo of solution 2f in toluene was taken under 365 nm of irradiation.
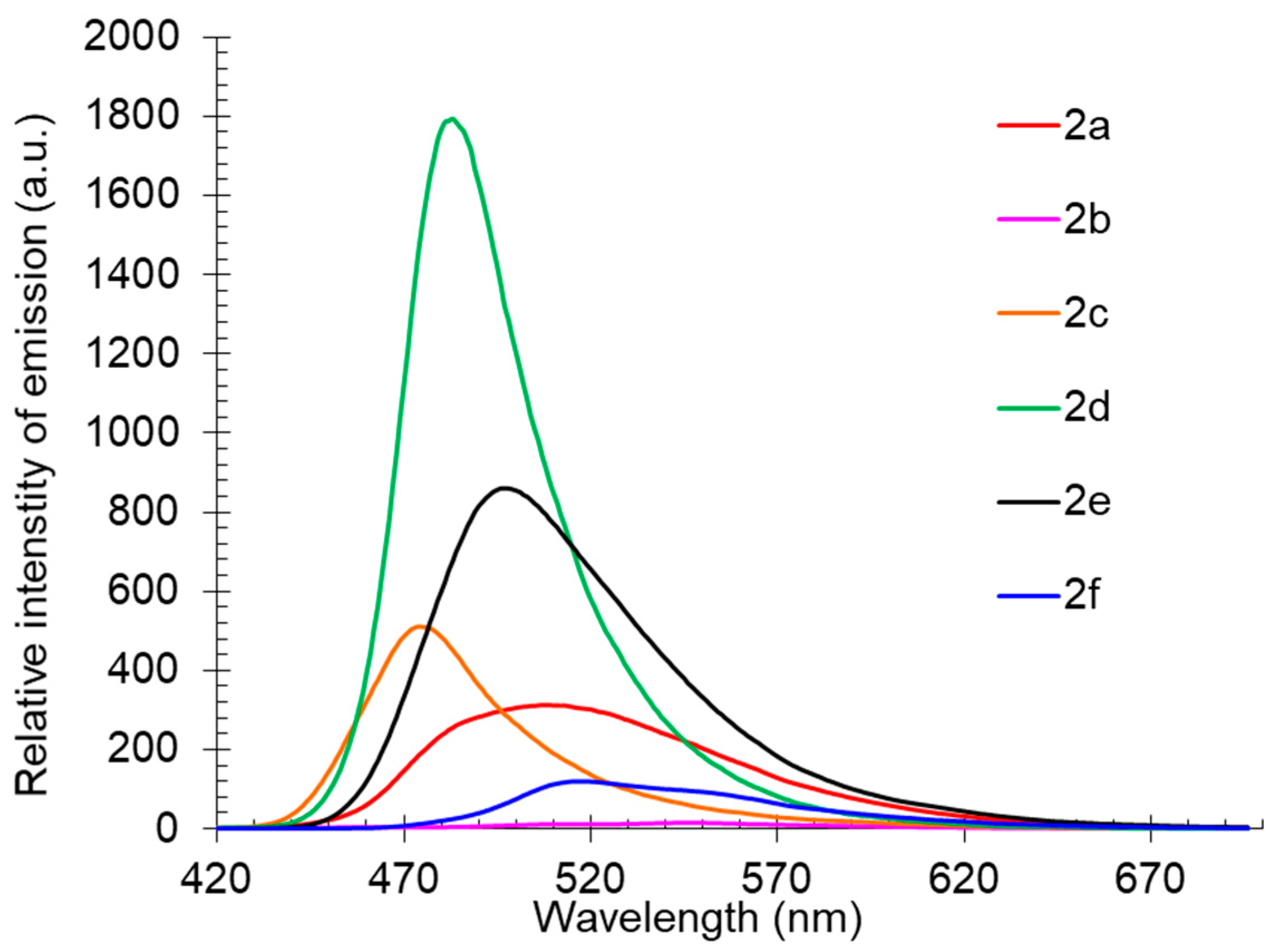
Compound 2 also showed good photoluminescence in the individual form in the crystalline state (Table 3, Figure 4). It was found that the emission maxima of the compounds lay in the range of 474–547 nm, corresponding to a wide range from the blue-green to yellow-orange regions of the visible spectrum, and in most cases, short-wavelength emissions were much more intense. These observations indicated that derivatives 2b and 2f, showing red-shifted emission maxima with pronounced shoulders, were prone to aggregation with subsequent fluorescence quenching, while compound 2d, on the contrary, demonstrated the highest photoluminescence intensity and a narrow emission band.
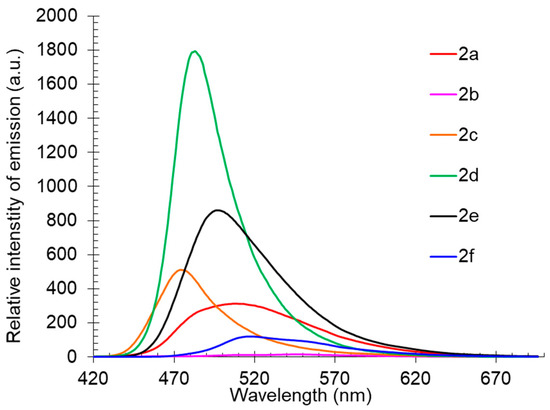
Figure 4.
Solid-state photoluminescence spectra of compound 2 in a powder.
2.2. Synthesis and Photophysical Properties of 2-(Butyl(methyl)amino)Pyridine-3,4-Dicarbonitriles 3
The next part of the presented study was the synthesis of compounds bearing a butyl substituent without the mobile N–H moiety. This proton can participate in amine–imine tautomerism and affects the optical properties of compound 2. The synthesis of target compound 3 bearing N-butyl-N-methylamine fragments was carried out using two approaches.
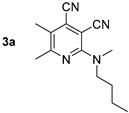
The first method was developed based on the NH alkylation of compound 2 with methyl iodide in the presence of sodium hydride (Scheme 2) in absolute DMF. The isolated yield of 2-(butyl(methyl)amino)pyridine-3,4-dicarbonitriles 3 was about 12–37%. The second preparation method was based on the halogen substitution reaction in pyridines. As a result of the reaction of 2-chloropyridine-3,4-dicarbonitriles 1 with N-butyl-N-methylamine in propan-2-ol in the presence of N,N-diisopropylethylamine (DIPEA), compounds 3a–f were synthesized with 71–96% yields (Scheme 2, Table 1).
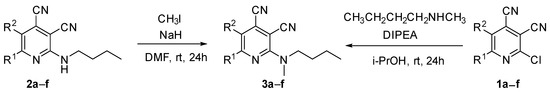
Scheme 2.
Synthesis of 2-(butyl(methyl)amino)pyridine-3,4-dicarbonitriles 3.
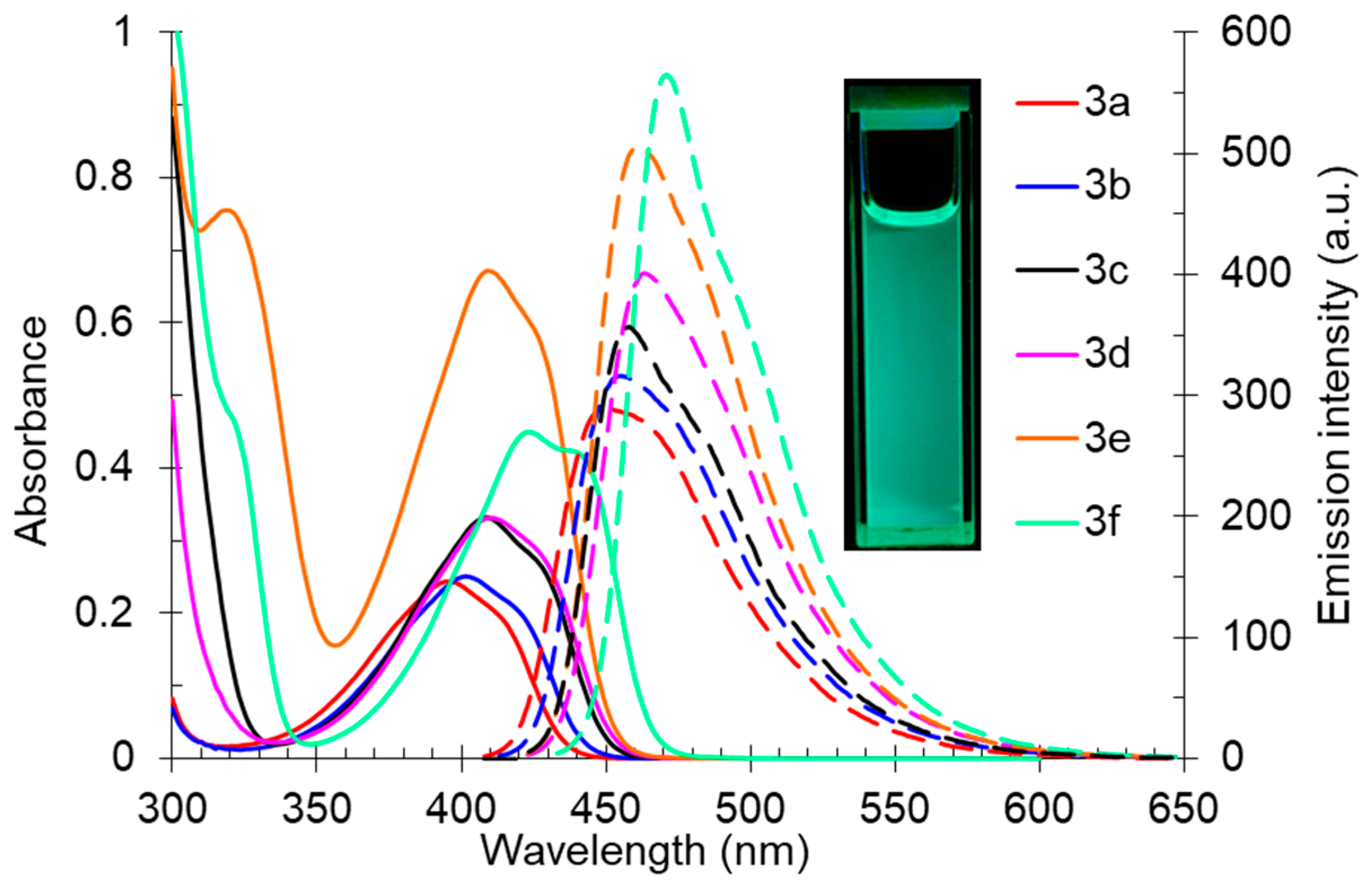
The synthesized 2-(butyl(methyl)amino)pyridine-3,4-dicarbonitriles 3 also exhibited a pronounced fluorescence in solution. The registered absorption spectra of compound 3 in toluene were characterized by maxima in the range of 395–439 nm, and the emission spectra showed an intense fluorescence band in the range of 449–471 nm, corresponding to the blue region of the spectra (Table 3, Figure 5). The shape of the photoluminescence spectra was characterized by a pronounced long-wavelength shoulder, indicating the probability of the existence of several radiating excited states. The Stokes shift values (Δλ) correlated with the observed photoluminescence quantum yield values (Φs). Thus, for compound 3a, significant nonradiative energy losses (3045 cm–1) were observed leading to a decrease in Φs to 36%. In turn, for the derivative 3f, which was characterized by a bathochromic shift in both the absorption and emission bands, the quantum yield reached 47%, and the Stokes shift was the smallest one of 32 nm (1548 cm−1).
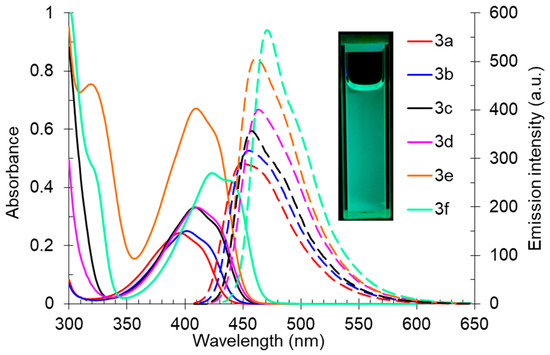
Figure 5.
UV-vis absorption (5 × 10−5 M, solid lines) and emission (5 × 10−5 M, dashed lines) spectra of compound 3 in toluene. A photo of solution 3f in toluene was taken under 365 nm of irradiation.
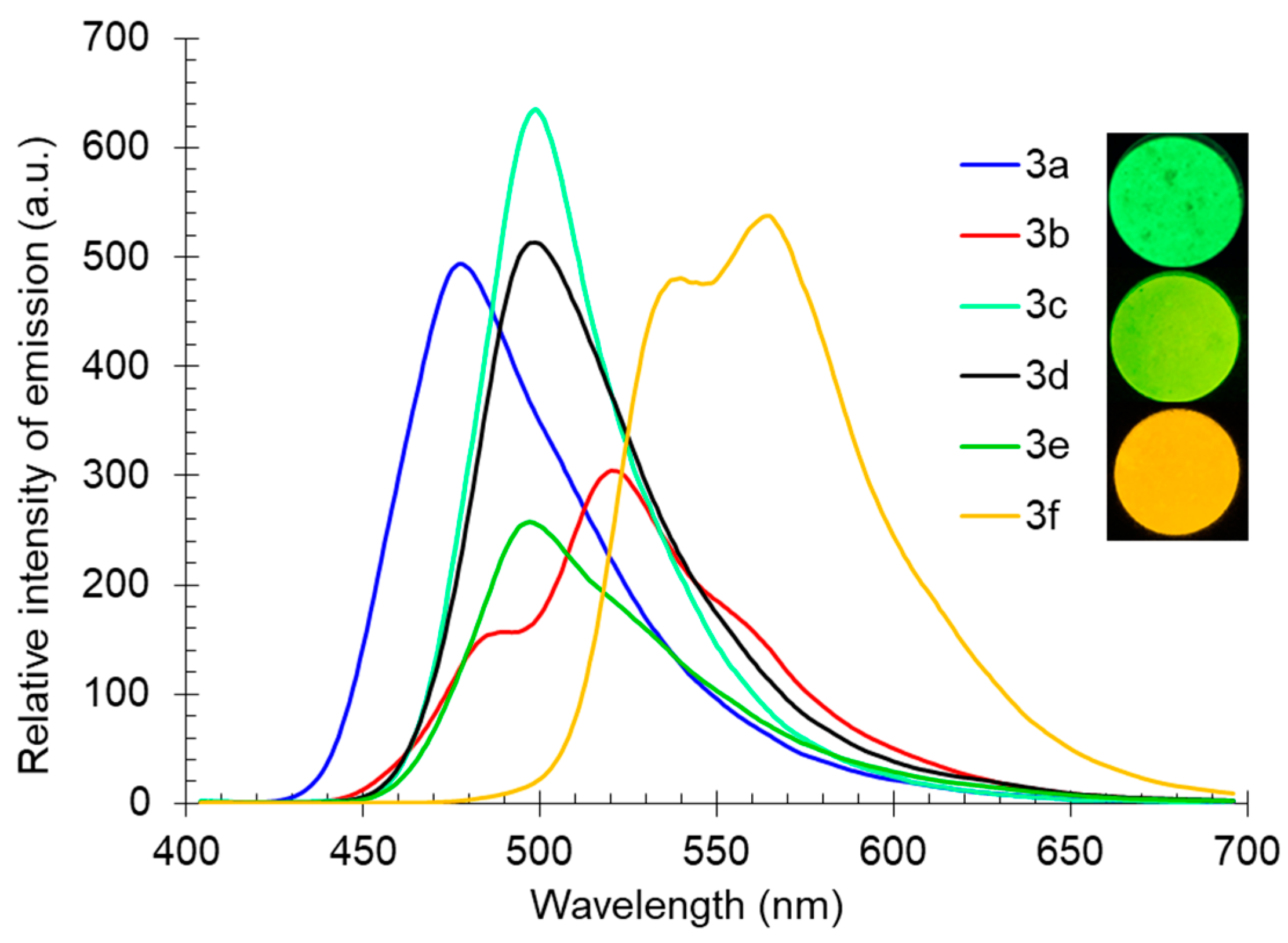
The fluorescence spectra of compound 3, registered at room temperature for powders of the studied substances, covered a wide range from the blue-green to yellow-orange spectral regions, with maxima in the range of 478–564 nm (Table 3, Figure 6). Compounds 3b and 3f, bearing a six-membered ring fused with pyridine, were characterized by the presence of several emission maxima, which were associated with different types of crystal packings in solid samples. The highest intensity of the solid-state photoluminescence upon excitation at 365 nm was noted for compounds 3a, 3c, 3d, and 3f.
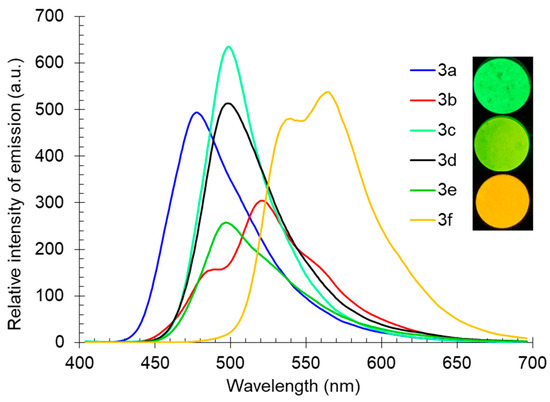
Figure 6.
Solid-state photoluminescence spectra of compound 3 in a powder. Photos of compounds 3c, 3e, and 3f were taken under 365 nm of irradiation.
2.3. Synthesis and Photophysical Properties of 2-(Dibutylamino)Pyridine-3,4-Dicarbonitriles 4
In the next step, the method for preparation of compounds bearing two bulky alkyl substituents was developed. It was found that the reaction of 2-chloropyridine-3,4-dicarbonitriles 1 with N,N-dibutylamine in propan-2-ol in the presence of N,N-diisopropylethylamine (DIPEA) gave 2-dibutylaminopyridine-3,4-dicarbonitriles 4a–d (Scheme 3, Table 1), with 43–77% yields. The method based on the N-alkylation of compound 2 with butyl halides gave extremely poor yields (less than 10%).
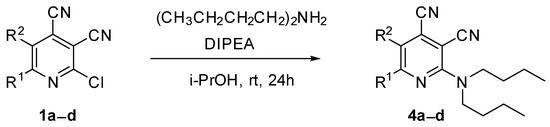
Scheme 3.
Synthesis of 2-(dibutylamino)pyridine-3,4-dicarbonitriles 4.
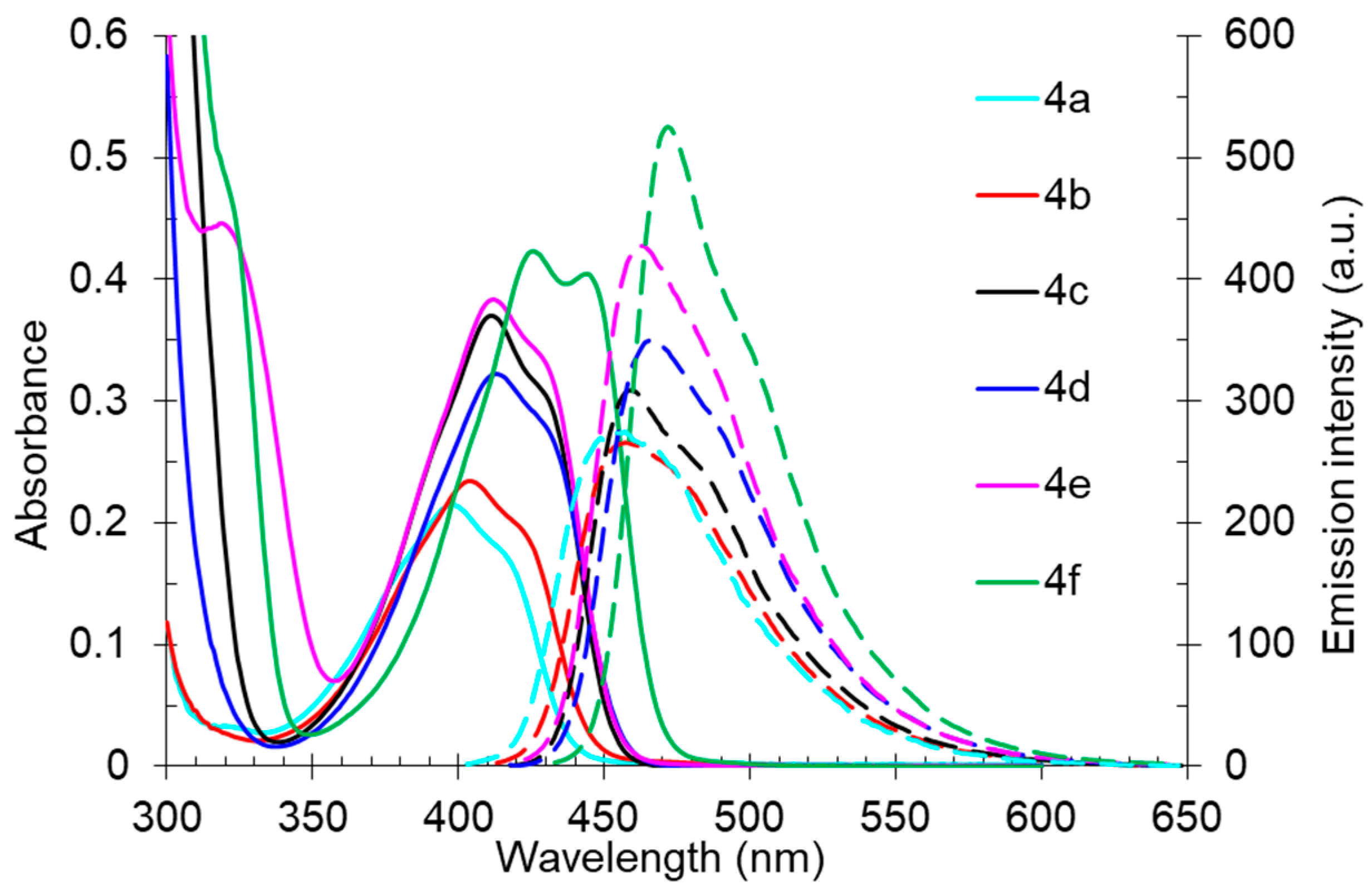
Studies of the photophysical properties of the 2-dibutylamino-substituted derivatives 4 showed that the absorption maxima of these compounds in toluene were in the range of 398–426 nm and were also characterized by intermediate values of light absorption coefficients (4300–8460 M−1 cm−1) (Table 3, Figure 7). The expected bathochromic shift in the absorption band along with an increase in intensity was observed for aryl-substituted derivatives 4c–f. Compound 4f, containing a spatially locked aromatic fragment, showed the biggest red shift and hyperchromic effect. The fluorescence bands of compound 4 were also in the blue region of the spectra, with maxima in the range of 456–472 nm, that also showed a bathochromic shift for aryl-substituted derivatives 4c–f. The photoluminescence quantum yields were in the range from 33% to 43%, and the highest emission intensity was also noted for compound 4f with fused cyclic fragments. The Stokes shift values (2288–3196 cm−1) showed the expected inverse correlation with the emission efficiency, and the shape of the emission bands was also characterized by a pronounced long-wavelength shoulder, probably caused by another radiative excited state.
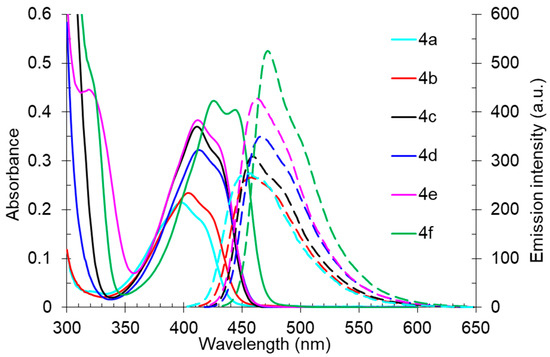
Figure 7.
UV-vis absorption (5 × 10−5 M, solid lines) and emission (5 × 10−5 M, dashed lines) spectra of compound 4 in toluene.
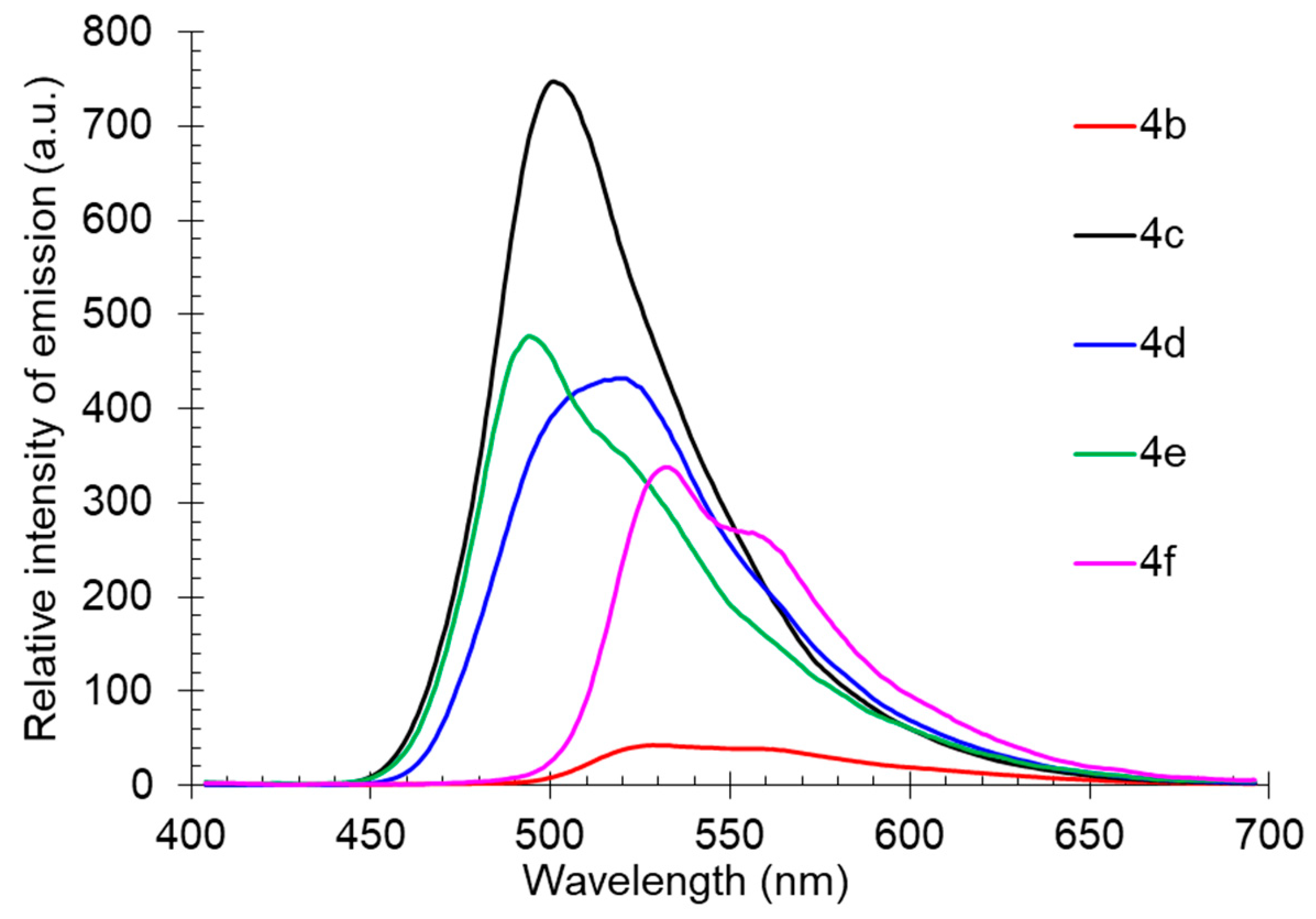
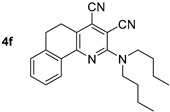
The solid-state photoluminescence was registered at room temperature for the powder samples of compounds 4b-f (compound 4a is liquid at rt). It was found that excitation by UV (365 nm) caused fluorescence of compound 4 with emission maxima in the green-yellow region of the spectra between 494–532 nm. Compound 4c showed the highest emission intensity; all other compounds observed slight quenching, probably caused by self-aggregation. It was supported by the shape of the fluorescence spectra showing a pronounced long-wavelength shoulder (Table 3, Figure 8).
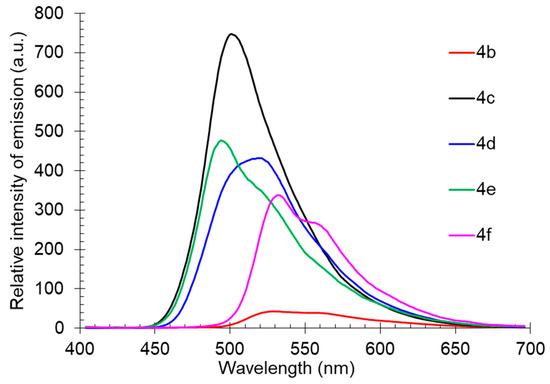
Figure 8.
Solid-state photoluminescence spectra of compound 4 in a powder.
2.4. Synthesis and Photophysical Properties of 2-(Diisobutylamino)Pyridine-3,4-Dicarbonitriles 5
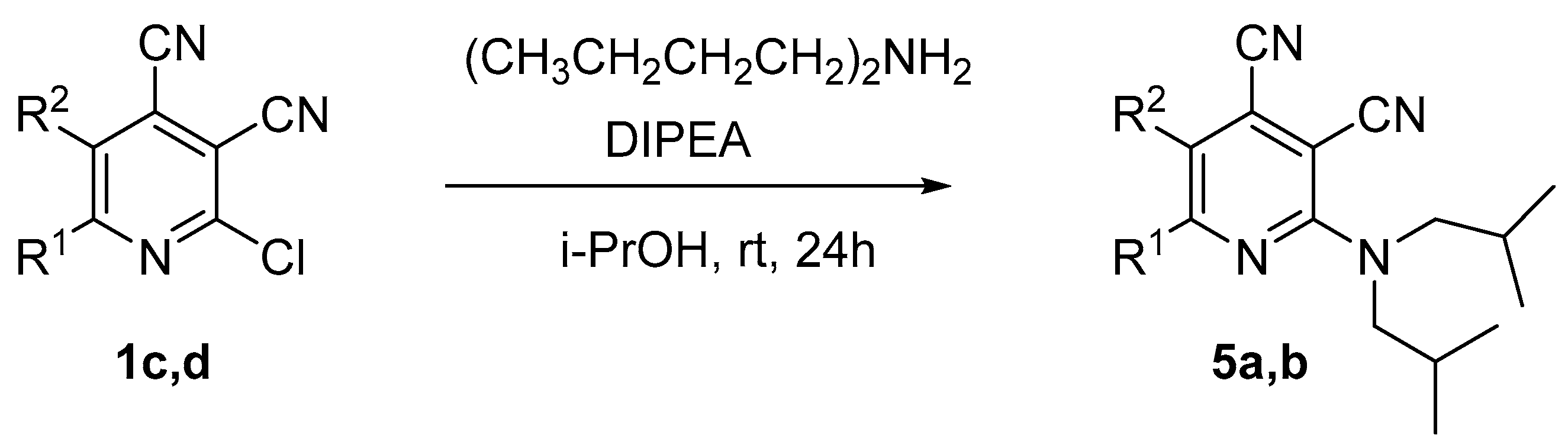
The next part of the presented study was the synthesis of compounds bearing branched alkyl substituents. It was found that the reaction of 2-chloropyridine-3,4-dicarbonitriles 1 with diisobutylamine in propan-2-ol in the presence of N,N-diisopropylethylamine (DIPEA) gave 2-diisobutylaminopyridine-3,4-dicarbonitrile derivatives 5a,b, with yields of 48–56% (Scheme 4, Table 4).
Scheme 4.
Synthesis of 2-(diisobutylamino)pyridine-3,4-dicarbonitriles 5.

Table 4.
Structure of starting compounds 1 and isolated products 5 and 6.
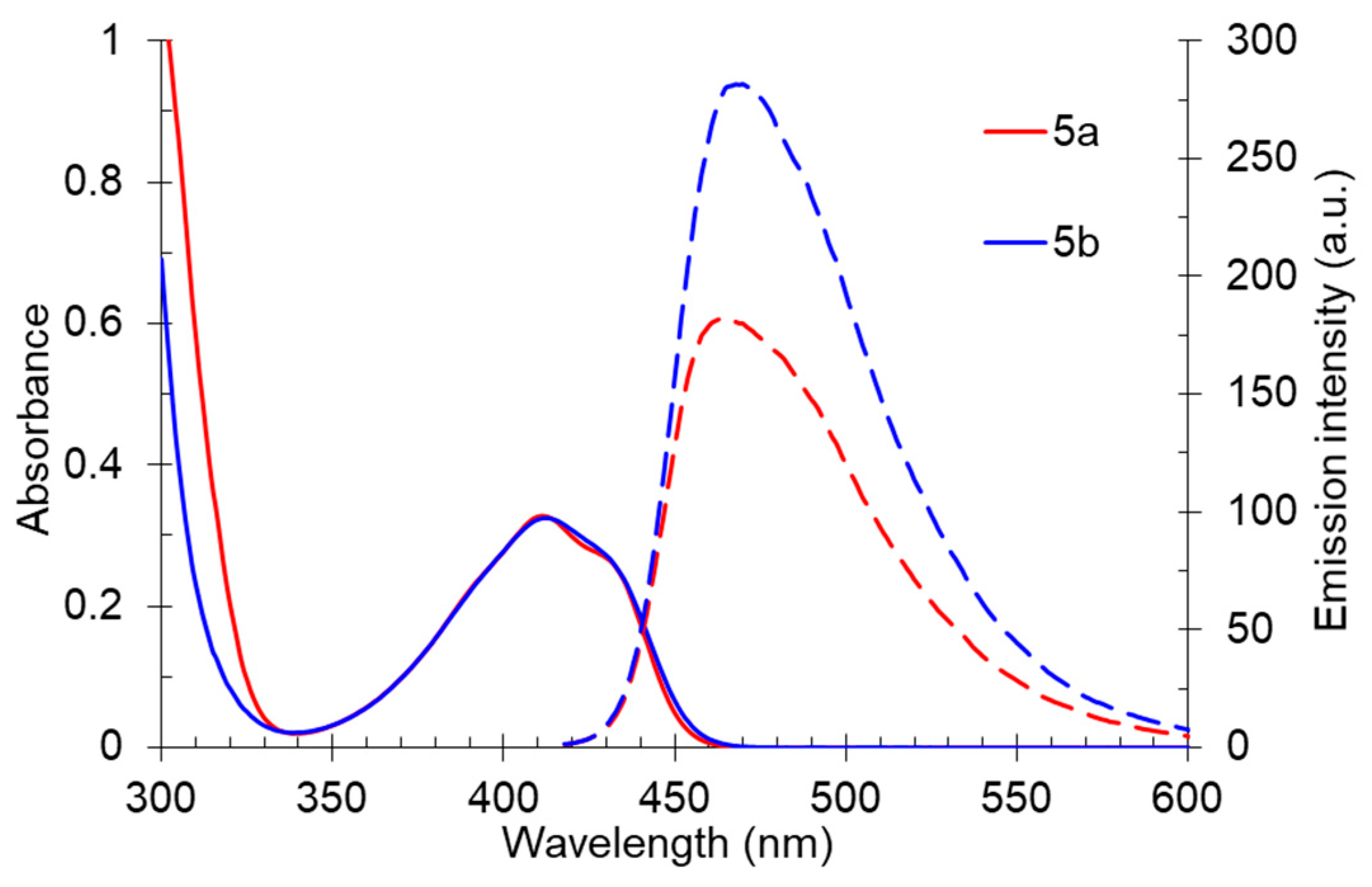
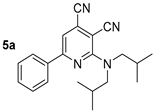
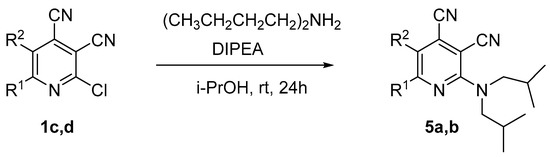
To study the effect of the branching alkyl fragment of the substituted amino group on the spectral properties, the electronic spectra of 2-diisobutylamino-substituted pyridines 5 were analyzed (Table 3, Figure 9). It was found that their absorption maxima were in a narrow range of 411–413 nm, and the emission maxima were between 464–470 nm. Moreover, it should be noted that the 5-methyl-substituted derivative 5b was characterized with a high photoluminescence quantum yield, reaching 33% in a toluene solution. For compound 5a, the fluorescence efficiency was 23%. Apparently, the presence of the methyl group at the C5 atom of the pyridine ring prevented the planarity of the molecule; as a result, the efficiency of conjugation between aromatic fragments was decreased. Apparently, it hindered the process of deactivation of the excited state through intramolecular charge transfer and increased the probability of radiative relaxation. The Stokes shift values (2779–2936 cm−1) also indicated a significant nonradiative loss of excitation energy, which was associated with an increase in the contribution of vibrational relaxation due to an increase in the number of mobile moieties.
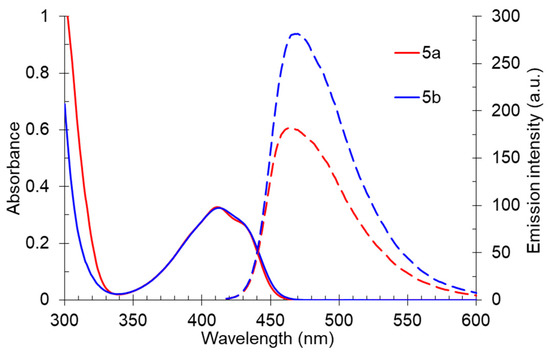
Figure 9.
UV-vis absorption (5 × 10−5 M, solid line) and emission (5 × 10−5 M, dashed line) spectra of compound 5 in toluene.
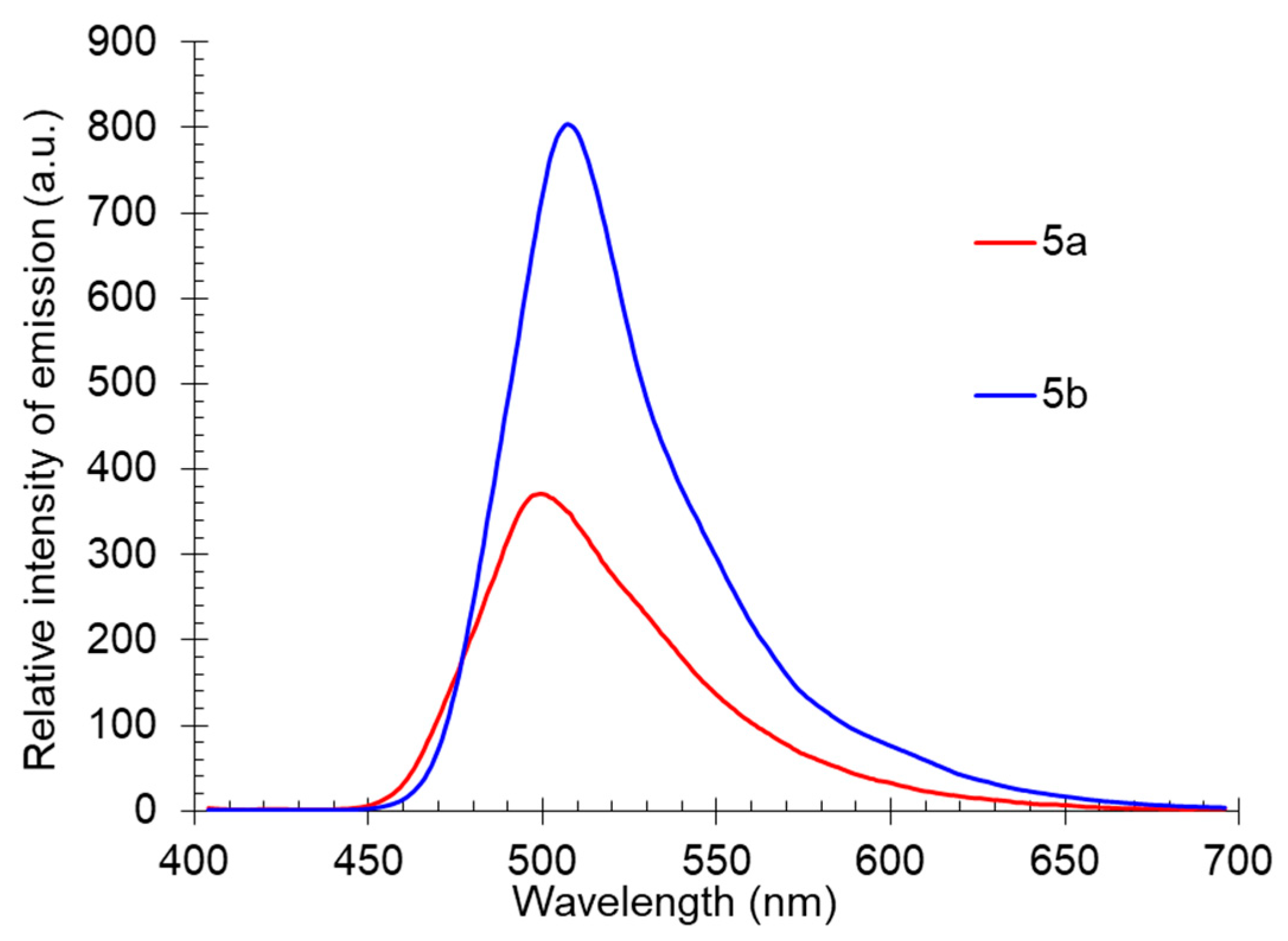
The solid-state photoluminescence spectra of compounds 5 were registered in powders at room temperature (excitation wavelength was 365 nm). The spectra demonstrated a similar dependence with solutions: the 5-methyl-substituted derivative 5b was characterized by a higher fluorescence intensity. The maxima of the solid-state emission bands of both compounds were in the yellow region of the spectra (500 nm and 507 nm) and did not show a pronounced “shoulder” (Table 3, Figure 10).
Figure 10.
Solid-state photoluminescence spectra of compound 5 in a powder.
2.5. Synthesis of N-Alkyl-2-(Butylamino)Pyridine-3,4-Dicarbonitriles 6
One of the tasks of the presented study was to establish the effects of the length of the alkyl substituent at the amino group on the photophysical properties of cinchomeronic dinitrile derivatives. Therefore, we directly prepared a series of N-substituted derivatives of 2-butylamino-5-methyl-6-phenylpyridine-3,4-dicarbonitrile 2d, 3d, and 4d and additional N-ethyl (6a) and N-propyl (6b) derivatives. They were obtained by the reaction of 2-butylamino-5-methyl-6-phenylpyridine-3,4-dicarbonitrile 2d with bromoethane or bromopropane in absolute DMF in the presence of sodium hydride (Scheme 5, Table 4).
Scheme 5.
Synthesis of 2-((alkyl)butylamino)pyridine-3,4-dicarbonitriles 6.
3. Discussion
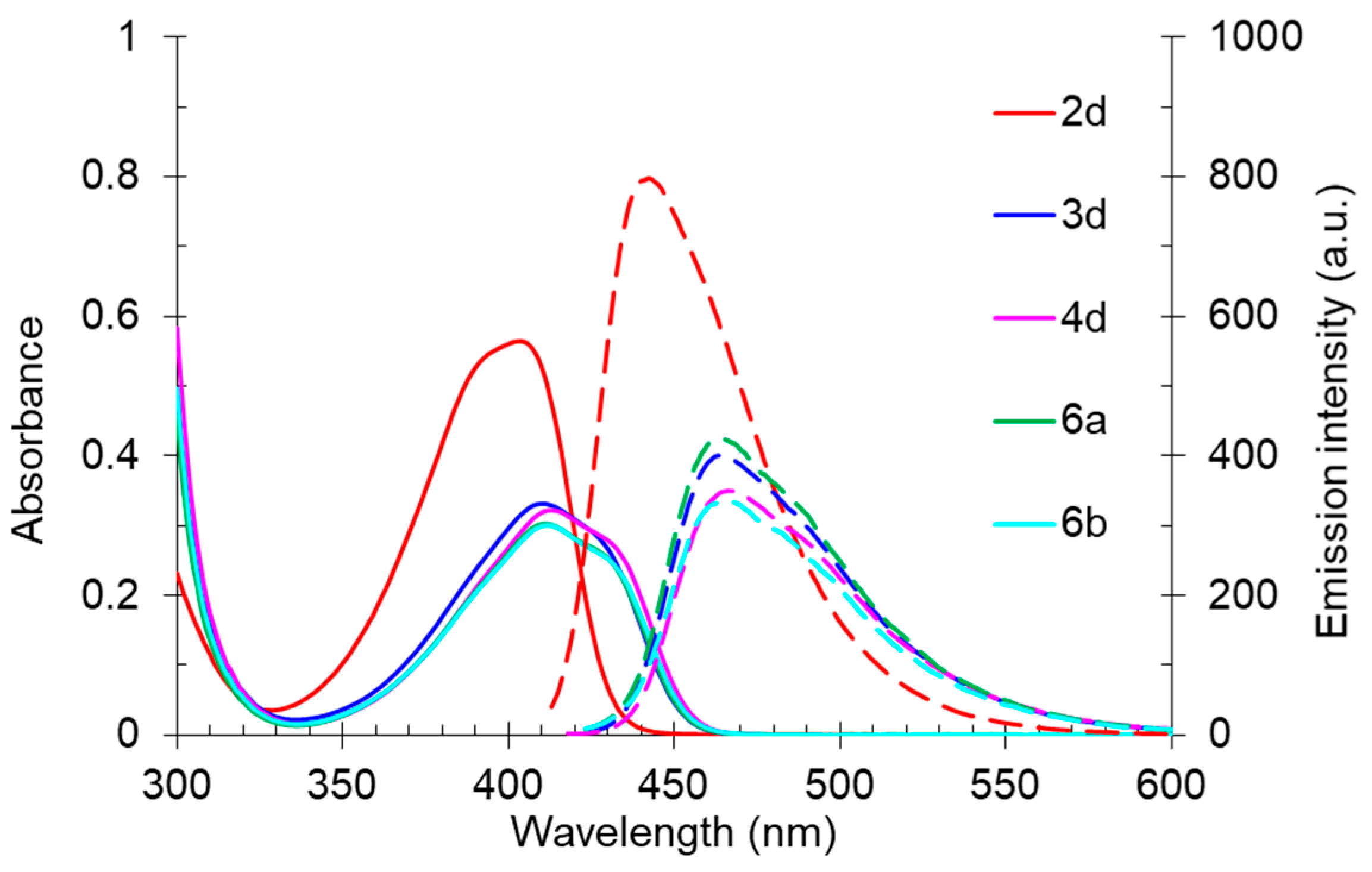
A study of the optical properties showed that the highest fluorescence intensity was observed for compound 2d, bearing a free NH fragment. Its photoluminescence quantum yield reached 60% in a toluene solution. A replacement of a hydrogen atom of NH moiety by alkyl substituents of various lengths led to a decrease in the photoluminescence efficiency, down to 37%, and that decrease occurred with an increase in the alkyl chain per carbon atom. Moreover, a slight bathochromic shift in the absorption bands from 403 nm (derivative 2d) to 410–413 nm (compounds 3d, 6a, 6b, 4d) also occurred (Figure 11). A similar trend was also observed for the emission band, shifting from 442 nm to the region of 463–466 nm.
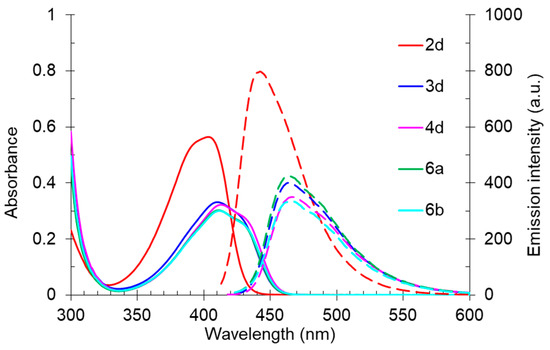
Figure 11.
UV-vis absorption (5 × 10–5 M, solid line) and emission (5 × 10–5 M, dashed line) spectra of compounds 2d, 3d, 4d, 6a, and 6b in toluene.
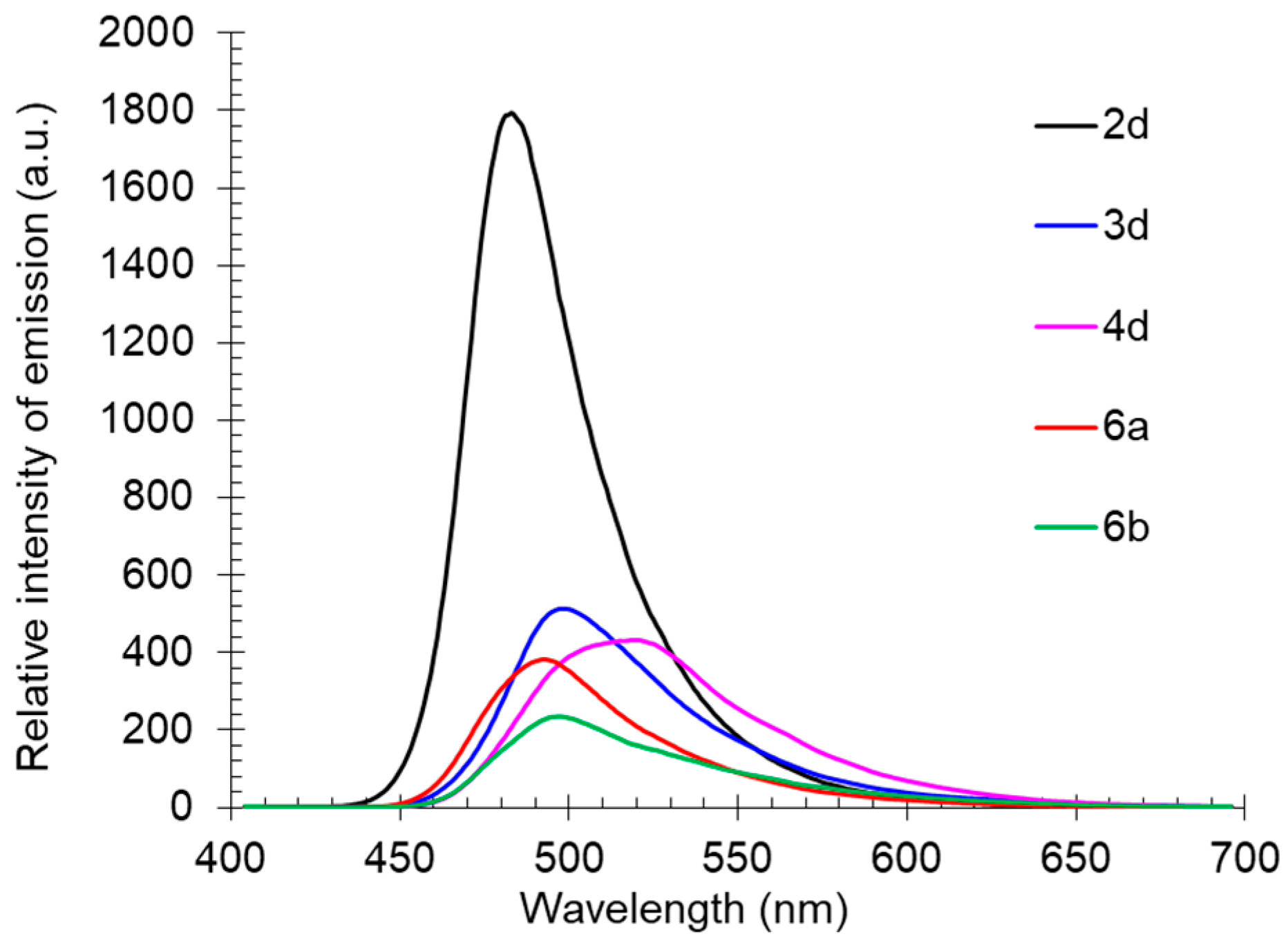
The solid-state photoluminescence spectra of compounds 2d, 3d, 6a, 6b, and 4d, containing various substituents at the nitrogen atom of the amino group, were characterized by emission maxima in the range of 483–519 nm. Compound 2d, with a free NH fragment was the most intense, similarly as in solution. The introduction of an alkyl substituent into the amino group led to a bathochromic shift in the emission band and caused a decrease in its intensity (Table 3, Figure 12).
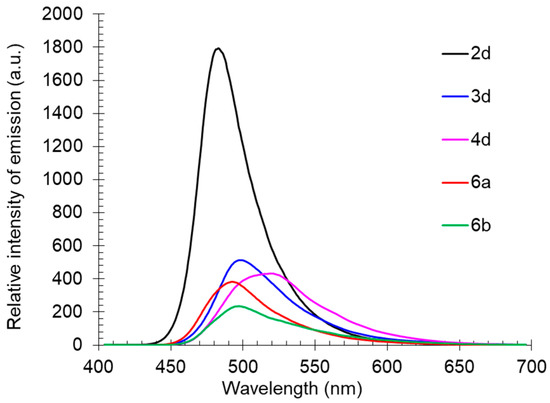
Figure 12.
Solid-state photoluminescence spectra of compounds 2d, 3d, 4d, 6a, and 6b in a powder.
Thus, it can be concluded that the presence of a free NH fragment in the studied compounds is essential for the intense photoluminescence both in solution and in solid state (dual-state emission). The introduction of a second substituent into the amino group, as well as an increase in its size, lead to a decrease in the fluorescence efficiency (Table 3, Figure 11).
4. Materials and Methods
The progress of reactions and the purity of products were monitored by thin-layer chromatography (TLC) on Sorbfil plates (spots were visualized under UV light, by treatment with iodine vapor, or by heating). Melting points were determined using an OptiMelt MPA100 device. The IR spectra were recorded on an FSM-2201 spectrometer with Fourier transforms from samples dispersed in mineral oil. The NMR spectra were measured a DMSO-d6 on Bruker AV-500 spectrometer using tetramethylsilane or residual solvent peak as the internal references. Copies of the NMR spectra are provided in supplementary materials. Elemental analyses were performed using a FlashEA 1112 CHN analyzer. The mass spectra (electron impact, 70 eV) were obtained on a Shimadzu GCMS-QP2020 using a direct-probe inlet. The UV spectra were recorded on an Agilent Cary 60 UV-Vis spectrophotometer using a standard quartz cuvette with a pathlength of 1 cm. The fluorescence spectra were recorded on an Agilent Cary Eclipse spectrofluorometer using a quartz cuvette with four optically clear sides. The relative fluorescence quantum yields in solution (Φs) were evaluated by the comparative method using quinine sulfate in 0.05 M H2SO4 as the standard compound with a known fluorescence efficiency (Φstd = 60%, excited at 380 nm) [46] by the creation of a calibration curve, plotting the area of fluorescence against the absorbance for different concentrations of the fluorophore. The solid-state emission spectra were registered at room temperature for sample powders using the Agilent Cary Eclipse solid sample holder. Photoluminescence quantum yields in the crystalline state (Φc) were determined by Wrighton’s method [47] using 365 nm of excitation.
4.1. General Procedure for the Preparation of 2-(Butylamino)Pyridine-3,4-Dicarbonitriles 2
An appropriate 2-chloropyridine-3,4-dicarbonitrile 1 (0.01 mol) was suspended in i-PrOH (5 mL), and then N-butylamine (0.80 g, 0.011 mol) and DIPEA (1.42 g, 0.011 mol) were added. The reaction mixture was stirred at 60–70 °C for 24 h. After reaction completion (TLC monitoring), the mixture was cooled, and the precipitated product was filtered off and washed with ice-cold water and i-PrOH. The resulting product was crystallized from i-PrOH, and then dried in a vacuum desiccator over CaCl2.
Compound 2a: 2-(Butylamino)-5,6-dimethylpyridine-3,4-dicarbonitrile. Yield 53%, mp 160–161 °C. IR (mineral oil, cm−1): 3377 (NH); 2233, 2214 (C≡N); 1591 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.89 (t, J = 7.4 Hz, 3H, CH2CH3), 1.25–1.33 (m, 2H, CH2CH3), 1.48–1.54 (m, 2H, CH2CH2), 2.25 (s, 3H, CH3), 2.40 (s, 3H, CH3), 3.34–3.39 (m, 2H, CH2NH), 7.38 (br t, 1H, NH). 13C NMR (125.76 MHz, DMSO-d6): δ 13.5, 15.8, 19.3, 23.7, 30.6, 40.2, 86.6, 114.5, 114.9, 121.0, 123.3, 156.1, 163.3. MS (EI, 70 eV): m/z (%) 228 ([M]+, 25), 185 ([M-C3H7]+, 100). Anal: Calcd for C13H16N4: C, 68.39; H, 7.06; N, 24.54. Found: C, 68.18; H, 7.03; N, 24.64.
Compound 2b: 2-(Butylamino)-5,6,7,8-tetrahydroquinoline-3,4-dicarbonitrile. Yield 54%, mp 149–150 °C. IR (mineral oil, cm−1): 3367 (NH); 2214 (C≡N); 1582 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.88 (t, J = 7.3 Hz, 3H, CH2CH3), 1.25–1.32 (m, 2H, CH2CH3), 1.45–1.54 (m, 2H, CH2), 1.71–1.79 (m, 4H, 2CH2), 2.66–2.72 (m, 4H, 2CH2), 3.33–3.37 (m, 2H, CH2NH), 7.36 (br t, 1H, NH). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 19.46, 21.6, 21.6, 25.9, 30.7, 33.0, 40.3, 87.9, 114.0, 114.8, 121.8, 123.9, 155.9, 163.4. MS (EI, 70 eV): m/z (%) 254 ([M]+, 22), 211 ([M-C3H7]+, 100). Anal: Calcd for C15H18N4: C, 70.84; H, 7.13; N, 22.03. Found: C, 70.51; H, 7.02; N, 22.13.
Compound 2c: 2-(Butylamino)-6-phenylpyridine-3,4-dicarbonitrile. Yield 65%, mp 151–152 °C. IR (mineral oil, cm−1): 3393 (NH); 2242, 2213 (C≡N); 1588 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.91 (t, J = 7.4 Hz, 3H, CH2CH3), 1.28–1.39 (m, 2H, CH2CH3), 1.54–1.65 (m, 2H, CH2), 3.47–3.54 (m, 2H, CH2NH), 7.50–7.55 (m, 3H, Ph), 7.76 (s, 1H, CHpyr), 7.82 (t, J = 5.6 Hz, 1H, NH), 8.11–8.17 (m, 2H, Ph). 13C NMR (125.76 MHz, DMSO-d6): δ 13.8, 19.6, 30.6, 40.6, 88.9, 110.2, 114.7, 115.3, 125.3, 127.3, 128.9, 131.2, 136.3, 157.7, 159.6. MS (EI, 70 eV): m/z (%) 276 ([M]+, 37), 233 ([M-C3H7]+, 100). Anal: Calcd for C17H16N4: C, 73.89; H, 5.84; N, 20.27. Found: C, 74.01; H, 5.81; N, 20.18.
Compound 2d: 2-(Butylamino)-5-methyl-6-phenylpyridine-3,4-dicarbonitrile. Yield 74%, mp 141–142 °C. IR (mineral oil, cm−1): 3361 (NH); 2231, 2217 (C≡N); 1586 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.86 (t, J = 7.4 Hz, 3H, CH2CH3), 1.25–1.32 (m, 2H, CH2CH3), 1.49–1.58 (m, 2H, CH2), 2.31 (s, 3H, CH3), 3.35–3.39 (m, 2H, CH2NH), 7.48–7.51 (m, 3H, Ph), 7.54–7.57 (m, 2H, Ph), 7.60 (t, J = 5.6 Hz, 1H, NH). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 17.4, 19.5, 30.7, 40.5, 89.1, 114.7, 114.8, 120.2, 125.8, 128.2, 128.8, 129.3, 138.5, 156.1, 162.2. MS (EI, 70 eV): m/z (%) 290 ([M]+, 43), 247 ([M-C3H7]+, 100). Anal: Calcd for C18H18N4: C, 74.46; H, 6.25; N, 19.30. Found: C, 74.59; H, 6.21; N, 19.24.
Compound 2e: 2-(Butylamino)-6-(4-methoxyphenyl)-5-methylpyridine-3,4-dicarbonitrile. Yield 63%, mp 125–126 °C. IR (mineral oil, cm−1): 3377 (NH); 2234, 2211 (C≡N); 1585 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.88 (t, J = 7.4 Hz, 3H, CH2CH3), 1.26-1.34 (m, 2H, CH2CH3), 1.51–1.57 (m, 2H, CH2), 2.35 (s, 3H, CH3), 3.36–3.42 (m, 2H, CH2NH), 3.83 (s, 3H, OCH3), 7.02–7.09 (m, 2H, C6H4), 7.50–7.61 (m, 3H, C6H4+NH). 13C NMR (125.76 MHz, DMSO-d6): δ 14.3, 18.3, 20.1, 31.4, 41.1, 55.9, 88.9, 114.2, 115.4, 115.6, 120.7, 126.3, 131.3, 131.4, 156.6, 160.9, 162.3. MS (EI, 70 eV): m/z (%) 320 ([M]+, 51), 277 ([M-C3H7]+, 100). Anal: Calcd for C19H20N4O: C, 71.23; H, 6.29; N, 17.49. Found: C, 71.44; H, 6.32; N, 17.38.
Compound 2f: 2-(Butylamino)-5,6-dihydrobenzo[h]quinoline-3,4-dicarbonitrile. Yield 81%, mp 161–162 °C. IR (mineral oil, cm−1): 3368 (NH); 2215 (C≡N); 1583 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.91 (t, J = 7.4 Hz, 3H, CH2CH3), 1.29–1.39 (m, 2H, CH2CH3), 1.56–1.63 (m, 2H, CH2), 2.86–2.96 (m, 4H, 2CH2), 3.45–3.49 (m, 2H, CH2NH), 7.30–7.44 (m, 3H, C6H4), 7.56 (br t, 1H, NH), 8.07–8.12 (m, 1H, C6H4). 13C NMR (125.76 MHz, DMSO-d6): δ 14.4, 20.24, 25.4, 27.1, 31.3, 41.2, 88.9, 114.8, 115.7, 122.1, 123.8, 126.1, 127.8, 128.9, 131.9, 132.7, 140.1, 155.9, 157.4. MS (EI, 70 eV): m/z (%) 302 ([M]+, 46), 259 ([M-C3H7]+, 100). Anal: Calcd for C19H18N4: C, 75.47; H, 6.00; N, 18.53. Found: C, 75.22; H, 5.97; N, 18.61.
4.2. General Procedure for the Preparation of 2-(Butyl(methyl)amino)Pyridine-3,4-Dicarbonitriles 3
Method A. An appropriate 2-butylaminopyridine-3,4-dicarbonitrile 2 (0.01 mol) was dissolved in dry DMF (10 mL), and then NaH (0.52 g, 0.013 mol, 60% in mineral oil) and methyl iodide (2.13 g, 0.015 mol) were added. The reaction mixture was stirred at room temperature for 24 h. After reaction completion (TLC monitoring), the mixture was diluted with water (50 mL) and neutralized by acetic acid. The precipitated solid was filtered off, washed with ice-cold water, and crystallized from i-PrOH, and then dried in a vacuum desiccator over CaCl2.
Method B. An appropriate 2-chloropyridine-3,4-dicarbonitrile 1 (0.01 mol) was suspended in i-PrOH (5 mL), and then N-butyl-N-methylamine (0.96 g, 0.011 mol) and DIPEA (1.42 g, 0.011 mol) were added. The reaction mixture was stirred at 60–70 °C for 24 h. After reaction completion (TLC monitoring), the mixture was cooled, and the precipitated product was filtered off and washed with ice-cold water and i-PrOH. The resulting product 3 was crystallized from i-PrOH, and then dried in a vacuum desiccator over CaCl2.
Compound 3a: 2-(Butyl(methyl)amino)-5,6-dimethylpyridine-3,4-dicarbonitrile. Yield 17% (method A), 71% (method B), mp 43–44 °C. IR (mineral oil, cm−1): 2232, 2206 (C≡N); 1578 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.90 (t, J = 7.4 Hz, 3H, CH2CH3), 1.25–1.32 (m, 2H, CH2CH3), 1.54–1.60 (m, 2H, CH2CH2), 2.28 (s, 3H, CH3), 2.41 (s, 3H, CH3), 3.19 (s, 3H, CH3N), 3.62 (t, J = 7.6 Hz, 2H, CH2N). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 16.0, 19.3, 23.7, 29.1, 37.9, 50.8, 85.8, 114.8, 116.9, 122.9, 125.3, 155.9, 162.4. MS (EI, 70 eV): m/z (%) 242 ([M]+, 19), 199 ([M-C3H7]+, 100). Anal: Calcd for C14H18N4: C, 69.39; H, 7.49; N, 23.12. Found: C, 69.18; H, 7.54; N, 23.04.
Compound 3b: 2-(Butyl(methyl)amino)-5,6,7,8-tetrahydroquinoline-3,4-dicarbonitrile. Yield 12% (method A), 84% (method B), mp 42–43 °C. IR (mineral oil, cm−1): 2231, 2212 (C≡N); 1571 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.90 (t, J = 7.4 Hz, 3H, CH2CH3), 1.25–1.32 (m, 2H, CH2CH3), 1.54–1.60 (m, 2H, CH2), 1.74–1.80 (m, 4H, 2CH2), 2.69–2.74 (m, 4H, 2CH2), 3.18 (s, 3H, CH3N), 3.61 (t, J = 7.6 Hz, 2H, CH2N). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 19.3, 21.5, 21.6, 26.0, 29.1, 32.9, 38.0, 50.8, 87.0, 114.2, 116.7, 123.5, 125.7, 155.8, 162.4. MS (EI, 70 eV): m/z (%) 268 ([M]+, 20), 225 ([M-C3H7]+, 100). Anal: Calcd for C16H20N4: C, 71.61; H, 7.51; N, 20.88. Found: C, 71.22; H, 7.55; N, 20.96.
Compound 3c: 2-(Butyl(methyl)amino)-6-phenylpyridine-3,4-dicarbonitrile. Yield 20% (method A), 87% (method B), mp 69–70 °C. IR (mineral oil, cm−1): 2240, 2205 (C≡N); 1588 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.92 (t, J = 7.4 Hz, 3H, CH2CH3), 1.29–1.39 (m, 2H, CH2CH3), 1.60–1.68 (m, 2H, CH2), 3.31 (s, 3H, CH3N), 3.72 (t, J = 7.7 Hz, 2H, CH2N), 7.50–7.54 (m, 3H, Ph), 7.86 (s, 1H, CHpyr), 8.10–8.14 (m, 2H, Ph). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 19.4, 29.0, 38.2, 51.2, 87.4, 111.4, 115.5, 116.7, 127.3, 127.5, 128.9, 131.2, 135.9, 157.0, 158.5. MS (EI, 70 eV): m/z (%) 290 ([M]+, 29), 247 ([M-C3H7]+, 100). Anal: Calcd for C18H18N4: C, 74.46; H, 6.25; N, 19.30. Found: C, 74.62; H, 6.21; N, 19.11.
Compound 3d: 2-(Butyl(methyl)amino)-5-methyl-6-phenylpyridine-3,4-dicarbonitrile. Yield 19% (method A), 96% (method B), mp 126–127 °C. IR (mineral oil, cm−1): 2237, 2212 (C≡N); 1584 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.89 (t, J = 7.4 Hz, 3H, CH2CH3), 1.25–1.33 (m, 2H, CH2CH3), 1.58–1.64 (m, 2H, CH2), 2.35 (s, 3H, CH3), 3.25 (s, 3H, CH3N), 3.65 (t, J = 7.7 Hz, 2H, CH2N), 7.48–7.52 (m, 3H, Ph), 7.57–7.60 (m, 2H, Ph). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 17.4, 19.3, 29.1, 38.1, 51.0, 87.8, 114.8, 116.7, 121.8, 127.7, 128.2, 128.9, 129.5, 138.2, 155.7, 161.2. MS (EI, 70 eV): m/z (%) 304 ([M]+, 31), 261 ([M-C3H7]+, 100). Anal: Calcd for C19H20N4: C, 74.97; H, 6.62; N, 18.41. Found: C, 74.69; H, 6.66; N, 18.31.
Compound 3e: 2-(Butyl(methyl)amino)-6-(4-methoxyphenyl)-5-methylpyridine-3,4-dicarbonitrile. Yield 37% (method A), 79% (method B), mp 118–119 °C. IR (mineral oil, cm−1): 2229, 2210 (C≡N); 1582 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.90 (t, J = 7.4 Hz, 3H, CH2CH3), 1.26–1.34 (m, 2H, CH2CH3), 1.58–1.64 (m, 2H, CH2), 2.39 (s, 3H, CH3), 3.25 (s, 3H, CH3N), 3.66 (t, J = 7.6 Hz, 2H, CH2N), 3.82 (s, 3H, OCH3), 7.03–7.06 (m, 2H, C6H4), 7.58–7.61 (m, 2H, C6H4). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 17.7, 19.3, 29.1, 38.1, 51.0, 55.3, 87.0, 113.7, 114.9, 116.9, 121.7, 127.6, 130.4, 130.7, 155.7, 160.3, 160.7. MS (EI, 70 eV): m/z (%) 334 ([M]+, 39), 291 ([M-C3H7]+, 100). Anal: Calcd for C20H22N4O: C, 71.83; H, 6.63; N, 16.75. Found: C, 71.57; H, 6.65; N, 16.60.
Compound 3f: 2-(Butyl(methyl)amino)-5,6-dihydrobenzo[h]quinoline-3,4-dicarbonitrile. Yield 14% (method A), 92% (method B), mp 135–136 °C. IR (mineral oil, cm−1): 2233, 2203 (C≡N); 1584 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.92 (t, J = 7.4 Hz, 3H, CH2CH3), 1.30–1.38 (m, 2H, CH2CH3), 1.60–1.66 (m, 2H, CH2), 2.87–2.98 (m, 4H, 2CH2), 3.28 (s, 3H, CH3N), 3.70 (t, J = 7.6 Hz, 2H, CH2N), 7.29–7.48 (m, 3H, C6H4), 8.08 (d, J = 7.7 Hz, 1H, C6H4). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 19.4, 24.8, 26.4, 29.0, 38.2, 51.2, 87.1, 114.3, 117.0, 123.0, 125.1, 125.5, 127.2, 128.3, 131.4, 131.8, 139.5, 154.4, 156.5. MS (EI, 70 eV): m/z (%) 316 ([M]+, 32), 273 ([M-C3H7]+, 100). Anal: Calcd for C20H20N4: C, 75.92; H, 6.37; N, 17.71. Found: C, 75.54; H, 6.39; N, 17.64.
4.3. General Procedure for the Preparation of 2-(Dibutylamino)Pyridine-3,4-Dicarbonitriles 4
An appropriate 2-chloropyridine-3,4-dicarbonitrile 1 (0.01 mol) was suspended in i-PrOH (5 mL), and then N,N-dibutylamine (1.42 g, 0.011 mol) and DIPEA (1.42 g, 0.011 mol) were added. The reaction mixture was stirred at 60–70 °C for 24 h. After reaction completion (TLC monitoring), the mixture was cooled, the solvent was removed using a rotary evaporator, and the residue was extracted with ethyl acetate. The extract was dried with CaCl2 and then purified with column chromatography (ethyl acetate/hexane, 1/1 v/v). Product 4 was isolated after solvent evaporation.
Compound 4a: 2-(Dibutylamino)-5,6-dimethylpyridine-3,4-dicarbonitrile. Yield 58%, yellow oil. IR (thin film, cm−1): 2233, 2208 (C≡N); 1580 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.91 (t, J = 7.4 Hz, 6H, 2CH2CH3), 1.27–1.34 (m, 4H, 2CH2CH3), 1.54–1.60 (m, 4H, 2CH2CH2), 2.29 (s, 3H, CH3), 2.41 (s, 3H, CH3), 3.60 (t, J = 7.7 Hz, 4H, (CH2)2N). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 16.0, 19.2, 23.7, 29.6, 49.3, 85.4, 114.9, 117.0, 122.8, 125.6, 154.9, 162.6. MS (EI, 70 eV): m/z (%) 284 ([M]+, 32), 241 ([M-C3H7]+, 56), 199 (100), 185 (73). Anal: Calcd for C17H24N4: C, 71.79; H, 8.51; N, 19.70. Found: C, 71.88; H, 8.47; N, 19.64.
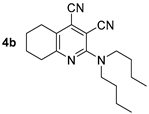
Compound 4b: 2-(Dibutylamino)-5,6,7,8-tetrahydroquinoline-3,4-dicarbonitrile. Yield 43%, mp 42–43 °C. IR (mineral oil, cm−1): 2232, 2203 (C≡N); 1572 (C=C). 1H NMR (500.13 MHz, DMSO-d6): 0.90 (t, J = 7.4 Hz, 6H, 2CH2CH3), 1.26–1.33 (m, 4H, 2CH2CH3), 1.53–1.59 (m, 4H, 2CH2), 1.72–1.80 (m, 4H, 2CH2), 2.67–2.74 (m, 4H, 2CH2), 3.57 (t, J = 7.7 Hz, 4H, (CH2)2N). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 19.2, 21.6, 21.6, 26.0, 29.6, 32.9, 49.3, 86.5, 114.2, 116.8, 123.2, 126.0, 154.7, 162.5. MS (EI, 70 eV): m/z (%) 310 ([M]+, 36), 267 ([M-C3H7]+, 66), 225 (100), 211 (74). Anal: Calcd for C19H26N4: C, 73.51; H, 8.44; N, 18.05. Found: C, 73.22; H, 8.49; N, 17.94.
Compound 4c: 2-(Dibutylamino)-6-phenylpyridine-3,4-dicarbonitrile. Yield 59%, mp 75–76 °C. IR (mineral oil, cm−1): 2240, 2202 (C≡N); 1587 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.92 (t, J = 7.4 Hz, 6H, 2CH2CH3), 1.31–1.38 (m, 4H, 2CH2CH3), 1.60–1.66 (m, 4H, 2CH2), 3.65 (t, J = 7.6 Hz, 4H, (CH2)2N), 7.48–7.53 (m, 3H, Ph), 7.83 (s, 1H, CHpyr), 8.07–8.11 (m, 2H, Ph). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 19.3, 29.6, 49.7, 86.9, 111.2, 115.5, 116.6, 127.2, 127.8, 128.9, 131.2, 136.0, 156.0, 158.6. MS (EI, 70 eV): m/z (%) 332 ([M]+, 51), 289 ([M-C3H7]+, 54), 247 (100), 233 (76). Anal: Calcd for C21H24N4: C, 75.87; H, 7.28; N, 16.85. Found: C, 75.51; H, 7.31; N, 16.80.
Compound 4d: 2-(Dibutylamino)-5-methyl-6-phenylpyridine-3,4-dicarbonitrile. Yield 77%, mp 77–78 °C. IR (mineral oil, cm−1): 2229, 2203 (C≡N); 1583 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.88 (t, J = 7.3 Hz, 6H, 2CH2CH3), 1.26–1.34 (m, 4H, 2CH2CH3), 1.57–1.64 (m, 4H, 2CH2), 2.34 (s, 3H, CH3), 3.61 (t, J = 7.6 Hz, 4H, (CH2)2N), 7.48–7.51 (m, 3H, Ph), 7.56–7.59 (m, 2H, Ph). 13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 17.4, 19.3, 29.6, 49.5, 87.3, 114.9, 116.8, 121.9, 128.0, 128.2, 128.8, 129.5, 138.2, 154.7, 161.3. MS (EI, 70 eV): m/z (%) 346 ([M]+, 50), 303 ([M-C3H7]+, 47), 261 (100), 247 (77). Anal: Calcd for C22H26N4: C, 76.27; H, 7.56; N, 16.17. Found: C, 76.44; H, 7.53; N, 16.08.
Compound 4e: 2-(Dibutylamino)-6-(4-methoxyphenyl)-5-methylpyridine-3,4-dicarbonitrile. Yield 61%, mp 76–77 °C. IR (mineral oil, cm−1): 2229, 2207 (C≡N); 1580 (C=C). 1H NMR (500.13 MHz, DMSO-d6): 0.90 (t, J = 7.4 Hz, 6H, 2CH2CH3), 1.26–1.36 (m, 4H, 2CH2CH3), 1.57–1.64 (m, 4H, 2CH2), 2.38 (s, 3H, CH3), 3.57–3.64 (m, 4H, (CH2)2N), 3.82 (s, 3H, OCH3), 7.02–7.06 (m, 2H, C6H4), 7.56–7.60 (m, 2H, C6H4).13C NMR (125.76 MHz, DMSO-d6): δ 13.7, 17.7, 19.3, 29.7, 49.5, 55.3, 86.5, 113.6, 115.0, 116.9, 121.4, 127.9, 130.4, 130.7, 154.6, 160.3, 160.8. MS (EI, 70 eV): m/z (%) 376 ([M]+, 46), 333 ([M-C3H7]+, 40), 291 (100), 277 (68). Anal: Calcd for C23H28N4O: C, 73.37; H, 7.50; N, 14.88. Found: C, 73.11; H, 7.54; N, 14.79.
Compound 4f: 2-(Dibutylamino)-5,6-dihydrobenzo[h]quinoline-3,4-dicarbonitrile. Yield 64%, mp 89–90 °C. IR (mineral oil, cm−1): 2238, 2201 (C≡N); 1584 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.93 (t, J = 7.4 Hz, 6H, 2CH2CH3), 1.31–1.41 (m, 4H, 2CH2CH3), 1.60–1.68 (m, 4H, 2CH2), 2.92–2.98 (m, 4H, 2CH2), 3.64–3.71 (m, 4H, (CH2)2N), 7.32–7.48 (m, 3H, C6H4), 8.07 (m, 1H, C6H4). 13C NMR (125.76 MHz, DMSO-d6): δ 13.8, 19.4, 24.9, 26.5, 29.7, 49.8, 86.7, 114.4, 117.0, 122.9, 125.3, 125.4, 127.3, 128.4, 131.4, 131.9, 139.7, 154.6, 155.5. MS (EI, 70 eV): m/z (%) 358 ([M]+, 58), 315 ([M-C3H7]+, 65), 273 (100), 259 (76). Anal: Calcd for C23H26N4: C, 77.06; H, 7.31; N, 15.63. Found: C, 76.87; H, 7.35; N, 15.57.
4.4. General Procedure for the Preparation of 2-(Diisobutylamino)Pyridine-3,4-Dicarbonitriles 5
An appropriate 2-chloropyridine-3,4-dicarbonitrile 1 (0.01 mol) was suspended in i-PrOH (5 mL), and then N,N-diisobutylamine (1.42 g, 0.011 mol) and DIPEA (1.42 g, 0.011 mol) were added. The reaction mixture was stirred at 60–70 °C for 24 h. After reaction completion (TLC monitoring), the mixture was cooled, and the precipitated product was filtered off and washed with ice-cold water and i-PrOH. The resulting product 5 was crystallized from i-PrOH, and then dried in a vacuum desiccator over CaCl2.
Compound 5a: 2-(Diisobutylamino)-6-phenylpyridine-3,4-dicarbonitrile. Yield 48%, mp 180–181 °C. IR (mineral oil, cm−1): 2242, 2215 (C≡N); 1586 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.91 (d, J = 6.6 Hz, 12H, 4CH3), 2.06–2.16 (m, 2H, 2CH(CH3)2), 3.67 (d, J = 7.4 Hz, 4H, (CH2)2N), 7.51–7.57 (m, 3H, Ph), 7.93 (s, 1H, CH), 8.11–8.16 (m, 2H, Ph). 13C NMR (125.76 MHz, DMSO-d6): δ 19.5, 26.8, 58.4, 87.9, 111.6, 115.5, 116.8, 127.3, 127.9, 129.1, 131.3, 136.0, 156.4, 158.6. MS (EI, 70 eV): m/z (%) 332 ([M]+, 16), 289 ([M-C3H7]+, 77), 233 (100). Anal: Calcd for C21H24N4: C, 75.87; H, 7.28; N, 16.85. Found: C, 75.66; H, 7.32; N, 16.80.
Compound 5b: 2-(Diisobutylamino)-5-methyl-6-phenylpyridine-3,4-dicarbonitrile. Yield 56%, mp 105–106 °C. IR (mineral oil, cm−1): 2207 (C≡N); 1582 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.86 (d, J = 6.6 Hz, 12H, 4CH3), 1.99–2.10 (m, 2H, 2CH(CH3)2), 2.36 (s, 3H, CH3), 3.57 (d, J = 7.4 Hz, 4H, (CH2)2N), 7.49–7.53 (m, 3H, Ph), 7.57–7.62 (m, 2H, Ph). 13C NMR (125.76 MHz, DMSO-d6): δ 17.5, 19.5, 26.7, 58.0, 88.4, 114.8, 116.8, 122.0, 128.0, 128.3, 128.8, 129.5, 138.2, 155.0, 161.3. MS (EI, 70 eV): m/z (%) 346 ([M]+, 14), 303 ([M-C3H7]+, 66), 247 (100). Anal: Calcd for C22H26N4: C, 76.27; H, 7.56; N, 16.17. Found: C, 76.04; H, 7.58; N, 16.09.
4.5. General Procedure for the Preparation of 2-(Butylamino)-5-Methyl-6-Phenylpyridine-3,4-Dicarbonitriles 6
The compound 2-(Butylamino)-5-methyl-6-phenylpyridine-3,4-dicarbonitrile 2d (2.9 g, 0.01 mol) was dissolved in dry DMF (10 mL), and then NaH (0.52 g, 0.013 mol, 60% in mineral oil) and bromoethane (1.64 g, 0.015 mol, for 6a) or bromopropane (1.85 g, 0.015 mol, for 6b) were added. The reaction mixture was stirred at room temperature for 24 h. After reaction completion (TLC monitoring), the mixture was diluted with water (50 mL) and neutralized by acetic acid. The precipitated solid was filtered off, washed with ice-cold water, and crystallized from i-PrOH, and then dried in a vacuum desiccator over CaCl2.
Compound 6a: 2-(Butyl(ethyl)amino)-5-methyl-6-phenylpyridine-3,4-dicarbonitrile. Yield 36%, mp 116–117 °C. IR (mineral oil, cm−1): 2235, 2209 (C≡N); 1582 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.90 (t, J = 7.4 Hz, 3H, CH2CH3), 1.21 (t, J = 6.9 Hz, 3H, CH2CH3), 1.29–1.36 (m, 2H, CH2CH3), 1.60–1.66 (m, 2H, CH2), 2.35 (s, 3H, CH3), 3.59–3.64 (m, 2H, CH2N), 3.69 (q, J = 7.0 Hz, 2H, CH2N), 7.49–7.53 (m, 3H, Ph), 7.57–7.61 (m, 2H, Ph). 13C NMR (125.76 MHz, DMSO-d6): δ 13.9, 14.3, 18.1, 20.0, 30.4, 45.1, 49.5, 87.9, 115.5, 117.4, 122.2, 128.6, 128.9, 129.4, 130.1, 138.8, 155.3, 162.0. MS (EI, 70 eV): m/z (%) 318 ([M]+, 40), 275 ([M-C3H7]+, 100), 261 (25), 247 (84). Anal: Calcd for C20H22N4: C, 75.44; H, 6.96; N, 17.60. Found: C, 75.21; H, 7.00; N, 17.55.
Compound 6b: 2-(Butyl(propyl)amino)-5-methyl-6-phenylpyridine-3,4-dicarbonitrile. Yield 29%, mp 78–79 °C. IR (mineral oil, cm−1): 2231, 2203 (C≡N); 1581 (C=C). 1H NMR (500.13 MHz, DMSO-d6): δ 0.85-0.90 (m, 6H, 2CH2CH3), 1.25–1.35 (m, 2H, CH2CH3), 1.56–1.69 (m, 4H, 2CH2), 2.35 (s, 3H, CH3), 3.51–3.67 (m, 4H, 2CH2N), 7.48–7.52 (m, 3H, Ph), 7.55–7.60 (m, 2H, Ph). 13C NMR (125.76 MHz, DMSO-d6): δ 10.6, 13.7, 17.4, 19.3, 20.9, 29.6, 49.5, 51.2, 87.3, 114.8, 116.7, 121.6, 128.0, 128.2, 128.8, 129.4, 138.2, 154.7, 161.3. MS (EI, 70 eV): m/z (%) 332 ([M]+, 40), 289 ([M-C3H7]+, 59), 261 (55), 247 (100). Anal: Calcd for C21H24N4: C, 75.87; H, 7.28; N, 16.85. Found: C, 75.63; H, 7.30; N, 16.78.
5. Conclusions
Thus, we have developed a facile approach to the synthesis of novel 2-(butylamino)pyridine-3,4-dicarbonitrile derivatives and have investigated their absorption, fluorescence, and solvatochromic properties. The synthesized compounds showed a unique property to be efficiently fluorescent both in solution and in solid state (dual-state emission). The highest fluorescence of the synthesized compounds was observed in nonpolar media with a quantum yield up to 63%. The strongest photoluminescence was noted for the butylaminocinchomeronic dinitrile derivative. The introduction of an additional substituent to the amino nitrogen atom led to the decrease in emissions in a row of N-substituted methyl, ethyl, propyl and butyl derivatives. All the 2-(butylamino)pyridine-3,4-dicarbonitrile derivatives also showed solid-state emissions from the blue to green regions of the spectra 478–564 nm.
Supplementary Materials
Copies of the NMR spectra for compounds 2—6 can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217144/s1.
Author Contributions
Conceptualization, O.V.E.; investigation, K.V.L., M.Y.I. and A.I.E.; data analysis, K.V.L. and M.Y.I.; writing—original draft preparation, O.V.E. and M.Y.I.; writing—review and editing, K.V.L., M.Y.I. and A.I.E.; supervision, O.V.E.; project administration, O.V.E.; funding acquisition, O.V.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed in the framework of state assignment of the Ministry of Science and Higher Education of the Russian Federation, project no. 0849–2020-0003.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of all the reported compounds are available from the authors.
References
- Belmonte-Vázquez, J.L.; Amador-Sánchez, Y.A.; Rodríguez-Cortés, L.A.; Rodríguez-Molina, B. Dual-State Emission (DSE) in Organic Fluorophores: Design and Applications. Chem. Mater. 2021, 33, 7160–7184. [Google Scholar] [CrossRef]
- Behera, S.K.; Park, S.Y.; Gierschner, J. Dual Emission: Classes, Mechanisms, and Conditions. Angew. Chemie Int. Ed. 2021, 60, 22624–22638. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cortés, L.A.; Navarro-Huerta, A.; Rodríguez-Molina, B. One Molecule to Light It All: The Era of Dual-State Emission. Matter 2021, 4, 2622–2624. [Google Scholar] [CrossRef]
- Stoerkler, T.; Pariat, T.; Laurent, A.D.; Jacquemin, D.; Ulrich, G.; Massue, J. Excited-State Intramolecular Proton Transfer Dyes with Dual-State Emission Properties: Concept, Examples and Applications. Molecules 2022, 27, 2443. [Google Scholar] [CrossRef]
- Förster, T.; Kasper, K. Ein Konzentrationsumschlag Der Fluoreszenz Des Pyrens. Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für Phys. Chemie 1955, 59, 976–980. [Google Scholar] [CrossRef]
- Qi, J.; Hu, X.; Dong, X.; Lu, Y.; Lu, H.; Zhao, W.; Wu, W. Towards More Accurate Bioimaging of Drug Nanocarriers: Turning Aggregation-Caused Quenching into a Useful Tool. Adv. Drug Deliv. Rev. 2019, 143, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Suman, G.R.; Pandey, M.; Chakravarthy, A.S.J. Review on New Horizons of Aggregation Induced Emission: From Design to Development. Mater. Chem. Front. 2021, 5, 1541–1584. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chemie Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Wang, X.; Kong, L.; Yang, J. Indolo[3,2-b]Carbazole Derivatives with High Fluorescent Emission Both in Solution and Aggregated States and Mechanical-Induced Emission Enhancement Characteristic. Dye. Pigment. 2021, 188, 109230. [Google Scholar] [CrossRef]
- Hou, R.; Zhao, B.; Xia, Y.; Li, D. Organic Fluorescent Compounds that Display Efficient Aggregation-Induced Emission Enhancement and Intramolecular Charge Transfer. Molecules 2018, 23, 1446. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, L.; Dang, D.; Zhi, Y.; Wang, X.; Meng, L. A Strategy of “Self-Isolated Enhanced Emission” to Achieve Highly Emissive Dual-State Emission for Organic Luminescent Materials. Chem.-A Eur. J. 2018, 24, 10383–10389. [Google Scholar] [CrossRef]
- Xi, W.; Yu, J.; Wei, M.; Qiu, Q.; Xu, P.; Qian, Z.; Feng, H. Photophysical Switching between Aggregation-Induced Phosphorescence and Dual-State Emission by Isomeric Substitution. Chem.-A Eur. J. 2020, 26, 3733–3737. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishna, P.; Iyer, P.K. Monosubstituted Dibenzofulvene-Based Luminogens: Aggregation-Induced Emission Enhancement and Dual-State Emission. J. Phys. Chem. C 2016, 120, 26556–26568. [Google Scholar] [CrossRef]
- Yu, F.; Yan, Q.; Liang, K.; Cong, Z.; Shao, Q.; Wang, Y.; Hong, L.; Jiang, L.; Ye, G.; Wang, H.; et al. Dual-State Emission and Solvatofluorochromism Properties of Facile Squaraine Dyes with Cis-3,5-Dimethylpiperidine. J. Lumin. 2021, 233, 117882. [Google Scholar] [CrossRef]
- Singh, V.D.; Kushwaha, A.K.; Singh, R.S. Achieving Flexibility/Rigidity Balance through Asymmetric Donor−Acceptor Scaffolds in Pursuit of Dual State Emission with Application in Acidochromism. Dye. Pigment. 2021, 187, 109117. [Google Scholar] [CrossRef]
- Singh, D.K.; Jang, K.; Kim, J.; Lee, J.; Kim, I. Intramolecular Electrophilic Cyclization Approach to 6-Substituted Naphtho[2,1-b]Benzofurans: Novel Dual-State Emissive Fluorophores with Blue Emission. ACS Comb. Sci. 2019, 21, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Narita, F.; Yokobori, H.; Iiduka, N.; Sugaya, T.; Nagasawa, A.; Fujihara, T. Substituent Effect on Emission of Flavonolate-Boron Difluoride Complexes: The Role of π-System for Dual-State (Solution and Solid) Emission. Dye. Pigment. 2021, 187, 109081. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Bryce, M.R. Dual Emission in Purely Organic Materials for Optoelectronic Applications. Mater. Horizons 2021, 8, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Xu, Y.; Xu, R.; Wang, Z.; Liu, D.; Shen, Q.; Yue, L.; Dang, D.; Meng, L. A Facilely Synthesized Dual-State Emission Platform for Picric Acid Detection and Latent Fingerprint Visualization. Chem.-A Eur. J. 2020, 26, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H.; Bu, X.; Fu, Y.; Wang, Z.; Liu, Q. Recent Advances in Dual-Emission Ratiometric Fluorescence Probes for Chemo/Biosensing and Bioimaging of Biomarkers. Coord. Chem. Rev. 2019, 383, 82–103. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small Molecule-Based Ratiometric Fluorescence Probes for Cations, Anions, and Biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhang, S.; He, X.; Huang, J.; Kong, L.; Yang, J.; Yang, J. Dual-State Emission Difluoroboron Derivatives for Selective Detection of Picric Acid and Reversible Acid/Base Fluorescence Switching. Anal. Methods 2021, 13, 2830–2835. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, Y.; Huang, J.; Kong, L.; Yang, J. High Dual-State Blue Emission of a Functionalized Pyrazoline Derivative for Picric Acid Detection. CrystEngComm 2021, 23, 221–226. [Google Scholar] [CrossRef]
- Cao, D.; Zhu, L.; Liu, Z.; Lin, W. Through Bond Energy Transfer (TBET)-Based Fluorescent Chemosensors. J. Photochem. Photobiol. C Photochem. Rev. 2020, 44, 100371. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, H.; Li, Y.; Xia, G.; Wang, H. D–A-Type Fluorophores with Efficient Dual-State Emission for Imaging at Ultralow Concentration. Mater. Chem. Front. 2022, 6, 155–162. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, L.; Xu, C.; Ren, T.-B.; Xu, S.; Lou, X.; Yuan, L.; Zhang, X.-B. An Integration Strategy to Develop Dual-State Luminophores with Tunable Spectra, Large Stokes Shift, and Activatable Fluorescence for High-Contrast Imaging. CCS Chem. 2022, 4, 2153–2164. [Google Scholar] [CrossRef]
- Zhang, G.; Palmer, G.M.; Dewhirst, M.W.; Fraser, C.L. A Dual-Emissive-Materials Design Concept Enables Tumour Hypoxia Imaging. Nat. Mater. 2009, 8, 747–751. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Q.; Tian, H. Photochromic Materials: More Than Meets The Eye. Adv. Mater. 2013, 25, 378–399. [Google Scholar] [CrossRef]
- Wöll, D.; Flors, C. Super-Resolution Fluorescence Imaging for Materials Science. Small Methods 2017, 1, 1700191. [Google Scholar] [CrossRef]
- Gierschner, J.; Varghese, S.; Park, S.Y. Organic Single Crystal Lasers: A Materials View. Adv. Opt. Mater. 2016, 4, 348–364. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, P.; Kumar, P.; Srivastava, R.; Pal, S.K.; Ghosh, S. Exploring an Emissive Charge Transfer Process in Zero-Twist Donor–Acceptor Molecular Design as a Dual-State Emitter. J. Phys. Chem. C 2016, 120, 12723–12733. [Google Scholar] [CrossRef]
- Wong, M.Y.; Zysman-Colman, E. Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M.P. Recent Advances in Organic Thermally Activated Delayed Fluorescence Materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef]
- Chen, X.-K.; Kim, D.; Brédas, J.-L. Thermally Activated Delayed Fluorescence (TADF) Path toward Efficient Electroluminescence in Purely Organic Materials: Molecular Level Insight. Acc. Chem. Res. 2018, 51, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Reineke, S.; Seidler, N.; Yost, S.R.; Prins, F.; Tisdale, W.A.; Baldo, M.A. Highly Efficient, Dual State Emission from an Organic Semiconductor. Appl. Phys. Lett. 2013, 103, 093302. [Google Scholar] [CrossRef]
- Bhalekar, S.; Kothavale, S.; Sekar, N. Yellow-Red Emitting, Methoxy Substituted Triphenylamine-Based Styryl Derivatives: Synthesis, Photophysical Properties, Viscosity Sensitivity, Aggregation Induced Emission, NLO Properties, and DFT Study. J. Photochem. Photobiol. A Chem. 2019, 384, 112027. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, L.; Li, Y.; Zhang, Y.; Li, T.; Qin, Y.; Song, Y.; Hou, H.; Li, K. Aggregation/Viscosity-Induced Emission and Third-Order Nonlinear Optical Signal Inversion in a TICT System. J. Phys. Chem. C 2020, 124, 22684–22691. [Google Scholar] [CrossRef]
- Ershov, O.V.; Shishlikova, M.A.; Ievlev, M.Y.; Belikov, M.Y.; Maksimova, V.N. DIPEA Catalyzed Step-by-Step Synthesis and Photophysical Properties of Thieno[2,3-b]Pyridine Derivatives. Tetrahedron 2019, 75, 130465. [Google Scholar] [CrossRef]
- Ershov, O.V.; Ievlev, M.Y.; Belikov, M.Y.; Naidenova, A.I.; Maksimova, V.N.; Tafeenko, V.A. Synthesis, Solution and Solid-State Fluorescence of 2-Diethylaminocinchomeronic Dinitrile Derivatives. RSC Adv. 2017, 7, 34886–34891. [Google Scholar] [CrossRef]
- Ershova, A.I.; Ievlev, M.Y.; Maksimova, V.N.; Belikov, M.Y.; Ershov, O.V. Synthesis, Solution and Solid-State Fluorescence of 2-(N -cycloamino)Cinchomeronic Dinitrile Derivatives. ChemistrySelect 2020, 5, 7243–7248. [Google Scholar] [CrossRef]
- Chunikhin, S.S.; Ershov, O.V.; Ievlev, M.Y.; Belikov, M.Y.; Tafeenko, V.A. Novel Chromophores of Cyanopyridine Series with Strong Solvatochromism and Near-Infrared Solid-State Fluorescence. Dye. Pigment. 2018, 156, 357–368. [Google Scholar] [CrossRef]
- Ershov, O.V.; Chunikhin, S.S.; Ievlev, M.Y.; Belikov, M.Y.; Tafeenko, V.A. Crystallographic Characterization of Ethylammonium Salts of Tetracyanopyridine (TCPy) and Fluorescence Determination of the Degree of Substitution of the Amino Nitrogen Atom Thereof. CrystEngComm 2019, 21, 5500–5507. [Google Scholar] [CrossRef]
- Dang, D.; Wang, X.; Wang, D.; Yang, Z.; Hao, D.; Xu, Y.; Zhang, S.; Meng, L. Fluorescent Organic Nanoparticles Constructed by a Facile “Self-Isolation Enhanced Emission” Strategy for Cell Imaging. ACS Appl. Nano Mater. 2018, 1, 2324–2331. [Google Scholar] [CrossRef]
- Ershov, O.V.; Ievlev, M.Y.; Belikov, M.Y.; Lipin, K.V.; Naydenova, A.I.; Tafeenko, V.A. Synthesis and Solid-State Fluorescence of Aryl Substituted 2-Halogenocinchomeronic Dinitriles. RSC Adv. 2016, 6, 82227–82232. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Wrighton, M.S.; Ginley, D.S.; Morse, D.L. Technique for the determination of absolute emission quantum yields of powdered samples. J. Phys. Chem. 1974, 78, 2229–2233. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).