Abstract
The COVID-19 pandemic has intensively disrupted global health, economics, and well-being. Andrographis paniculata (Burm. f.) Nees has been used as a complementary treatment for COVID-19 in several Asian countries. This review aimed to summarize the information available regarding A. paniculata and its constituents, to provide critical points relating to its pharmacological properties, safety, and efficacy, revealing its potential to serve as a source of lead compounds for COVID-19 drug discovery. A. paniculata and its active compounds possess favorable antiviral, anti-inflammatory, immunomodulatory, and antipyretic activities that could be beneficial for COVID-19 treatment. Interestingly, recent in silico and in vitro studies have revealed that the active ingredients in A. paniculata showed promising activities against 3CLpro and its virus-specific target protein, human hACE2 protein; they also inhibit infectious virion production. Moreover, existing publications regarding randomized controlled trials demonstrated that the use of A. paniculata alone or in combination was superior to the placebo in reducing the severity of upper respiratory tract infection (URTI) manifestations, especially as part of early treatment, without serious side effects. Taken together, its chemical and biological properties, especially its antiviral activities against SARS-CoV-2, clinical trials on URTI, and the safety of A. paniculata, as discussed in this review, support the argument that A. paniculata is a promising natural source for drug discovery regarding COVID-19 post-infectious treatment, rather than prophylaxis.
1. Introduction
Since 2019, Coronavirus disease (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as a global challenge, particularly in terms of the rapid increase in critically ill patients, leading to morbidity and mortality. Up until May 2022, over 500 million infected cases and over 6 million deaths have been confirmed worldwide [1]. SARS-CoV-2 initiates its infection process via interaction with the human angiotensin-converting enzyme 2 (ACE2) receptors through its receptor-binding domain (RBD) [2], and uses the transmembrane protease serine 2 (TMPRSS2) for priming the spike protein [3]. COVID-19 patients can be asymptomatic or suffer from mild respiratory symptoms. Unfortunately, many patients suffering from SARS-CoV-2 infection subsequently developed acute respiratory distress syndrome (ARDS) and cytokine release syndrome (CRS), which led to life-threatening multi-organ failures that were considered to be the leading cause of death [4]. Although COVID-19 vaccinations have proved to be an effective prevention method that has helped to reduce the numbers of new cases and mitigate the severity of infected cases, the contagious nature of this virus and the emergence of new SARS-CoV-2 variants have given rise to concerns that this epidemic will be prolonged. Since no gold-standard therapeutics are available, researchers are urgently seeking possible solutions to tackle this pandemic situation. Several natural products and traditional medicines have been reviewed and considered promising prophylactic agents, treatments, or lead compounds for drug discovery due to their potential properties and tolerable toxicity [5]. In this review, we discuss Andrographis paniculata (Burm. f) Nees, a herbal medicine that has been used traditionally to alleviate flu and respiratory syndrome and has proven effective for its antiviral, anti-inflammatory, and immunomodulatory activity. Although the phytochemical and pharmacological profiles of A. paniculata have previously been reviewed [6,7,8], this article aims to provide valuable and up-to-date scientific data regarding A. paniculata and its constituents relating to COVID-19. The extract of A. paniculata and the andrographolide derivatives are among natural lead compounds that have been selected and evaluated in recent in silico and in vitro models for their potential in COVID-19 management.
Andrographis paniculata (Burm. f) Nees (synonyms A. paniculata var. glandulosa Trimen., Justicia paniculata Burm. f), belonging to the Acanthaceae family, is an annual herbaceous plant that is native to India and Sri Lanka; it has also been cultivated or naturalized in various other areas of the world (China, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Thailand, Vietnam, and the Caribbean) as shown in Figure 1 [9]. It is commonly known as the “king of bitter”, or green Chiretta in English. It is also known as “Kalmegh” in Hindi, “Chanxinlian” in Chinese, “fah tha lai” in Thai, and “Hempedubumi” in Malaysian. The aerial part of A. paniculata has been effectively utilized as a medicinal herb in many traditional systems for centuries, used to cure fever and to treat the common cold, respiratory symptoms, diabetes, and skin infections [10]. A. paniculata is described in several official materia medica and national pharmacopeia, such as the Chinese herbal pharmacopeia, Indian herbal pharmacopeia, Thai herbal pharmacopeia and British herbal pharmacopeia, and appears in the United States pharmacopeia as a dietary supplement. Furthermore, the World Health Organization included A. paniculata as a medicinal plant in a WHO monograph on widely used medicinal plants that was intended to monitor quality control and herbal medicine usage [11]. In Thailand, the Ministry of Public Health has chosen A. paniculata as one of the essential medicinal plants included in the Thailand National List of Essential Medicines [12], used in hospitals and public health services.

Figure 1.
Morphological characteristics of Andrographis paniculata. (A): Aerial part, (B): fruits and flowers, (C): close-up of the flower, and (D): fruits.
2. Materials and Methods
This study was conducted by reviewing the published information in the national pharmacopeias, books, published articles, and electronic databases such as the Web of Science, Scopus, PubMed, Google Scholar, Elsevier, and so on. The extensive results were explored for subsequent analyses and summaries of the botanical and chemical profiles, as well as the scientific data, of A. paniculata and its related compounds in terms of its pharmacological properties in the context of COVID-19. Furthermore, we have also gathered information on the safety profiles of A. paniculata for this article.
3. Results
3.1. Traditional Uses
The aerial parts and roots of A. paniculata are utilized in many countries for different medicinal purposes, in Asia as well as Europe. The crude drug from A. paniculata, known as Kalmegh in India, has been commonly used as a primary component in more than 26 Ayurvedic formulations [10,13]. In addition, it is reported that the drug has been employed to treat all types of fever, particularly intermittent fevers [14]. Obtained from India and Southeast Asia for over century A. paniculata (Chuanxinlian in Chinese) has been used in Chinese medicine to clear heat and dampness; according to the traditional theory of Chinese medicine, it counters fire on account of its cooling properties [15]. In Southeast Asian countries, e.g., Thailand and Malaysia, it has been utilized for the treatment of diabetes and hypertension in Malaysian folk medicine [8]. Conversely, in Thai traditional medicine, A. paniculata has been given to patients suffering from fever and common cold symptoms. It is also used in Scandinavian countries for curing fevers and the symptoms of the common cold [10].
3.2. Chemical Constituents
The chemical substances that are found in A. paniculata have been studied extensively, as shown in Table 1. It is reported that the plant contains many diterpenoids, lactones, and flavonoids. Nevertheless, the concentration and composition of its phytochemicals vary, according to geography, plant parts, season, and phenological growth stage [16]. The main active compound of A. paniculata is andrographolide, which is found in the whole plant, leaves, stem, and roots. The characteristics of andrographolide are a colorless and bitter ent-labdane diterpene lactone substance [17]. Actually, this substance was first isolated by Boorsma, who named it andrographide. Then, in 1911, Gorter proved the structure of andrographolide and gave it that name (Figure 2C) [17,18]. Andrographolide can be extracted from all parts of the plant but it is most highly concentrated in the leaves [10,19,20,21,22,23]. There are several studies proving that the highest amount of andrographolide is found in the vegetative stage before flowering (about 130 days after initial cultivation) [22,24,25]. Andrographolide possesses various pharmacological properties, such as anti-allergic, anti-bacterial, anticancer, anti-diabetic, anti-dyslipidemic, anti-inflammatory, anti-leishmanial, anti-viral, antipyretic, analgesic, hepatoprotective, and neuroprotective activities [26,27,28,29,30,31,32,33]. The other ent-labdane diterpenoids from this plant, i.e., neoandrographolide (Figure 2F), isoandrographolide (Figure 2E), 14-deoxy-11, and 12-didehydroandrographolide (Figure 2H), also exhibit pharmacological activities [24]. In addition, the findings of this study indicate that a number of flavonoids have been reported in the past three decades. The isolation and elucidation of flavonoids were mostly performed using the root, but they were also found in the aerial parts of the plant. Previous studies revealed that the flavonoids show biological activity, including anti-proliferative and anti-platelet aggregation properties [24,34,35]. Besides this, xanthone, quinic acid and its derivative, sitosterol, and polysaccharide were reported as minor chemical substances found in A. paniculata [36,37,38].

Table 1.
Chemical constituents of Andrographis paniculata: ent-labdane diterpenoids, flavonoids, and other substances.
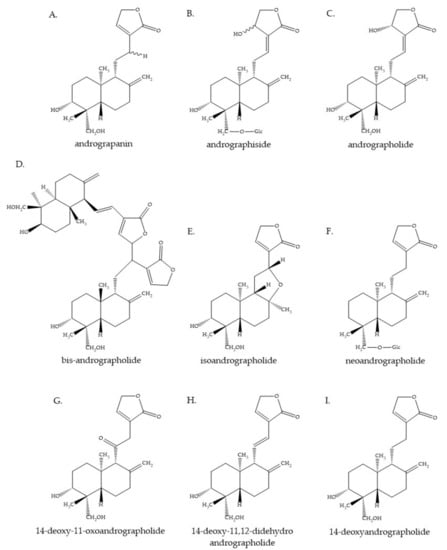
Figure 2.
Some of the chemical constituents of Andrographis paniculata. (A): Andrograpanin, (B): andrographiside, (C): andrographolide, (D): bis-andrographolide, (E): isoandrographolide, (F): neoandrographolide, (G): 14-deoxy-11-oxoandrographolide, (H): 14-deoxy-11,12-didehydroandrographolide, and (I): 14-deoxyandrographolide.
3.3. Pharmacological Activities: Anti-SARS-CoV-2 and Other Anti-Virus
Since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been identified as the causative pathogen of the COVID-19 outbreak, antiviral drugs have been at the top of the list for COVID-19 management, to reduce viral shedding and control the spread. To date, there is no specific antivirus against SARS-CoV-2. Certain conventional antiviral agents, i.e., lopinavir/ritonavir, favipiravir, and remdesivir have been utilized in many countries during the pandemic [84]. However, their effectiveness, specificity, limitations, and the adverse effects of existing treatments that have occurred in several cases have led to an urgent need for effective drugs for the prevention and treatment of COVID-19. Many studies had previously confirmed the virucidal activity of A. paniculata against different viral strains, including the influenza A virus (IAV), human immunodeficiency virus (HIV), Hepatitis B and C, Herpes Simplex virus I, Epstein-Barr virus, human papillomavirus, and Chikungunya virus [85].
3.3.1. In Silico Analysis of Potential Anti-SARS-CoV-2 Agents from A. paniculata Phytochemicals
Due to the rapid spread of COVID-19, scientists around the world are racing against time to find potential anti-SARS-CoV-2 agents. The in silico approach is often used to identify drugs or potential compounds, to save time and costs. Several studies have employed in silico approaches, particularly molecular docking, and molecular dynamics (MD) simulations, to study the effects of AP phytochemicals on SAR-CoV-2’s vital proteins and the proteins involved in the process of infecting the human body. In this paper, we will focus on the in silico analysis of A. paniculata phytochemicals on five potential drug targets: the spike (S) glycoprotein, 3-chymotrypsin-like protease (3CLpro), papain-like protease (PLpro), and RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2 and human angiotensin-converting enzyme 2 (hACE2) [86].
The SAR-CoV-2 S-glycoprotein, a trimeric class I fusion protein, is marked because it plays a pivotal role in SARS-Cov-2 attachment via its binding to hACE2. S-glycoprotein contains two domains, S1 and S2. The S1 receptor-binding domain (RBD) binds to the peptidase extracellular domain (PD) of hACE2; then, cleavage by the host proteases at the S1–S2 inter-domain protease site leads to the fusion of the S2-mediated virus and host cell membrane [87,88]. The S-glycoprotein is a key target for vaccines, therapeutic antibodies, small-molecule drugs, and diagnostics [86]. Recently, Hiremath et al. conducted an in silico docking analysis using AutoDock Vina software on 14 A. paniculata phytochemicals, including andrographolide, in terms of their being SAR-CoV-2 inhibitors and revealed that various A. paniculata phytochemicals can bind SARS-CoV-2 proteins, especially the RBD of the S-glycoprotein. Isoandrographolide (Figure 2E) is the best substance for binding to the S-glycoprotein, with the lowest binding energy (−9.1 kcal/mol). Besides this, other A. paniculata phytochemicals, comprising 19-O-acetyl-14-deoxy-11,12-didehydroandrographolide, neoandrographolide (Figure 2F), and 5-hydroxy-7,8,2′,5′-tetramethoxy flavone, also showed low binding energy to the S-glycoprotein. The results indicated that andrographolide (Figure 2C), the major active ingredient, binds to S-glycoprotein with a binding energy of −7.9 kcal/mol. The findings of Hiremath et al. also showed that there are no significant differences in the binding affinities of these substances with closed- and open-state S-glycoprotein [89]. Previously, Lakshmi et al. conducted their study in a similar way, but instead added a 200 ns MD simulation and used the same open-state S-glycoprotein structure (PDB ID: 6VYB). This work only studied three of the major active ingredients of A. paniculata: andrographolide, bis-andrographolide, and caffeic acid. The results showed that andrographolide is one of the top 10 substances that bind to open-state S-glycoprotein, out of 47 bioactive compounds taken from ten traditional medicinal plants [82]. The research by Dey et al. on the effect of andrographolide and 14-deoxy-11,12-didehydroandrographolide (Figure 2H), using AutoDock 4.0 software, showed that both substances bind equally well to S-glycoprotein and tend to be more binding to S-glycoprotein than 3CLpro and PLpro [90]. From the above results, it can be seen that the best bioactive compound under consideration that is likely to inhibit the S-glycoprotein is isoandrographolide. However, andrographolide, a major active ingredient, also tends to bind and inhibit the activity of S-glycoprotein.
The next protein target that should be mentioned after S-glycoprotein is the metallopeptidase enzyme, hACE2. As previously mentioned, the research shows that the inhibition of hACE2 or the inhibition of the binding of the S-protein to the PD of hACE2 is a promising method of treating SAR-CoV-2 infection. The study by Alazmi et al. explores the same direction as another study by Srivastava et al. Both studies use molecular docking and continue with MD simulation, but they each used a different 3D structure of hACE2 (PDB ID: 6M17) and the model, based on the BLAST parameters for the Alazmi and Srivastava studies, respectively. However, both studies showed that andrographolide is a promising hACE2 inhibitor. Alazmi et al. found that andrographolide is the best compound, out of over 200,000 natural compounds, for hACE2 interaction, demonstrating the lowest binding energy, as calculated using the MM-PBSA/MM-GBSA tool. Likewise, Srivastava and colleagues reported that andrographolide is one of the best three compounds out of the 30 natural and synthetic compounds that demonstrate in vitro and in vivo antiviral activity [81,91].
The development of SAR-CoV-2 protease inhibitors is another potential method for treating SAR-CoV-2 infection, in particular, two cysteine proteases, the 3-chymotrypsin-like protease (3CLpro) or main protease (Mpro) and the papain-like protease (PLpro). These proteases take part in the cleavage of polyproteins to produce the non-structural proteins 2–16 (nsp2−16), which are involved in the replication-transcription complex and are critical for viral replication and transcription in the host cell [86]. Since the structures of 3CLpro and Plpro have been identified and are in the Protein Data Bank (PDB), many studies have been used on these structures to study the binding between the AP phytochemicals and the active site of these proteases. Sukardiman et al. found that among 45 phytochemicals from A. paniculata, the flavonoid glycosides 5,4′-dihydroxy-7-O-β-d-pyran-glycuronate butyl ester and andrographolide glycoside 3-O-β-d-glucopyranosyl-andrographolide showed good binding energy with 3CLpro and the highest similarity of interaction types with amino acid residues, compared to the co-crystal ligands of structure PDB ID: 6LU7 and indinavir, an HIV protease inhibitor [92]. Other substances, including andrographolide, bis-andrographolide (Figure 2D), andrographiside (Figure 2B), andrograpanin (Figure 2A), and neoandrographolide (Figure 2F) have also been reported to bind to 3CLpro [82,93,94,95,96,97]. Interestingly, the docking analysis conducted by Shi et al. showed that andrographolide binds to 3CLpro with a covalent linkage, which is an ideal springboard for developing cysteine protease inhibitors [97]. Similarly, a number of A. paniculata phytochemicals have been studied for their properties as PLpro inhibitors. However, previous studies have not been used on the SAR-CoV-2 PLpro structure, but instead used the PLpro structures of other coronaviruses or PLpro structures developed from homology modeling. Therefore, the accuracy of the study results is one of the important factors that should be considered, since using a PLpro protein structure from another coronavirus yields less accurate results than using the PLpro protein structure of SAR-CoV-2 itself. Elsewhere, 14-deoxy-11,12-didehydroandrographolide has been reported to be better than andrographolide for binding to PLpro, as analyzed by molecular docking [90]. Moreover, Murugan et al. also reported that neoandrographolide not only binds to 3CLpro but also binds to PLpro better than lopinavir, an HIV protease inhibitor [95]. Nevertheless, further in silico studies of the effects of A. paniculata phytochemicals using specific SAR-CoV-2 PLpro structures are needed.
The last protein drug target of SAR-CoV-2 infection discussed in this article is RdRp. RdRp or nsp12 is a crucial polymerase enzyme for genome replication and the gene transcription of SAR-CoV-2. as well as in other RNA viruses. This enzyme is the drug target of remdesivir, a nucleotide prodrug of an adenosine analog and a drug that is currently being used to treat patients with SAR-CoV-2 infection [86,98,99]. The molecular dockings and molecular dynamics simulation analysis of Sharma et al. showed that 5 bioactive compounds from A. paniculata, namely, andrographolide, hydroandrographolide, isoandrographolide, neoandrographolide, and oxoandrographolide, bind to RdRp but are not as successful as 3CLpro. The study by Sharma has the advantage of using the specific RdRp structure of SAR-CoV-2 (PDB ID: 6M71), while other structures were not used. Therefore, Sharma’s study offers a more realistic reflection of RdRp behavior [100]. Another study by Murugan et al., who used the homology model of RdRp, showed contradictory results. Neoandrographolide was reported as a potential compound for treating SAR-CoV-2 infection and binds to RdRp better than remdisevir and the main protease [95].
3.3.2. In Vitro Studies of the Anti-SARS-CoV-2 Activity of A. paniculata Extract and Its Components
Although there are many in silico studies of individual small molecules from A. paniculata regarding anti-SARS-CoV-2 activity, there are limitations in vitro and a lack of in vivo studies. Sa-ngiamsuntorn and colleagues from Thailand recently published the results of studies on the anti-SARS-CoV-2 activity of the ethanolic extracts of A. paniculata and andrographolide in human lung epithelial cells. The results showed that A. paniculata ethanolic extract and andrographolide could inhibit the infectious virion production, with IC50 of 0.036 µg/mL and 0.034 µM, respectively [101], while a previous study by Shi et al. that studied its activity, both in silico and in vitro, found that andrographolide could inhibit the 3CLpro activities of SARS-CoV-2, with an IC50 of 15 µM [97]. Both studies show that andrographolide is more successful in inhibiting 3CLpro than in inhibiting infectious virion production. It can be surmised that andrographolide has multiple mechanisms of action and is also involved in multiple steps of the viral life cycle, as predicted by the in silico studies.
The review above indicates that the ethanolic extracts of A. paniculata and andrographolide are effective in inhibiting the production of the infectious virion and could be synergistic with modern drugs. In terms of the use of A. paniculata extracts and andrographolide as a prophylactic agent to prevent viral entry into the host cells, although in silico studies have demonstrated that several compounds extracted from A. paniculata could bind to human ACE2, further in vitro or in vivo studies should confirm this.
3.3.3. Antiviral Activity
The traditional use of A. paniculata for respiratory infections had led to both in vitro and in vivo studies on anti-influenza activity. An in vitro assay demonstrated that andrographolide and its derivatives were active against various subtypes of Influenza A (IAV) viruses, including H1N1, H3N2, H9N2, and H5N1 [102,103,104,105]. Andrographolide was reported to ameliorate the H1N1 virus-induced cell mortality of an infected human bronchial epithelial cell line (16HBE) by inhibiting the viral-induced activation of the retinoic acid-inducible gene-I (RIG-I)-like receptor (RLR) signaling pathway [102]. Cai et al. found that 14-deoxy-11,12-dehydroandrographolide (DAP), another major constituent of A. paniculata, was more active than andrographolide against IAV H5N1 in vitro by means of inhibiting viral replication, restraining the nuclear export of vRNP complexes, and attenuating the immune dysregulation induced by virus infection [105]. An in vivo study conducted using the prophylaxis and treatment model revealed that AL-1 (14-a-lipoyl andrographolide), a synthetic derivative of andrographolide, was a potent antiviral agent against IAV subtypes H1N1, H9N2, and H5N1 by interfering with viral hemagglutinin and blocking viral binding to cellular targets [103].
Inhibitory activity against the HIV virus in the H9 cell line of A. paniculata aqueous extract was reported at nontoxic concentrations, with the growth of the H9 cells [106]. Andrographolide, a diterpene lactone compound, served as the main active component of its anti-HIV effect, along with its derivatives, such as 14-deoxy-11,12-didehydroandrographolide, dehydroandrographolide succinic acid monoester (DASM), the oxime of 3-keto derivative, amide derivatives of andrographolic acid, and the 12-ester of 12-hydroxy-14-deoxy-13,14-dehydroandrographolide [48,107,108]. One proposed mechanism involved the proprotein convertases -1 and -7 and the extract’s furin inhibition capacity, leading to the suppression of the proteolytic processing of glycoprotein gp160 by HIV, which is PC-mediated [109]. Moreover, the semisynthetic derivatives of andrographolide were evaluated in vitro, revealing that the 3-nitrobenzylidene derivative was more active against HIV, while the 2′,6′-dichloro-nicotinoyl ester derivative showed a higher therapeutic index compared to andrographolide. Computational studies exhibited inhibition capacity regarding andrographolide and its semisynthetic analogs on the gp120-mediated cell fusion of HL2/3 cells (expressing gp120 on its surface) with TZM-bl cells (expressing CD4 and co-receptors CCR5 and CXCR4) [107]. A phase I clinical trial using 10 mg/kg andrographolide was conducted in HIV patients; it showed no significant decrease in viral load but a significant increase in the CD4+ lymphocyte level, which may be a result of the inhibition of HIV-induced cell-cycle dysregulation [110].
The anti-herpes simplex virus 1 (HSV-1) activity of andrographolide and its derivatives, including neoandrographolide, 14-deoxyandrographolide (DAD), 14-deoxy-11,12-didehydroandrographolide, and 3,19-isopropylideneandrographolide (IPAD), was revealed in the in vitro studies, which suggest that these compounds possessed inhibitory effects on viral entry and replication [111,112]. The ethanolic extract of A. paniculata and the major compound andrographolide was reported to be active against the Epstein–Barr virus (EBV) by inhibiting the expression of EBV lytic proteins, transcription factors (Rta and Zta), and early antigen diffuse (EA-D), leading to the prevention of mature viral particles produced during the viral lytic cycle in P3HR1 cells [113]. Andrographolide and its dehydroandrographolide derivatives exhibited anti-HBV activity by inhibiting the secretion of the viral envelope antigen, HBsAg and HBeAg, as well as HBV DNA replication [77]. The anti-hepatitis C virus (HCV) of andrographolide was evaluated, showing that andrographolide could suppress HCV replication via the nuclear factor erythroid 2-related factor 2 (Nrf2)–mediated HO-1 (Nrf2–HO-1) signaling pathway, by activating p38 MAPK phosphorylation. Moreover, it acted as an adjuvant to the standard therapy IFN-α, an inhibitor targeting the HCV NS3/4A protease (telaprevir), and NS5B polymerase (PSI-7977) [114]. An in silico study demonstrated that andrographolide strongly and stably interacted with HCV NS3-4A protease and two drug-resistant mutants, namely, R155K and D168A, which had the least energy compared to a conventional medicine, Asunaprevir [115]. Andrographolide and 14-deoxy-11-oxoandrographolide (Figure 2G) possessed significant inhibitory activity against dengue viral protein NS5 in silico [116]. An in vivo assay confirmed the dengue viral inhibitory property of the methanolic and ethanolic extracts of A. paniculata [117,118]. The anti-Chikungunya virus (CHIKV) activity of andrographolide was examined in vitro, demonstrating an inhibitory effect on CHIKV infection by inhibiting protein synthesis and viral genome replication [119]. Gupta et al. confirmed the antiviral activity of andrographolide against CHIKV in vitro and in vivo, proposing that the inhibition of virus propagation and virus-induced inflammation, as well as the activation of the host’s innate immunity, might serve as mechanisms of action for anti-CHIKV [120]. Moreover, andrographolide and its derivatives, namely, 14-deoxy-11,12-didehydroandrographolide (14-DDA) and 3,19-isopropylidene andrographolide (IPAD), were active against human papillomavirus-16 (HPV16) infection, which is a high-risk human papillomavirus (HR-HPV) subtype causing cervical cancer; this is due to their abilities to inhibit E6 oncoprotein expression and restore p53 expression [121,122]. The overall virucidal bioactivity of A. paniculata against a diverse group of viruses from various families via different mechanisms has suggested that this plant has the potential for novel antiviral drug discovery.
3.4. Pharmacological Activity Relating to the COVID-19 Illness
3.4.1. Immunomodulatory Activity
SARS-CoV-2 infection can cause many different clinical features with varying degrees of severity, ranging from mild to life-threatening, depending on the individual’s immune system. Innate and adaptive immune responses are known to play an important role in repelling viral invaders. In addition, an imbalanced or impaired immune system may contribute to the development of disease manifestations, in terms of severity or opportunistic infection [123]. Hence, an immunity-boosting agent could be a promising strategy to combat or promote the effective treatment of SARS-CoV-2 infection.
A. paniculata, along with its constituents, diterpene lactones, have been reported to exert immunomodulatory activities via numerous pathways for over two decades. It has been proposed that the immunostimulant properties of A. paniculata are responsible for a wide range of pharmacological effects, especially its anti-infectious, anti-inflammatory, and anticancer activity [10,48]. In 1993, Puri et al. revealed the in vivo immunostimulant activity of A. paniculata ethanolic extract and the purified diterpenes, andrographolide, and neoandrographolide. The stimulation of both antigen-specific and nonspecific immune responses was observed, resulting in the enhancement of humoral and cell-mediated immune responses to sheep red blood cells (SRBC), as well as the macrophage migration index (MMI) phagocytosis and the proliferation of splenic lymphocytes in treated mice. It was noteworthy that the ethanolic extract was more active than purified andrographolide, suggesting that other constituents in A. paniculata might lead to a synergistic effect toward the stimulant activity of ethanolic extract [124]. Correspondingly, Wang et al. found that andrographolide exhibited an in vitro and in vivo modulatory effect on both innate and adaptive immune responses by regulating macrophage phenotypic polarization and Ag-specific antibody production, as well as antigen-specific IL-4-producing splenocytes. In this study, andrographolide showed an inhibitory effect on the phosphorylation of ERK 1/2 (the MAPK signaling pathway) and AKT (the PI3K signaling pathway) in macrophages treated with andrographolide, suggesting that the ERK 1/2 and AKT pathways may contribute to the regulatory activity of andrographolide on macrophage activation and polarization [125].
In addition, the immune-boosting activity of three diterpene compounds isolated from a methanolic extract, i.e., andrographolide (Figure 2C), 14-deoxyandrographolide (Figure 2I), and 14-deoxy-11,12-didehydroandrographolide (Figure 2H), at a low concentration were reported in an in vitro study showing an enhancement of the proliferation of human peripheral blood lymphocytes (HPBLs) and interleukin-2 (IL-2) induction in HPBLs [126]. This study supports the finding in a study of normal and type-2 diabetic mice, which revealed that the methanolic leaf extract of A. paniculata led to the increment of blood and splenic lymphocyte count, as well as peritoneal macrophage count [127]. These results are consistent with an in vitro study showing that the ethanolic extract of A. paniculata, which contains andrographolide as a major component, was able to induce lymphocyte cell proliferation at low concentrations (1–16 μg/mL) [128]. A study in male albino rats also showed that the chronic administration of A. paniculata leaf aqueous extract, at 250 mg/kg and 500 mg/kg, significantly increased white blood cell, lymphocyte, and monocyte counts, as well as serum IL-6 and TNF-α, and decreased the neutrophil and eosinophil counts. However, in this study, there was evidence that a 1 g/kg dosage of A. paniculata aqueous extract might contribute to anemia, multiple myeloma, and autoimmunity [129].
The immunomodulatory effects of mixtures and commercial medications containing A. paniculata and diterpene active compounds were investigated in several studies. In China, an in vitro study on LianBiZhi (LBZ), an injection including andrographolide as a primary active compound, confirmed that andrographolide had effects on both antigen-specific and nonspecific immune function by enhancing IFN-alpha, IFN-gamma, TNF-alpha, and IL-8 in peripheral blood mononuclear cells (PBMCs), as well as macrophage phagocytotic function and augmented natural killer cell cytotoxicity, damaging the K562 cell lines [130]. In Europe, the commercial preparation containing A. paniculata extract called “Kan Jang” and pure andrographolide was reported to inhibit the spontaneous proliferation of human peripheral blood lymphocyte (PBL) and stimulated the formation of interferon-gamma (INF-γ), tumor necrosis factor-alpha (TNF-α), and certain immune activation markers, such as neopterin (Neo) and β-2-microglobulin (β2MG), which could support the anti-infectious and anti-inflammatory effects of these agents. When at the equivalent amount of andrographolide, Kan Jang showed better immunostimulant activity than pure andrographolide, which could also be explained by the synergistic effect of the active components in A. paniculata [131]. Moreover, HN-02, a mixture containing andrographolides andrographolide (88 ± 5%) plus 14-deoxyandrographolide and 14-deoxy-11,12-didehydroandrographolide together (12 ± 3%), showed potent immunomodulatory activity in both in vivo and in vitro experimental models by modulating the altered immune responses during antigen interaction and ameliorating cyclophosphamide-induced immune suppression. Interestingly, over 30 days of HN-02 administration in mice, the total WBC count and the relative weight of the spleen and thymus significantly increased, supporting the argument that the substance could stimulate the humoral and cellular pathway of the immune system, both directly and indirectly [132].
A recent study evaluating the potential of A. paniculata as an immunostimulant for COVID-19 patients using a combination synergy analysis, based on network pharmacology, suggested that the compounds in A. paniculata possessed immune-protective and antiviral properties via different pathways, including the toll-like receptor pathway, the PI3/AKT pathway, and the MAP kinase pathways, which could offer benefits against SARS-CoV-2 and upper respiratory tract infections [133].
3.4.2. Anti-Inflammatory Activity
In severe cases of SARS-CoV-2 infection, pulmonary inflammation, as well as respiratory failure from acute respiratory distress syndrome (ARDS), and multiorgan failure, are commonly found and have been identified as the major causes of death. An exaggerated release of pro-inflammatory cytokines, such as TNF-α, IL-6, IL-1, IL-8, and MCP-1, or “cytokine storm syndrome”, has been observed in severe COVID-19 patients. The rise of pro-inflammatory mediators (e.g., prostaglandins and leukotrienes) or a so-called “macrophage-derived eicosanoid storm” have also been detected, leading to the induction of hyperinflammation and mortality [4,134]. Since viral-induced hyperinflammation is known to affect disease progression and prognosis, the resolution of inflammation is a necessary strategy, in addition to anti-viral agents, in the management of critical COVID-19 patients who are suffering from a cytokine storm [4,135]. Moreover, a recent review showed that several herbal medicines used in acute respiratory infection treatment possessed anti-inflammatory activity and some of them showed favorable in vitro activities against SARS-CoV-2 induced inflammation via different pathways, such as by reducing pro-inflammatory cytokines and suppressing NF-kB [5,136].
A. paniculata extract and its bioactive components, as well as the related synthetic compounds, have been studied for their anti-inflammatory activities against endogenous or exogenous causes. Different mechanisms of action were proposed as being responsible for the anti-inflammatory activity of a major compound, andrographoide [24], such as inhibiting intercellular adhesion molecule-1 (ICAM-1) expression and endothelial-monocyte adhesion, induced by tumor necrosis factor-α (TNF) [137], down-regulating the PI3K/Akt signaling pathway, and down-streaming target nuclear factor (NF)-κB activation [138], reducing proinflammatory proteins by blocking the DNA binding of NF-κB [139], suppressing NF-κB and nitric oxide (NO) [85], and modulating macrophage and neutrophil activity [10]. Not only andrographolide but also the diterpenoids isolated from A. paniculata, namely, dehydroandrographolide and neoandrographolide, also exhibited anti-inflammatory activities by affecting cyclooxygenase (COX)-1 and -2 and down-regulating the expression of genes associated with inflammation response, including cytokines and cytokine receptors, chemokines, JAK/STAT signaling, TLRs family, and NF-κB [140]. Moreover, the crude extract of A. paniculata showed potent inhibitory activities on pro-inflammatory (NO, IL-1 beta, and IL-6) and inflammatory (PGE2 and TXB2) mediators [141]. Several studies have been conducted, suggesting that the anti-inflammatory effect of A. paniculata extract and pure compounds were beneficial in breast, colon, and lung cancer, rheumatoid arthritis, and angiogenesis [85]. The anti-inflammatory properties of andrographolide and the related compounds also suggested protective effects against toxicity in several organs and cells, such as cyclophosphamide (CTX)-induced intestinal toxicity [142], lipopolysaccharide-induced neurotoxicity, and liver and hepatorenal toxicity [10,143]. In these studies, the lowered levels of proinflammatory cytokine TNF-alpha and other cytokines, such as IFN gamma, IL-2, and GMCSF, were observed [142].
Interestingly, a recent in vivo study on the anti-influenza activity of andrographolide in post-infection treatment revealed that andrographolide improved the survival rate and lung pathology, as well as demonstrating decreased viral loads and the expression of inflammatory cytokines via NF-kB and the JAK-STAT signaling pathway [144]. Moreover, several studies also reported that andrographolide possessed anti-pulmonary inflammation activity [7]. An in vivo assay using a mouse allergic asthma model concluded that andrographolide could reduce allergen-induced inflammation, cellular infiltration in the airway, and airway hyper-responsiveness by inhibiting NF-κB expression in the lung and suppressing the NF-κB expressed in the nucleus of airway epithelial cells [145]. According to the study by Tan et al., andrographolide prevented lung inflammation, induced by non-typeable Haemophilus influenza infection (NTHi) in a cigarette smoke-exposed mouse model, by decreasing lung cellular infiltrates and the expression of cytokines and chemokines, including TNF-α, IL-1β, CXCL1/KC, 8-OHdG, matrix metalloproteinase-8 (MMP-8), and MMP-9, as well as regulating the expression of Nrf2 and its downstream genes [146]. Ko et al. examined nine Chinese traditional medicines that are typically used in inflammation and viral infection and found that A. paniculata extracts showed the greatest potency in inhibiting the secretion of RANTES, a chemotactic cytokine, in influenza A virus (H1N1)-infected human bronchial epithelial cells (A549) with an IC50 of 1.2 ± 0.4 μg/m [147]. In addition, andrographolide sulfonate, a water-soluble form of andrographolide, could improve acute lung injury (ALI) induced by lipopolysaccharide (LPS), via NF-κB and the MAPK-mediated inflammatory responses [148].
In light of the anti-inflammatory activities of A. paniculata, andrographolide and the related compounds that involve various types of targets and pathways, including cytokines, chemokines, adhesion molecules, nitric oxide, lipid mediators, transcription factors NF-κB, AP-1, and HIF-1, and signaling pathways such as PI3K/Akt, MAPK, and JAK/STAT [149,150], together with its anti-pulmonary inflammation property, A. paniculata could be a promising herbal medicine that should be considered as an alternative or complementary treatment to alleviate COVID-19 disease progression.
3.4.3. Cold/Flu
A. paniculata has been widely used in many countries, especially in China, India, Southeast Asia, and Scandinavia, from generation to generation for the prevention and treatment of the common cold, flu, and related symptoms. Scientific data has revealed that A. paniculata and its active constituents show significant antipyretic activity. An in vivo study reported that A. paniculata ethanolic extract at 500 mg/kg, administered via an intragastric route, showed a significant capability in reducing fever that was comparable to that of 200 mg/kg of a standard treatment, aspirin [151], while 300 mg/kg oral doses of andrographolide presented an antipyretic activity that was comparable to the same amount of aspirin [152]. Although a previous study from Deng discovered that andrographolide, neoandrographolide, and dehydroandrographolide were active constituents in relieving fever [153], a recent study found that the synthetic compounds 3,19-isopropylidenyl-andrographolide and 3,19-dipalmitoyl-14-deoxy-11,12-didehydroandrographolide were more active than their parent compounds, andrographolide and 14-deoxy-11,12-didehydroandrographolide, in terms of antipyretic as well as analgesic and anti-inflammatory activity, with no serious toxicity at the administered doses. The increment of lipophilicity was suggested to improve the pharmacokinetic properties [154].
A number of clinical studies were carried out that confirm the efficacy of A. paniculata, in the form of dried leaves or commercial preparations against flu and the common cold, associated with uncomplicated acute upper respiratory tract infection in different target groups [155,156,157,158]. This effectiveness might be attributed to the immunomodulatory and anti-inflammatory activities of A. paniculata [10]. Systematic reviews and the meta-analyses of randomized controlled trials suggest that the use of A. paniculata extract alone or in combination was beneficial in early treatment or as an alternative therapy for uncomplicated acute upper respiratory tract infection. A. paniculata was superior to the placebo in terms of reducing the severity of uncomplicated acute upper respiratory tract infections [159,160]. The mean difference in the reduction in symptom severity scores was 2.13 points (95% CI 1.00–3.26 points, p = 0.0002). The difference in effect between A. paniculata and the placebo was 10.85 points (95% CI 10.36–11.34 points, p < 0.0001). A high dose of A. paniculata was reported to be more active than a low dose, with no serious side effects [159]. These results are consistent with those of a recent systemic review and meta-analysis involving 33 randomized controlled trials (7175 patients). The study identified that A. paniculata, alone or in combination with standard therapy, was superior to the placebo in terms of improving acute upper respiratory tract infection (ARTI) symptoms, including cough (n = 596, standardized mean difference (SMD): −0.39, 95% confidence interval CI (−0.67, −0.10)) and sore throat (n = 314, SMD: −1.13, 95% CI (−1.37, −0.89)). Moreover, when compared to the placebo, standard treatment, and other alternative herbal remedies, A. paniculata was more effective in alleviating the overall ARTI symptoms. Using A. paniculata could also reduce the duration of coughs and sore throats, and shortened the recovery time when compared to standard therapy [161]. According to existing data from the clinical study and systematic reviews, A. paniculata was considered a promising and safe natural product in the management of ARTI. However, variations in the active constituents due to the origin of the plant and the part used, as well as the quality of manufacturing and the standardization of preparation, have raised controversial issues regarding the quality of existing studies and any recommendation regimen of A. paniculata preparations. So far, no serious adverse events have been reported; only a few cases of mild and infrequent adverse events were observed, including unpleasant sensations in the chest [159], intensified headaches, or gastrointestinal side effects [161]. More details on the adverse effects will be discussed later in the following section.
3.5. Safety Data of Andrographis paniculata
3.5.1. Adverse Effects
The administration of A. paniculata at the usual recommended dosage showed safety and good tolerability, with no adverse effects reported during the studies [157,162,163,164,165,166]. Adverse effects reported in the short-term studies of the clinical trial have been mild, infrequent, and spontaneously recovered from [155,159,167,168]. Thamlikitkul and his Thai colleagues performed a trial on 152 patients with pharyngotonsillitis symptoms, randomized into three groups to receive a low dose of A. paniculata (3 g/day), a high dose (6 g/day), and paracetamol as placebo control [155]. Side effects found in this study included nausea, vomiting, abdominal discomfort, dizziness, drowsiness, and malaise. Among the clinical trial of A. paniculata products sold in the markets, the incidence of adverse effects was reported; for example, the adverse effects of KalmCold™ (total andrographolide, 38.40% w/w) were epistaxis, diarrhea, urticaria, and vomiting [167], while for Paractin® (total andrographolide, 30% w/w), they were headache, diarrhea, nausea, stomach discomfort, fatigue, cramp, and pruritus/rash [169]. Furthermore, in a phase I clinical trial study conducted on 13 HIV-positive patients and 5 healthy volunteers, the andrographolide regimen was 5 mg/kg for 3 weeks, increasing to 10 mg/kg for 3 weeks, and to 20 mg/kg for a final 3 weeks [110]. The adverse events investigated during the trial were diarrhea, dizziness, dysgeusia, eyes becoming sensitive to the light, fatigue, headache, heartburn, lymphadenopathy, nausea, pruritus/rash, tender lymph nodes, a decreased sex drive, decreased short-term memory, and anaphylactic reaction. The VigiAccess database (www.vigiaccess.org, accessed on 11 March 2020)), a platform for the general public to access information regarding pharmacovigilance and medicine safety, managed by the WHO Collaborating Center for International Drug Monitoring, Uppsala Monitoring Center (UMC) showed the reports of adverse reactions of A. paniculata usage in a total of 274 records from 2004–2020. The adverse events most frequently reported were: skin and subcutaneous tissue disorders, i.e., urticaria, rash, pruritus (183 reports), immune system disorders, i.e., anaphylactic reaction, hypersensitivity (37 reports), general disorder, and administration site conditions, i.e., face edema, fatigue, chest discomfort (34 reports), gastrointestinal disorders, i.e., nausea, vomiting, diarrhea, lip swelling (32 reports), and others. Focusing on the acute hypersensitivity reaction, Farah et al. suggested that the product label should contain warnings about the possibility of allergies from A. paniculata-based products [170].
3.5.2. Toxicity
Usually, a traditional dose of A. paniculata powder is 9–15 mg, once a day, which contains andrographolide at an amount of 90–150 mg [155]; in Chinese traditional medicine and orthodox medicine in Thailand and India, this has long been recognized as a safe amount [8]. However, when determining the safety of patients, scientists attempted to establish the toxicity data of A. paniculata. Toxicity studies were designed with both animals and humans. When conducted in HIV-infected patients and healthy volunteers, no toxicity was observed in an acute toxicity study of dose-escalating andrographolide, at 5 mg/kg body weight and three times a day (TID) for 3 weeks, 10 mg/kg body weight TID for a further 3 weeks, and 20 mg/kg body weight for a final 3 weeks [110]. In the animal model, Panossian et al. reported that the oral administration of A. paniculata standardized extract (andrographolide, 4.6%, and 14-deoxo-andrographolide, 2.3%) in doses of 200, 600, and 2000 mg/kg given to Wistar rats did not show toxicity [171]. Moreover, in female rats treated with standardized A. paniculata alcoholic extract (andrographolide > 30% w/w) at 5000 mg/kg, there were no treatment-necessitating toxic effects [172]. In 2009, it was revealed that mice that were intraperitoneally administered isopropylidene andrographolide and 14-deoxy-11,12-didehydro-3,19 dipalmitoyl andrographolide at 0.5, 1, 4, 8, 50, or 100 mg/kg were able to survive [154]. Bothiraja et al. reported that although the mice were treated with andrographolide at the maximum dose (5 g/kg), no dead mice were found [173]. Oral administration of the first true-leaf ethanolic extract of A. paniculata contained high levels of 14-deoxy-andrographolide but low andrographolide levels at 5000 mg/kg in mice; the results showed that all treated animals survived [174]. The median lethal dose (LD50) of A. paniculata extract and its andrographolide was tested in several studies, as shown in Table 2. In order to perform a subacute toxicity test, Wistar rats consumed andrographolide at 250 mg and 500 mg/kg for 21 successive days [173]. The result demonstrated that significant alterations of behavior, biochemicals, body weight gain, food intake, hematology, histopathology, mortality, and vital organ weight were undetectable.

Table 2.
The median lethal dose (LD50) data of Andrographis paniculata extract and its constituents.
3.5.3. Contraindication
A. paniculata is widely used in many countries at all stages of human life for various ailments. However, the scientific data that confirm the safety of usage of this herb among children, pregnant and lactating women, and the elderly are still ambiguous. In Ayurvedic traditional medicine, it was declared that A. paniculata is edible during pregnancy for a short time. To support the previous information, in 1999, pregnant rats in the first 19 days of pregnancy consumed extracts of A. paniculata leaves in 200, 600, and 2000 mg/kg doses (a higher dose than the usual therapeutic dose in humans at 30-, 90-, and 300-fold, respectively), undergoing an investigation of the content of the blood progesterone hormone, a necessary hormone for gestation [171]. The alteration of progesterone levels in the blood plasma of rats was not found; it was assumed that the human therapeutic dose of A. paniculata extract did not have an effect on the progesterone-mediated termination of pregnancy. Conversely, Satika et al. revealed that 85 female rats exposed to the water extract of A. paniculata (1 g/kg) for 4, 6, and 8 weeks exhibited a reduction in the female sex hormones: follicle-stimulating hormone, luteinizing hormone, estrogen, and progesterone, in a time-dependent fashion [179]. Zoha et al. studied the antifertility of A. paniculata in mice [180]. In a study giving 2 g per kilogram of the dried A. paniculata, orally fed only to female mice daily for six weeks, after mating, there were no pregnant mice. This result implies that A. paniculata probably inhibits ovulation in female mice. Furthermore, Noordalilati and other Malaysian colleagues (2005) found that when a 50% ethanolic extract of A. paniculata (andrographolide 1.33%) at 10 and 100 mg/kg of extracts, fed from day 6 to day 15 of pregnancy to Sprague Dawley rats, potentially teratogenic and toxic effects to the fetuses were related to abnormal features in the fetuses, such as micrognathia and exencephaly [181]. However, there is no information regarding the clinical trial study on pregnant women. Therefore, the usage of A. paniculata during pregnancy should be avoided, especially during early pregnancy [182]. In addition, information regarding its safety among breastfeeding women, children, and the elderly is insufficient; therefore, these groups should not use it, nor should those people who are allergic to plants in the Acanthaceae family [11].
3.5.4. Drug Interactions
Because A. paniculata has been commonly used in various counties in Asia and Europe, the interaction between this herbal medicine and modern drugs, which may cause adverse drug reactions (ADRs) and toxicities, brings up numerous safety concerns. In the metabolism process of xenobiotics and drugs, there are several enzymes related to the metabolic steps in phase I, i.e., cytochrome P450 (CYP), and phase II, i.e., UDP-glucuronosyltransferase, sulfotransferase, and glutathione-S-transferase [183]. The concomitant administration of A. paniculata with drugs will probably induce or inhibit the metabolic enzymes in the intestine and liver and affect the pharmacokinetic changes. The extract of A. paniculata, as well as its major compound, andrographolide, underwent studies on their interaction with the CYP family, both in vitro and in vivo (Table 3). It has been reported that approximately 50% of the drug metabolism is taken up by CYP3A4 [184]. In the in vitro studies, it was found that the ethanolic and methanolic extracts, andrographolide and 14-deoxy-11,12-didehydroandrographolide, inhibited the activity of the CYP 3A4 isozyme [185,186,187]. As a consequence, the concurrent usage of A. paniculata with prescribed medicines that are metabolized by CYP3A4 (i.e., chemotherapeutics, antihistamine, benzodiazepine, calcium channel blocker, and statins) causes the potential risk of toxicity. Drugs with a narrow therapeutic index, such as digoxin, phenytoin, theophylline, and warfarin are drugs with a small difference between therapeutic and toxic levels; therefore, a small fluctuation in drug concentration in the blood circulation can lead to serious therapeutic failure or adverse drug reactions. The metabolism of theophylline and warfarin is involved with the CYP1A2 enzyme. It is reported that in the in vitro studies, an ethanolic extract of A. paniculata and andrographolide, with its derivative, exhibited the inhibition of CYP1A2 activity [185,188]. Therefore, taking these medications with the herb probably interacts with the metabolism, which is a contributor to the potential risk of toxicity. Furthermore, A. paniculata and its major compounds are able to inhibit the CYP2C isoform (CYP2C9, 2C11, 2C19), and CYP 2D6, as shown in Table 3. On the other hand, some studies have revealed that the alcoholic extract and andrographolide can induce activities in the CYP1A1 and CYP2B isozyme, both in vitro and in vivo ([188,189,190,191]). In phase II drug metabolism, it was found that both the ethanolic and methanolic extracts, including andrographolide and the derivatives, showed the inhibition of UDP-glucuronyltransferases or the UGT isoforms (UGT1A3, UGT1A8, UGT2B7, UGT1A1, UGT1A6, UGT1A7, and UGT1A10) [192,193,194].

Table 3.
The interaction of Andrographis paniculata with cytochrome P450 enzymes and the proteins associated with metabolism.
Pharmacokinetic and pharmacodynamic studies of the interaction between A. paniculata and various therapeutic drugs were performed, both in vitro and in vivo (Table 3). The pharmacokinetics of warfarin, one of the narrow therapeutic-index drugs, was investigated in male Sprague-Dawley rats that were co-administered with warfarin (0.5 mg/kg) and andrographolide (30 mg/kg/day for 7 days) [195]. It is reported that andrographolide was able to increase the systemic level of warfarin via interference with enzymes in the metabolic process. Moreover, Chien and Chinese colleagues revealed that the phytochemical components in A. paniculata possibly interact with theophylline and inhibit CYP1A2, causing a delay in drug elimination [196]. In terms of the interaction between A. paniculata and non-steroidal anti-inflammatory drugs (NSAIDs), it was found that andrographolide demonstrates anti-arthritic activity through the mechanism of reducing pro-inflammatory mediators (COX-2, iNOS, and cytokines) [197]; therefore, the andrographolide manifested synergistic anti-arthritic activity with NSAIDs, such as naproxen [198] and etoricoxib [199]. Conversely, co-administered A. paniculata extract and andrographolide with nabumetone showed a reduction in anti-arthritic activity [198]. In addition, andrographolide feasibly influenced the CYP1A2 enzyme in the metabolism of NSAIDs. Only one study was performed on the pharmacokinetic and pharmacodynamic interaction of andrographolide and hypoglycemic drugs. Samala and Veeresham reported that the concurrent use of andrographolide and glyburide was capable of changing the pharmacokinetic and pharmacodynamic parameters because andrographolide contributes to the inhibition of CYP3A4, which is responsible for glyburide metabolism and improves absorption [200]. It was found that andrographolide demonstrated the inhibition of tumor growth [201], the suppression of colitis-associated colon cancer [202,203], and the induction of apoptosis in different cancer cells [204], as well as strengthening the cytotoxic effect of several chemotherapy drugs [62,205]. In 2009, Yang et al. reported that andrographolide can synergistically induce the apoptosis of 5-fluorouracil (5-FU) via the augmentation of caspase-8, p53 activity, and the significant alteration of the Bax conformation in hepatocellular carcinoma (SMMC-7721) [206]. In a subsequent study, it was reported that andrographolide exhibits an increase in apoptosis and Bax level when treated with 5-FU in human colorectal cancer [207]. In addition, an investigation was also performed on andrographolide’s effect on anticancer activity in non-small-cell lung cancer (NSCLC) (A549), co-treated with paclitaxel. The combination of andrographolide and paclitaxel showed a significant synergistic anticancer effect on A549 cells in vitro and in vivo because of the accumulation of reactive oxygen species [208]. Studies of the anticancer effect of the co-administration of andrographolide and cisplatin in the cisplatin-resistant ovarian cancer cell line A2780cisR found that the synergism of anticancer activity and the percentage of apoptotic cell death in ovarian cancer cell lines was observed [209].
4. Discussion
Since the initial rapid outbreak and the spread of COVID-19, scientists around the world have been searching for a vaccine. Medicines utilizing both chemicals and herbal substances have been sought to decrease the number of cases and eliminate this epidemic. A. paniculata is one of the most popular therapeutic herbs and is recommended in combination with modern medicine in many countries, such as China and Thailand. For a limited period of time, in silico studies have been the most widely used method for studying the effects of A. paniculata and its constituents. The therapeutic targets were divided into two parts: virus-specific target proteins and human-specific target proteins. It was discovered that the active ingredient isolated from A. paniculata was more likely to act on virus-specific target proteins. In particular, 3CLpro was investigated in silico, and the results were confirmed by an in vitro study. However, the results showed that the IC50 of andrographolide when inhibiting 3CLpro was greater than its IC50 when inhibiting infectious virion production [97,101]. Therefore, it was hypothesized that andrographolide was likely to act on other virus-specific target proteins as well; however, further in vitro and in vivo studies will be needed to confirm this hypothesis. When looking at the results of a study on hACE2 where human-specific target proteins were examined, it was reported that andrographolide binds to hACE2 with some instability, while the docking binding energy does not reach the cut-off value that should be reached for a potential compound [91]. This finding also contradicts another study reporting that andrographolide stably binds to hACE2 [81]. As a result, more research on the influence of A. paniculata and its constituents on hACE2 is needed, particularly via in vitro and in vivo trials.
Although the scientific evidence behind using A. paniculata to treat patients who have suffered from COVID-19 is still unclear, the national health committees of some countries have attempted to use A. paniculata as a supportive therapy to cope with the pandemic situation. For example, in Xiyanping, China, a marketed injection of A. paniculata extract containing andrographolides has been suggested in the China National Health Commission guidelines, to treat patients who have been infected with COVID-19 in progressive stages [214]. The Xiyanping injection program showed reductions in inflammation and the improvement of virus clearance and related symptoms, such as cough, fever, and rales in the lungs [214,215]. Recently (June 2021), A. paniculata has been listed in the guidelines of the National List of Essential Medicines (NLEM) to manage the COVID-19 pandemic in Thailand. The oral dosage (pills and capsules) of A. paniculata and its extract, with andrographolide at 180 mg per day, was recommended for patients infected with COVID-19 and exhibiting mild symptoms to avoid progression to intensive symptoms [12]. In India, the Ministry of AYUSH launched a recommendation to utilize A. paniculata in an Ayurvedic procedure for COVID-19 treatment and to boost immunity [216]. These efforts are intended to support the policies of the national health care systems, to resolve the problematic situations arising from COVID-19 in those countries. A recent clinical trial conducted in Thailand showed that 180 mg of A. paniculata extract, taken daily for five consecutive days, helped to reduce the symptoms and duration of COVID-19 more successfully than andrographolide at 60 mg/day and standard treatment, with mild adverse effects (diarrhea and stomach ache) [217].
5. Conclusions
In conclusion, in situations where modern medicines and herbal medicines are urgently needed, A. paniculata could serve as a promising source of lead compounds for drug discovery in the post-infectious treatment of COVID-19, rather than a prophylactic. In the meantime, the use of A. paniculata products should be given under the supervision of physicians or pharmacists, in terms of its toxicity, adverse effects, use by specific people, and allergies, including interactions between A. paniculata and modern medicines. Furthermore, in light of the high demand for A. paniculata in the context of health benefits in this situation, the quality of A. paniculata products, in terms of their safety and efficacy, is inevitably a matter of concern.
Author Contributions
Conceptualization, A.I. and W.A.; writing—original draft preparation, A.I., W.A. and W.Y.; writing—review and editing, A.I., W.A. and P.S.-a.; visualization, A.I.; supervision, W.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand for the supporting information resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 9 May 2022).
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Krüger, N.; Müller, M.; Drosten, C.; Pöhlmann, S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Huang, J.; Tao, G.; Liu, J.; Cai, J.; Huang, Z.; Chen, J.-x. Current Prevention of COVID-19: Natural Products and Herbal Medicine. Front. Pharmacol. 2020, 11, 588508. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Moh Qrimida, A.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life 2021, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, S.-R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci Nutr. 2019, 59, S17–S29. [Google Scholar] [CrossRef] [PubMed]
- Joselin, J.; Jeeva, S. Andrographis paniculata: A Review of its Traditional Uses, Phytochemistry and Pharmacology. Med Aromat Plants 2014, 3, 15. [Google Scholar] [CrossRef]
- Jiaqi, H.; Yunfei, D.; Daniel, T. ANDROGRAPHIS Wallich ex Nees in Wallich. Fl. China 2011, 19, 473–474. [Google Scholar]
- Jarukamjorn, K.; Nemoto, N. Pharmacological Aspects of Andrographis paniculata on Health and Its Major Diterpenoid Constituent Andrographolide. J. F Health Sci. 2008, 54, 370–381. [Google Scholar] [CrossRef]
- WHO. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2002; Volume 2. [Google Scholar]
- National Drug Selection Committees. Thai National List of Essential Medicine. 2021; pp. 46–47. Available online: https://pubmiddleware.mims.com/resource/document/82AE6301-4B56-473D-A76E-ADA40096A174/pdf/MIMS_1_2022_NLEM_Web.pdf?client=MIMS%20Publication-Topic&email=&country=Thailand&referenceId=Thailand%20National%20List%20of%20Essential%20Medicines%20(NLEM) (accessed on 5 July 2022).
- Mishra, S.K.; Sangwan, N.S.; Sangwan, R.S. Andrographis paniculata (Kalmegh): A Review. Pharm. Rev 2007, 1, 16. [Google Scholar]
- Panossian, A.; Wikman, G. Efficacy of Andrographis paniculata in Upper Respiratory Tract Infectious Diseases and the Mechanism of Action. In Evidence and Rational Based Research on Chinese Drugs; Springer: Vienna, Austria, 2012; p. 138. [Google Scholar]
- Bensky, D.; Gamble, A.; Kaptchuk, T. Chinese Herbal Medicine: Materia Medica; Revised ed.; Eastland Press: Washington, DC, USA, 1993. [Google Scholar]
- Sareer, O.; Ahmad, S.; Umar, S. Andrographis paniculata: A critical appraisal of extraction, isolation and quantification of andrographolide and other active constituents. Nat. Prod. Res. 2014, 28, 2081–2101. [Google Scholar] [CrossRef]
- Tang, W.; Eisenbrand, G. Andrographis paniculata (Burm. f.) Nees. In Chinese Drugs of Plant Origin; Springer: Berlin/Heidelberg, Germany, 1992; p. 1056. [Google Scholar]
- Chakravarti, R.; Chakravarti, M.D. Andrographolide, the Active Constituent of Andrographis paniculata Nees. A Preliminary Communication. Ind. Med. Gaz 1951, 86, 96. [Google Scholar]
- Chao, W.-W.; Lin, B.-F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Medi. 2010, 5, 1–15. [Google Scholar] [CrossRef]
- Cheung, H.; Cheung, C.; Kong, C. Determination of bioactive diterpenoids from Andrographis paniculata by micellar electrokinetic chromatography. J. Chromatogr. A 2001, 930, 171–176. [Google Scholar] [CrossRef]
- Matsuda, T.; Kuroyanagi, M.; Sugiyama, S.; Umehara, K.; Ueno, A.; Nishi, K. Cell differentiation-inducing diterpenes from Andrographis paniculata Nees. Chem. Pharm. Bull. 1994, 42, 1216–1225. [Google Scholar] [CrossRef]
- Pholphana, N.; Rangkadilok, N.; Thongnest, S.; Ruchirawat, S.; Ruchirawat, M.; Satayavivad, J. Determination and variation of three active diterpenoids in Andrographis paniculata (Burm. f.) Nees. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2004, 15, 365–371. [Google Scholar] [CrossRef]
- Sharma, A.; Lal, K.; Handa, S.S. Standardization of the Indian crude drug Kalmegh by high pressure liquid chromatographic determination of andrographolide. Phytochem. Anal. 1992, 3, 129–131. [Google Scholar] [CrossRef]
- Hossain, M.; Urbi, Z.; Sule, A.; Rahman, K. Andrographis paniculata (Burm. f.) Wall. ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014, 2014, 274905. [Google Scholar] [CrossRef]
- Pandey, A.; Mandal, A. Variation in morphological characteristics and andrographolide content in Andrographis paniculata (Burm. f.) Nees of Central India. Iran. J Energy Environ. 2010, 1, 165–169. [Google Scholar]
- Kandanur, S.G.S.; Tamang, N.; Golakoti, N.R.; Nanduri, S. Andrographolide: A natural product template for the generation of structurally and biologically diverse diterpenes. Eur. J. Med. Chem. 2019, 176, 513–533. [Google Scholar] [CrossRef]
- Kumar, M.P.; Mamidala, E.; Al-Ghanim, K.A.; Al-Misned, F.; Mahboob, S. Evaluation of the andrographolides role and its indoleamine 2, 3-dioxygenase inhibitory potential and attendant molecular mechanism against STZ-induced diabetic rats. Saudi J. Biol. Sci. 2020, 27, 713–719. [Google Scholar] [CrossRef]
- Luo, S.; Li, H.; Liu, J.; Xie, X.; Wan, Z.; Wang, Y.; Zhao, Z.; Wu, X.; Li, X.; Yang, M. Andrographolide ameliorates oxidative stress, inflammation and histological outcome in complete Freund’s adjuvant-induced arthritis. Chem.-Biol. Interact. 2020, 319, 108984. [Google Scholar] [CrossRef]
- Liu, W.; Fan, T.; Li, M.; Zhang, G.; Guo, W.; Yang, X.; Jiang, C.; Li, X.; Xu, X.; Tang, A. Andrographolide potentiates PD-1 blockade immunotherapy by inhibiting COX2-mediated PGE2 release. Int. Immunopharmacol. 2020, 81, 106206. [Google Scholar] [CrossRef]
- Geng, J.; Liu, J.; Yuan, X.; Liu, W.; Guo, W. Andrographolide triggers autophagy-mediated inflammation inhibition and attenuates chronic unpredictable mild stress (CUMS)-induced depressive-like behavior in mice. Toxicol. Appl. Pharmacol. 2019, 379, 114688. [Google Scholar] [CrossRef]
- Wang, D.-P.; Chen, S.-H.; Wang, D.; Kang, K.; Wu, Y.-F.; Su, S.-H.; Zhang, Y.-Y.; Hai, J. Neuroprotective effects of andrographolide on chronic cerebral hypoperfusion-induced hippocampal neuronal damage in rats possibly via PTEN/AKT signaling pathway. Acta Histochem. 2020, 122, 151514. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, C.; Xie, N.; Xu, Y.; Liu, L.; Liu, N. Treatment with andrographolide sulfonate provides additional benefits to imipenem in a mouse model of Klebsiella pneumoniae pneumonia. Biomed. Pharmacother. 2019, 117, 109065. [Google Scholar] [CrossRef]
- Paemanee, A.; Hitakarun, A.; Wintachai, P.; Roytrakul, S.; Smith, D.R. A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomed. Pharmacother. 2019, 109, 322–332. [Google Scholar] [CrossRef]
- Wu, T.-S.; Chern, H.-J.; Damu, A.G.; Kuo, P.-C.; Su, C.-R.; Lee, E.-J.; Teng, C.-M. Flavonoids and ent-labdane diterpenoids from Andrographis paniculata and their antiplatelet aggregatory and vasorelaxing effects. J. Asian Nat. Prod. Res. 2008, 10, 17–24. [Google Scholar] [CrossRef]
- Chen, L.-X.; He, H.; Xia, G.; Zhou, K.-L.; Qiu, F. A new flavonoid from the aerial parts of Andrographis paniculata. Nat. Prod. Res. 2014, 28, 138–143. [Google Scholar] [CrossRef]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. A bitter plant with a sweet future? A comprehensive review of an oriental medicinal plant: Andrographis paniculata. Phytochem. Rev. 2012, 11, 39–75. [Google Scholar] [CrossRef]
- Chua, L.S.; Yap, K.C.; Jaganath, I.B. Comparison of total phenolic content, scavenging activity and HPLC-ESI-MS/MS profiles of both young and mature leaves and stems of Andrographis paniculata. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef]
- Maity, G.N.; Maity, P.; Dasgupta, A.; Acharya, K.; Dalai, S.; Mondal, S. Structural and antioxidant studies of a new arabinoxylan from green stem Andrographis paniculata (Kalmegh). Carbohydr. Polym. 2019, 212, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chou, G.X.; Wang, Z.T. A new diterpene from the leaves of Andrographis paniculata Nees. Fitoterapia 2010, 81, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Li, R.T.; Xiao, W.L.; Xu, G.; Lin, Z.W.; Zhao, Q.S.; Sun, H.D. ent-Labdane diterpenoids from Andrographis paniculata. J. Nat. Prod. 2006, 69, 319–322. [Google Scholar] [CrossRef]
- Chen, L.-X.; Qiu, F.; Wei, H.; Qu, G.-X.; Yao, X.-S. Nine New ent-Labdane Diterpenoids from the Aerial Parts of Andrographis paniculata. Helv. Chim. Acta 2006, 89, 2654–2664. [Google Scholar] [CrossRef]
- Wen, Q.; Jin, X.; Lu, Y.; Chen, D.F. Anticomplement ent-labdane diterpenoids from the aerial parts of Andrographis paniculata. Fitoterapia 2020, 142, 104528. [Google Scholar] [CrossRef]
- Balmain, A.; Connolly, J.D. Minor diterpenoid constituents of Andrographis paniculata Nees. J. Chem. Soc. Perkin Trans. 1973, 4, 1247–1251. [Google Scholar] [CrossRef]
- Pramanick, S.; Banerjee, S.; Achari, B.; Das, B.; Sen, A.K., Sr.; Mukhopadhyay, S.; Neuman, A.; Prangé, T. Andropanolide and isoandrographolide, minor diterpenoids from Andrographis paniculata: Structure and X-ray crystallographic analysis. J. Nat. Prod. 2006, 69, 403–405. [Google Scholar] [CrossRef]
- Fujita, T.; Fujitani, R.; Takeda, Y.; Takaishi, Y.; Yamada, T.; Kido, M.; Miura, I. On the Diterpenoids of Andrographis paniculata: X-Ray Crystallographic Analysis of Andrographolide and Structure Determination of New Minor Diterpenoids. Chem. Pharm. Bull. 1984, 32, 2117–2125. [Google Scholar] [CrossRef]
- Kulyal, P.; Tiwari, U.K.; Shukla, A.; Gaur, A.K. Chemical constituents isolated from Andrographis paniculata. Indian J. Chem. 2010, 49B, 356–359. [Google Scholar]
- Reddy, M.K.; Reddy, M.V.; Gunasekar, D.; Murthy, M.M.; Caux, C.; Bodo, B. A flavone and an unusual 23-carbon terpenoid from Andrographis paniculata. Phytochemistry 2003, 62, 1271–1275. [Google Scholar] [CrossRef]
- Niranjan Reddy, V.L.; Malla Reddy, S.; Ravikanth, V.; Krishnaiah, P.; Venkateshwar Goud, T.; Rao, T.P.; Siva Ram, T.; Gonnade, R.G.; Bhadbhade, M.; Venkateswarlu, Y. A new BIS-Andrographolide Ether from Andrographis paniculata Nees and evaluation of anti-HIV activity. Nat. Prod. Res. 2005, 19, 223–230. [Google Scholar] [CrossRef]
- Siripong, P.; Kongkathip, B.; Preechanukool, K.; Picha, P.; Tunsawan, K.; Taylor, W. Cytotoxic diterpenoid constituents from Andrographis paniculata Nees Leaves. J. Sci. Soc. Thail. 1992, 18, 187–194. [Google Scholar] [CrossRef]
- Zou, Q.Y.; Li, N.; Dan, C.; Deng, W.L.; Peng, S.L.; Ding, L.S. A new ent-labdane diterpenoid from Andrographis Paniculata. Chin. Chem. Lett. 2010, 21, 1091–1093. [Google Scholar] [CrossRef]
- My, N.; Hanh, T.; Cham, P.; Cuong, N.; Huong, T.; Quang, T.; Nam, N.; Minh, C. Andropaniosides A and B, two new ent-labdane diterpenoid glucosides from Andrographis paniculata. Phytochem. Lett. 2020, 35, 37–40. [Google Scholar] [CrossRef]
- Radhika, P.; Prasad, Y.R.; Sowjanya, K. A new diterpene from the leaves of Andrographis paniculata Nees. Nat. Prod. Commun. 2012, 7, 485–486. [Google Scholar] [CrossRef]
- Koteswara Rao, Y.; Vimalamma, G.; Venkata Rao, C.; Tzeng, Y.-M. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry. 2004, 65, 2317–2321. [Google Scholar] [CrossRef]
- Geethangili, M.; Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in jurkat cells. Phytother. Res. PTR 2008, 22, 1336–1341. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Wen, T.; Liu, F.-F.; Tian, H.-Y.; Chun-Lin, F.A.N.; Huang, X.-J.; Ye, W.-C.; Wang, Y. Two new diterpenoid lactones isolated from Andrographis paniculata. Chin. J. Nat. Med. 2017, 15, 458–462. [Google Scholar] [CrossRef]
- Arifullah, M.; Namsa, N.D.; Mandal, M.; Chiruvella, K.K.; Vikrama, P.; Gopal, G.R. Evaluation of anti-bacterial and anti-oxidant potential of andrographolide and echiodinin isolated from callus culture of Andrographis paniculata Nees. Asian Pac. J. Trop. Biomed. 2013, 3, 604–610. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, B. Isolation and structure of two new diterpenoid glucosides from Andrographis paniculata Nees. Acta Pharm. Sin. 1982, 17, 435–440. [Google Scholar]
- Chen, L.; Zhu, H.; Wang, R.; Zhou, K.; Jing, Y.; Qiu, F. ent-Labdane Diterpenoid Lactone Stereoisomers from Andrographis paniculata. J. Nat. Prod. 2008, 71, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.P.; Yang, Z.J.; Zhao, W.Y.; Zhang, R.Y.; Li, H.; Chen, L.X. A novel 15-spiro diterpenoid dimer from Andrographis paniculata with inhibitory potential against human carboxylesterase 2. Bioorg. Chem. 2020, 97, 103680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Wang, G.-C.; Ye, W.-C.; Li, Q.; Zhou, G.-X.; Yao, X.-S. New Diterpenoids from Andrographis paniculata (Burm. f.) Nees. J. Integr. Plant Biol. 2006, 48, 1122–1125. [Google Scholar] [CrossRef]
- Zhou, K.-L.; Chen, L.-X.; Zhuang, Y.-L.; Wang, N.-L.; Yao, X.-S.; Qiu, F. Two new ent-labdane diterpenoid glycosides from the aerial parts of Andrographis paniculata. J. Asian Nat. Prod. Res. 2008, 10, 939–943. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, G.-D.; Ong, C.-S.; Ong, C.-N.; Shen, H.-M. Andrographolide sensitizes cancer cells to TRAIL-induced apoptosis via p53-mediated death receptor 4 up-regulation. Mol. Cancer Ther. 2008, 7, 2170–2180. [Google Scholar] [CrossRef]
- Ma, X.C.; Gou, Z.P.; Wang, C.Y.; Yao, J.H.; Xin, X.L.; Lin, Y.; Liu, K.X. A new ent-labdane diterpenoid lactone from Andrographis paniculata. Chin. Chem. Lett. 2010, 21, 587–589. [Google Scholar] [CrossRef]
- Ji, L.L.; Wang, Z.; Dong, F.; Zhang, W.B.; Wang, Z.T. Andrograpanin, a compound isolated from anti-inflammatory traditional Chinese medicine Andrographis paniculata, enhances chemokine SDF-1alpha-induced leukocytes chemotaxis. J. Cell. Biochem. 2005, 95, 970–978. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.T.; Ge, B.X. Andrograpanin, isolated from Andrographis paniculata, exhibits anti-inflammatory property in lipopolysaccharide-induced macrophage cells through down-regulating the p38 MAPKs signaling pathways. Int. Immunopharmacol. 2008, 8, 951–958. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.T. Chemical constituents from roots of Andrographis paniculata. Yao Xue Xue Bao = Acta Pharm. Sin. 2011, 46, 317–321. [Google Scholar]
- Li, W.; Xu, X.; Zhang, H.; Ma, C.; Fong, H.; van Breemen, R.; Fitzloff, J. Secondary metabolites from Andrographis paniculata. Chem. Pharm. Bull. 2007, 55, 455–458. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Sato, M.; Ueno, A.; Nishi, K. Flavonoids from Andrographis paniculata. Chem. Pharm. Bull. 1987, 35, 4429–4435. [Google Scholar] [CrossRef]
- Xu, C.; Chou, G.-X.; Wang, C.-H.; Wang, Z.-T. Rare noriridoids from the roots of Andrographis paniculata. Phytochemistry 2012, 77, 275–279. [Google Scholar] [CrossRef]
- Wang, G.-C.; Wang, Y.; Williams, I.D.; Sung, H.H.-Y.; Zhang, X.-Q.; Zhang, D.-M.; Jiang, R.-W.; Yao, X.-S.; Ye, W.-C. Andrographolactone, a unique diterpene from Andrographis paniculata. Tetrahedron Lett. 2009, 50, 4824–4826. [Google Scholar] [CrossRef]
- Cava, M.P.; Chan, W.R.; Stein, R.P.; Willis, C.R. Andrographolide: Further transformations and stereochemical evidence; the structure of isoandrographolide. Tetrahedron 1965, 21, 2617–2632. [Google Scholar] [CrossRef]
- Kleipool, R.J.C. Constituents of Andrographis paniculata Nees. Nature 1952, 169, 33–34. [Google Scholar] [CrossRef]
- Weiming, C.; Xiaotian, L. Deoxyandrographolide-19beta-D-glucoside from the leaves of Andrographis paniculata. Planta Med. 1982, 45, 245–246. [Google Scholar] [CrossRef]
- Chan, W.R.; Taylor, D.R.; Willis, C.R.; Bodden, R.L.; Fehlhaber, H.W. The structure and stereochemistry of neoandrographolide, a diterpene glucoside from Andrographis paniculata nees. Tetrahedron 1971, 27, 5081–5091. [Google Scholar] [CrossRef]
- Allison, A.J.; Butcher, D.N.; Connolly, J.D.; Overton, K.H. Paniculides A, B, and C, bisabolenoid lactones from tissue cultures of Andrographis paniculata. Chem. Commun. 1968, 23, 1493. [Google Scholar] [CrossRef]
- Jalal, M.A.; Overton, K.; Rycroft, D. Formation of three new flavones by differentiating callus cultures of Andrographis paniculata. Phytochemistry 1979, 18, 149–151. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.-B.; Huang, X.-Y.; Geng, C.-A.; Zhao, Y.; Wang, L.-J.; Guo, R.-H.; Liang, W.-J.; Zhang, X.-M.; Chen, J.-J. Synthesis, structure–activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg. Med. Chem. Lett. 2014, 24, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Du, G.; Zhou, B.; Zong, K.; Ma, B. Study on spectroscopy of two flavonyl glycosides from Andrographis paniculata. Front. Chem. China 2008, 3, 178–181. [Google Scholar] [CrossRef]
- Gupta, K.K.; Taneja, S.C.; Dhar, K.L.; Atal, C.K. Flavonoids of Andrographis paniculata. Phytochemistry 1983, 22, 314–315. [Google Scholar] [CrossRef]
- Radhika, P.; Prasad, Y.R.; Lakshmi, K.R. Flavones from the Stem of Andrographis paniculata Nees. Nat. Prod. Commun. 2010, 5. [Google Scholar] [CrossRef]
- Alazmi, M.; Motwalli, O. Molecular basis for drug repurposing to study the interface of the S protein in SARS-CoV-2 and human ACE2 through docking, characterization, and molecular dynamics for natural drug candidates. J. Mol. Model. 2020, 26, 1–10. [Google Scholar] [CrossRef]
- Alagu Lakshmi, S.; Shafreen, R.M.B.; Priya, A.; Shunmugiah, K.P. Ethnomedicines of Indian origin for combating COVID-19 infection by hampering the viral replication: Using structure-based drug discovery approach. J. Biomol. Struct. Dyn 2020, 39, 4597–4609. [Google Scholar] [CrossRef]
- Dua, V.K.; Ojha, V.P.; Roy, R.; Joshi, B.C.; Valecha, N.; Devi, C.U.; Bhatnagar, M.C.; Sharma, V.P.; Subbarao, S.K. Anti-malarial activity of some xanthones isolated from the roots of Andrographis paniculata. J. Ethnopharmacol. 2004, 95, 247–251. [Google Scholar] [CrossRef]
- Şimşek Yavuz, S.; Ünal, S. Antiviral treatment of COVID-19. Turk. J. Med. Sci. 2020, 50, 611–619. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.P.; Ganju, L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017, 162, 611–623. [Google Scholar] [CrossRef]
- Gil, C.; Ginex, T.; Maestro, I.; Nozal, V.; Barrado-Gil, L.; Cuesta-Geijo, M.Á.; Urquiza, J.; Ramírez, D.; Alonso, C.; Campillo, N.E. COVID-19: Drug targets and potential treatments. J. Med. Chem. 2020, 63, 12359–12386. [Google Scholar] [CrossRef]
- Yang, J.; Petitjean, S.J.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Suryamohan, K.; Diwanji, D.; Stawiski, E.W.; Gupta, R.; Miersch, S.; Liu, J.; Chen, C.; Jiang, Y.-P.; Fellouse, F.A.; Sathirapongsasuti, J.F. Human ACE2 receptor polymorphisms and altered susceptibility to SARS-CoV-2. Commun. Biol. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Hiremath, S.; Kumar, H.V.; Nandan, M.; Mantesh, M.; Shankarappa, K.; Venkataravanappa, V.; Basha, C.J.; Reddy, C.L. In silico docking analysis revealed the potential of phytochemicals present in Phyllanthus amarus and Andrographis paniculata, used in Ayurveda medicine in inhibiting SARS-CoV-2. 3 Biotech 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Khanal, P.; Dey, Y.N.; Patil, R.; Chikhale, R.; Wanjari, M.M.; Gurav, S.S.; Patil, B.M.; Srivastava, B.; Gaidhani, S.N. Combination of system biology to probe the anti-viral activity of andrographolide and its derivative against COVID-19. RSC Adv. 2021, 11, 5065–5079. [Google Scholar] [CrossRef]
- Srivastava, N.; Garg, P.; Srivastava, P.; Seth, P.K. A molecular dynamics simulation study of the ACE2 receptor with screened natural inhibitors to identify novel drug candidate against COVID-19. PeerJ 2021, 9, e11171. [Google Scholar] [CrossRef]
- Sukardiman, M.E.; Pratama, M.R.F.; Poerwono, H.; Siswodihardjo, S. The coronavirus disease 2019 main protease inhibitor from Andrographis paniculata (Burm. f) Ness. J. Adv. Pharm. Technol. Res. 2020, 11, 157. [Google Scholar] [CrossRef]
- Enmozhi, S.K.; Raja, K.; Sebastine, I.; Joseph, J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach. Biomol. Struct. Dyn. 2020, 39, 3092–3098. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Murugan, N.A.; Pandian, C.J.; Jeyakanthan, J. Computational investigation on Andrographis paniculata phytochemicals to evaluate their potency against SARS-CoV-2 in comparison to known antiviral compounds in drug trials. J. Biomol. Struct. Dyn. 2020, 39, 4415–4426. [Google Scholar] [CrossRef]
- Rajagopal, K.; Varakumar, P.; Baliwada, A.; Byran, G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): An in silico approach. Future J. Pharm. Sci. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Shi, T.-H.; Huang, Y.-L.; Chen, C.-C.; Pi, W.-C.; Hsu, Y.-L.; Lo, L.-C.; Chen, W.-Y.; Fu, S.-L.; Lin, C.-H. Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem. Biophys. Res. Commun. 2020, 533, 467–473. [Google Scholar] [CrossRef]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, W.; Xu, H.E. RNA-dependent RNA polymerase: Structure, mechanism, and drug discovery for COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 47–53. [Google Scholar] [CrossRef]
- Sharma, A.; Vora, J.; Patel, D.; Sinha, S.; Jha, P.C.; Shrivastava, N. Identification of natural inhibitors against prime targets of SARS-CoV-2 using molecular docking, molecular dynamics simulation and MM-PBSA approaches. J. Biomol. Struct. Dyn. 2020, 40, 3296–3311. [Google Scholar] [CrossRef] [PubMed]
- Sa-ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Khemawoot, P. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J. Nat. Prod 2021, 84, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Dai, C.Q.; Jiang, Z.Y.; Li, E.Q.; Chen, C.; Wu, X.L.; Chen, J.; Liu, Q.; Zhao, C.L.; He, J.X.; et al. Andrographolide as an anti-H1N1 drug and the mechanism related to retinoic acid-inducible gene-I-like receptors signaling pathway. Chin. J. Integr. Med. 2014, 20, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xue, H.J.; Ye, W.C.; Fang, B.H.; Liu, Y.H.; Yuan, S.H.; Yu, P.; Wang, Y.Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, C.; Sun, H.; Liu, Q.; Huang, J.; Sheng, L.; Lin, B.; Wang, J.; Chen, L. The semi-synthesis of novel andrographolide analogues and anti-influenza virus activity evaluation of their derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 769–773. [Google Scholar] [CrossRef]
- Cai, W.; Li, Y.; Chen, S.; Wang, M.; Zhang, A.; Zhou, H.; Chen, H.; Jin, M. 14-Deoxy-11,12-dehydroandrographolide exerts anti-influenza A virus activity and inhibits replication of H5N1 virus by restraining nuclear export of viral ribonucleoprotein complexes. Antivir. Res. 2015, 118, 82–92. [Google Scholar] [CrossRef]
- Shihman Chang, R.; Yeung, H.W. Inhibition of growth of human immunodeficiency virus in vitro by crude extracts of Chinese medicinal herbs. Antivir. Res. 1988, 9, 163–175. [Google Scholar] [CrossRef]
- Uttekar, M.M.; Das, T.; Pawar, R.S.; Bhandari, B.; Menon, V.; Nutan; Gupta, S.K.; Bhat, S.V. Anti-HIV activity of semisynthetic derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur. J. Med. Chem. 2012, 56, 368–374. [Google Scholar] [CrossRef]
- Chang, R.S.; Ding, L.; Gai-Qing, C.; Qi-Choa, P.; Ze-Lin, Z.; Smith, K.M. Dehydroandrographolide Succinic Acid Monoester as an Inhibitor against the Human Immunodeficiency Virus. Proc. Soc. Exp. Biol. Med. 1991, 197, 59–66. [Google Scholar] [CrossRef]
- Basak, A.; Cooper, S.; Roberge, A.G.; Banik, U.K.; Chrétien, M.; Seidah, N.G. Inhibition of proprotein convertases-1, -7 and furin by diterpines of Andrographis paniculata and their succinoyl esters. Biochem. J. 1999, 338 Pt 1, 107–113. [Google Scholar] [CrossRef]
- Calabrese, C.; Berman, S.H.; Babish, J.G.; Ma, X.; Shinto, L.; Dorr, M.; Wells, K.; Wenner, C.A.; Standish, L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 2000, 14, 333–338. [Google Scholar] [CrossRef]
- Wiart, C.; Kumar, K.; Yusof, M.Y.; Hamimah, H.; Fauzi, Z.M.; Sulaiman, M. Antiviral properties of ent-labdene diterpenes of Andrographis paniculata nees, inhibitors of herpes simplex virus type 1. Phytother. Res. 2005, 19, 1069–1070. [Google Scholar] [CrossRef]
- Suebsasana, S.; Pientong, C.; Ekalaksananan, T.; Thongchai, S.; Aromdee, C. A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Med. Chem. 2011, 7, 237–244. [Google Scholar] [CrossRef]
- Lin, T.-P.; Chen, S.-Y.; Duh, P.-D.; Chang, L.-K.; Liu, Y.-N. Inhibition of the Epstein–Barr Virus Lytic Cycle by Andrographolide. Biol. Pharm. Bull. 2008, 31, 2018–2023. [Google Scholar] [CrossRef]
- Lee, J.C.; Tseng, C.K.; Young, K.C.; Sun, H.Y.; Wang, S.W.; Chen, W.C.; Lin, C.K.; Wu, Y.H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014, 171, 237–252. [Google Scholar] [CrossRef]
- Chandramohan, V.; Kaphle, A.; Chekuri, M.; Gangarudraiah, S.; Bychapur Siddaiah, G. Evaluating Andrographolide as a Potent Inhibitor of NS3-4A Protease and Its Drug-Resistant Mutants Using In Silico Approaches. Adv. Virol. 2015, 2015, 972067. [Google Scholar] [CrossRef]
- Nithya, P.; Jeyaram, C.; Sundaram, K.M.; Chandrasekar, A.; Ramasamy, M.S. Anti-Dengue Viral Compounds from Andrographis paniculata by Insilico Approach. World J. Altern. Med. 2014, 1, 10–16. [Google Scholar]
- Ramalingam, S.; Karupannan, S.; Padmanaban, P.; Vijayan, S.; Sheriff, K.; Palani, G.; Krishnasamy, K.K. Anti-dengue activity of Andrographis paniculata extracts and quantification of dengue viral inhibition by SYBR green reverse transcription polymerase chain reaction. Ayu 2018, 39, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.I.C.; Ling, A.P.K.; Koh, R.Y.; Chye, S.M.; Voon, K.G.L. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement. Altern. Med. 2012, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Kaur, P.; Lee, R.; Ramphan, S.; Kuadkitkan, A.; Wikan, N.; Ubol, S.; Roytrakul, S.; Chu, J.H.; Smith, D. Activity of andrographolide against chikungunya virus infection. Sci. Rep. 2015, 5, 14179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mishra, K.; Dash, P.; Parida, M.; Ganju, L.; Singh, S. Andrographolide inhibits chikungunya virus infection by up-regulating host innate immune pathways. Asian Pac. J. Trop. Med. 2018, 11, 214–221. [Google Scholar] [CrossRef]
- Fangkham, S.; Ekalaksananan, T.; Aromdee, C.; Seubsasana, S.; Kongyingyoes, B.; Patarapadungkit, N.; Pientong, C. The effect of andrographolide on Human papillomavirus type 16 (HPV16) positive cervical cancer cells (SiHa). Int. J. Infect. Dis. 2012, 16, e80. [Google Scholar] [CrossRef][Green Version]
- Ekalaksananan, T.; Sookmai, W.; Fangkham, S.; Pientong, C.; Aromdee, C.; Seubsasana, S.; Kongyingyoes, B. Activity of Andrographolide and Its Derivatives on HPV16 Pseudovirus Infection and Viral Oncogene Expression in Cervical Carcinoma Cells. Nutr. Cancer 2015, 67, 687–696. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Puri, A.; Saxena, R.; Saxena, R.P.; Saxena, K.C.; Srivastava, V.; Tandon, J.S. Immunostimulant Agents from Andrographis paniculata. J. Nat. Prod. 1993, 56, 995–999. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Dong, S.-f.; Liu, C.-h.; Italiani, P.; Sun, S.-h.; Xu, J.; Boraschi, D.; Ma, S.-p.; Qu, D. Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol. Sin. 2010, 31, 191–201. [Google Scholar] [CrossRef]
- Kumar, R.A.; Sridevi, K.; Kumar, N.V.; Nanduri, S.; Rajagopal, S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef]
- Radhika, P.; Annapurna, A.; Rao, S.N. Immunostimulant, cerebroprotective & nootropic activities of Andrographis paniculata leaves extract in normal & type 2 diabetic rats. Indian J. Med. Res. 2012, 135, 636–641. [Google Scholar]
- Churiyah; Pongtuluran, O.B.; Rofaani, E.; Tarwadi. Antiviral and Immunostimulant Activities of Andrographis paniculata. HAYATI J. Biosci. 2015, 22, 67–72. [Google Scholar] [CrossRef]
- Bukoye, O.; Musbau, A. Immune Modulation Potentials of Aqueous Extract of Andrographis paniculata Leaves in Male Rat. Researcher 2011, 3, 48–57. [Google Scholar]
- Peng, G.Y.; Zhou, F.; Ding, R.L.; Li, H.D.; Yao, K. Modulation of lianbizi injection (andrographolide) on some immune functions. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2002, 27, 147–150. [Google Scholar]
- Panossian, A.; Davtyan, T.; Gukassyan, N.; Gukasova, G.; Mamikonyan, G.; Gabrielian, E.; Wikman, G. Effect of andrographolide and Kan Jang—Fixed combination of extract SHA-10 and extract SHE-3—On proliferation of human lymphocytes, production of cytokines and immune activation markers in the whole blood cells culture. Phytomed. Int. J. Phytother. Phytopharm. 2002, 9, 598–605. [Google Scholar] [CrossRef]
- Naik, S.R.; Hule, A. Evaluation of immunomodulatory activity of an extract of andrographolides from Andographis paniculata. Planta Med. 2009, 75, 785–791. [Google Scholar] [CrossRef]
- Banerjee, S.; Kar, A.; Mukherjee, P.; Haldar, P.; Sharma, N.; Katiyar, C. Immunoprotective potential of Ayurvedic herb Kalmegh (Andrographis paniculata) against respiratory viral infections—LC–MS/MS and network pharmacology analysis. Phytochem. Anal. 2021, 32, 629–639. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Huang, S.; Gartung, A.; Cortés-Puch, I.; Sime, P.J.; Phipps, R.P.; Serhan, C.N.; Hammock, B.D. Inflammation resolution: A dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020, 39, 337–340. [Google Scholar] [CrossRef]
- Calò Carducci, F.I.; De Ioris, M.A.; Agrati, C.; Carsetti, R.; Perrotta, D.; D’Argenio, P.; De Benedetti, F.; Notari, S.; Rossi, P.; Campana, A. Hyperinflammation in Two Severe Acute Respiratory Syndrome Coronavirus 2-Infected Adolescents Successfully Treated With the Interleukin-1 Inhibitor Anakinra and Glucocorticoids. Front. Pediatr. 2020, 8. [Google Scholar] [CrossRef]
- Ma, Q.; Pan, W.; Li, R.; Liu, B.; Li, C.; Xie, Y.; Wang, Z.; Zhao, J.; Jiang, H.; Huang, J.; et al. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol. Res. 2020, 158, 104850. [Google Scholar] [CrossRef]
- Habtemariam, S. Andrographolide inhibits the tumour necrosis factor-α-induced upregulation of ICAM-1 expression and endothelial-monocyte adhesion. Phytother. Res. 1998, 12, 37–40. [Google Scholar] [CrossRef]
- Chen, H.-W.; Lin, A.-H.; Chu, H.-C.; Li, C.-C.; Tsai, C.-W.; Chao, C.-Y.; Wang, C.-J.; Lii, C.-K.; Liu, K.-L. Inhibition of TNF-α-Induced Inflammation by Andrographolide via Down-Regulation of the PI3K/Akt Signaling Pathway. J. Nat. Prod. 2011, 74, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.A.; Romero, A.; Figueroa, J.; Cortés, P.; Concha, I.I.; Hancke, J.L.; Burgos, R.A. Andrographolide interferes with binding of nuclear factor-κB to DNA in HL-60-derived neutrophilic cells. Br. J. Pharmacol. 2005, 144, 680–686. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Suthisisang, C.; Dhepakson, P.; Herunsalee, A. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm.f.) Nees and their effects on gene expression. Int. Immunopharmacol. 2010, 10, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, C.V.; Gupta, A.; Agarwal, A. Effect of an extract of Andrographis paniculata leaves on inflammatory and allergic mediators in vitro. J. Ethnopharmacol. 2010, 129, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Sheeja, K.; Kuttan, G. Ameliorating effects of Andrographis paniculata extract against cyclophosphamide-induced toxicity in mice. Asian Pac. J. Cancer Prev. 2006, 7, 609–614. [Google Scholar] [PubMed]
- Freedland, S.; Abrahamsson, P.-A. Androgen deprivation therapy and side effects: Are GnRH antagonists safer? Int. J. Nutr. Pharmacol. Neurol. Dis. 2013, 3, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, L.; Wu, W.; Yang, J.; Yang, Z.; Liu, S. Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAK-STAT signaling pathway. Microbes Infect. 2017, 19, 605–615. [Google Scholar] [CrossRef]
- Li, J.; Luo, L.; Wang, X.; Liao, B.; Li, G. Inhibition of NF-kappaB expression and allergen-induced airway inflammation in a mouse allergic asthma model by andrographolide. Cell Mol. Immunol. 2009, 6, 381–385. [Google Scholar] [CrossRef]
- Tan, W.S.; Peh, H.Y.; Liao, W.; Pang, C.H.; Chan, T.K.; Lau, S.H.; Chow, V.T.; Wong, W.S. Cigarette Smoke-Induced Lung Disease Predisposes to More Severe Infection with Nontypeable Haemophilus influenzae: Protective Effects of Andrographolide. J. Nat. Prod. 2016, 79, 1308–1315. [Google Scholar] [CrossRef]
- Ko, H.C.; Wei, B.L.; Chiou, W.F. The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus-infected human epithelial cells. J. Ethnopharmacol. 2006, 107, 205–210. [Google Scholar] [CrossRef]
- Peng, S.; Hang, N.; Liu, W.; Guo, W.; Jiang, C.; Yang, X.; Xu, Q.; Sun, Y. Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-κB pathways. Acta Pharm. Sin. B 2016, 6, 205–211. [Google Scholar] [CrossRef]
- Lim, J.C.; Chan, T.K.; Ng, D.S.; Sagineedu, S.R.; Stanslas, J.; Wong, W.S. Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer. Clin. Exp. Pharmacol. Physiol. 2012, 39, 300–310. [Google Scholar] [CrossRef]
- Burgos, R.A.; Alarcón, P.; Quiroga, J.; Manosalva, C.; Hancke, J. Andrographolide, an Anti-Inflammatory Multitarget Drug: All Roads Lead to Cellular Metabolism. Molecules 2020, 26, 5. [Google Scholar] [CrossRef]
- Vedavathy, S.; Rao, K.N. Antipyretic activity of six indigenous medicinal plants of Tirumala Hills, Andhra Pradesh, India. J. Ethnopharmacol. 1991, 33, 193–196. [Google Scholar] [CrossRef]
- Madav, S.; Tripathi, C.; Tandan, S.K.; Mishra, S. Analgesic, antipyretic, and antiulcerogenic effects of andrographolide. Indian J. Pharm. Sci. 1995, 57, 121–125. [Google Scholar]
- Deng, W.L. Preliminary studies on the pharmacology of the Andrographis product dihydroandrographolide sodium succinate. Newsl. Chin. Herb. Med. 1978, 8, 26–28. [Google Scholar]
- Suebsasana, S.; Pongnaratorn, P.; Sattayasai, J.; Arkaravichien, T.; Tiamkao, S.; Aromdee, C. Analgesic, antipyretic, anti-inflammatory and toxic effects of andrographolide derivatives in experimental animals. Arch. Pharmacal Res. 2009, 32, 1191–1200. [Google Scholar] [CrossRef]
- Thamlikitkul, V.; Dechatiwongse, T.; Theerapong, S.; Chantrakul, C.; Boonroj, P.; Punkrut, W.; Ekpalakorn, W.; Boontaeng, N.; Taechaiya, S.; Petcharoen, S.; et al. Efficacy of Andrographis paniculata, Nees for pharyngotonsillitis in adults. J. Med. Assoc. Thail. = Chotmaihet Thangphaet 1991, 74, 437–442. [Google Scholar]
- Panossian, A.; Hovhannisyan, A.; Mamikonyan, G.; Abrahamian, H.; Hambardzumyan, E.; Gabrielian, E.; Goukasova, G.; Wikman, G.; Wagner, H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomed. Int. J. Phytother. Phytopharm. 2000, 7, 351–364. [Google Scholar] [CrossRef]
- Cáceres, D.D.; Hancke, J.L.; Burgos, R.A.; Sandberg, F.; Wikman, G.K. Use of visual analogue scale measurements (VAS) to asses the effectiveness of standardized Andrographis paniculata extract SHA-10 in reducing the symptoms of common cold. A randomized double blind-placebo study. Phytomed. Int. J. Phytother. Phytopharm. 1999, 6, 217–223. [Google Scholar] [CrossRef]
- Spasov, A.A.; Ostrovskij, O.V.; Chernikov, M.V.; Wikman, G. Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother. Res. PTR 2004, 18, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Poolsup, N.; Suthisisang, C.; Prathanturarug, S.; Asawamekin, A.; Chanchareon, U. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: Systematic review of randomized controlled trials. J. Clin. Pharm. Ther. 2004, 29, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Coon, J.; Ernst, E. Andrographis paniculata in the Treatment of Upper Respiratory Tract Infections: A Systematic Review of Safety and Efficacy. Planta Med. 2004, 70, 293–298. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Wu, R.-H.; Logue, M.; Blondel, C.; Lai, L.Y.W.; Stuart, B.; Flower, A.; Fei, Y.-T.; Moore, M.; Shepherd, J.; et al. Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0181780. [Google Scholar] [CrossRef]
- Melchior, J.; Spasov, A.; Ostrovskij, O.; Bulanov, A.; Wikman, G. Double-blind, placebo-controlled pilot and phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan jang) in the treatment of uncomplicated upper-respiratory tract infection. Phytomed. Int. J. Phytother. Phytopharm. 2000, 7, 341–350. [Google Scholar] [CrossRef]
- Amaryan, G.; Astvatsatryan, V.; Gabrielyan, E.; Panossian, A.; Panosyan, V.; Wikman, G. Double-blind, placebo-controlled, randomized, pilot clinical trial of ImmunoGuard®–a standardized fixed combination of Andrographis paniculata Nees, with Eleutherococcus senticosus Maxim, Schizandra chinensis Bail. and Glycyrrhiza glabra L. extracts in patients with Familial Mediterranean Fever. Phytomed. Int. J. Phytother. Phytopharm. 2003, 10, 271–285. [Google Scholar]
- Mkrtchyan, A.; Panosyan, V.; Panossian, A.; Wikman, G.; Wagner, H. A phase I clinical study of Andrographis paniculata fixed combination Kan Jang™ versus ginseng and valerian on the semen quality of healthy male subjects. Phytomed. Int. J. Phytother. Phytopharm. 2005, 12, 403–409. [Google Scholar] [CrossRef]
- Caceres, D.; Hancke, J.; Burgos, R.; Wikman, G. Prevention of common colds with Andrographis paniculata dried extract. A pilot double blind trial. Phytomed. Int. J. Phytother. Phytopharm. 1997, 4, 101–104. [Google Scholar] [CrossRef]
- Hancke, J.; Burgos, R.; Caceres, D.; Wikman, G. A double-blind study with a new monodrug Kan Jang: Decrease of symptoms and improvement in the recovery from common colds. Phytother. Res. 1995, 9, 559–562. [Google Scholar] [CrossRef]
- Saxena, R.; Singh, R.; Kumar, P.; Yadav, S.; Negi, M.; Saxena, V.; Joshua, A.; Vijayabalaji, V.; Goudar, K.; Venkateshwarlu, K. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold™) in patients with uncomplicated upper respiratory tract infection. Phytomed. Int. J. Phytother. Phytopharm. 2010, 17, 178–185. [Google Scholar] [CrossRef]
- Ulbricht, C.; Seamon, E. Natural Standard Herbal Pharmacotherapy an Evidence Based Approach; Mosby Elsevier: St. Louis, MI, USA, 2009. [Google Scholar]
- Burgos, R.; Hancke, J.; Bertoglio, J.; Aguirre, V.; Arriagada, S.; Calvo, M.; Cáceres, D. Efficacy of an Andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: A prospective randomized placebo-controlled trial. Clin. Rheumatol. 2009, 28, 931–946. [Google Scholar] [CrossRef]
- Farah, M.; Meyboom, R.; Ploen, M. Acute hypersensitivity reactions to Andrographis paniculata containing products, as reported in international pharmacovigilance. Drug Saf. 2008, 31, 885. [Google Scholar]
- Panossian, A.; Kochikian, A.; Gabrielian, E.; Muradian, R.; Stepanian, H.; Arsenian, F.; Wagner, H. Effect of Andrographis paniculata extract on progesterone in blood plasma of pregnant rats. Phytomed. Int. J. Phytother. Phytopharm. 1999, 6, 157–161. [Google Scholar] [CrossRef]
- Chandrasekaran, C.; Thiyagarajan, P.; Sundarajan, K.; Goudar, K.S.; Deepak, M.; Murali, B.; Allan, J.J.; Agarwal, A. Evaluation of the genotoxic potential and acute oral toxicity of standardized extract of Andrographis paniculata (KalmCold™). Food Chem. Toxicol. 2009, 47, 1892–1902. [Google Scholar] [CrossRef]
- Bothiraja, C.; Pawar, A.P.; Shende, V.S.; Joshi, P.P. Acute and subacute toxicity study of andrographolide bioactive in rodents: Evidence for the medicinal use as an alternative medicine. Comp. Clin. Pathol. 2013, 22, 1123–1128. [Google Scholar] [CrossRef]
- Worasuttayangkurn, L.; Nakareangrit, W.; Kwangjai, J.; Sritangos, P.; Pholphana, N.; Watcharasit, P.; Rangkadilok, N.; Thiantanawat, A.; Satayavivad, J. Acute oral toxicity evaluation of Andrographis paniculata-standardized first true leaf ethanolic extract. Toxicol. Rep. 2019, 6, 426–430. [Google Scholar] [CrossRef]
- Chang, H.; But, P.; Wang, L. Chuanxinlian. In Pharmacology and Applications of Chinese Material Medica; Chang, H., But, P., Eds.; World Scientific Publishing Co. Pte. Ltd: Singapore, 1987; Volume 2, pp. 913–926. [Google Scholar]
- Rana, A.C.; Avadhoot, Y. Hepatoprotective effects of Andrographis paniculata against carbon tetrachloride-induced liver damage. Arch. Pharmacal Res. 1991, 14, 93–95. [Google Scholar] [CrossRef]
- Burgos, R.A.; Caballero, E.E.; Sánchez, N.S.; Schroeder, R.A.; Wikman, G.K.; Hancke, J.L. Testicular toxicity assessment of Andrographis paniculata dried extract in rats. J. Ethnopharmacol. 1997, 58, 219–224. [Google Scholar] [CrossRef]
- Handa, S.S.; Sharma, A. Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J. Med. Res. 1990, 92, 276–283. [Google Scholar]
- Sakila, S.; Begum, N.; Kawsar, S.; Begum, Z.; Zola, M. Relationship of anti-fertility effects of Andrographis paniculata and hormonal assay in female rats. Bangladesh J. Med. Sci 2009, 8, 10–14. [Google Scholar] [CrossRef]
- Zoha, M.; Hussain, A.; Choudhury, S. Antifertility effect of Andrographis paniculata in mice. Bangladesh Med. Res. Counc. Bull. 1989, 15, 34–37. [Google Scholar]
- Noordalilati, M.N.; Sembulingam, K.; Chan, K.L.; Siti Amrah, S. The teratogenic effect of standardised Andrographis paniculata extract in sprague dawley rats. Malays. J. Med. Sci 2007, 14, 91. [Google Scholar]
- Mills, S.Y.; Bone, K. The Essential Guide to Herbal Safety; Elsevier Health Sciences: London, UK, 2004. [Google Scholar]
- Singh, A.; Zhao, K. Herb–drug interactions of commonly used Chinese medicinal herbs. Int. Rev. Neurobiol. 2017, 135, 197–232. [Google Scholar] [PubMed]
- Pandit, S.; Kanjilal, S.; Awasthi, A.; Chaudhary, A.; Banerjee, D.; Bhatt, B.; Narwaria, A.; Singh, R.; Dutta, K.; Jaggi, M. Evaluation of herb-drug interaction of a polyherbal Ayurvedic formulation through high throughput cytochrome P450 enzyme inhibition assay. J. Ethnopharmacol. 2017, 197, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.P.; Kuroyanagi, M.; Sulaiman, S.F.; Muhammad, T.S.T.; Tan, M.L. Andrographolide and 14-deoxy-11, 12-didehydroandrographolide inhibit cytochrome P450s in HepG2 hepatoma cells. Life Sci. 2011, 88, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Abd-Rashid, B.A.; Ismail, Z.; Ismail, R.; Mak, J.W.; Pook, P.C.; Er, H.M.; Ong, C.E. In vitro determination of the effect of Andrographis paniculata extracts and andrographolide on human hepatic cytochrome P450 activities. J. Nat. Med. 2011, 65, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Hou, X.-L.; Takahashi, K.; Chen, L.-X.; Azuma, J.; Kang, N. Andrographolide inhibits the expression and metabolic activity of cytochrome P450 3A4 in the modified Caco-2 cells. J. Ethnopharmacol. 2012, 141, 709–713. [Google Scholar] [CrossRef]
- Jaruchotikamol, A.; Jarukamjorn, K.; Sirisangtrakul, W.; Sakuma, T.; Kawasaki, Y.; Nemoto, N. Strong synergistic induction of CYP1A1 expression by andrographolide plus typical CYP1A inducers in mouse hepatocytes. Toxicol. Appl. Pharmacol. 2007, 224, 156–162. [Google Scholar] [CrossRef]
- Kondo, S.; Chatuphonprasert, W.; Jaruchotikamol, A.; Sakuma, T.; Nemoto, N. Cellular glutathione content modulates the effect of andrographolide on β-naphthoflavone-induced CYP1A1 mRNA expression in mouse hepatocytes. Toxicology 2011, 280, 18–23. [Google Scholar] [CrossRef]
- Chatuphonprasert, W.; Remsungnen, T.; Nemoto, N.; Jarukamjorn, K. Different AhR binding sites of diterpenoid ligands from Andrographis paniculata caused differential CYP1A1 induction in primary culture in mouse hepatocytes. Toxicol. Vitr. 2011, 25, 1757–1763. [Google Scholar] [CrossRef]
- Jarukamjorn, K.; Don-in, K.; Makejaruskul, C.; Laha, T.; Daodee, S.; Pearaksa, P.; Sripanidkulchai, B.-o. Impact of Andrographis paniculata crude extract on mouse hepatic cytochrome P450 enzymes. J. Ethnopharmacol. 2006, 105, 464–467. [Google Scholar] [CrossRef]
- Ismail, S.; Aziah Hanapi, N.; Ab Halim, M.R.; Uchaipichat, V.; Mackenzie, P.I. Effects of Andrographis paniculata and Orthosiphon stamineus extracts on the glucuronidation of 4-methylumbelliferone in human UGT isoforms. Molecules 2010, 15, 3578–3592. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Sun, D.-X.; Cao, Y.-F.; Ai, C.-Z.; Qu, Y.-Q.; Hu, C.-M.; Jiang, C.; Dong, P.-P.; Sun, X.-Y.; Hong, M. Herb–drug interaction prediction based on the high specific inhibition of andrographolide derivatives towards UDP-glucuronosyltransferase (UGT) 2B7. Toxicol. Appl. Pharmacol. 2014, 277, 86–94. [Google Scholar] [CrossRef]
- Uchaipichat, V. In vitro inhibitory effects of major bioactive constituents of Andrographis paniculata, Curcuma longa and Silybum marianum on human liver microsomal morphine glucuronidation: A prediction of potential herb-drug interactions arising from andrographolide, curcumin and silybin inhibition in humans. Drug Metab. Pharmacokinet. 2018, 33, 67–76. [Google Scholar]
- Zhang, X.; Zhang, X.; Wang, X.; Zhao, M. Influence of andrographolide on the pharmacokinetics of warfarin in rats. Pharm. Biol. 2018, 56, 351–356. [Google Scholar] [CrossRef]
- Chien, C.-F.; Wu, Y.-T.; Lee, W.-C.; Lin, L.-C.; Tsai, T.-H. Herb–drug interaction of Andrographis paniculata extract and andrographolide on the pharmacokinetics of theophylline in rats. Chem.-Biol. Interact. 2010, 184, 458–465. [Google Scholar] [CrossRef]
- Carretta, M.D.; Alarcón, P.; Jara, E.; Solis, L.; Hancke, J.L.; Concha, I.I.; Hidalgo, M.A.; Burgos, R.A. Andrographolide reduces IL-2 production in T-cells by interfering with NFAT and MAPK activation. Eur. J. Pharmacol. 2009, 602, 413–421. [Google Scholar] [CrossRef]
- Balap, A.; Lohidasan, S.; Sinnathambi, A.; Mahadik, K. Herb-drug interaction of Andrographis paniculata (Nees) extract and andrographolide on pharmacokinetic and pharmacodynamic of naproxen in rats. J. Ethnopharmacol. 2017, 195, 214–221. [Google Scholar] [CrossRef]
- Balap, A.; Atre, B.; Lohidasan, S.; Sinnathambi, A.; Mahadik, K. Pharmacokinetic and pharmacodynamic herb–drug interaction of Andrographis paniculata (Nees) extract and andrographolide with etoricoxib after oral administration in rats. J. Ethnopharmacol. 2016, 183, 9–17. [Google Scholar] [CrossRef]
- Samala, S.; Veeresham, C. Pharmacokinetic and pharmacodynamic interaction of boswellic acids and andrographolide with glyburide in diabetic rats: Including its PK/PD modeling. Phytother. Res. 2016, 30, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Kumar, R.A.; Deevi, D.S.; Satyanarayana, C.; Rajagopalan, R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J. Exp. Ther. Oncol. 2003, 3, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, Y.; Liu, W.; Wu, X.; Guo, L.; Cai, P.; Wu, X.; Wu, X.; Shen, Y.; Shu, Y. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy 2014, 10, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, W.; Guo, L.; Gu, Y.; Cai, P.; Xie, N.; Yang, X.; Shu, Y.; Wu, X.; Sun, Y. Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. Int. Immunopharmacol. 2014, 20, 337–345. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Choon-Nam, O.; Shen, H.-M. Critical role of pro-apoptotic Bcl-2 family members in andrographolide-induced apoptosis in human cancer cells. Biochem. Pharmacol. 2006, 72, 132–144. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, S.-E.; Tan, S.-H.; Cao, R.; Chen, Y.; Xia, D.; Zhu, X.; Yang, X.-F.; Ong, C.-N.; Shen, H.-M. Andrographolide sensitizes cisplatin-induced apoptosis via suppression of autophagosome-lysosome fusion in human cancer cells. Autophagy 2012, 8, 338–349. [Google Scholar] [CrossRef]
- Yang, L.; Wu, D.; Luo, K.; Wu, S.; Wu, P. Andrographolide enhances 5-fluorouracil-induced apoptosis via caspase-8-dependent mitochondrial pathway involving p53 participation in hepatocellular carcinoma (SMMC-7721) cells. Cancer Lett. 2009, 276, 180–188. [Google Scholar] [CrossRef]
- Wang, W.; Guo, W.; Li, L.; Fu, Z.; Liu, W.; Gao, J.; Shu, Y.; Xu, Q.; Sun, Y.; Gu, Y. Andrographolide reversed 5-FU resistance in human colorectal cancer by elevating BAX expression. Biochem. Pharmacol. 2016, 121, 8–17. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, B.; Gao, F.; Lan, M. Synergistic anticancer effects of andrographolide and paclitaxel against A549 NSCLC cells. Pharm. Biol. 2016, 54, 2629–2635. [Google Scholar] [CrossRef][Green Version]
- Yunos, N.M.; Mutalip, S.S.; Jauri, M.H.; Yu, J.Q.; Huq, F. Anti-proliferative and pro-apoptotic effects from sequenced combinations of andrographolide and cisplatin on ovarian cancer cell lines. Anticancer Res. 2013, 33, 4365–4371. [Google Scholar]
- Pekthong, D.; Martin, H.; Abadie, C.; Bonet, A.; Heyd, B.; Mantion, G.; Richert, L. Differential inhibition of rat and human hepatic cytochrome P450 by Andrographis paniculata extract and andrographolide. J. Ethnopharmacol. 2008, 115, 432–440. [Google Scholar] [CrossRef]
- Pekthong, D.; Blanchard, N.; Abadie, C.; Bonet, A.; Heyd, B.; Mantion, G.; Berthelot, A.; Richert, L.; Martin, H. Effects of Andrographis paniculata extract and Andrographolide on hepatic cytochrome P450 mRNA expression and monooxygenase activities after in vivo administration to rats and in vitro in rat and human hepatocyte cultures. Chem. Biol. Interact. 2009, 179, 247–255. [Google Scholar] [CrossRef]
- Hanapi, N.A.; Juzaili, A.z.; Sabariah, I.; Sharif, M. Evaluation of Selected Malaysian Medicinal Plants on Phase I Drug Metabolizing Enzymes, CYP2C9, CYP2D6 and CYP3A4 Activities in vitro. Int. J. Pharmacol. 2010, 6. [Google Scholar] [CrossRef]
- Kar, A.; Pandit, S.; Mukherjee, K.; Bahadur, S.; Mukherjee, P.K. Safety assessment of selected medicinal food plants used in Ayurveda through CYP450 enzyme inhibition study. J. Sci. Food Agric. 2017, 97, 333–340. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Lv, L.; Zhou, Y.-L.; Xie, L.-D.; Xu, Q.; Zou, X.-F.; Ding, Y.; Tian, J.; Fan, J.-L.; Fan, H.-W.; et al. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial. Phytother. Res. 2021, 35, 4401–4410. [Google Scholar] [CrossRef]
- Wang, C.; Sun, S.; Ding, X. The therapeutic effects of traditional Chinese medicine on COVID-19: A narrative review. Int. J. Clin. Pharm. 2021, 43, 35–45. [Google Scholar] [CrossRef]
- Ghosh, A.; Nundy, S.; Mallick, T.K. How India is dealing with COVID-19 pandemic. Sens. Int. 2020, 1, 100021. [Google Scholar] [CrossRef]
- Rattanaraksa, D.; Khempetch, R.; Poolwiwatchaikool, U.; Nimitvilai, S.; Loatrakul, O.; Srimanee, P. The Efficacy and Safety of Andrographis paniculate Extractfor Treatment of COVID-19 Patients with Mild Symptoms, Nakhonpathom Hospital. Reg. 4-5 Med. J. 2022, 40, 269–282. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).