Abstract
Metal sulfide electrocatalyst is developed as a cost-effective and promising candidate for hydrogen evolution reaction (HER). In this work, we report a novel Mo-doped Cu2S self-supported electrocatalyst grown in situ on three-dimensional copper foam via a facile sulfurization treatment method. Interestingly, Mo-Cu2S nanosheet structure increases the electrochemically active area, and the large fleecy multilayer flower structure assembled by small nanosheet facilitates the flow of electrolyte in and out. More broadly, the introduction of Mo can adjust the electronic structure, significantly increase the volmer step rate, and accelerate the reaction kinetics. As compared to the pure Cu2S self-supported electrocatalyst, the Mo-Cu2S/CF show much better alkaline HER performance with lower overpotential (18 mV at 10 mA cm−2, 322 mV at 100 mA cm−2) and long-term durability. Our work constructs a novel copper based in-situ metal sulfide electrocatalysts and provides a new idea to adjust the morphology and electronic structure by doping for promoting HER performance.
1. Introduction
Nowadays, in order to alleviate the non-renewable fossil energy and the associated serious environmental pollution, clean and sustainable energy has been vigorously pursued all over the world. Hydrogen gas, as a green, renewable, high calorific value of combustions and pollution-free energy carrier was widely concerned [1,2,3,4]. Among the current three main methods of hydrogen production, water electrolysis is considered to be an ideal pathway with zero-emission, high purity, and abundant water reserves. It is well known that noble metal platinum (Pt) and platinum-based materials offer the most efficient and stable electrocatalytic activity [5,6]. However, the scarcity and high cost of platinum (Pt) seriously limit its practical deployment. Thus, it is urgent to develop new high-performance and cost-effective electrocatalysts for hydrogen evolution reaction (HER).
Benefitting from the d-orbital electrons and vacant d-orbitals simultaneously existing in transition metal, it is easy to lose or capture electrons and has strong capable of redox reaction, which can bring down the activation energy of the water-splitting-related intermediates generated and thus promote the electrocatalytic process. Until now, transition metal-based electrocatalysts, including phosphides [7,8,9,10,11], (hydro) oxides [12,13], selenides [14,15], etc., have attracted much attention. Among them, transition metal sulfides (TMS) have been exploited by untiring effort as a considerably promising electrocatalyst in view of their advantages of economy and excellent electrochemical performance on water splitting [16,17,18,19]. Compared with Fe, Co and Ni, Cu was recognized as having favorable stability, excellent electrical conductivity and cost effectiveness; therefore, copper sulfide has been widely investigated in energy storage and conversion fields, such as battery [20,21], capacitor [22,23] and photocatalysis [24,25]. For example, Zhu et al. synthesized hierarchical cuprous sulfide nanosheets to modify nanowires on copper foam as a non-binder conversion cathode material for lithium/magnesium hybrid battery [20]. Li et al. successfully exploited multistage hybrid cuprous sulfide (Cu2S) nanoparticles anchoring on graphene to improve capacitive energy storage [22]. Wang et al. constructed cuprous sulfide nanoparticles for photocatalytic reduction of carbon dioxide on amorphous CuSX matrix and successfully boosted the catalysis of cuprous sulfide with hybrid structure [24]. However, cuprous sulfide (Cu2S) is rarely used in the realm of electrochemical hydrogen evolution [26,27,28]. Zhang et al. proposed a “d-orbital complementarity” principle for the synthesis of vanadium-doped cobalt phosphide (V-CoP) as an efficient electrocatalyst towards HER [29]. Inspired by the “d-orbital complementarity” principle, we aim to develop a simple and universal method such as doping the early transition metal Mo to cuprous sulfide, which possesses the late transition metal copper. We aim to modulate the electronic structure and nano-structure, increase the specific surface area of catalysts, and enhance catalytic activity. Thus, the high-cost challenge may be alleviated, making it promising in use in large-scale and highly efficient water electrolysis.
In this report, molybdenum doped Cu2S multilayer nanosheets grown in situ on copper foam (CF) were synthesized by one-step hydrothermal route, denoted as Mo-Cu2S/CF. The results show that the molybdenum doping changed the nano-structure of the catalyst and formed a fluffy multilayer flower-like structure, leading to enlarging more active surface area, and promoted the optimization of electronic structure for improved intrinsic activity. The optimized Mo-Cu2S/CF exhibited extraordinarily low HER overpotential and robust stability in alkaline solution which only required 18 mV and 322 mV overpotential for obtaining a high current density of 10 and 100 mA cm−2, and worked stably for at least 20 h. Our work shows an innovative way to design and fabricate potential metal sulfide electrocatalytic materials for hydrogen production in water splitting.
2. Results and Discussion
Molybdenum doped Cu2S multilayer nanosheets grown in situ on copper foam (Mo-Cu2S/CF) is fabricated by a facile one-step sulfurization hydrothermal method with sulfur source thioacetamide in a hydrothermal system at 180 °C for 24 h, as illustrated in Figure 1. The surface of CF was coarsened by sulfurization etching, which was more conducive to the growth of self-supported electrode, and the Cu2+ ions from CF are precipitated into the solution reacting with S2- ions, meanwhile with the successful doping of molybdenum source, Mo-Cu2S nanosheet array was successfully grown on the CF. The SEM images in Figure 2 clearly reveal that hydrothermal reaction time has a significant impact on the structure and morphology of Mo-Cu2S/CF. When the hydrothermal reaction time is 6 h (Figure 2A), the CF surface is completely covered with the agglomerated balls. With the increase of reaction time 12 h (Figure 2B), small numbers of nanosheets can be observed on the surface of the agglomeration. Extending reaction time to 24 h (Figure 2C), the nanosheet with curved edge can be observed clearly. Obviously, the puffy multilayer nanosheet structure expands the contact area, facilitates the electron transfer rate and the electrolyte in and out. As the reaction time is prolonged to 36 h (Figure 2D), the nanosheets gradually disappear, which is not conducive to the electrolysis reaction to some extent. The phase of Mo-Cu2S/CF-6 h, Mo-Cu2S/CF-12 h, Mo-Cu2S/CF-24 h, Mo-Cu2S/CF-36 h remains Mo-Cu2S, as demonstrated in Figure S1.
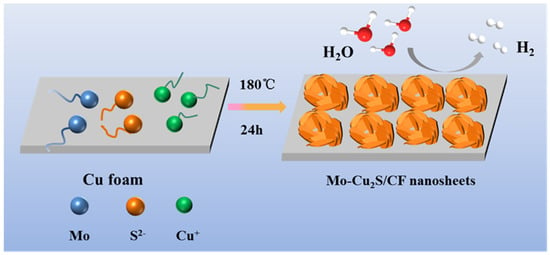
Figure 1.
Schematic illustration of the construction of Mo-Cu2S/CF.

Figure 2.
SEM images of Mo-Cu2S/CF (A) 6 h; (B) 12 h; (C) 24 h; (D) 36 h.
Figure 3A displays the X-ray diffraction (XRD) spectra of Mo-Cu2S/CF and Cu2S/CF exhibited three typical diffraction peaks at 38.33°, 45.17°, 47.76° matches well with Cu2S (PDF#83-1462), and the two obvious peaks at 43.41° and 50.56° belong to the substrate Cu (PDF#65-9743). This reveals that Cu2S has good crystallinity, and an “all in one” structured Cu2S/CF is successfully synthesized. The TEM image of Mo-Cu2S/CF is observed in Figure 3B, Mo-Cu2S/CF is composed of many stacked nanosheet structures, which is consistent with SEM images. The HRTEM was also conducted in Figure 3C, a clear lattice spacing ~0.24 nm and ~0.274 nm corresponding to (111) and (034) planes of Cu2S (PDF#72-1071) and (PDF#83-1462) respectively consists well with the XRD results. Further, the EDS mapping images of Mo-Cu2S/CF clearly confirms that Cu, Mo, and S elements are uniformly dispersed over the entire area of nanosheet, resulting in the formation of Mo-doped Cu2S material (Figure 3D).
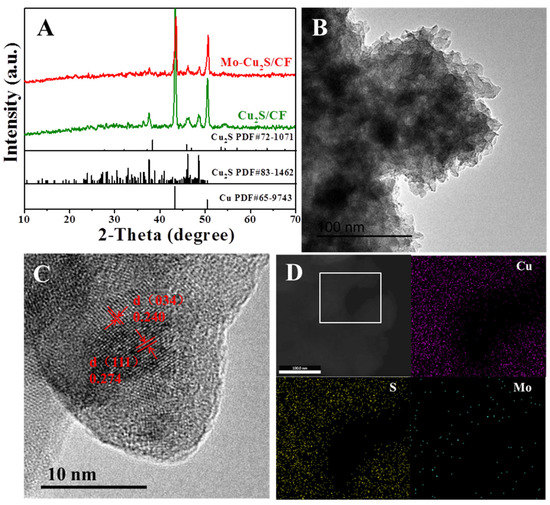
Figure 3.
(A) XRD patterns of Mo-Cu2S/CF and Cu2S/CF; (B) TEM image; (C) the HRTEM image of Mo-Cu2S/CF; (D) the elemental mapping images.
As a comparison, the SEM, TEM and HRTEM images of Cu2S/CF are performed in Supporting Information. The SEM image of Cu2S/CF (Figure S2A) displays a block structure with much larger size compared to Mo-Cu2S/CF, and this is evident in the TEM image (Figure S2B). To further determine the microstructure of Cu2S/CF, the HRTEM image of Cu2S/CF is conducted (Figure S2C), and visible lattice fringes with spacing of 0.274 nm are matched with Cu2S (111) plane. The corresponding elemental mapping images (EDX) of Cu2S/CF indicate that Cu and S are uniformly distributed over the whole Cu2S/CF, implying a homogeneous sulfurization (Figure S2D–F). By comparing Figure 3, Figures S2, S3 and Table S1, it is easy to draw a conclusion that Mo element was successfully induced and played a crucial role in the formation of nanosheet structure, reduced the size of Cu2S which is conducive to fully exposing active sites on the catalyst surface and making the hydrogen evolution process more efficient. Compared with massive block structures of Cu2S/CF, Mo-Cu2S/CF with the large fleecy multilayer flower-like morphologies assembled by asmall nanosheet obviously exposes more active sites and facilitates the flow of electrolyte in and out, effectively improving the efficiency of electrocatalytic hydrogen evolution [30].
In addition, X-ray photoelectron spectroscopy (XPS) measurements were utilized to elucidate the electronic structure and chemical states on the surface of Mo-Cu2S/CF and Cu2S/CF samples. Cu, S, C, O elements of Mo-Cu2S/CF and Cu2S/CF are clearly observed in Figure 4A, while the Mo 3d signal appears in Mo-Cu2S/CF, indicating that Mo is successfully doped in the Cu2S/CF sample. Oxygen signal originates from the surface oxidation in the air. For the case of high-resolution of Cu 2p (Figure 4B), the spectrum clearly displays the Cu 2p3/2 peaks at 932.43 eV and 934.18 eV [31], which corresponds to Cu+ and Cu2+ states, respectively. The peaks of Cu 2p1/2 located at 952.28 and 954.34 eV belong to states of Cu+ and Cu2+. The satellite peaks at 941.32, 944.09 and 962.38 eV confirmed the existence of Cu2+, which is caused by exposure to air [32,33,34]. To be noticed, Cu 2p3/2 at 932.43 and 934.18 eV exhibit negative shifts of ~0.21, ~0.65 eV, respectively, compared with Cu2S/CF (952.28 and 954.34 eV), which could lead to the buildup of negative charges on and therefore favoring the adsorption of H* intermediates [35,36,37]. The in-situ Cu2+ reduction into Cu+ ions could be caused by S2- ions reducing agents of Mo-Cu2S/CF [38]. Meanwhile, we observed that the characteristic peak of Cu+ 2p3/2 increases conspicuously in intensity along with the intensity area enlarging from 1.74% to 11.78% compared with Cu2S/CF. These results demonstrate that Mo-Cu2S/CF has better HER performance because Cu+ species have easier electron transfer than Cu2+ [27]. Figure 4C shows the comparison of S 2p XPS spectrum among Mo-Cu2S/CF and Cu2S/CF. There are two characteristic peaks with energy of 161.7 and 162.94 eV that can be assigned to S 2p3/2 and S 2p1/2 [39], indicating the formation of metal sulfides. The weak peak at 168.52 eV corresponds to SO42-, which roots in the surface oxidation of Cu2S in the air [40]. The binding energies of S 2p peaks in Mo-Cu2S/CF have positive shifts of ~0.11, ~0.13 eV, as compared to Cu2S/CF (161.59 and 162.81 eV). Figure 4D demonstrates the successful synthesis of Mo-Cu2S/CF because the two characteristic peaks at 230.23 and 232.11 eV belong to Mo4+ 3d5/2 and Mo4+ 3d3/2, respectively. Additionally, the higher energy peak at 235.15 eV is indexed to Mo6+, because of the slight oxidation of the sample when exposed to air. From the binding energy deviation of Cu and S, we can conclude that electrons are transferred from S to Cu, indicating that the doping of Mo changes the electronic structure of Cu2S/CF successfully, and confirming strong electronic interaction between Mo and Cu2S, which can lead to enhancing electrical conductivity of the material [28,40]. The doping of molybdenum changes the content of Cu+, the increasing Cu+ is more conducive to hydrogen evolution reaction.
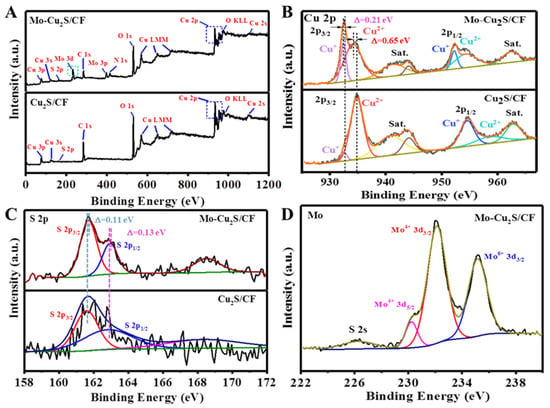
Figure 4.
(A) Survey; (B) Cu 2p, (C) S 2p XPS spectra of Mo-Cu2S/CF and Cu2S/CF; (D) Mo XPS spectra of Mo-Cu2S/CF.
The electrocatalytic HER activity of Mo-Cu2S/CF was tested in Figure 5. Linear sweep voltammetry (LSV) measurements were recorded in 0.1M KOH solution at scan rate of 5 mV s−1. For comparison, the electrochemical hydrogen evolution tests of four samples with different hydrothermal times are carried out respectively (Figure S4). Mo-Cu2S/CF-24 exhibits best electrocatalytic activity towards HER, with quite a small overpotential of 18 and 322 mV to deliver a current density of 10 and 100 mA cm−2. In order to verify that the HER activity is driven by the catalytic site on Mo-Cu2S/CF, the blank CF and Cu2S/CF are tested respectively. As shown in Figure 5A, Mo-Cu2S/CF exhibits remarkable HER activity in alkaline media, which requires only a quite small overpotentials of 18/322 mV to deliver the current densities of 10/100 mA cm−2, and significantly outperforms Cu2S/CF (277/445 mV). It was worth noting that the electrocatalytic HER performance over the resultant Mo-Cu2S was better than that of most of previously reported Cu2S-based electrocatalyst for HER (Table S2) [26,27,41,42,43,44,45,46,47]. Furthermore, the HER kinetics of electrocatalysts are studied in the reference of Tafel slopes in Figure 5B. The Tafel slope of Mo-Cu2S/CF is 171 mV dec−1, smaller than that of Cu2S/CF (181 mV dec−1) and pure CF (420 mV dec−1), suggesting that Mo-Cu2S/CF proceeds the rapidest HER kinetics among them. Electrochemical impedance spectroscopy (EIS) is then examined to reveal electron transfer rates during the HER process. The corresponding Nyquist plots are shown in Figure 5C. Mo-Cu2S/CF exhibits a smaller charge transfer resistance compared to Cu2S/CF and pure CF, which indicates that Mo-Cu2S/CF shows the faster electron transfer and HER kinetics than Cu2S/CF and pure CF. Electrochemical specific surface area (ECSA) is also an important parameter to estimate the electrochemical properties, which is proportional to double-layer capacitance (Cdl). The Cdl values of different electrodes are calculated by fitting of the plots of current density at different scan rates. As manifested in Figure 5D, the Cdl of Mo-Cu2S/CF (58 mF cm−2) is much higher than that of Cu2S/CF (50 mF cm−2) and pure CF (0.04 mF cm−2), meaning that Mo-Cu2S/CF possess more active surface area, generally resulting in even more active sites and normally better catalytic activity [48].
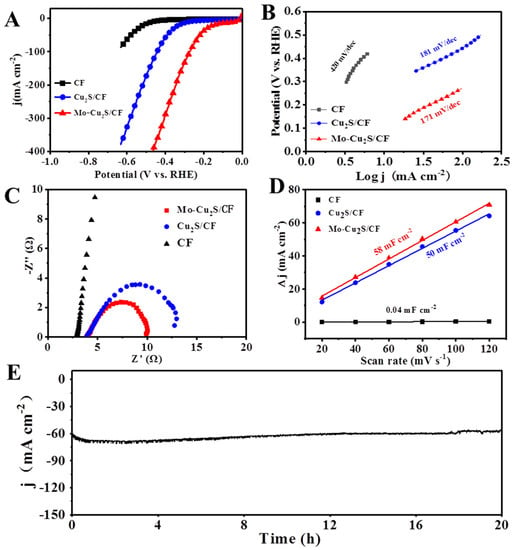
Figure 5.
(A) LSV polarization curves of Mo-Cu2S/CF, Cu2S/CF and CF in1.0 M KOH at scan rate of 5 mVs−1; (B) Tafel curves; (C) EIS Nyquist plots; (D) Cdl plots for the estimation of the ECSA; (E) chronoamperometric curve (I-t) obtained for HER with Mo-Cu2S/CF at the current density of 60 mA cm−2 in 1 M KOH.
Meanwhile, the highly durability of Mo-Cu2S/CF is investigated by chronopotentiometry, and Mo-Cu2S/CF displays a negligible attenuation of overpotential after 20 h at 60 mA cm−2, proving its excellent stability. The XRD pattern in Figure S5 signifies that the phase remains Mo-Cu2S after 20 h HER electrocatalysis. Meanwhile, the TEM, HRTEM and elementals mapping images after 20 h HER test (Figure S6), the microstructure and composition of the catalyst were well-maintained, confirming the outstanding structural stability after long-term HER electrocatalysis.
From the above tests, it is inferred that the excellent HER activity of Mo-Cu2S/CF has the following three aspects: (I) using foam copper (CF) as the substrate is conducive to the uniform distribution of Mo-Cu2S on the surface, and can effectively inhibit the interlayer aggregation of the nanosheets. Besides, the “all in one” self-supporting electrode improves the catalytic stability and activity avoids the influence of the binder between the catalyst surface and the electrolyte. (II) multilayer nanosheets of Mo-Cu2S/CF increases the contact area with electrolyte in the process of HER; this structure provides more active sites, which makes the hydrogen evolution reaction more efficient. (III) Mo doping increases the charge transfer rate, which is beneficial to the electrocatalytic process.
3. Experimental Section
3.1. Reagents and Materials
Sodium molybdate dishydrate (NaMoO4·2H2O) and thioacetamide (C2H5NS) were analytical grade and bought from Sinopharm Group Chemical Reagent Co., Ltd. (Beijing, China). KOH, C3H2O and CH3CH2OH were purchased from Tianjin Kemeiou Reagent Co., Ltd. (Tianjin, China) The copper foam (CF, with 1 mm thickness) was obtained from Suzhou Jiashide foam metal Co., Ltd. (Suzhou, China).
3.2. Synthesis of Mo-Cu2S/CF Samples
The Mo-Cu2S/CF samples were prepared by one-step hydrothermal method. Typically, 0.25 mmol of Na2MoO4·2H2O and 1.25 mmol of C2H5NS were dissolved in 30 mL ultrapure water under stirring treatment. A piece of 1 cm × 5.5 cm copper foam (CF) was cleaned with acetone and 3 M hydrochloric acid for 15 min in each to remove the impurities on the surface. It was then immersed in ethanol and deionized water 5 min respectively to wash several times alternately. Subsequently, the obtained mixture was added into a 50 mL polyphenylene autoclave, which was heated at 180 °C oven for 24 h, and then cooled to indoor temperature. Finally, the resulting materials was obtained and repeatedly rinsed with water and ethanol several times, subsequently dried at 60 °C for 10 h in vacuum. In addition, so as to further investigate the impact of hydrothermal reaction time on the structure and catalytic performance, three other samples by changing the reaction time (6 h, 12 h and 36 h) were synthesized, which were denoted as Mo-Cu2S/CF -6, Mo-Cu2S/CF -12 and Mo-Cu2S/CF -36.
3.3. Synthesis of Cu2S/CF Samples
In the synthesis of Cu2S/CF, the reaction conditions were the same as those of Mo-Cu2S/CF, except that Na2MoO4·2H2O was not added in the raw material.
3.4. Electrochemical Measurements
Relevant electrochemical measurements were conducted in a typical three-electrode electrochemical system and performed on the CHI660E B17060 electrochemical workstation (Chenhua Instrument Co., LTD. Shanghai). The as-prepared Mo-Cu2S/CF and Cu2S/CF served as the working electrode, as well as extremely saturated calomel reference electrode (SCE) and graphite carbon counter electrode. For the electrochemical measurements, the prepared Mo-Cu2S/CF material exposed a 0.3 cm × 0.4 cm area as a working electrode. The HER measurements were carried out in 1 M KOH, and the potential vs. SCE was converted into reversible hydrogen electrode (RHE) according with the equation of Evs RHE = Evs SCE + 0.242 + 0.059 pH. The linear sweep voltammetry (LSV) curve was performed at the scan rate of 5 mV s−1 and iR-corrected. Tafel slopes were derived from the LSV curves. Electrochemical impedance spectroscopy (EIS) was measured at a voltage generated by Faraday current with a frequency range from 10−2 to 105 Hz. The stability measurement was evaluated with I-T curve under a constant voltage. Electrochemical specific surface area (ECSA) was obtained by conducting cyclic voltammetry (CV) under the different scanning speeds (20, 40, 60, 80, 100 and 120 mV s−1), which is proportional to the Cdl and also an important factor in electrochemical measurements.
3.5. Materials Characterization
X-ray diffraction (XRD) data were performed by the Rigaku D/max-2200pc. The microstructure and morphology were monitored by field emission scanning electron microscopy (FESEM, Hitachi, S4800), the transmission electron microscopy and high-resolution TEM were texted on Tecnai G2 F20S-TWIN. X-ray photoelectron spectroscopy (XPS) was obtained on an XIS SUPRA.
4. Conclusions
In summary, a novel Mo-doped Cu2S nanosheets (Mo-Cu2S/CF) grown in situ on copper foam (CF) has been successfully synthesized by a simple one-step hydrothermal method. Experimental results provide evidence that Mo doping can regulate the catalyst morphology and modulate the electronic structure, thus enhancing the hydrogen evolution activity. The over potential is only 322 mV in the alkaline condition (1 m KOH) at current density 100 mA cm−2, and the stability could be maintained for at least 20 h. Therefore, the optimized Mo-Cu2S/CF catalyst exhibiting remarkable electrocatalytic activity provides a direction for the development of transition metal sulfide self-supported electrode for HER.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27185961/s1, Figure S1. XRD of Mo-Cu2S/CF-6 h; Mo-Cu2S/CF-12 h; Mo-Cu2S/CF-24 h; Mo-Cu2S/CF-36 h, Figure S2. (A–C) SEM, TEM and HRTEM images, (D–F) The corresponding elementals mapping images of Cu2S/CF, Figure S3. STEM-EDX spectrum of the Mo-Cu2S/NF, Figure S4. Polarization curves of Mo-Cu2S/CF-6 h; Mo-Cu2S/CF-12 h; Mo-Cu2S/CF-24 h; Mo-Cu2S/CF-36 h, Figure S5. XRD of Mo-Cu2S/CF after HER test, Figure S6. (A–B) TEM and HRTEM images, (C–F) The corresponding elementals mapping images of Mo-Cu2S/CF after 20 h HER test, Table S1. The atomic percentage of the Mo-Cu2S/NF, Table S2. Comparison of the electrocatalytic activity of Mo-Cu2S with previously reported Cu2S-based electrocatalysts in 1.0 M KOH electrolyte.
Author Contributions
Conceptualization: L.F. and J.H.; methodology: Y.X. and L.F.; software: Y.X. and X.L.; validation: Y.X. and D.H.; formal analysis: Y.X.; M.N. and G.X; investigation: Y.X.; data curation: R.X.; writing—original draft preparation: Y.X.; writing—review and editing: L.F. and J.H.; funding acquisition: J.H.; L.F.; L.C.; Y.X. and G.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 22179074, 52172049, 52073166), Science and Technology Resource Sharing Platform of Shaanxi Province (No.2020PT-022), Agricultural Science and Technology Innovation Drive project of Shaanxi Agricultural Department (No. NYKJ-2022-XA-08), Scientific Research Project of Education Department of Shaanxi Province (No. 22JK0297) and Open Project of Key Laboratory of Auxiliary Chemistry and Technology for Chemical Industry, Ministry of Education, Shaanxi University of Science and Technology (No. KFKT2020-06).
Conflicts of Interest
There is no conflict to declare.
References
- Meloni, E.; Iervolino, G.; Ruocco, C.; Renda, S.; Festa, G.; Martino, M.; Palma, V. Electrified hydrogen production from methane for PEM fuel cells feeding: A review. Energies 2022, 15, 3588. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Iervolino, G.; Ruocco, C.; Renda, S.; Festa, G.; Palma, V. The route from green H2 production through bioethanol reforming to CO2 catalytic conversion: A Review. Energies 2022, 15, 2383. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Sun, X. Recent progress on earth abundant electrocatalysts for hydrogen evolution reaction (HER) in alkaline medium to achieve efficient water splitting—A review. J. Energy Chem. 2019, 33, 111–160. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Cao, L.; Kajiyoshi, K.; Li, K.; Feng, Y.; Fu, C.; Kou, L.; Feng, L. V-doping triggered formation and structural evolution of dendritic Ni3S2@NiO core−shell nanoarrays for accelerating alkaline water splitting. ACS Sustain. Chem. Eng. 2020, 8, 6222–6233. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, P.; Jiang, H.; Mu, J.; Meng, L.; Su, X.; Wang, Y.; Lin, Y.; Zhu, Y.; Song, L.; et al. Synergistic effect of platinum single atoms and nanoclusters boosting electrocatalytic hydrogen evolution. CCS Chem. 2020, 2, 2539–2547. [Google Scholar] [CrossRef]
- Guan, Y.; Feng, Y.; Wan, J.; Yang, X.; Fang, L.; Gu, X.; Liu, R.; Huang, Z.; Li, J.; Luo, J.; et al. Ganoderma-like MoS2/NiS2 with single platinum atoms doping as an efficient and stable hydrogen evolution reaction catalyst. Small 2018, 14, 1800697. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Garcia, A.; Su, D.; Sun, S. Sea urchin-like cobalt–iron phosphide as an active catalyst for oxygen evolution reaction. Nanoscale 2016, 8, 3244–3247. [Google Scholar] [CrossRef]
- Ma, B.; Yang, Z.; Chen, Y.; Yuan, Z. Nickel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res. 2019, 12, 375–380. [Google Scholar] [CrossRef]
- Liu, M.; Li, J. Cobalt phosphide hollow polyhedron as efficient bifunctional electrocatalysts for the evolution reaction of hydrogen and oxygen. ACS Appl. Mater. Interfaces 2016, 8, 2158–2165. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, B.; Chen, Y. Iron phosphides supported on three-dimensional iron foam as an efficient electrocatalyst for water splitting reactions. J. Mater. Sci. 2019, 54, 14872–14883. [Google Scholar] [CrossRef]
- Chen, S.; Dai, J.; Ren, F.; Xu, H.; Du, Y. 3D hollow nanoflowers assembled by ultrathin molybdenum-nickel phosphide nanosheets as robust electrocatalysts for oxygen evolution reaction. J. Colloid Interface Sci. 2019, 536, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Xu, F.; Wang, C.; Meng, W.; Grice, C.; Yan, Y. Layered Na1−xNiyFe1−yO2 double oxide oxygen evolution reaction electrocatalyst for highly efficient water-splitting. Energy Environ. Sci. 2017, 10, 121–128. [Google Scholar] [CrossRef]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, J.; Zhou, K.; Yang, L.; Ke, Y.; Tang, Z.; Chen, S. CoSe2 nanoparticles embedded defective carbon nanotubes derived from MOFs as efficient electrocatalyst for hydrogen evolution reaction. Nano Energy 2016, 28, 143–150. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Huang, J.; Chen, J.; Wen, Z. Oxygen-incorporated amorphous cobalt sulfide porous nanocubes as high-activity electrocatalysts for the oxygen evolution reaction in an alkaline/neutral medium. Angew. Chem. Int. Ed. 2017, 56, 4858–4861. [Google Scholar] [CrossRef]
- Lukowski, M.; Daniel, A.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. the Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, R.; Wen, Z.; Ci, S.; Chang, J.; Mao, S.; Chen, J. Superior electrocatalysis for hydrogen evolution with crumpled graphene/tungsten disulfide/tungsten trioxide ternary nanohybrids. Nano Energy 2018, 47, 66–73. [Google Scholar] [CrossRef]
- Staszak-Jirkovský, J.; Malliakas, C.; Lopes, P.; Danilovic, N.; Kota, S.; Chang, K.; Genorio, B.; Strmcnik, D.; Stamenkovic, V.; Kanatzidis, M.; et al. Design of active and stable Co–Mo–Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 2016, 15, 197–203. [Google Scholar] [CrossRef]
- Zhu, G.; Xia, G.; Yu, X. Hierarchical 3D cuprous sulfide nanoporous cluster arrays self-assembled on copper foam as a binder free cathode for hybrid magnesium-based batteries. Small 2021, 17, 2101845. [Google Scholar] [CrossRef]
- Gong, J.; Jain, P. Room-temperature superionic-phase nanocrystals synthesized with a twinned lattice. Nat. Commun. 2019, 10, 3285. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, B.; Lv, X.; Li, Y.; Wang, L. Synthesis of cuprous sulfide nanoparticles anchored graphene for enhanced capacitive energy storage. Appl. Surf. Sci. 2016, 370, 508–513. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Bai, J.; Liu, F.; Wang, Z.; Wu, W.; Bradley, R.; Li, L.; Ruan, H.; Guo, S. Boosting high-rate sodium storage of CuS via a hollow spherical nanostructure and surface pseudocapacitive behavior. ACS Appl. Energy Mater. 2021, 4, 8901–8909. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Wang, L.; Yang, Z.; Zhu, Q.; Liu, Y.; Fang, W.; Gong, X.; Liu, Y.; Liu, X.; et al. CuSx-mediated two reaction systems enable biomimetic photocatalysis in CO2 reduction with visible light. J. Energy Chem. 2022, 65, 497–504. [Google Scholar] [CrossRef]
- Li, J.; Yuan, L.; Li, S.; Tang, Z.; Xu, Y. One-dimensional copper-based heterostructures toward photo-driven reduction of CO2 to sustainable fuels and feedstocks. J. Mater. Chem. A 2019, 7, 8676–8689. [Google Scholar] [CrossRef]
- Ma, B.; Yang, Z.; Yuan, Z.; Chen, Y. Effective surface roughening of three-dimensional copper foam via sulfurization treatment as a bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 2019, 44, 1620–1626. [Google Scholar] [CrossRef]
- Yang, D.; Cao, L.; Huang, J.; Liu, Q.; Li, G.; He, D.; Wang, J.; Feng, L. Vanadium-doped hierarchical Cu2S nanowall arrays assembled by nanowires on copper foam as an efficient electrocatalyst for hydrogen evolution reaction. Scr. Mater. 2021, 196, 113756. [Google Scholar] [CrossRef]
- Thi, L.; Din, N.; Dinh, C.; Nguyen, N.; Manh, T.; Nguyen, V.; Tac, D.; Hai, L. Three-dimensional heterostructures of Co@CuxS core–shell nanowire arrays as efficient bifunctional electrocatalysts for overall water splitting. Colloids Surf. A Physicochem. Eng. Aspects 2021, 611, 125779. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, Z.; Ye, G.; Chen, G.; Miao, J.; Zhou, X.; Zhu, X.; Cao, X.; Sun, X. “d-Electron Complementation” induced V-Co phosphide for efficient overall water splitting. Adv. Energy Mater. 2021, 11, 2101758. [Google Scholar] [CrossRef]
- Adamson, W.; Jia, C.; Li, Y.; Zhao, C. Cobalt oxide micro flowers derived from hydrothermal synthesised cobalt sulphide pre-catalyst for enhanced water oxidation. Electrochim. Acta 2020, 355, 136802. [Google Scholar] [CrossRef]
- Shen, C.; Sun, L.; Koh, Z.; Wang, Q. Cuprous sulfide counter electrodes prepared by ion exchange for high-efficiency quantum dot-sensitized solar cells. J. Mater. Chem. A 2014, 2, 2807. [Google Scholar] [CrossRef]
- Liu, P.; Hensen, E. Highly efficient and robust Au/MgCuCr2O4 catalyst for gasphase oxidation of ethanol to acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, K.; Shanks, B. Active species of copper chromite catalyst in C–O hydrogenolysis of 5-methylfurfuryl alcohol. J. Catal. 2012, 285, 235–241. [Google Scholar] [CrossRef]
- Severino, F.; Laine, J.; Fierro, J.; Agudo, A. Nature of Copper Active Sites in the Carbon Monoxide Oxidation on CuAl2O4 and CuCr2O4 Spinel Type Catalysts. J. Catal. 1988, 177, 82–95. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Liu, J.; Zhou, Y.; Yu, D.; Kuei, J.; Han, F.; Li, Q.; Chen, J.; Huang, Y. Facile synthesis of silk-cocoon S-rich cobalt polysulfide as an efficient catalyst for hydrogen evolution reaction. Energy Environ. Sci. 2018, 9, 2467–2475. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Zhang, X.; You, T. Defect- and S-rich ultrathin MoS2 nanosheets embedded N-doped carbon nanofibers for efficient hydrogen evolution. J. Mater. Chem. A 2015, 3, 15927–15934. [Google Scholar] [CrossRef]
- Deng, Z.; Li, L.; Ding, W.; Xiong, K.; Wei, Z. Synthesized ultrathin MoS2 nanosheets perpendicular to graphene for catalysis of hydrogen evolution reaction. Chem. Commun. 2015, 51, 1893–1896. [Google Scholar] [CrossRef]
- Hu, D.; Wang, X.; Yang, H.; Liu, D.; Wang, Y.; Guo, J.; Wu, T. Host-guest electrocatalyst with cage-confined cuprous sulfide nanoparticles in etched chalcogenide semiconductor zeolite for highly efficient oxygen reduction reaction. Electrochim. Acta 2018, 282, 877–885. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Zhao, Y.; Li, K.; Zhang, N.; Yang, D.; Feng, L.; Feng, L. Tuning coupling interface of ultrathin Ni3S2@NiV-LDH heterogeneous nanosheet electrocatalysts for improved overall water splitting. Nanoscale 2019, 11, 8855–8863. [Google Scholar] [CrossRef]
- Feng, J.; Xu, H.; Dong, Y.; Ye, S.; Tong, Y.; Li, G. FeOOH/Co/FeOOH hybrid nanotube arrays as high-performance electrocatalysts for the oxygen evolution reaction. Angew. Chem. 2016, 128, 3758–3762. [Google Scholar] [CrossRef]
- Marimuthu, T.; Yuvakkumar, R.; Ravi, G.; Zheng, Y.; Bi, Z.; Xu, X.; Xu, G.; Velauthapillai, D. One-step fabrication of copper sulfide catalysts for HER in natural seawater and their bifunctional properties in freshwater splitting. Fuel 2022, 322, 124073. [Google Scholar] [CrossRef]
- Xie, N.; Ma, D.; Wu, Y.; Zhu, Q. Hierarchical Cu2S hollow nanowire arrays for highly efficient hydrogen evolution reaction. Sustain. Energy Fuels 2021, 5, 2633–2639. [Google Scholar]
- Lv, L.; Li, Z.; Wan, H.; Wang, C. Achieving low-energy consumption water-to-hydrogen conversion via urea electrolysis over a bifunctional electrode of hierarchical cuprous sulfide@nickel selenide nanoarrays. J. Colloid Interface Sci. 2021, 592, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.; Nagaraja, H. In Situ Synthesis of Copper Sulfide-Nickel Sulfide Arrays on Three-Dimensional Nickel Foam for Overall Water Splitting. ChemistrySelect 2020, 5, 2455–2464. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Xu, H.; Zhao, J. Bifunctional Cu2S–Co(OH)2 nanotube array/Cu foam electrocatalyst for overall water splitting. Electrochim. Acta 2019, 316, 8–18. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, T.; Wang, J.; Guo, F.; Zheng, Y. Hierarchical Cu2S NRs@CoS core-shell structure and its derivative towards synergistic electrocatalytic water splitting. Electrochim. Acta 2018, 296, 1035–1041. [Google Scholar] [CrossRef]
- Yang, L.; Yao, Y.; Zhu, G.; Ma, M.; Wang, W.; Wang, L.; Zhang, H.; Zhang, Y.; Jiao, Z. Co doping of worm–like Cu2S: An efficient and durable heterogeneous electrocatalyst for alkaline water oxidation. J. Alloys Compd. 2018, 762, 637–642. [Google Scholar] [CrossRef]
- He, D.; Cao, L.; Huang, J.; Kajiyoshi, K.; Wu, J.; Wang, C.; Liu, Q.; Yang, D.; Feng, L. In-situ optimizing the valence configuration of vanadium sites in NiV-LDH nanosheet arrays for enhanced hydrogen evolution reaction. J. Energy Chem. 2020, 47, 263–271. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).