Abstract
The chemical components and medicinal properties of different medicinal parts of Angelica sinensis are often used as medicine after being divided into the head, body and tail of Angelica sinensis. In this study, the chemical components of different medicinal parts in different periods were analyzed by GC-MS for the first time, and the differences of the accumulation rules of chemical components in different medicinal parts of Angelica sinensis were obtained. This study demonstrated that the differences of composition accumulation in different medicinal parts of Angelica sinensis were mainly reflected in the types and relative contents of compounds. The study found that the number of compounds in different medicinal parts of Angelica sinensis in each period were different and the change rules of the same compound in different medicinal parts were also different. The number of compounds in the tail of Angelica sinensis was the least in April, and the largest in October. The content of ligustilide in the body of Angelica sinensis was higher in April and was the highest in the tail in October. The relative content of butylidenephthalide in the head was the highest in October. The relative contents of senkyunolide A and butylphthalide in the head were decreased in October, while the contents in the body and tail increased, indicating that the compounds that accumulate in the head may transfer to the body and tail in later stages of growth. This study clarified the differences in the accumulation of chemical components in different medicinal parts of Angelica sinensis, which could provide a theoretical basis for the reasons for the differences of chemical components in the different medicinal parts.
1. Introduction
Angelica sinensis Radix (A. sinensis) is the dried root of the Angelica Sinensis (Oliv.) Diels [1]. It has the effect of replenishing blood, activating blood circulation and regulating irregular menstruation [2,3]. During the Ming and Qing Dynasties, doctors clearly divided A. sinensis into head (the upper part of the root), body (the taproot) and tail (lateral roots). It is divided into different parts and used as medicine. The main function of the head is to stop bleeding, the body has a strong effect of replenishing blood and the tail focuses on promoting blood circulation and removing blood stasis. There is a high correlation between the difference in the medicinal properties of the different parts of A. sinensis and the basis of the chemical substances [4]. Studies have found that the content of the same substance in the different medicinal parts was different, the content of volatile oil and ferulic acid in the tail of A. sinensis was the highest, and the content of total tannins in head was the highest [5]. Other researchers determined the content of ferulic acid in different medicinal parts of A. sinensis by HPLC and also reached the conclusion that the content of ferulic acid in the tail of A. sinensis was the highest [6].
The accumulation dynamics of chemical components in medicinal plants is closely related to growth and development. A. sinensis is a perennial herb with a total growth cycle of three years with two winters (about 800 days), mainly in three stages: grow–seedling stage, medicine formation period, and reserve–seed period [7]. The first year is the grow–seedling stage, which mainly increases the length of roots to meet the needs of water and inorganic salts in the later growth process [8]. At this stage, the content of Z-ligustilide gradually increased and Z-butenylphthalide decreased; in the early stage of the second year, the stems and leaves grew vigorously, and the accumulated assimilates were transported to the roots through photosynthesis. The contents of Z-ligustilide and Z-butenylphthalein decreased with the growth of stems and leaves and gradually increased after the stems and leaves began to wither. During the reserve–seed period in the third year, the biological yield gradually decreased: these two components first increased with the growth of stems and leaves and then decreased sharply to the lowest at the bolting and flowering stages. In the process of seed maturation to harvest, the content increased briefly and then decreased [9,10,11,12,13,14].
There are obvious differences in the chemical components of different medicinal parts of A. sinensis, and the components and pharmacological activities change greatly in different periods [15]. At present, there is no report on the accumulation law of chemical components in different medicinal parts of A. sinensis in different periods. Therefore, GC-MS technology was used to study the accumulation dynamics of different medicinal parts of A. sinensis and to analyze the differences of chemical components in different medicinal parts in each period to clarify the rules of chemical component accumulation and mutual transformation in the head, body and tail of A. sinensis.
2. Materials and Methods
2.1. Materials and Reagents
The plant materials of A. sinensis used in this experiment were fresh materials that were collected from Xizhai village, Xizhai Town, Min County, Dingxi City, Gansu Province on April 15 (seedling stage), June 6 (leaf growing stage), August 24 (root enlargement stage), September 10 (late root expansion stage) and October 24 (harvest stage) in 2021 and were brought back in an ice box. The voucher specimens (No. GAUAB-AS-20210415, No. GAUAB-AS-20210606, No. GAUAB-AS-20210824, No. GAUAB-AS-20210910 and No. GAUAB-AS-20211025) were deposited in the herbarium of Department of Chinese herbal medicine, Agronomy building of Gansu Agricultural University, Lanzhou, China. The n-Hexane (Lot 110-54-3, Chromatographic grade) was purchased from Shenzhen Dongmao Chemical Reagent Co., Ltd. (Shenzhen, China).
2.2. Sample Solution Preparation
The fresh materials in different periods were divided into head, body and tail (see the left photo of Figure 1). They were subsequently dried to constant weight in a freeze dryer and then crushed. The n-hexane (25 mL) was added to the sample prepared by precisely weighing 2.5 g of the plant material and soaking for 1 h, then ultrasonically extracted for 30 min, the extraction repeated twice and then the filtrate was combined. The combined filtrate was concentrated at 40 °C for 5 min in a rotary evaporator, the concentrate dissolved with n-hexane and adjusted to 5 mL. Finally, it was filtered with a 0.22 μm microporous membrane to obtain A. sinensis sample solution [16].
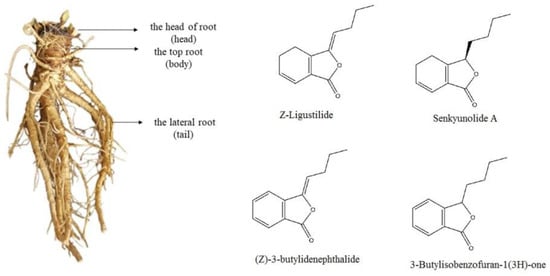
Figure 1.
The photo of A. sinensis root and structures of main components.
2.3. GC–MS Conditions
2.3.1. GC–MS Column
The column used was a DB-23 (30 m × 0.25 mm × 0.25 μm). The carrier gas was high purity helium, and its flow rate was 1.0 mL/min. The initial temperature was maintained at 60 °C for 3 min, and then raised to 270 °C at a rate of 10 °C/min. All samples were injected in split mode at 270 °C. The split injection was 5:1.
2.3.2. Mass Spectrum Conditions
An electron impact ion source was used, with full scanning mode (mass range m/z 50–650), ion source temperature 230 °C, interface temperature 250 °C, quadrupole temperature 150 °C, electronic energy 70 eV and solvent delay time 3 min. The ion detection mode selected ion monitor was selected.
2.4. Data Analysis
GC-MS was used to perform a full ion scan of the compound, and the total ion current map of different medicinal parts of A. sinensis in different periods was obtained. The compound was qualitatively analyzed by searching the NIST14 standard mass spectrometry library, and according to the peak area normalization method the relative content of each component was calculated.
3. Results and Discussion
3.1. Analysis of Chemical Components in the Head of A. sinensis in Different Periods
The sample solution of the head of A. sinensis in different periods was analyzed by GC-MS. The GC-MS chromatogram in different periods was shown in Figure 2. The chemical composition and relative concentrations were obtained using the peak area normalization method (Table 1).
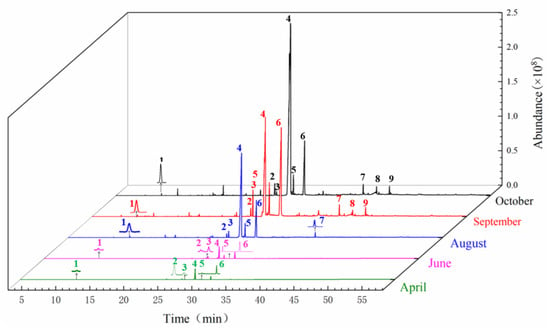
Figure 2.
Total ion chromatogram from the head of root of A. sinensis in different periods. Note: 1: α-Pinene; 2: Z-Butylidenephthalide; 3: 3-Butylisobenzofuran-1(3H)-one; 4: (E)-Ligustilide; 5: Senkyunolide A; 6: (Z)-Ligustilide; 7: Panaxynone; 8: γ-Sitosterol; 9: Senkyunolide H.

Table 1.
Analysis of chemical components in the head of A. sinensis in different periods.
A total of 12 compounds were identified in April: (Z)-ligustilide, (E)-ligustilide, 3-butylisobenzofuran-1(3H)-one, senkyunolide A, α-pinene, etc., among them, the component with the highest relative content was (Z)-ligustilide, which was 1.16%. In June, 20 components were identified, 3-carene, p-cymene, azulene, α-himachalene, panaxynone, ethyl iso-allocholate, 6-undecanone and tetradecane were newly added compounds. A total of 42 compounds were identified in August, mainly increasing α-acorenol, β-copaene, β-chamigrene, patchoulene, β-bisabolene, (+)-bicyclogermacrene, α-longipinene, trans-isoeugenol and other phenolic compounds. In September, 11 compounds including (9Z,12Z)-9,12-Octadecadienoic acid, (Z)-9-octadecenoic acid methyl ester and β-farnesene were newly added. In October, phenolic compounds such as cis-β-ocimene, β-ylangene, thujopsene-(I2), phthalides and vitamin E were added. It was found that August was the peak period of accumulation of phenolic compounds, oleic acid compounds accumulated more in September, and October was the period of accumulation of rare volatile oil compounds; it was also the key period of rapid increase in content.
The relative contents of the compounds in different periods were analyzed by the peak area normalization method, and the accumulation dynamics of four important active components (see the right photo of Figure 1) in the head of A. sinensis were analyzed. As shown in Figure 3, the relative content of (Z)-ligustilide increased gradually from April to October and increased rapidly from September to October. The relative content of (Z)-3-butylidenephthalide decreased by 0.0082% in June, and then showed a gradual upward trend. The relative contents of senkyunolide A and 3-butylisobenzofuran-1(3H)-one reached the maximum value of 3.6762% and 0.7921% in September, and the contents decreased in the harvest period. Combining the changes of compounds in each period, (Z)-ligustilide was the highest content component from seedling stage to harvest stage, and the relative content was up to 72.2466% in harvest stage, which was the main core component in the head of A. sinensis in each period.
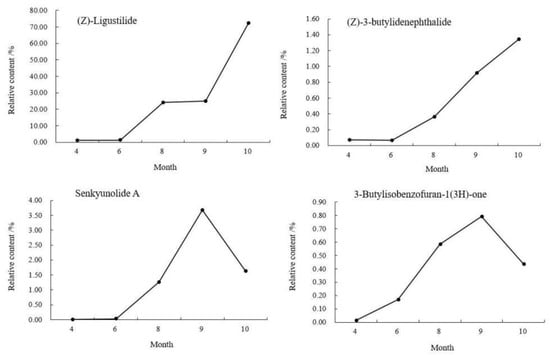
Figure 3.
Changes of chemical components in the head of A. sinensis in different periods.
3.2. Analysis of Chemical Components in the Body of A. sinensis at Different Periods
The GC-MS chromatogram of A. sinensis of the body of A. sinensis in different periods was shown in Figure 4. The chemical composition and relative concentrations were obtained using the peak area normalization method (Table 2). The types of chemical components in the body of A. sinensis varied greatly in different periods.
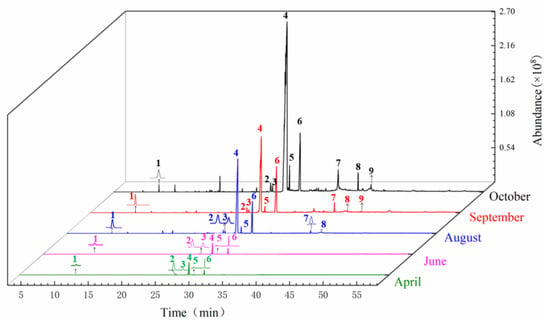
Figure 4.
Total ion chromatogram from the body of A. sinensis in different periods. Note: The compounds represented by peaks 1~9 are the same as the compounds in Figure 2.

Table 2.
Analysis of chemical components in the body of A. sinensis in different periods.
In April, the compounds in the body were the same as those in the head of A. sinensis. They mainly contain Z-ligustilide, (Z)-3-butylidenephthalide, senkyunolide A and other ester compounds and a small amount of olefin compounds. In June, 12 components were added, and 4 compounds were added compared with the head in the same period: 1,2,6,6-tetramethylcyclohexa-1,3-diene, α-acorenol and trans-Isoeugenol. A total of 48 components were identified in the body of A. sinensis in August. In addition to a large number of phenolic components added in the same period, vitamin E, γ-sitosterol, (Z,Z)-9,12-octadecadienoic acid, (3R,3aR,7R,8aS)-3,8,8-trimethyl-6-methyleneoctahydro-1H-3a,7-methanoazulene, etc. were also added. In September, 11 new compounds were added in the body of A. sinensis, mainly including cis-β-ocimene, thujopsene-(I2), (+)-cuparene, 11,14-eicosadienoic acid-methyl ester, carveol and 9-hexadecenoic acid. In October, 68 compounds were identified, and the relative content of Z-ligustilide was the highest, at 71.3681%. Seven new compounds were added, including β-ylangene, 9-hexadecenoic acid, cis-5,8,11,14,17-eicosapentaenoic acid and campesterol.
As shown in Figure 5, it was found that from April to June, the contents of Z-ligustilide, (Z)-3-butylidenephthalide and senkyunolide A in the body of A. sinensis decreased by 0.2584%, 0.0235% and 0.0183%, 3-butylisobenzofuran-1(3H)-one increased by 0.0092%. The aboveground part grew vigorously in June, and these three reduced compounds might be involved in the transformation of the compounds when the aboveground part grew. The content of senkyunolide A decreased by 0.0525% in September; the other three compounds showed an increasing trend from June to October, entered a rapid accumulation period in September and reached the maximum value in October. The relative contents of Z-ligustilide, (Z)-3-butylidenephthalide, senkyunolide A and 3-butylisobenzofuran-1(3H)-one were: 71.3681%, 0.9806%, 1.5863% and 0.5095%.
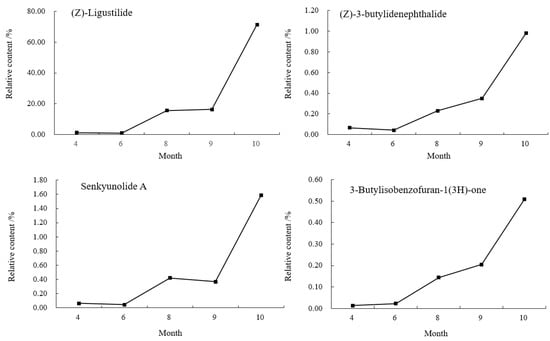
Figure 5.
Changes of chemical components in the body of A. sinensis in different periods.
3.3. Analysis of Chemical Components in the Tail of A. sinensis at Different Periods
The GC-MS chromatogram of A. sinensis of the tail of A. sinensis in different periods was shown in Figure 6. The chemical composition and relative concentrations were obtained using the peak area normalization method (Table 3).
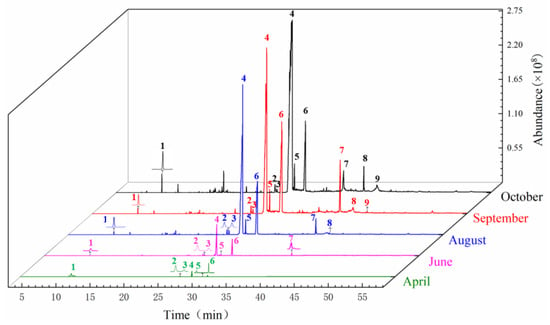
Figure 6.
Total ion chromatogram from the tail of A. sinensis in different periods. Note: The compounds represented by peaks 1~9 are the same as the compounds in Figure 2.

Table 3.
Analysis of chemical composition in the tail of A. sinensis in different periods.
In April, a total of 11 compounds such as (Z)-ligustilide, (E)-ligustilide, (Z)-3-butylidenephthalide, senkyunolide A, α-pinene, arteannuin b, etc. were identified in the tail of A. sinensis. Compared with April, 20 new compounds were added in June, such as 3-carene, β-copaene, β-chamigrene, α-himachalene, p-cymene, (Z)-9-octadecenoic acid, methyl ester, 6-undecanone, panaxynone, carveol, α-acorenol, etc. A total of 55 compounds were identified in August, including olefin compounds such as cis-β-ocimene, cis-β-farnesene, β-bisabolene, α-longipinene, limonen-6-ol, pivalate, 2,5-Octadecadiynoic acid, methyl ester, (Z,Z)-9,12-Octadecadienoic acid ethyl ester and organic acids such as 9-Hexadecenoic acid, (Z,Z)-9,12-Octadecadienoic acid, etc. In September, 7 new compounds, such as thujopsene-(I2) and (+)-cuparene were added. A total of 69 compounds were identified in October, and 7 new compounds were added: including 9-hexadecenoic acid, 9-octadecen-12-ynoic acid methyl ester, 10,13-octadecadiynoic acid methyl ester, pentylbenzene, α-elemene, β-guaiene, and β-ylangene.
As shown in Figure 7, in the tail of A. sinensis, Z-ligustilide, (Z)-3-butylidenephthalide, senkyunolide A and 3-butylisobenzofuran-1(3H)-one were also selected to analyze the relative content change trend. The results showed that the contents of these four compounds showed a gradual upward trend from April to October. The growth was relatively slow from April to June, entered the period of rapid growth after June, and reached the highest value in October. The relative contents were 73.4925%, 0.8135%, 1.4591% and 0.3314%, respectively.
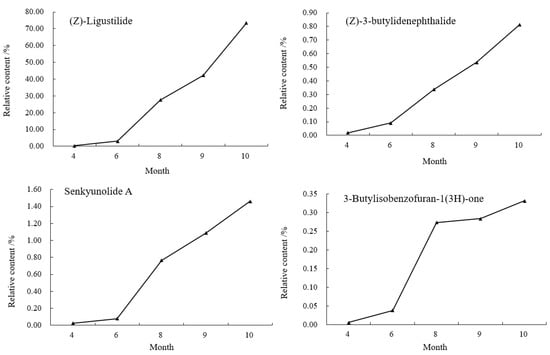
Figure 7.
Changes of chemical components in the tail of A. sinensis in different periods.
3.4. Analysis of the Differences in the Accumulation of Compounds in Different Medicinal Parts of A. sinensis
The number of compounds identified in different medicinal parts of A. sinensis in different periods was shown in Table 4. From April to October, the number of compounds in the head, body and tail of A. sinensis showed an increasing trend. In April, there was one less compound in the tail than in the head and body. In June, there were 11 and 7 more compounds in tail than head and body, respectively. It mainly contained olefin compounds such as β-chamigrene and 1,2,6,6-tetramethylcyclohexa-1,3-diene and ester compounds such as (Z)-9-octadecenoic acid, methyl ester and 7,10-octadecadienoic acid, etc. Since then, the species of compounds in the tail of A. sinensis were always the highest. During the harvest period, the number of species gradually reached a balance, and the number of compounds in head, body and tail were 61, 68 and 69, respectively, and there were differences in the types of components. In the harvest period, 6-epi-shyobunol only existed in the head of A. sinensis, eicosapentaenoic acid was found only in the body of A. sinensis, and 10,13-Octadecadiynoic acid and α-Elemen were only found in the tail of A. sinensis. 9-Hexadecenoic acid, 9-Octadecen-12-ynoic acid methyl ester, limonen-6-ol, pivalate, (+)-cuparene, carveol and (Z,Z)-9,12-octadecadienoic acid were not found in the head but existed in body and tail of A. sinensis.

Table 4.
The number of chemical compounds of A. sinensis in different parts in different periods.
The relative content changes of the four main active components in A. sinensis in different medicinal parts were shown in Figure 8. In April, the content of Z-ligustilide in the body of A. sinensis was the highest, 0.8323% higher than that in the tail. From June, the content of Z-ligustilide in the tail was gradually higher than that in the head and body. From June to October, the relative content remained as tail > head > body. During the rapid accumulation of content in September, the difference of relative content in head, body and tail gradually decreased and tended to balance.
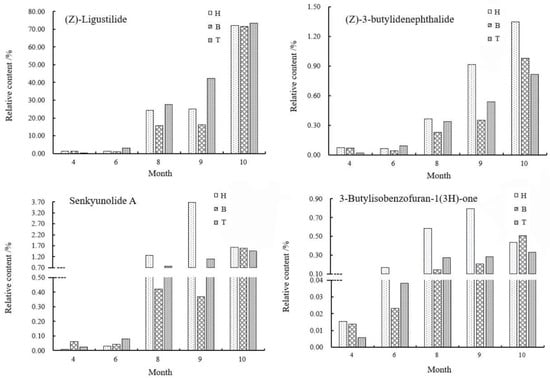
Figure 8.
Changes of chemical components in different medicinal parts in different periods. Note: H, head; B, body; T, tail.
As one of the important active components in A. sinensis, the accumulation law of (Z)-3-butylidenephthalide was different from that of Z-ligustilide. In April, the relative content of (Z)-3-butylidenephthalide in the head was higher, which was 0.0159% higher than that in the tail. In August, the content in the head was higher, and from August to October, the relative content gap between the head and the body and the tail gradually increased, and the highest content in the head was 1.3462% during the harvest period, which was 0.5327% higher than that in the tail. Researchers [17] analyzed the chemical components of different parts by GC-MS and found that the relative contents of compounds in different parts were different. Z-ligustilide was the main compound, which was consistent with the results of our study. Ligustilide compounds affect platelet aggregation or thrombosis [18], and the content of Z-ligustilide in tail was the highest, which was consistent with the effect of tail focusing on promoting blood circulation.
In April, the content of senkyunolide A in the body was higher; the content in the head had always been the highest since August and reached the highest value of 3.6762% in September. In October, the content in the head of A. sinensis decreased by 2.0498%, while the content in the body and tail increased by 1.2184% and 0.3708%, respectively, which may be caused by the transfer of senkyunolide A from head to body and tail.
From April to September, the content of 3-Butylisobenzofuran-1(3H)-one in the head was always the highest and decreased by 0.3047% in the head and increased by 0.3046% and 0.0477% in the tail by the harvest period. The relationship of the content of 3-Butylisobenzofuran-1(3H)-one at the harvest period was: body > head > tail. In September, the aboveground parts withered gradually, the required nutrients decreased gradually and a large number of assimilates transferred to the roots. At this time, the contents of (Z)-3-butylidenephthalide, senkyunolide A and 3-butylisobenzofuran-1(3H)-one decreased in the head and increased in the body and tail, which can confirm this inference. It was speculated that there was a phenomenon that the compounds that accumulated in the head transferred to the body and tail during the accumulation of effective components in different medicinal parts. Overall, the accumulation and distribution of compounds were consistent with the efficacy of different medicinal parts of A. sinensis and the accumulation of the types and contents of the compounds reached the maximum in October, which was consistent with the traditional harvesting period [19].
The content changes of the same compound in different medicinal parts of A. sinensis in different periods may be caused by some related factors during growth and development such as environmental factors, temperature and solar radiation, which would accelerate the synthesis of a compound, thus slowing down the synthesis of other related compounds [20,21]. But the specific mechanism is not clear at present.
4. Conclusions
In summary, we revealed the differences of accumulation dynamics in different medicinal parts of A. sinensis by GC-MS for the first time, which were mainly reflected in the component types and relative contents. The number of compounds contained in different medicinal parts of A. sinensis showed an increasing trend from April to October, but the number of compounds in different medicinal parts was different in each period and the types of components were also different. This study revealed the accumulation rule of chemical constituents in different medicinal parts of A. sinensis and provided a theoretical basis for the differences in compounds and medicinal properties in different medicinal parts.
Author Contributions
Conceptualization, Y.C. and Q.L.; data curation, Y.C.; formal analysis, Y.C.; investigation, Y.C. and Q.L.; methodology, Y.C. and Q.L.; project administration, Q.L. and D.Q.; supervision, Q.L. and D.Q.; validation, Y.C.; writing—original draft, Y.C.; writing—review and editing, D.Q. and Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31860102), Research Program Sponsored by Gansu Provincial Key Laboratory of Aridland Crop Science, Gansu Agricultural University (No. GSCS-2018-3), Discipline Construction Fund Project of Gansu Agricultural University (GSAU-XKJS-2018-086), FuXi Young Talents Introduction Projects of Gansu Agricultural University (GSAU-RCZX201704) and Outstanding Graduate Student Innovation Star Project in Gansu Province(2021CXZX-399).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
There are no conflict of interest to declare.
Sample Availability
Samples of the plant materials of Angelica sinensis are available from the authors.
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; Volume 1, pp. 274–275. [Google Scholar]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, Z.; Zhao, H.; Ju, D.; Chen, Y.; Chen, X.; Zhang, J. Ferulic acid promotes endothelial cells proliferation through up-regulating cyclin D1 and VEGF. J. Ethnopharmacol. 2011, 137, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Duan, J.A.; Shang, R.X.; Hua, Y.Q.; Qian, D.W. Study on chemical materials and drug nature association of efficacy orientation of different parts from Angelicae sinensis Radix. Chin. Tradit. Herb. Drugs 2014, 45, 3208–3212. [Google Scholar]
- Su, B.H. Study on Effective Chemical Constituents in Different Parts of Angelica Sinensis. China J. Pharm. Econ. 2019, 14, 34–37. [Google Scholar]
- Huang, R. Analysis and research on the content of active components in different medicinal parts of Angelica sinensis. Inn. Mong. J. Tradit. Chin. Med. 2017, 36, 117–118. [Google Scholar]
- Gao, X.; Guo, F.; Chen, Y.; Bai, G.; Liu, Y.; Jin, J.; Wang, Q. Full-length transcriptome analysis provides new insights into the early bolting occurrence in medicinal Angelica sinensis. Sci. Rep. 2021, 11, 13000. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.Q.; Hu, Z.H. Research on the Growth and Development of Angelica Root. J. Chin. Med. Mater. 1982, 1, 2–6. [Google Scholar]
- An, Z.; Guo, F.; Chen, Y.; Bai, G.; Chen, Z. Rhizosphere bacterial and fungal communities during the growth of Angelica sinensis seedlings cultivated in an Alpine uncultivated meadow soil. PeerJ 2020, 8, e8541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.R. Eco-Driver Factors Research of Quality Variation for Angelica Sinensis Radix. Master’s Thesis, Peking Union Medical College, Beijing, China, 2021; pp. 38–47. [Google Scholar]
- Sun, H.M. Study on Resources Survey and Quality Characteristics of Angelica sinensis. Master’s Thesis, China Union Medical College, Beijing, China, 2010; pp. 36–38. [Google Scholar]
- Ling, F.; Xiao, X.F.; Liu, C.X.; Xin, H.E. Recent advance in studies on Angelica sinensis. Chin. Herb. Med. 2012, 4, 12–25. [Google Scholar]
- Lin, H.M.; Liu, X.Z.; Liu, X.R.; Wang, X.Z. Effects of cultivation methods on dry matter accumulating and growth dynamics of Angelica sinensis. Chin. Herb. Med. 2007, 38, 257–261. [Google Scholar]
- Sa, R.N.; Wang, Y.L.; Zhu, S.Q. Study on the Dynamic Variations of Z-ligustilide and n-Butylidenephthalide Contentin Essential Oil of Radix Angelicae Sinensis from Different Growth Period. J. Chin. Med. Mater. 2012, 35, 1138–1142. [Google Scholar]
- Zhang, W.L.; Zheng, K.Y.; Zhu, K.Y.; Zhan, J.Y.; Bi, C.W.; Chen, J.P.; Dong, T.X.; Choi, C.Y.; Lau, T.W.; Tsim, K.W. Chemical and biological assessment of angelica roots from different cultivated regions in a chinese herbal decoction danggui buxue tang. Evid.-Based Complement. Altern. Med. 2013, 2013, 483286. [Google Scholar]
- Liu, L.J.; Deng, S.F.; Sun, J.Y.; Xing, D.M.; Wang, Y.P. Comparison of Chemical Constituents of the Essential Oil from Different Parts of Angelica sinensis by GC-MS. Chem. World 2020, 61, 426–432. [Google Scholar]
- Wu, H.Y.; Hua, Y.L.; Guo, Y.S.; Wei, Y.M. Extraction and Analysis of Volatile Oil in Different Parts of Radix Angelicae Sinensis from Min County in Gansu. Nat. Prod. Res. Dev. 2012, 24, 1225–1229. [Google Scholar]
- Choi, E.S.; Yoon, J.J.; Han, B.H.; Jeong, D.H.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Ligustilide attenuates vascular inflammation and activates Nrf2/HO-1 induction and, NO synthesis in HUVECs. Phytomedicine 2018, 38, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Guo, S.J.; Wang, W.N.; Wei, G.X.; Ma, G.Y.; Ma, X.D. Diversity and Bioactivity of Endophytes From Angelica sinensis in China. Front. Microbiol. 2020, 11, 1489. [Google Scholar] [CrossRef]
- Ge, Y.L.; Qian, D.W.; Duan, J.A.; Song, B.S.; Su, S.L.; Shang, R.X.; Yan, H.; He, Z.P. Studies on the dynamic accumulations of Radix Angelicae Sinensis volatile constituents in different regions and harvest times and the appropriate harvest time. Chin. J. Pharm. Anal. 2009, 29, 517–523. [Google Scholar]
- Lin, G.S.; Ma, C.J.; Li, X.Y.; Liu, K. Studies on the Stability of Ligustilide and the Analysis of Its Isomerized Products by GC-MS. Chin. Herb. Med. 2000, 6, 7–9. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).