Abstract
Tungsten trioxide (WO3) is mainly studied as an electrochromic material and received attention due to N-type oxide-based semiconductors. The magnetic, structural, and optical behavior of pristine WO3 and gadolinium (Gd)-doped WO3 are being investigated using density functional theory. For exchange-correlation potential energy, generalized gradient approximation (GGA+U) is used in our calculations, where U is the Hubbard potential. The estimated bandgap of pure WO3 is 2.5 eV. After the doping of Gd, some states cross the Fermi level, and WO3 acts as a degenerate semiconductor with a 2 eV bandgap. Spin-polarized calculations show that the system is antiferromagnetic in its ground state. The WO3 material is a semiconductor, as there is a bandgap of 2.5 eV between the valence and conduction bands. The Gd-doped WO3’s band structure shows few states across the Fermi level, which means that the material is metal or semimetal. After the doping of Gd, WO3 becomes the degenerate semiconductor with a bandgap of 2 eV. The energy difference between ferromagnetic (FM) and antiferromagnetic (AFM) configurations is negative, so the Gd-doped WO3 system is AFM. The pure WO3 is nonmagnetic, where the magnetic moment in the system after doping Gd is 9.5599575 μB.
1. Introduction
The exclusive ability to induce bistable electrical and optical characteristics in WO3 with different excitation sources makes it very promising among significant technological devices [1,2,3,4]. The element that has three oxygen atoms is called the perovskite-like structure. WO3 has useful applications in optical and spintronic devices [5,6,7]. It is used for the construction of semiconductor-based gas sensors (SGS) and electrochromic devices such as high-temperature superconductors (HTS), smart windows, solar cells, and water-splitting applications. The addition of electrons/holes could alter the characteristics of WO3. Additionally, the possibility of ion intercalation/deintercalation arises in several possible applications in rechargeable batteries. Especially, the photocatalytic activities and SGS properties of WO3 can be modified by doping transitional elements such as Au, Pd, and Pt. Rare earth and transition metal-doped WO3 systems show interesting magnetic properties that usually do not exist in undoped WO3 [8,9,10]. In this study, the magnetic, electronic, and optical properties of Gd-doped WO3 are investigated by using first principle calculations. Here is a brief description of previous works related to WO3. The monoclinic WO3 is the most communal and stable phase of WO3 with space group P21/n. The unit cell comprises 8W atoms and 24O atoms and holds 8O atoms at the corner in somewhat distorted cubic arrangements [11,12].
For the RT monoclinic, the direct bandgap calculated by using generalized gradient approximation (GGA) was increased initially as the volume decreased but, after that, decreases again with a further decrease in the volume. The cubic structure space groups Pm-3m contain basic structural characteristics but ignore distortion. The indirect bandgap of the cubic is smaller than the bandgap of the RT monoclinic. Low-temperature (LT) monoclinic structures with space groups Pc are other distorted forms of WO3. The unit cell of LT monoclinic contains 4W atoms and 12O atoms. The bandgap at a low-temperature (LT) monoclinic is, to some extent, greater than the RT monoclinic. This shows a direct bandgap with the conduction band minimum (CBM) and valence band maximum (VBM). The triclinic structures have space groups P-1 and contain 8W and 24O atoms. The bandgap of the triclinic is a direct bandgap. The orthorhombic structure of WO3 with a space group of Pmnb also has distorted oxygen-octahedrons [13,14]. The unit cell consists of 8W and 24O atoms. The bandgap is larger than the cubic and tetragonal ones but smaller than the monoclinic and triclinic structures [15].
The current studies showed that the assimilation of Gd3+ ion and other rare earth element ions in large bandgap semiconductor fallouts in boosting ferromagnetic properties inspired scientists on the way to rare earth elemental ion doping in various oxide nanomaterials for spintronics applications. Especially, the Gd3+ ion has more potential due to its optical and magnetic properties [16]. It was reported that WO3 is translucent in visible light, but strong absorption arises in near-infrared regions because of the phonon–electron interaction [17]. The reflectance and transmittance of WO3 material were noted in the range 400–2600 nm at Ptot = 10 and 30 mTorr. The dielectric function of the monoclinic and triclinic are comparable and cannot be separated by their dispersion relation. The optical gap is 2.5 to 2.6 eV, which is less than that of TiO2, and absorbs adequate visible radiations to produce a photocurrent. According to UV–Vis diffuse reflectance, WO3, bare light absorbs at a wavelength of less than 460 nm, which gives an energy bandgap of 2.6 eV. In Gd-WO3, 4% absorption significantly transfers towards a longer wavelength from 460 to 470 nm along a bandgap of 2.64 eV. This perception is associated with the reality that hybridization occurs between O 2p and Gd 4f/5d orbitals alternatively and is incorporated into the WO3 lattice [18].
Bullet, Stashans, and Lunnel observed the effect of the interaction of alkali ions on the cubic, room temperature monoclinic and Perovskite structure of WO3 [19]. The WO3 electronic structure and sub-stoichiometry are informally connected to the properties of the structure. O 2p orbitals are present in the valence band, and W 5d orbitals are present in the conduction band. The phase transition outcome in the W 5d states causes changes in the energy gap. They concluded that the upward shift in the W 5d states changes the ideal cubic structure to a monoclinic structure and increases Eg from 1.5 to 2.45 eV [20].
The bandgap value of WO3 changes experimentally from 2.6 to 3.2 eV. This is due to the changes in the structure of WO3 [21]. The significance of the surface area and the interphase boundary is of great importance [22,23,24,25,26,27]. The insufficient oxygen WO3-x is connected to the effect on the electrical properties and color, and the color changes from greenish to yellowish in WO3. In nanostructure WO3 films processed by reactive sputtering, Eg has linked to the O2 sputtering pressure and the vacancy of the O concentration. For the various phases of WO3 alternations in Eg with d orbital occupancy, the points of the VBM and CBM have been recognized. The calculation of cubic WO3 with the density functional theory (DFT) computations underreported at about 0.6 eV correlated with the value of the experimental value of 2.6 eV. In the dispersion of the band close to the region Nd–gap, the γ-WO3, δ-WO3, β-WO3, and ε-WO3 phases are observed, which are less by the initial calculations, which is due to the small difference in the lattice constant and short difference in the bond angle [17,28].
Even though only insufficient theoretical/experimental research has been done to discover the electronic/magnetic properties of RT monoclinic WO3, it is needed to explore the bandgap, energy band, and electron/hole reshuffle. Momentum density studies of WO3 have not been conducted up to now. WO3 exists in more than one crystalline form. The most common structure of WO3 is cubic, as for ReO3 [29,30,31]. The crystal structure of WO3 depends on the temperature when the temperature rises above 770 °C, its crystal shape is tetragonal; between 330 °C and 740 °C, it is orthorhombic; between 17 °C and 33 °C, it is monoclinic; and between −50 °C and 17 °C, it is triclinic [32,33,34]. Momentum density studies of WO3 have been not conducted to date. Even though only insufficient theoretical/experimental research has been done to discover the electronic/magnetic properties of RT monoclinic WO3, it is needed to explore bandgap, energy band, and electron/hole reshuffle. In the present work, the electronic, magnetic, and optical properties of Gd-doped WO3 are investigated by using first principle calculations. GGA+U approximation is applied to the orthorhombic structure to calculate the density of the states and optical and magnetic properties of WO3.
2. Simulation and Calculations
The full potential linear augmented plane wave (FPLAPW) method with the WEIN2k code is used to calculate the optical, electrical, and magnetic properties of pure WO3 and Gd-doped WO3 [35]. The lattice parameters of pure WO3 are: a = 7.303, b = 7.5389, and c = 7.6896 A, and those of Gd-doped WO3 are a = 7.303, b = 7.5389, and c = 15.3724 A. The space group number of WO3 is 14P21/n, and K-mesh points 21 × 21 × 10 were used. The electronic charge density “ECD” expanded up to Gmax = 12. In the interstitial region “IR”, the plane wave “PW” cut-off value is Kmax = 6.5/RMT. The size of the supercell is 21 × 21 × 10, and it contains 16 atoms. The optical and magnetic properties of the orthorhombic-like structure WO3 are calculated using GGA+U approximation. The element that has three oxygen atoms is called the perovskite-like structure. The orthorhombic structure is shown in Figure 1.
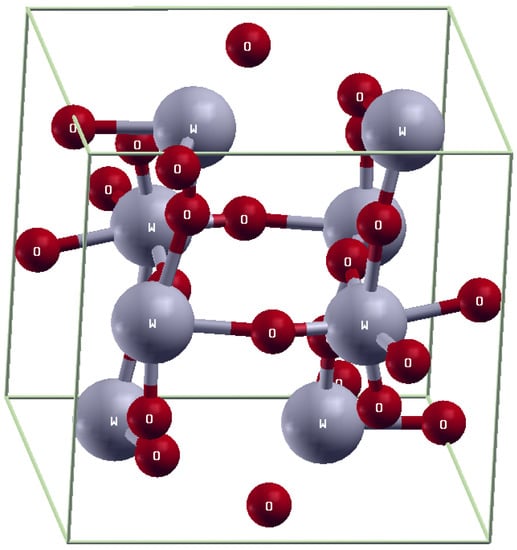
Figure 1.
Orthorhombic structure of WO3.
3. Results and Discussion
Figure 2 represents the total density of the states of WO3 before and after the doping of Gd, a rare earth metal. There is no absolute energy taken along the x-axis but the Fermi energy. Fermi energy is an approach in quantum mechanics that usually mentions the highest filled states of single particles in a quantum system of noninteracting fermions at absolute zero temperature. In Figure 2a, no states reside in the Fermi level for pure WO3. In Figure 2b, after the doping of Gd, some states reside in the Fermi level when the system is ferromagnetic (FM), which means that all the unpaired electrons have the same spin directions. In Figure 2c, some states reside in the Fermi level after the doping of Gd when the system has antiferromagnetic materials (AFM), which means that all the unpaired electrons have a spin moment in the antiparallel direction.
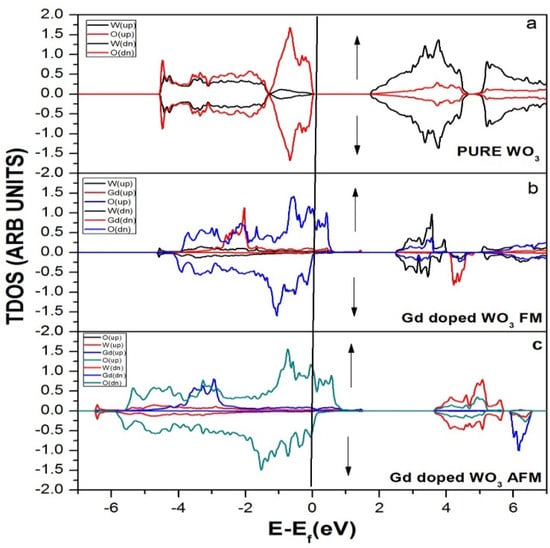
Figure 2.
Density of the states (DOS) of (a) pure WO3, (b) Gd-doped WO3 FM, and (c) Gd-doped WO3 AFM by using GGA+U approximation.
Figure 3 represents the partial density of the states. GGA+U approximation is used for the calculation of Gd-doped WO3. For calculating the partial density of the states, W has the states of s, p, d, and f, and O has s and p, while Gd has the s, p, d, and f states. Many clear states reside in the bandgap after the doping of the Gd rare earth metal. In the case of pure WO3, no states exist in the bandgap, as shown in Figure 3a, but after the doping of Gd, some states reside that cross the Fermi level, as shown in Figure 3b,c. Both calculations are done by using the GGA+U approximations. According to Figure 4, the material is a semiconductor, as there is a bandgap of 2.5 eV between the valence band and conduction band [36]. Figure 4 represents the band structure of pure WO3, which shows the direct bandgap. Figure 4a is the spin-up band, and Figure 4b is the spin-down direction.
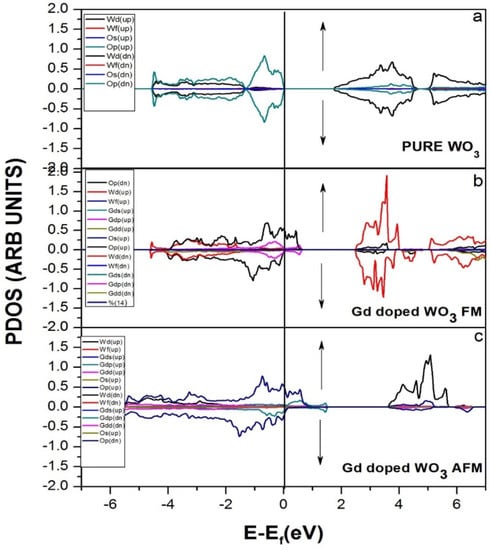
Figure 3.
Projected DOS (PDOS) of (a) pure WO3, (b) Gd-doped WO3 FM, and (c) Gd-doped WO3 AFM by using GGA+U approximation.
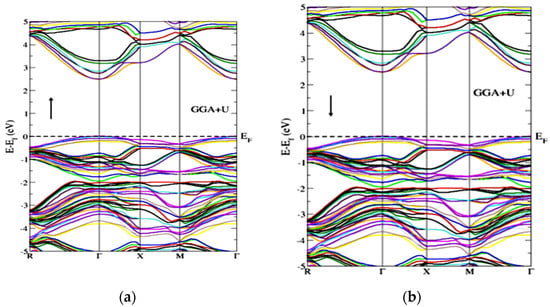
Figure 4.
Band structure of pure WO3 for the (a) spin-up and (b) spin-down directions by using GGA+U approximation.
Figure 5 represents the band structure of Gd-doped WO3 for spin-up and spin-down in the FM configuration. It shows that few states cross the Fermi level, which means that the material is metal or semimetal. Figure 5 represents the gap inside the conduction band. From Figure 5, after the doping of Gd, WO3 becomes the degenerate semiconductor. The degenerate semiconductor shows a metallic character after high doping.
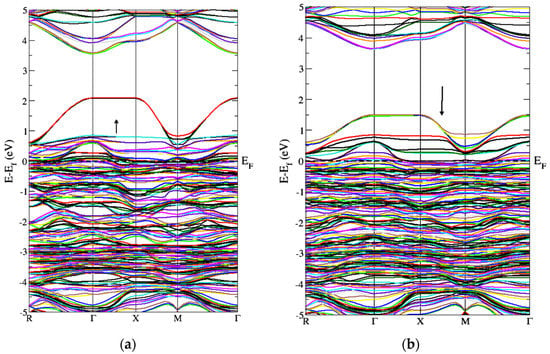
Figure 5.
Band structure of Gd-doped WO3 for FM calculations (a) spin-up and (b) spin-down.
Figure 6 represents the band structure of the Gd-doped WO3 by AFM calculations. Figure 6 is for the spin-up and spin-down directions after WO3 doping by Gd. As the states lie inside the Fermi level, it shows a metallic character after the doping of the Gd metal. Figure 6 also shows that, after the doping of Gd, the WO3 semiconductor becomes a degenerate semiconductor. As the energy difference between the FM and AFM configurations is negative, the Gd-doped WO3 system is AFM, according to the calculations, and the energy is calculated in millielectron volts. The pure WO3 is nonmagnetic, where the magnetic moment in the system after doping Gd is 9.5599575 μB (Table 1).
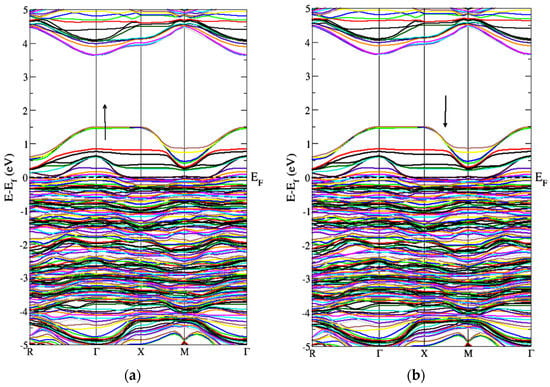
Figure 6.
Band structure of Gd-doped WO3 for AFM calculations (a) spin-up and (b) spin-down.

Table 1.
Magnetic properties of Gd-doped WO3.
Figure 7 represents the absorption coefficient of WO3 and Gd-doped WO3. Figure 7a indicates that, in pure WO3, the absorption increases gradually at energy 2 eV, which is the threshold energy for absorption and is equivalent to the bandgap energy. However, after the doping of the Gd rare earth element, there is a clear difference in the absorption of WO3, as the curve starts rising from 0 eV, as shown in Figure 7b,c, which means that the material becomes metallic or semi-metallic.
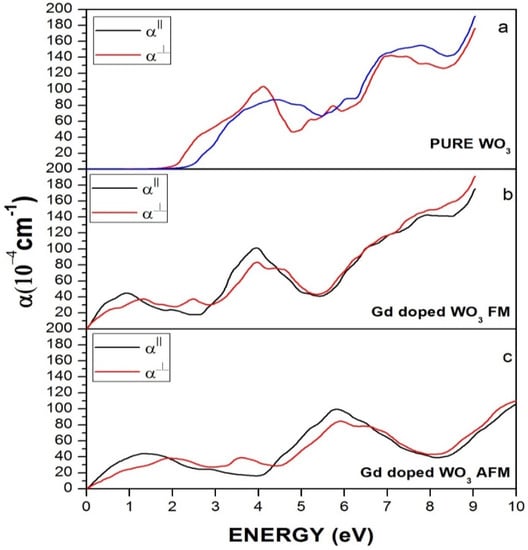
Figure 7.
Absorption of (a) pure WO3, (b) Gd-doped WO3 for FM, and (c) Gd-doped WO3 for the AFM configuration.
Figure 8 represents the real part of the dielectric function for pure and doped WO3 in FM and AFM configurations. In Figure 8a, the perpendicular component lies in the negative region in the energy range 6–7 eV, which shows that the material behaves reflective in this energy range and is transparent in the rest of the energy region. However, after the doping of Gd, as shown in Figure 7b,c, the states mostly lie above zero points, and very few states are below zero points. A clear reduction in the states below zero points happened. Figure 8b is FM Gd-doped WO3, and Figure 8c is Gd-doped WO3 in the AFM configuration. Gd causes a reduction of the states below zero points, which shows that the material became nonreflective.
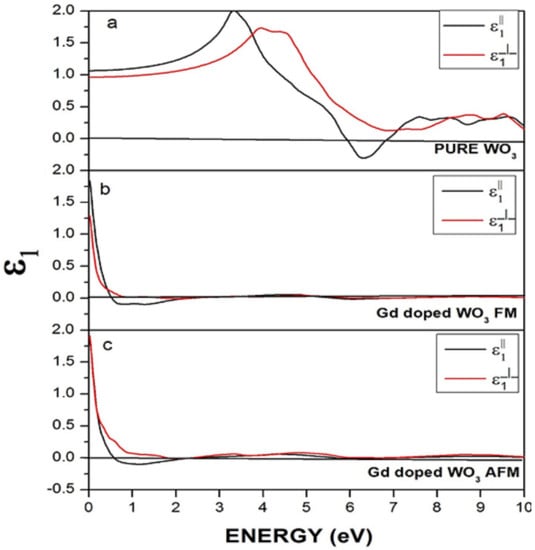
Figure 8.
Real dielectric function of (a) pure WO3, (b) Gd-doped WO3 for FM, and (c) Gd-doped WO3 for the AFM configuration.
A material’s complex dielectric function consists of two parts: real ε1(ω) and imaginary ε2(ω) (Figure 9). The absorption of photons is represented by the imaginary part of the dielectric function and electronic transition from valence towards the conduction band. The equation for ε2(ω) can be written as:
where M represents the dipole matrix, the free electron is represented by m, for the initial and final states, the i and j symbols are used, the ith state Fermi distribution is represented by fi, and Ei and Ej are the energy of the free electrons in the initial and final states.
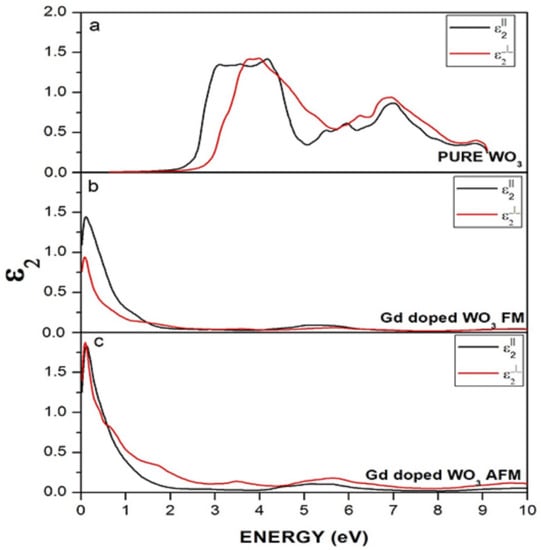
Figure 9.
Imaginary dielectric function of (a) WO3, (b) Gd-doped WO3 for FM, and (c) Gd-doped WO3 for the AFM configuration.
4. Conclusions
In the present work, the electronic, magnetic, and optical properties of pure WO3 and Gd-doped WO3 were calculated by using the GGA+U approximation for exchange–correlation energy. The energy difference between the FM and AFM configurations showed that the Gd-doped WO3 system is AFM. Pure WO3 is a nonmagnetic semiconductor with a bandgap of 2.5 eV. The system becomes a degenerate semiconductor after the doping of the rare earth element Gd in WO3. In the real dielectric function of WO3, the perpendicular component lies in the negative region in the energy range 6–7 eV, which shows that the material behaves as reflective in this energy range and is transparent in the rest of the energy region. The spin-polarized calculations showed that the system is antiferromagnetic in its grounded state. The WO3 material is a semiconductor, as there is a bandgap of 2.5 eV between the VB and CB. Pure WO3 is nonmagnetic, where the magnetic moment in the system after doping Gd is 9.5599575 μB.
Author Contributions
Conceptualization, writing—original draft Preparation, A.B., T.A.A. and M.R.; methodology, writing—original draft Preparation, S.I. and H.A.; software, writing review and editing, funding, M.A.Q. and Z.A.; validation, project administration, critical revision, M.M.A.-A., E.B.E. and R.A.P.; writing review and editing, resources, funding, E.A. and A.-E.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4320141DSR46). This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R7), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dören, R.; Leibauer, B.; Lange, M.A.; Schechtel, E.; Prädel, L.; Panthöfer, M.; Mondeshki, M.; Tremel, W. Gram-scale selective synthesis of WO3−x nanorods and (NH4)xWO3 ammonium tungsten bronzes with tunable plasmonic properties. Nanoscale 2021, 13, 8146–8162. [Google Scholar] [CrossRef]
- Prasad, U.; Young, J.L.; Johnson, J.C.; McGott, D.L.; Gu, H.; Garfunkeld, E.; Kannan, A.M. Enhancing interfacial charge transfer in a WO3/BiVO4 photoanode heterojunction through gallium and tungsten co-doping and a sulfur modified Bi2O3 interfacial layer. J. Mater. Chem. A 2021, 9, 16137–16149. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Li, L.; Gu, Y.; Kim, B.-H.; Huang, J. Synthesis of vertical WO3 nanoarrays with different morphologies using the same protocol for enhanced photocatalytic and photoelectrocatalytic performances. RSC Adv. 2021, 11, 23700–23706. [Google Scholar] [CrossRef]
- Deng, C.-B.; Zhang, M.; Lan, T.; Zhou, M.-J.; Wen, Y.; Zhong, J.; Sun, X.-Y. Spectroscopic investigation on Eu3+-doped TeO2-Lu2O3-WO3 optical glasses. J. Non-Cryst. Solids 2021, 554, 120565. [Google Scholar] [CrossRef]
- Shen, L.; Zheng, J.; Xu, C. Enhanced electrochromic switches and tunable green fluorescence based on terbium ion doped WO3 films. Nanoscale 2019, 11, 23049–23057. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.B.; Sagir, M. Carbon nanodots and rare metals (RM = La, Gd, Er) doped tungsten oxide nanostructures for photocatalytic dyes degradation and hydrogen production. Sep. Purif. Technol. 2019, 209, 94–102. [Google Scholar] [CrossRef]
- Palanisamy, G.; Bhuvaneswari, K.; Bharathi, G.; Pazhanivel, T.; Grace, A.N.; Pasha, S.K. Construction of magnetically recoverable ZnS–WO3–CoFe2O4 nanohybrid enriched photocatalyst for the degradation of MB dye under visible light irradiation. Chemosphere 2021, 273, 129687. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, J.; Ruan, M.; Guo, Z. An effective strategy of constructing a multi-junction structure by integrating a heterojunction and a homojunction to promote the charge separation and transfer efficiency of WO3. J. Mater. Chem. A 2020, 8, 6256–6267. [Google Scholar] [CrossRef]
- Gopakumar, G.; Nair, S.V.; Shanmugam, M. Assessing the role of plasma-engineered acceptor-like intra- and inter-grain boundaries of heterogeneous WS2–WO3 nanosheets for photocurrent characteristics. Nanoscale Adv. 2020, 2, 2276–2283. [Google Scholar] [CrossRef]
- Gong, H.; Hao, X.; Jin, Z.; Ma, Q. WP modified S-scheme Zn0.5Cd0.5S/WO3 for efficient photocatalytic hydrogen production. New J. Chem. 2019, 43, 19159–19171. [Google Scholar]
- Çoban, Ö.; Gür, E.; Tüzemen, S. Platinum activated WO3 optical hydrogen sensors. Mater. Today Proc. 2021, 46, 6913–6915. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, C.; Lin, S.; Li, H.; Feng, Y.; Gao, X. Oxygen vacancy modified Bi2MoO6/WO3 electrode with enhanced photoelectrocatalytic degradation activity toward RhB. Fuel 2021, 285, 119171. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Harish, S.; Archana, J.; Navaneethan, M. Fabrication of novel hybrid Z-Scheme WO3@g-C3N4@MWCNT nanostructure for photocatalytic degradation of tetracycline and the evaluation of antimicrobial activity. Chemosphere 2022, 287, 132050. [Google Scholar] [CrossRef]
- Muller, O.; Gibot, P. Optical limiting properties of templated Cr2O3 and WO3 nanoparticles. Opt. Mater. 2019, 95, 109220. [Google Scholar] [CrossRef]
- Huda, M.N.; Yan, Y.; Moon, C.-Y.; Wei, S.-H.; Al-Jassim, M.M. Density-functional theory study of the effects of atomic impurity on the band edges of monoclinic WO3. Phys. Rev. B 2008, 77, 195102. [Google Scholar] [CrossRef]
- Vijayaprasath, G.; Murugan, R.; Hayakawa, Y.; Ravi, G. Optical and magnetic studies on Gd doped ZnO nanoparticles synthesized by co-precipitation method. J. Lumin. 2016, 17, 375–383. [Google Scholar] [CrossRef]
- Deb, S.K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol. Energy Mater. Sol. Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Guo, R.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Enhanced photocatalytic activity and ferromagnetism in Gd doped BiFeO3 nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic tungsten oxide films: Review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, D.; Niu, F.; Wang, S.; Qin, L.; Huang, Y. Enhanced visible light photocatalytic activity of Gd-doped BiFeO3 nanoparticles and mechanism insight. Sci. Rep. 2016, 6, 26467. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, W.; Chan, Y.; Leung, C.; Mak, C.; Ploss, B. Studies of rare-earth-doped BiFeO3 ceramics. Int. J. Appl. Ceram. Technol. 2011, 8, 1246–1253. [Google Scholar] [CrossRef]
- Iqbal, S. Spatial Charge Separation and Transfer in L-Cysteine Capped NiCoP/CdS Nano-Heterojunction Activated with Intimate Covalent Bonding for High-Quantum-Yield Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2020, 274, 119097. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Anwer, S.; Ali, S.; Saeed, A.; Irfan, R.M.; Li, H.; Javed, M.; Raheel, M.; Shoaib, M. Shape and phase-controlled synthesis of specially designed 2D morphologies of l-cysteine surface capped covellite (CuS) and chalcocite (Cu2S) with excellent photocatalytic properties in the visible spectrum. Appl. Surf. Sci. 2020, 526, 146691. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Anwer, S.; Shoaib, M.; Liu, G.; Li, H.; Raheel, M.; Javed, M.; Khalid, B. Designing novel morphologies of l-cysteine surface capped 2D covellite (CuS) nanoplates to study the effect of CuS morphologies on dye degradation rate under visible light. Cryst. Eng. Comm. 2020, 22, 4162–4173. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Ali, S.; Ahmad, Z.; Javed, M.; Irfan, R.M.; Ahmad, N.; Qamar, M.A.; Liu, G.; Akbar, M.B. Critical role of the heterojunction interface of silver decorated ZnO nanocomposite with sulfurized graphitic carbon nitride heterostructure materials for photocatalytic applications. J. Alloys Compd. 2021, 858, 158338. [Google Scholar] [CrossRef]
- Irfan, R.M.; Tahir, M.H.; Khan, S.A.; Shaheen, M.A.; Ahmed, G.; Iqbal, S. Enhanced photocatalytic H2 production under visible light on composite photocatalyst (CdS/NiSe nanorods) synthesized in aqueous solution. J. Colloid Interface Sci. 2019, 557, 1–9. [Google Scholar] [CrossRef]
- Hussain, W.; Malik, H.; Bahadur, A.; Hussain, R.A.; Shoaib, M.; Iqbal, S.; Green, I.R.; Badshah, A.; Li, H. Synthesis and Characterization of CdS Photocatalyst with Different Morphologies: Visible Light Activated Dyes Degradation Study. Kinet. Catal. 2018, 59, 710–719. [Google Scholar] [CrossRef]
- Pradhan, S.; Das, J.; Rout, P.; Das, S.; Mishra, D.; Sahu, D.; Srinivasu, V.; Nayak, B.; Verma, S.; Roul, B. Defect driven multiferroicity in Gd doped BiFeO3 at room temperature. J. Magn. Magn. Mater. 2010, 322, 3614–3622. [Google Scholar] [CrossRef]
- Biltz, W.; Lehrer, G.A.; Meisel, K. Zeitschrift für anorganische und allgemeine Chemie. Rheniumtrioxyd II Mitt. 1932, 207, 113–120. [Google Scholar]
- Ablat, A.; Wu, R.; Mamat, M.; Li, J.; Muhemmed, E.; Si, C.; Wu, R.; Wang, J.-O.; Qian, H.; Ibrahim, K. Structural analysis and magnetic properties of Gd doped BiFeO3 ceramics. Ceram. Int. 2014, 40, 14083–14089. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ramu, S.; Sudharani, A.; Ramanadha, M.; Murali, G.; Vijayalakshmi, R. Enhanced magnetic and dielectric properties of Gd doped BiFeO3: Er nanoparticles synthesized by sol-gel technique. Phys. E Low-Dimens. Syst. Nanostructures 2020, 115, 113689. [Google Scholar] [CrossRef]
- Salje, E.K.; Rehmann, S.; Pobell, F.; Morris, D.; Knight, K.S.; Herrmannsdörfer, T.; Dove, M.T. Crystal structure and paramagnetic behaviour of. J. Phys. Condens. Matter 1997, 9, 6563. [Google Scholar] [CrossRef]
- Kehl, W.; Hay, R.G.; Wahl, D. The structure of tetragonal tungsten trioxide. J. Appl. Phys. 1952, 23, 212–215. [Google Scholar] [CrossRef]
- Tanisaki, S. Crystal structure of monoclinic tungsten trioxide at room temperature. J. Phys. Soc. Jpn. 1960, 15, 573–581. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.K.H.; Kvasnicka, D. WIEN2k: An augmented plane wave+ local orbitals program for calculating crystal properties. Mater. Trans. 2001, 45, 1991–1993. [Google Scholar]
- Bak, T.; Nowotny, J.; Rekas, M.; Sorrell, C.C. Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int. J. Hydrogen Energy 2002, 27, 991–1022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).