Abstract
A new effective method for the synthesis of imidazo[1,5-b]pyridazines derivatives (yields = 68–89%) by the interaction of 1,2-diamino-4-phenylimidazole with DMAD, in methanol and in the presence of a catalytic amount of acetic acid, is proposed. The course of reaction has been examined by classical organic methods, HPLC-MS analysis, and quantum-chemical calculations.
1. Introduction
Several natural biologically active systems (from chlorophyll to hemoglobin) and many natural and synthetic drugs (from penicyllin to sulfadiazine) contain in their structure heterocyclic systems (mono- or poly-cyclic) holding different hetero-atoms (from nitrogen to oxygen or sulphur).
This is likely one of the reasons that so many chemists study the syntheses, the reactivities, andthe properties of several heterocyclic compounds.
Thus, in the past fewdecades we have studied rearrangements [1,2,3,4], heterocyclicring-openings [5,6,7,8,9], and the different behaviors [10,11,12] of many heterocyclic compounds, also gaininguseful information on their pharmacological activities (cardiovascular [13,14,15], antitumor [16,17,18], and anti-MDR1 [19,20] effectshave been evaluated).
We have focusedour attention onboth mono- and poly-cyclic systems. Now, we are turning our interest to the study of a reaction able to "build" a new ring on an already existing one.
Among the reagents employed in this field, special attention has been devoted to the use of dimethyl acetylenedicarboxylate (1, DMAD). It represents a versatile synthetic reagent useful for the discovery of new methods in combinatorial chemistry and multicomponent reactions, able to produceheterocyclic compounds. Being an electron-deficient acetylene derivative, it can act as a Michael acceptor or participate in cycloaddition reactions (Diels-Alder, 1,3-dipolar and [2 + 2] cycloaddition) depending on the chosen reagents/conditions. The ability of DMAD to form zwitterions in multicomponent processes is quite important. The interactions of DMAD with amino azoles, leading to the formation of different heterocyclic systems from structurally similar initial bi-nucleophilic substrates, depend on experimental conditions and on reagents used. In fact, the observed different results can depend onthe used solvent, onthe catalyst, and onthe nature of the substituents present in the used substrates.
For example, it was found that N,N- and N,S-dinucleophiles, such as amino- and mercaptotriazoles 2a and 2b, respectively [21,22], by interaction with DMAD in dichloromethane, led to the formation of 4,7-dihydro-s-triazolo[1,5-a]pyrimidinones 4a, [1,2,4]triazolo[5,1-b][1,3]thiazinones 4b and dihydro[1,2,4]triazolo[4,3-a]pyrimidinones 4c with high antibacterial activity [23,24] (Scheme 1). In a first step, an addition ofDMAD at the multiple bond by the amino or mercapto group of the starting triazole occurs, then an intramolecular cyclization on the NH fragment due to the “far” ester group happens. A similar process, involving N-substituted aminotriazoles 6, [25] aminoimidazoles 7 and 10, [26,27] aminopyrazoles 3, [28] 2-amino-3-carboethoxyindoles 12b [29], and indoline-2-thiones 12a [30] leads to the formation of [1,2,4]triazolo[4,3-a]pyrimidinones 8, imidazo[1,2-a]pyrimidinones 9 and 11, pyrazolo[1,5-a]pyrimidinones 5, pyrimido[3,2-a]indolones 14, and thiopyrano[2,3-b]indolones 13.
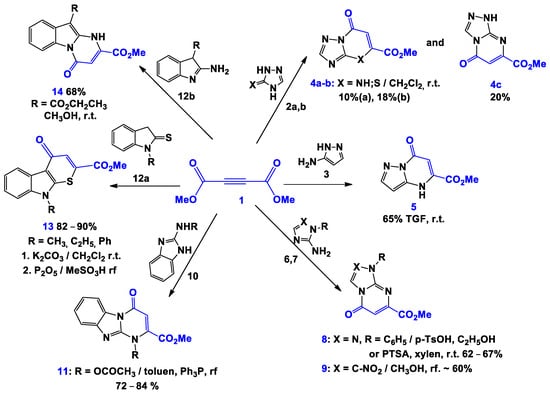
Scheme 1.
Interaction of DMAD (1) with N,N- and S,N-dinucleophiles.
With some dinucleophiles, the intermediates initially formed by the additionon the multiple bonds of DMAD can undergo cyclization at the nearest ester group. Such reaction has been described for 2-hydrazinobenzimidazoles 15 [31] and 3,4-dihydropyrimidine-2-thiones 16a–c, [32,33,34] with the formation of dihydrobenzo[4,5]imidazo[2,1-c][1,2,4]triazines 17 and dihydrothiazolo[3,2-a]pyrimidines 18, respectively (Scheme 2).
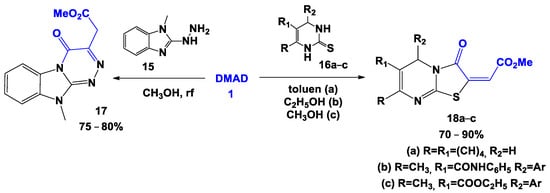
Scheme 2.
Formation of cyclic products due to the “nearest” carboxyl group.
Ueda et al. [35] found that when DMAD was reacted with 7-alkyl-8-amino-1,3-dimethylxanthines amines 19 in methanol, 5-alkyl-1,3-dimethyl-9-methoxycarbonyl-1,2,3,4-tetrahydro-5N,7N-pyrimido[1,2-e]purine-2,4,7-triones 20 and 7-alkyl-1,3-dimethyl-1,2,3,6-tetrahydro-8-(1,2,4,5,6,7-hexamethoxycarbonyl-3-azatricyclo[2.2.1.02,6]heptyl)purine-2,6-diones 21 were formed (Scheme 3). However, the reaction, performedin dimethylformamide, led to the formation of the mixture of diazocino- and diazepinopurines 22–24.
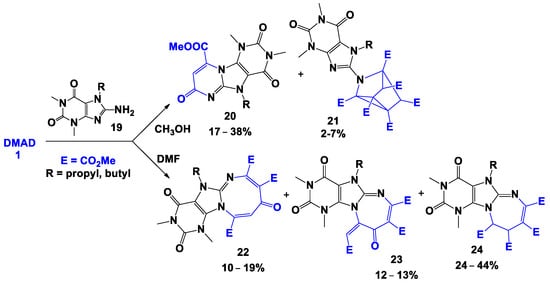
Scheme 3.
Reactions of xanthines with DMAD.
It has also been observed that 1,3-substituted 5-aminopyrazole-4-carbonitriles 25 [36] (in the presence of potassium carbonate, in DMSO) and N,N-diisopropylamidinylpyrazolimines 26 [37] (in the presence of catalytic amounts of zinc chloride in acetonitrile) interact with DMAD (in case 26 DEtAD 1* has been used), inducingan Aza-Diels–Alder reaction, leading to the formation of the relevant 4-aminopyrazolo[3,4-b]pyridine-5,6-dicarboxylates 27 and 1H-pyrazolo[3,4-b]pyridine-4,5-dicarboxylates 28 (Scheme 4), respectively.
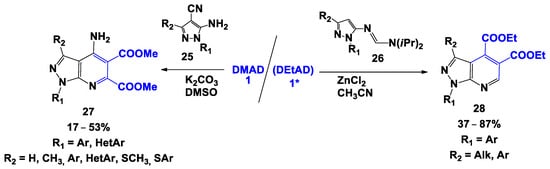
Scheme 4.
Cyclization of DMAD with the involvement of C,N-dinucleophiles and systems containing nucleophilic and electrophilic groups.
It was discovered that when1-substituted 5-aminopyrazoles 30 (prolonged boiling in acetic acid), [38] 1-methyl-2-aminoindoles 29 (prolonged boiling in methanol), [29] 3-aminocoumarin 31, 3-aminochromone 32 (in methanol and diphenyl ether) [39], and aminouracil 33 (methanol at room temperature) [40,41] were used, DMAD acts as a Michael acceptor, promoting the formation of the corresponding heterocyclic systems (Scheme 5).
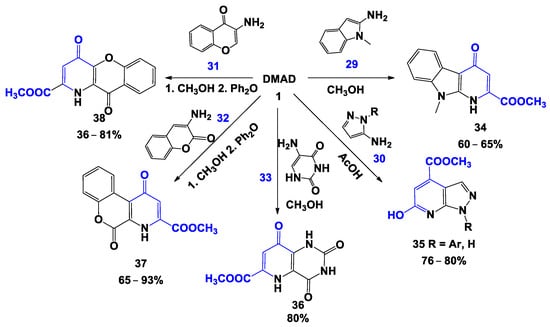
Scheme 5.
Cyclization of DMAD with the involvement of C,N-dinucleophiles.
Reactions with polynucleophiles are quite complex. In fact, Miyamoto [42] observed that the course of the reaction of DMAD 1 with 2-amino-4-R-1-arylideneaminoimidazoles 39 in benzene depends on the nature of the present substituents and the temperature of the reaction. For example, interaction with 2-amino-1- benzylidenamino-4-methylimidazole 39a in boiling benzene led to the formation of dimethyl-2-amino-1-benzylideneamino-1H-pyrrole-3,4-dicarboxylate 40a and methyl 1-benzylidenamino-4-methyl-5-oxo-1H-imidazole[1,2-a]pyrimidine-7-carboxylate 41. Vice versa, in the instance of 2-amino-4-aryl-1-arylideneaminoimidazoles 39b, the previously described pyrroles 40b in a mixture with benzonitriles 42 were isolated after boiling. Moreover, performing the reaction at room temperature the formation of dimethyl-1- (2-amino-1-benzylideneamino-4-aryl-1N-imidazol-5-yl)fumarates 43 was observed (Scheme 6).
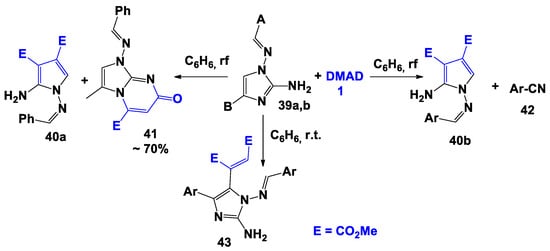
Scheme 6.
Interaction of DMAD with 2-amino-4-R-1-arylideneaminoimidazoles.
Looking at the complex aforementioned results, we have focusedour attention onthe study of the reaction of DMAD with non-symmetric polynucleophiles deriving from 1,2-diaminoimidazoles: the influence of reaction conditions on the regioselectivity will be examined using classical organic synthesis and quantum chemical calculations.
2. Results and Discussion
Considering the fact that the observed course of reaction of DMAD 1 with some nucleophiles depends on the nature of the solvent, first we have investigated and selected the optimal reaction conditions, examining the possible interactions of 1 with 1,2-diamino-4-phenylimidazole 44a. Based on the literature data and considering the solubility of the starting diamine, we have chosen thepossible solvents benzene, dioxane, chloroform, methylene chloride, methanol, and ethanol. The composition of the reaction mixture was assessed using the absolute normalization method based on HPLC-MS analysis. The signals were interpreted on the basis of preliminary calculated weights, in the form of molecular ions with [M+H]+ of all the possible initial, intermediate, and expected final compounds. Samples were treated for 0, 60, and 120 min. It should also be noted that under the conditions of our HPLC-MS experiment, DMAD was not protonated or detected at a given wavelength. The results obtained are portrayedin Table 1.

Table 1.
Content of 1,2-diamino-4-phenylimidazole determined by the reaction mass (%).
As can be observed, when solvents such as benzene, dioxane, chloroform, or methylene chloride were used, the highest conversion of the starting diamine after 2 h of boiling is about 37–65%. In this case, the mass spectra of the reactionsindicatesignals of the formed intermediates and products of their intramolecular cyclization.
A different behavior was observed when methanol or ethanol were used as solvents: complete conversion of 1,2-diamino-4-phenylimidazole occurred in 2 h. However, using ethanol, the formation of the target reaction product was significantly low, in line with an ongoing process of transesterification of the ester group. Moreover, the formation of a mixture of ethyl and methyl esters makes more complex the identification/analysis of the products of reaction.
We have previously observed that heterocyclization reactions involving diaminoimidazole are accelerated by acid catalysis (for example, by acetic acid) [43,44,45]. In this case, we have observedthat the addition of acetic acid, using methanol as a solvent significantly affected the reactivity. In fact, as presented in Table 1, the addition of catalytic amounts of acetic acid allows a complete conversion of the reactant in 60 min. It should be noted that, on the contrary, conducting a reaction in a mixture of acetic acid and methanol (1:1) reduces the reactivity of the reagents and leads to the formation of several by-products.
Thus, it was discoveredthat the reaction between dimethylacetylenedicarboxylate 1 and 1,2-diamino-4-phenylimidazoles 44a–e proceeds most smoothly when methanol with the addition of a catalytic amount of acetic acid was used as solvent. In this case, the complete conversion of the starting diamine and the maximum yield of the target product were achieved after an hour of boiling the reagents.
Considering the polynucleophilic nature of the starting 1,2-diaminoimidazoles 44a–e, Scheme 7 reports the structures of all possible intermediates of their interaction with DMAD 1. Thus, during the first step, the nucleophilic addition of 44a–e on the triple bond of 1 due to the nucleophilic CH, NH, and NH2 groups, in addition to the nitrogen atom of the cycle, can lead to the formation of the intermediates A–D. The resulting Michael adducts can undergo intramolecular cyclization, due to the “near” or “far” carboxyl group, with the formation of the corresponding products 45–50.
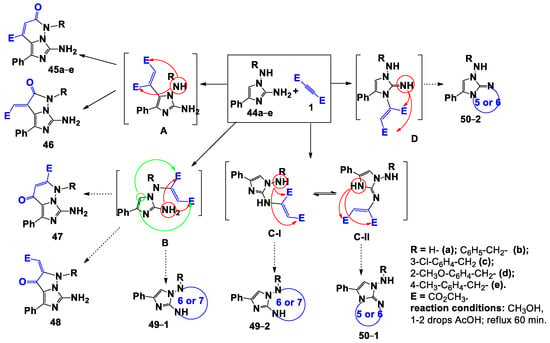
Scheme 7.
Possible ways of interaction of 1,2-diaminoimidazoles with DMAD.
To gain information on the real course of the reaction, we have examined the 1H-NMR spectra of the products of reaction. We have observed that, in them, the characteristic CH signals of the imidazole ring fragment and of the amino group of the hydrazine fragment were absent; this observation allowed us to exclude the possibility that compounds 49 and 50 were formed. A further confirmation of this hypothesis was derived from the presence of the amino group signals at C-7 (6). Moreover, the spectra of the isolated compounds contained signals of the protons of the pyridazine ring at δ = 5.9–6.3 ppm, while the spectra of compound 45a, in which R was a hydrogen atom, contained the characteristic signal of the amide proton at δ = 11.75 ppm. Thus, it was possible to also exclude the structures 46 and 48. At this point, we can confirmthat only the structures 45 and 47 are possible for the obtained products of reaction.
The key criterion for choosing between these two structures (45 and 47) was the presence of cross peaks in the NOESY spectrum between the protons of the methyl group of the ester fragment and the ortho-protons of the aromatic ring present on C-5 of the imidazole ring, as presentedin Figure 1 for compound 45b. Thus, we can conclude that the reaction of1,2-diaminoimidazoles 44a–e with DMAD produces the methyl esters of 7-amino-1,2-dihydro-1-R-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylic acids 45a–e.
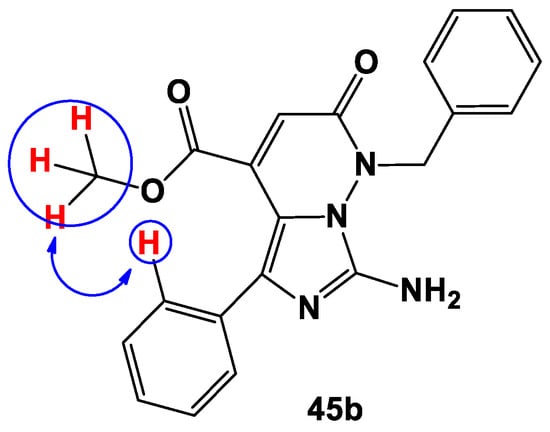
Figure 1.
Key interactions for compound 45b in the NOESY spectra.
Of course, the structures of all the obtained compounds (45a–e) have been confirmed by the collected spectroscopic data (1H- and 13C-NMR, in addition to HRMS data). All of the examined reactions occurred with good/excellent yields (68–89%).
To obtainan additional confirmation of the structure of the obtained imidazopyridazines 45a–e and as a further step of our research, we have carried out the transformation of methyl 7-amino-1,2-dihydro-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylate 45a into 7-amino-5-phenylimidazo[1,5-b]pyridazin-2-one 52 (Scheme 8). Thus, by alkaline hydrolysis 45a producedthe relevant carboxylic acid 51, which, in turn, by refluxing in diphenyl ether in the presence of copper acetate, furnished by decarboxylation (pathway A) the 7-amino-5-phenylimidazo[1,5-b]pyridazin-2-one 52. The structure of isolated compounds 51 and 52 was proven by 1H- and 13C-NMR spectroscopy and HRMS.
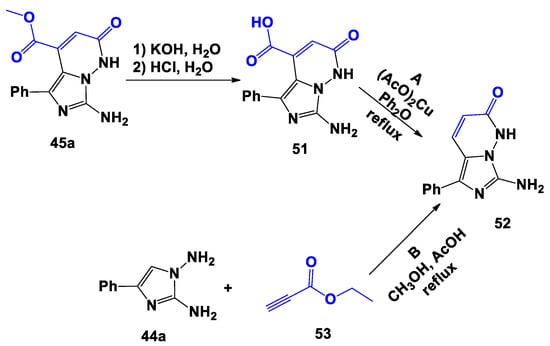
Scheme 8.
Alternative syntheses of 7-amino-5-phenylimidazo[1,5-b]pyridazin-2-one (52).
The structure of imidazopyridazine 52 was also confirmed by the NOESY spectrum, in which cross-interaction between the CH proton of the pyridazine ring and ortho-protons of the aromatic ring was observed (Figure 2). This kind of interaction is impossible for this couple of protons in all alternative structures 46–48. Therefore, the proven structure of imidazopyridazine 52 has further confirmed that in the course of the interaction between 1,2-diaminoimidazoles 44a–e and DMAD, methyl esters of 7-amino-1,2-dihydro-1-R-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylic acids 45a–e have been obtained.
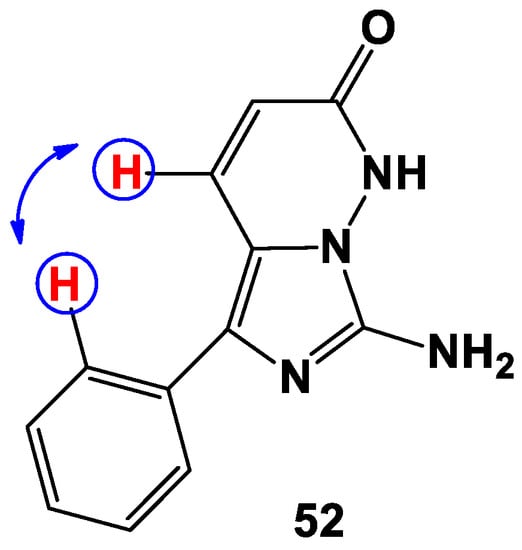
Figure 2.
Key interactions concerning compound 52 in the NOESY spectra.
The structure of compound 52 was further confirmed by preparing it with an alternative synthesis, id est by treating the 1,2-diamino-4-phenylimidazole 44a with ethyl propargylate 53 (Scheme 8, pathway B). As in the instance of the reaction with DMAD, the optimal condition was boiling for 60 min the mixture of reagents in methanol with the addition of a couple of drops of acetic acid under constant stirring.
To obtainfurther information on the course of the reaction between 1,2-diaminoimidazoles 44 and DMAD 1, we have calculated the minimum energy paths (MEP) for the reaction. Quantum-chemical DFT calculations were performed using B3LYP/6-311++G(d, p) basis and considering the solvation effects by the polarizable continuum model (PCM) in methanol.
Firstly, we examined the interaction of the simplest 4-phenyl-1H-imidazole-1,2-diamine 44a and DMAD 1, leading to the formation of 45a.
In the first step of this process, anucleophilic attack of carbon atom C-5 of imidazole on a carbon atom of the triple bond of DMAD 1 occurred with the formation of the bipolar σ-complex 54, which was 23.3 kcal/mol higher in energy comparedto the starting reagents (Figure 3).
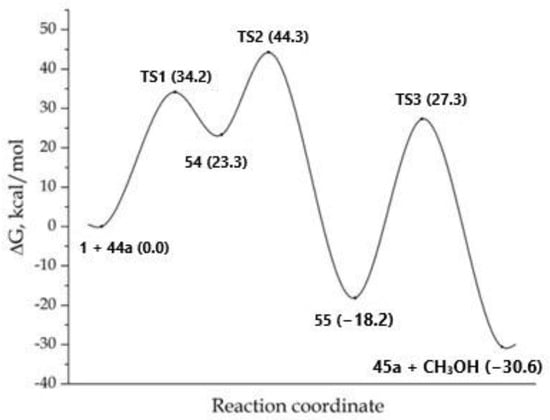
Figure 3.
MEP for the reaction concerning the formation of product 45a. The total energy of the starting reagents 1 and 44a was taken as the reference point.
Now we will discuss the change in the relative free energy ΔG. During the second step of the process, a 1,3-hydrogen shift occurred and the covalent σ-complex 55 was formed: although the formation of this structure required an activation energy of 21.0 kcal/mol, 55 is produced, which is much more stable. In the final step, an intramolecular nucleophilic attack by the nitrogen atom of the amino group on the carbonyl carbon atom occurred, followed by the elimination of the methanol molecule and the formation of the final product 45a. The formation of 45a was highly exothermic (a gain of 30.6 kcal/mol occurred), and it required the overcoming of a very large energy barrier (45.5 kcal/mol) in the last step (TS3) (Scheme 9).
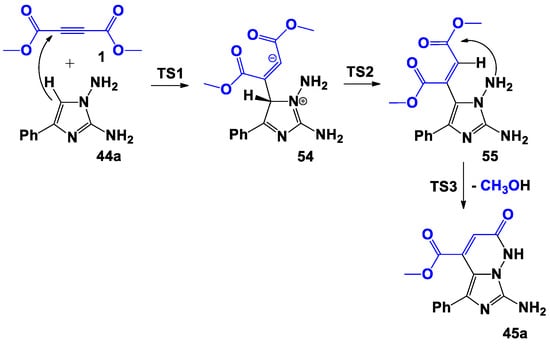
Scheme 9.
Proposed bimolecular formation mechanism of 45a.
Then we considered the reaction proceeded via the intermediate D, theoretically leading to the formation of product 50-2 (Scheme 7 and Scheme 10).
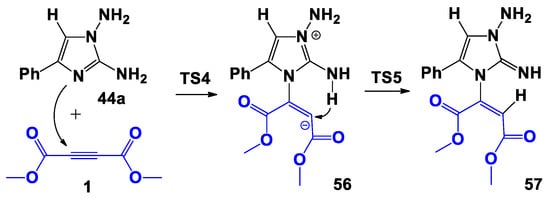
Scheme 10.
Proposed bimolecular formation mechanism of 57.
During the first step of the studied process, a nucleophilic attack by N-3 of imidazole on a carbon atom of the triple bond of DMAD 1 occurred with the formation of the bipolar σ-complex 56. As in the previous instance, the formation of a non-classical structure is energetically unfavorable; in fact, structure 56 was 20.2 kcal/mol higher in energy than the initial reagents (Figure 4). During the second step, a 1,5-hydrogen shift occurred and the compound 57 was formed. It should be noted that 57 appears to be the final product; in fact, further cyclization into product 50-2 was not observed. In general, this path was weakly endothermic: 57 was energetically less favorable than the initial reagents, then the formation of complex D was unlikely.
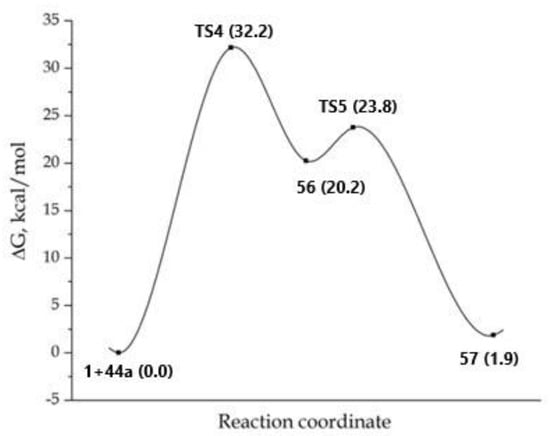
Figure 4.
MEP for the reaction concerning the formation of adduct 57. The total energy of the starting reagents 1 and 44a was taken as the reference point.
Now the formation of a complex of type C (leading to products 49-2 or 50-1) will be examined (Scheme 7 and Scheme 11).
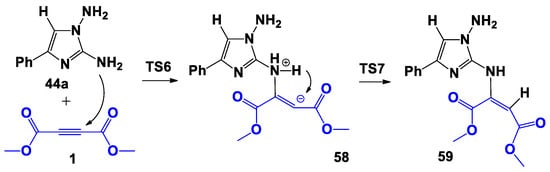
Scheme 11.
Proposed bimolecular formation mechanism of 59.
Now, during the first step of the reaction, anucleophilic attack of the nitrogen atom of the amino group linked at the C-2 atom of imidazole occurred on a carbon atom of the triple bond of DMAD 1 with the formation of the bipolar σ-complex 58: it was 33.4 kcal higher in energy than the starting reagents (Figure 5). The following 1,3-hydrogen shift led to the formation of the covalent adduct 59. However, as in the previously examined process (Scheme 10), the following cyclization into products 49-2 or 50-1 did not exist on the potential energy surface (PES).
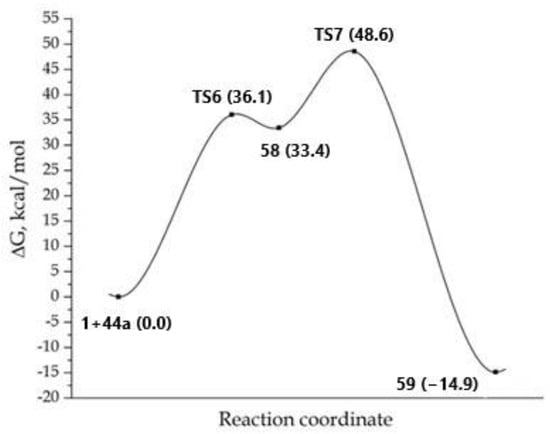
Figure 5.
MEP for the reaction concerning the formation of adduct 59. The total energy of starting reagents 1 and 44a was taken as the reference point.
The processes involving the formation of type B complexes proceeded through the formation of a bipolar σ-complex. Zwitterionic adduct 60 was formed by the nucleophilic attack of the N-amino group of imidazole 44a on a carbon atom of the triple bond of DMAD 1 (Scheme 12), then giving the covalent adduct 61 as a result of the 1,3-hydrogen shift. The formation of adduct 61 from DMDA 1 and 44 was energetically favorable and was accompanied by an energy gain of 17.3 kcal/mol (Figure 6). The maximum barrier of the process 1 + 44→61 concerns the first stage (TS8) and required an activation energy of 38.2 kcal/mol. As in the two previous cases, the formation of a cyclic structure from adduct 61 was not suggested by PES.
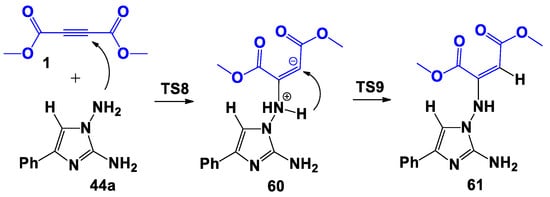
Scheme 12.
Proposed bimolecular formation mechanism of 61.
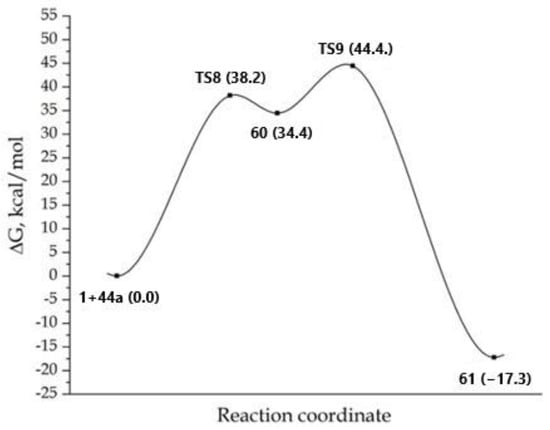
Figure 6.
MEP for the reaction concerning the formation of adduct 61. The total energy of the starting reagents 1 and 44a was taken as the reference point.
In conclusion, calculations for the bimolecular pathways of interaction of 4-phenyl-1H-imidazole-1,2-diamine 44a and DMAD 1 indicatedthat between the hypothetical cyclic products 45–50, only the formation of a cyclic system 45a appeared as a possible path of reaction. In addition, data of quantum-chemical calculations on the course of the bimolecular processes indicatedthat the open-chain covalent adducts 59 and 61 could be formed, but their formation occurred with an energy barrier in the first stage higher than in the instance of the formation of adduct 54.
Since, according to the results of the experiments carried out using an alcohol as solvent of reaction, re-esterification products were recorded, we concludedthat the interaction of 4-phenyl-1H-imidazole-1,2-diamine 44a and DMAD 1 can develop not only via a bimolecular process, but also as a trimolecular process with the involvement of an alcohol molecule. For this, we performed the quantum-chemical calculation of paths involving methanol.
Firstly, we have considered the trimolecular process concerning the formation of the target product 45a (Scheme 13, Figure 7). At the molecular level, the methanol molecule affects all steps of the process. Thus, in the first step, during the formation of the bipolar σ-complex 62, the proton of the hydroxyl of methanol was coordinated to the carbon atom charged negatively inthe multiple bond. However, no stabilization of the zwitterionic structure (compared with the process on Scheme 9) occurred; in fact, the adduct 62 was less energy-efficient by 27.1 kcal/mol compared to the starting reagents. Furthermore, the lowbarrier of the 1,3-hydrogen shift which led to 63 was performed through a five-center transition state (TS11), in which the molecule of methanol acted as a bifunctional catalyst.
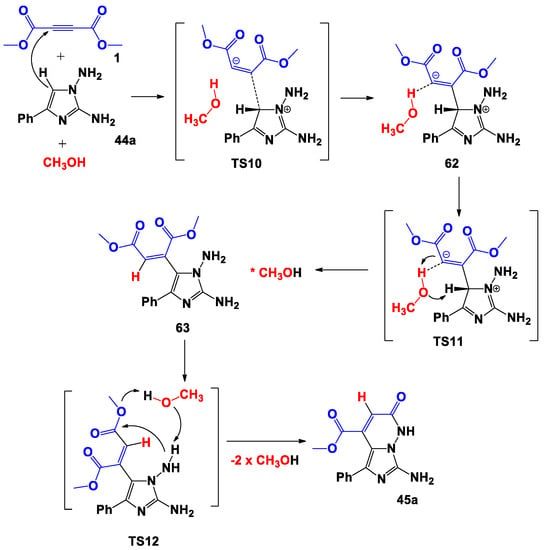
Scheme 13.
Proposed trimolecular formation mechanism of 45a.
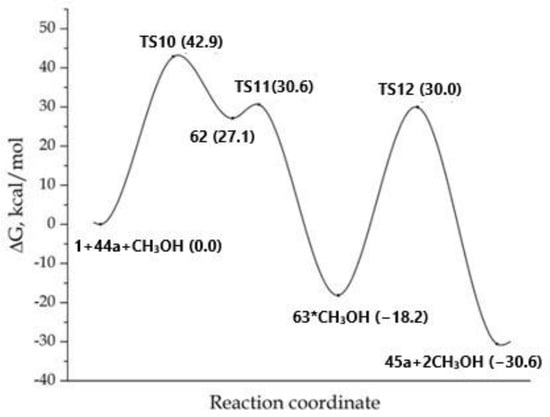
Figure 7.
MEP for the trimolecular reaction concerning the formation of product 45a. The total energy of the starting reagents 1, 44a and methanol was taken as the reference point.
Finally, the covalent 63 was transformed into product 45a through the transition state TS12, in which the molecule of methanol again performed the function of a bifunctional catalyst and participated in the formal transfer of a proton from the N-amino group to the ester oxygen atom. Despite the participation of the methanol in the proton transfer, the formation of product 45a from 63 proceeded with a barrier of 48.2 kcal/mol, which was higher than in the process portrayedin Scheme 9.
The addition of a methanol molecule in the process presented in Scheme 10 causes an effect at the molecular level (Scheme 14). Thus, when the bipolar intermediate 64 was formed from the initial reagents, the methanol coordinated the proton of the hydroxy group to the carbon atom of the double bond.
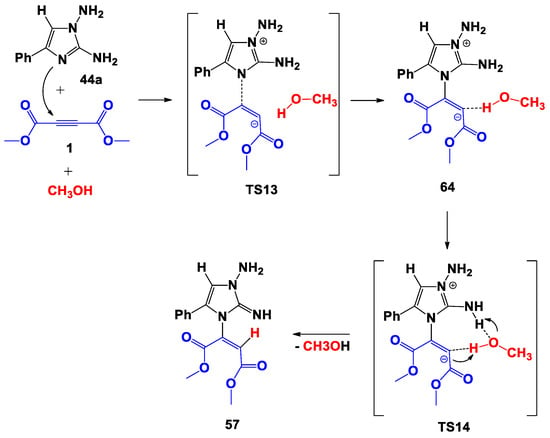
Scheme 14.
Proposed trimolecular formation mechanism of 57.
Despite the coordination of the zwitterionic 56 with a molecule of methanol in complex 64, this was 28.1 kcal/mol higher in energy than the initial reagents, which in turn means 7.9 kcal/mol higher in energy than for 56 in Scheme 10 (compare Figure 4 and Figure 8).
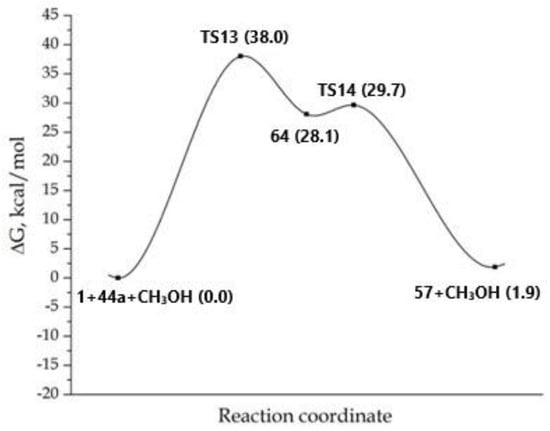
Figure 8.
MEP for the trimolecular reaction concerning the formation of adduct 57. The totalenergy of the starting reagents 1, 44a and methanol was taken as the reference point.
The methanol molecule facilitated the 1,5-proton transfer, being included in the eighth-center transition state (TS14), performing the function of a bifunctional catalyst. As before, the process of the formation of structure 57 was endothermic.
When the methanol molecule was involved in the processes reported in Scheme 11 and Scheme 12, we observed a similar pattern (see Supplementary Materials). It was coordinated with the zwitterion, destabilizing it. However, during the following step of the process, the methanol acted as a bifunctional catalyst facilitating the transfer of the hydrogen atom. In this case, the reactions, as in the case of the bimolecular pathway, stop at the stage of the formation of the covalent adducts.
According to the calculation data, a pattern analogous to the trimolecular processes with the involvement of methanol was observed in the case of the participation of acetic acid in the trimolecular process at the molecular level. Thus, for the process of formation of the compound 45a from the initial compounds, the barrier of the first stage of the formation of the zwitterionic associate was 40.4 kcal/mol, and the zwitterionic associate itself was 26.3 kcal/mol higher in energy than the initial reagents. The covalent adduct into which it rearranged, as a result of electron transfer, was 9.0 kcal/mol higher in energy comparedto the starting reagents. The trimolecular process with the involvement of acetic acid, as in the case of the participation of methanol, TS was higher in energy than in the corresponding bimolecular processes. Obviously, in all the cases considered, the involvement of a polar protic solvent at the molecular level can make worse the course of reactions. However, the covalent and zwitterionic intermediates in trimolecular processes were higher in energy than the intermediates infinitely separated from the solvent molecules, which means that the reaction can proceed by bimolecular pathways without switching to the trimolecular ones. Then, the experimentally observed ease of processes in polar solvents is explained exclusively by the dielectric constant (polarity of the medium). In fact, the higher the polarity of the medium, the higher the stabilizationenergy of zwitterionic or ionic processes [46].
When switching from the gas-phase calculations to the PCM medium accounting for the process in Scheme 9, we observed a reduction in the barrier values. Thus, the barrier of the first stage (TS1) is only 27 kcal/mol (see Supporting Materials) and the thermodynamic gain of the whole process increased to 39.3 kcal/mol.
Thus, based on quantum chemical calculations, the following conclusions can be drawn:
- -
- the predominant cyclic product of the interaction of diaminoimidazoles 44 with DMAD 1 is imidazopyridazines 45;
- -
- open-chain nucleophilic addition products of diaminoimidazoles 44 to DMAD 1 at positions other than the C-2 carbon atom, which are less preferable kinetically, can participate in a further cascade of processes leading to the formation of difficultly separable mixtures not involved in further intramolecular cyclization;
- -
- interaction of diaminoimidazoles 44 with DMAD 1 is complicated in the instance that methanol or acetic acid participate to the reaction. In fact, the participation of the molecules of the third component in the formation of the primary bipolar σ-complexes can destabilize them. Conversely, during the following steps of the process, they can facilitate the transfer of protons because of the formation of classical covalent adducts;
- -
- the fact that all stationary points in the instance of trimolecular processes are higher in energy than the sum energies of the corresponding points and solvent molecules in case of bimolecular processes makes trimolecular processes alternative, but not real, processes.
- -
- polar solvents can facilitate the formation processes of type 45 systems due to their polarity (ε value), stabilizing polar intermediates, and not due to their direct involvement in the processes.
3. Materials and Methods
3.1. General
1H NMR, 13C NMR, NOESY spectra were recorded on BrukerDRX-500 devices (500.13 and 125.75 MHz, respectively) in DMSO-d6 and TFA-d with internal TMS standard. Melting points were taken on a Stuart SMP30 device (Cole-Parmer Ltd., St. Neots, UK). HPLC/MS spectra were recorded on an Agilent Infinity 1260 chromatograph (Agilent Technologies, Palo Alto, CA, USA) with MS interface Agilent 6230 TOFLC/MS. Conditions for the separation: mobile phase MeCN/H2O + 0.1% FA (formic acid), gradient elution (first CH3CN:H2O (60:40), then for 5 min to 90% CH3CN), column—Poroshell 120 EC-C18 (4.6 × 50 mm, 2.7 μm), thermostat 23–28 °C, flow rate of 0.3–0.4 mL/min electrospray ionization (capillary—3.5 kV; fragmentor +191 V; OctRF +66 V—positive polarity). The course of the reaction and the purity of the obtained compounds were controlled by TLC on Merck TLC Silica gel 60 F254 plates in a 20:1 CHCl3–MeOH system (visualization under UV light). The commercially available reagents were purchased from Acros Organics (Geel, Belgium).
Quantum chemical DFT calculations were carried out in the 6–311++G(d,p) triple-zeta basis using a B3LYP functional [47]. This basis has provideda good account of itself in reproduction of vibrational frequencies, geometry, and minimum energy reaction paths [48,49,50]. Full geometry optimization of molecular structures corresponding to stationary points of the potential energy surface was carried out as high as a gradient value of 10−7 hartree/bohr according to the Gaussian 09 software package (Wallingford, CT, USA) [51] using the Silver cluster of the Research Institute of Physical and Organic Chemistry at SFedU. The nature of stationary points was determined relying on the calculation of normal vibration frequencies (the Hessian matrix). MEPs were plotted using gradient descent from transition states in forward and reverse directions of transition vectors. In order to search for transition states, linear and quadratic synchronous transit methods were used [52,53].
3.2. General Procedure for the Synthesis of Methyl 7-Amino-1,2-dihydro-1-R-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylates (45a–e)
Acetylene dicarboxylic acid dimethyl ester 1 (5 mmol) was added dropwise to 5 mmol of diaminoimidazole 44a–e dissolved in 5 mLof methanol containing catalytic amounts of acetic acid (2–3 drops). After adding 1, the reaction mixture was refluxed for 60 min. The precipitated crystals were filtered off and recrystallized from MeOH.
3.2.1. Methyl 7-Amino-1,2-dihydro-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylate (45a)
Yield (124.4 mg, 89%), orange. Mp:239–241 °C.1H NMR (500 MHz, DMSO-d6, δ ppm): 11.75 (br.s, 1H), 7.35–7.39 (m, 2H), 7.26–7.32 (m, 3H), 6.23 (s, 1H), 5.91 (s, 2H), 3.41 (s, 3H). 13C{1H} NMR (125.8 MHz, DMSO-d6, δ ppm): 164.9, 158.4, 143.7, 135.4, 134.1, 129.2, 127.8, 127.6, 126.7, 111.0, 104.5, 52.2. HRMS (ESI) m/z: [M+H+] calc. for C14H12N4O3, 285.0982, found 285.0980.
3.2.2. Methyl 7-Amino-1-benzyl-1,2-dihydro-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylate (45b)
Yield (127.1 mg, 68%), orange. Mp: 153–155 °C. 1H NMR 500 MHz, DMSO-d6, δ ppm): 7.30–7.38 (m, 3H), 7.23–7.29 (m, 3H), 7.20 (d, J = 7.2, 2H), 7.09 (d, J = 7.2, 2H), 6.71 (s, 2H), 5.97 (s, 1H), 5.42 (s, 2H), 3.27 (s, 3H). 13C{1H} NMR (125.8 MHz, DMSO-d6, δ ppm): 164.7, 164.5, 146.8, 137.4, 135.5, 135.4, 133.9, 128.6, 128.1, 128.0, 127.9, 127.7, 112.5, 108.9, 52.3, 47.8. HRMS (ESI), m/z: [M+H+] calc. for C21H18N4O3, 375.1453, found 375.1452.
3.2.3. Methyl 7-Amino-1-(3-chlorobenzyl)-1,2-dihydro-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylate (45c)
Yield (145 mg, 71%), yellow. Mp: 237–239 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.30–7.40 (m, 5H), 7.21–7.24 (m, 2H), 7.12 (s, 1H), 7.01–7.04 (m, 1H), 6.73 (s, 2H), 5.99 (s, 1H), 5.42 (s, 2H), 3.28 (s, 3H). 13C{1H} NMR (125.8 MHz, DMSO-d6, δ ppm): 164.5, 164.3, 146.7, 137.7, 137.5, 135.5, 133.8, 133.0, 130.5, 128.0, 128.0, 129.9, 127.5, 126.1, 112.4, 108.8, 52.2, 47.2. HRMS (ESI), m/z: [M+H+] calc. forC21H17ClN4O3409.1061, found 409.1060.
3.2.4. Methyl 7-Amino-1-(2-methoxybenzyl)-1,2-dihydro-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylate (45d)
Yield (175.7 mg, 87%), red. Mp: 185–187 °C.1H NMR (500 MHz, DMSO-d6, δ ppm): 7.29–7.38 (m, 3H), 7.17–7.21 (m, 3H), 7.13 (dd, J = 1.65, J = 8.5, 1H), 6.87 (d, J = 7.9, 1H), 6.79 (t, J = 7.5, 1H), 6.64 (s, 2H), 5.89 (s, 1H), 5.30 (s, 2H), 3.64 (s, 3H), 3.29 (s, 3H). 13C{1H} NMR (125.8 MHz, DMSO-d6, δ ppm): 165.2, 164.7, 157.6, 147.3, 137.3, 135.1, 134.1, 130.7, 129.5, 127.9, 127.8, 127.8, 122.3, 119.7, 112.3, 110.2, 108.9, 54.9, 52.2, 46.8. HRMS (ESI), m/z: [M+H+] calc. for C22H20N4O4 405.1558, found 405.1557.
3.2.5. Methyl 7-Amino-1-(4-methylbenzyl)-1,2-dihydro-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylate (45e)
Yield (149.4 mg, 77%), orange. Mp: 170–172 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 7.30–7.38 (m, 4H), 7.20 (d, J = 7.2, 1H), 7.07 (d, J = 7.8, 2H), 6.98 (d, J = 7.7, 2H), 6.68 (s, 2H), 5.96 (s, 1H), 5.37 (s, 2H), 3.27 (s, 3H), 2.21 (s, 3H). 13C{1H} NMR (125.8 MHz, DMSO-d6, δ ppm): 164.5, 164.4, 146.7, 137.2, 137.2, 135.3, 133.9, 132.3, 129.1, 128.0, 127.9, 127.9, 127.6, 112.4, 108.9, 52.2, 47.3, 20.5. HRMS (ESI), m/z: [M+H+] calc. for C22H20N4O3 389.1609, found 389.1608.
3.3. Synthesis of 7-Amino-1,2-dihydro-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylic Acid (51)
Potassium hydroxide (5.5 mmol),dissolved in 5 mLof water, was added to 5 mmol of 45a. The mixture was boiled for 2 h, until 45a completely disappeared (control by TLC). After cooling, the mixture was acidified with hydrochloric acid. The formed precipitate was filtered off.
7-Amino-1,2-dihydro-2-oxo-5-phenylimidazo[1,5-b]pyridazine-4-carboxylicAcid (51)
Yield (124.2 mg, 92%),lightyellow. Mp: >300 °C. 1HNMR (500 MHz, DMSO-d6,δppm): 11.72 (br.s, 1H), 7.40–7.43 (m, 2H), 7.29–7.34 (m, 3H), 7.22 (t, J = 7.3, 1H), 6.17 (s, 1H), 5.94 (s, 2H). 13C{1H} NMR (125.8 MHz, DMSO-d6, δ ppm): 166.3, 158.8, 143.3, 137.1, 134.6, 127.8, 127.5, 126.5, 111.3, 103.9. HRMS (ESI), m/z: [M+H+] calc. for C13H10N4O3 271.0826, found 271.0825.
3.4. Synthesis of 7-Amino-5-phenylimidazo[1,5-b]pyridazin-2(1H)-one (52)
3.4.1. Method A
Acid 51 (5 mmol) was dissolved in 5 mLof diphenyl ether under constant heating and stirring, and 15 mmol of copper (II) acetate were added. The reaction mixture was boiled for 3 h. The formed precipitate was filtered off, washed with water, and then recrystallized from a mixture of i-PrOH/DMF, 2:1.
3.4.2. Method B
Ethyl propionate 53 (5 mmol) was added dropwise to 5 mmol of diaminoimidazole 44a, dissolved in 5 mL of methanol in the presence of catalytic amounts of acetic acid (2–3 drops) under constant stirring and heating at 40 °C for 60 min. The formed precipitate was filtered off and recrystallized from a mixture of i-PrOH/DMF, 2:1.
3.4.3. 7-Amino-5-phenylimidazo[1,5-b]pyridazin-2(1H)-one (52)
Yields (36.2 mg, 32% (method A) and 84.8 mg, 75% (method B)), yellow. Mp.: 271–273 °C. 1H NMR (500 MHz, DMSO-d6, δ ppm): 11. 35 (br. s, 1H), 8.08 (d, J = 9.7, 1H), 7.79 (d, J = 7.5, 2H), 7.38 (t, J = 7.6, 2H), 7.20 (t, J = 7.3, 1H), 6.08 (d, J = 9.6, 1H), 5.75 (s, 2H). 13C{1H} NMR (125.8 MHz, DMSO-d6, δ ppm): 159.0, 143.2, 134.9, 129.7, 128.6, 126.6, 125.8, 125.1, 115.7, 104.4. HRMS (ESI), m/z: [M+H+] calc. for C12H10N4O 227.0928, found 227.0926.
4. Conclusions
A new approach for the synthesis of 7-amino-1,2-dihydro-1-R-2-oxo-5-phenylimidazo[1,5-b] pyridazine-4-carboxylic acids and their esters (45a–e) from readily available 1,2-diamino-4-phenylimidazoles and dimethylacetylene dicarboxylate (DMAD) was suggested. DFT calculations indicatedthat this synthesis proceeds via the formation of low-barrier intermediates. The use of a protic solvent (methanol and few drops of acetic acid) accelerates the course of the processes; this factis probably not due to their involvement as catalysts, but to a change in the dielectric constant of the medium, able to stabilizepolar intermediates. DFT results confirm the experimental results, moreover, the observed course of the reaction (formation of compounds 45a–e) is confirmed by spectroscopic results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27103326/s1. Copies of 1H, 13C NMR, NOESY and HPLC/MS spectre; HPLC/MS analysis of reaction masses; methods of quantum chemical calculation; calculated transition states geometries; cartesian coordinates of all stationary point (PDF).
Author Contributions
D.Y.V. and K.S.S. supervised the experiment and provided technical guidance and wrote the original draft. D.A.M. and T.N.I. designed and synthesized all of the novel compounds. M.A.P. performed spectral and chromatographic analysis. O.N.B. and A.V.L. performed DFT calculations and described the results. A.G. and D.S. performed writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation grant No. 18-74-10097, https://rscf.ru/project/18-74-10097 (accessed on 4 August 2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Spinelli, D.; Frenna, V.; Corrao, A.; Vivona, N. Mononuclear heterocyclic rearrangements. Part 2. Substituent effects on the rate of rearrangement of some arylhydrazones of 3-benzoyl-5-phenyl-1,2,4-oxadiazole into 2-aryl-4-benzoyl-5-phenyl-1,2,3-triazole, at pS+ 3.80. J. Chem. Soc. Perkin Trans. 1978, 2, 19–22. [Google Scholar] [CrossRef]
- D’Anna, F.; Frenna, V.; Macaluso, G.; Marullo, S.; Morganti, S.; Pace, V.; Spinelli, D.; Tavani, C. On the rearrangement in dioxane/water of Z-arylhyydrazones of 5-amino-3-benzoyl-1,2,4-oxadiazole into (2-aryl.5-phenyl-2H-1,2,3-triazol-4-yl)ureas: Substituent effects on the different reaction pathways. J. Org. Chem. 2006, 71, 5616–5624. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.Y.; Romanov, P.S.; Shikhaliev, K.S.; Polunin, E.V.; Firgang, S.I. Novel variant of the anrorc rearrangement of [1,2,4]triazolo[1,5-a]pyrimidines and pyrimido-[1,2-a]benzimidazole. Chem. Heterocycl. Compd. 2012, 47, 1309–1311. [Google Scholar] [CrossRef]
- Didenko, V.V.; Ledenyova, I.V.; Shestakov, A.S.; Shikhaliev, K.S. First example of an anrorc rearrangement of a pyrazolo[5,1-c][1,2,4]triazine involving a side chain. Chem. Heterocycl. Compd. 2010, 46, 770–772. [Google Scholar] [CrossRef]
- Dell’Erba, C.; Spinelli, D.; Leandri, G. Ring-opening reaction in the thiophen series: Reaction between 3,4-dinitrothiophen an secondary amines. J. Chem. Soc. Chem. Commum. 1969, 10, 549. [Google Scholar] [CrossRef]
- Dell’Erba, C.; Gabellini, A.; Novi, M.; Petrillo, G.; Tavani, C.; Cosimelli, B.; Spinelli, D. Ring opening of 2-substituted 4-nitrothiophenes with pyrrolidine. Access to new functionalized nitro-unsaturated building blocks. Tetrahedron 2001, 57, 8159–8165. [Google Scholar] [CrossRef]
- Vandyshev, D.Y.; Shikhaliev, K.S.; Kokonova, A.V.; Potapov, A.Y.; Kolpakova, M.G.; Sabynin, A.L.; Zubkov, F.I. A novel method for the synthesis of pyrimido[1,2-a]benzimidazoles. Chem. Heterocycl. Compd. 2016, 52, 493–497. [Google Scholar] [CrossRef]
- Kovygin, Y.A.; Shikhaliev, K.S.; Krysin, M.Y.; Potapov, A.Y.; Ledenyova, I.V.; Kosheleva, Y.A.; Vandyshev, D.Y. Cascade recyclization of N-arylitaconimides as a new approach to the synthesis of polyfunctional octahydroquinolines. Chem. Heterocycl. Compd. 2019, 55, 748–754. [Google Scholar] [CrossRef]
- Vandyshev, D.Y.; Shikhaliev, K.S.; Potapov, A.Y.; Firgang, S.I.; Krysin, M.Y. New method for synthesis of the hetero-cyclic system-1,2,3,4-tetrahydro-imidazo[5,1-f][1,2,4]triazin-7-amine. Chem. Heterocycl. Compd. 2014, 4, 587–589. [Google Scholar] [CrossRef]
- Spinelli, D.; Guanti, G.; Dell’Erba, C. Transmission of substituent effects in systems with bonds of different orders. Kinetics of the reactions of 3-bromo-2-nitro-4-X-thiophens and 3-bromo-4-nitro-2-X-thiophenes with sodium benzenethiolate in methanol. J. Chem. Soc. Perkin Trans. 1972, 2, 441–445. [Google Scholar] [CrossRef]
- Spinelli, D.; Noto, R.; Consiglio, G. Linear free Energy ortho-correlations in the thiophen series, Part II. Acid dissociation of some 3-substituted thiophen-2-carboxylic acids in water. J. Chem. Soc. Perkin Trans. 1976, 2, 747–751. [Google Scholar] [CrossRef]
- Dell’Erba, C.; Mele, A.; Novi, M.; Petrillo, G.; Sancassan, F.; Spinelli, D. On the nature of resonance interactions in subsituted benzenes. Part 3. A13C nuclear magnetic resonance study of substituent effects in 4-substituted benzamides and methyl benzoates in dimethylsulphoxide. J. Chem. Soc. Perkin Trans. 1990, 2, 2055–2058. [Google Scholar] [CrossRef]
- Carosati, E.; Budriesi, R.; Cosimelli, B.; Ioan, P.; Ugenti, M.P.; Spinelli, D.; Chiarini, A. Discovery of novel and cardioselective diltiazem-like calcium channel blockers via virtual screening. J. Med. Chem. 2008, 51, 5552–5565. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.Y.; Paponov, B.V.; Podoplelova, N.A.; Panteleev, M.A.; Polikarchuk, V.A.; Ledenyova, I.V.; Shikhaliev, K.S. Synthesis and study of new 2H-pyranoquinolin-2-one-based inhibitors of blood coagulation factors Xa and XIa. Russ. Chem. Bull. 2021, 70, 492–497. [Google Scholar] [CrossRef]
- Novichikhina, N.P.; Skoptsova, A.A.; Shestakov, A.S.; Potapov, A.Y.; Kosheleva, E.A.; Kozaderov, O.A.; Shikhaliev, K.S. Synthesis and Anticoagulant Activity of New Ethylidene and Spiro Derivatives of Pyrrolo[3,2,1-ij]quinolin-2-ones. Russ. J. Org. Chem. 2020, 56, 1550–1556. [Google Scholar] [CrossRef]
- Dell’Erba, C.; Chiavarina, B.; Fenoglio, C.; Petrillo, G.; Cordazzo, C.; Boncompagni, E.; Spinelli, D.; Viale, M. Inhibition of cell proliferation, cytoxicity and induction of apoptosis of 1,4-bis(1-naphthyl)-2,3-dinitro-1,3-butadiene in gastrointestinal tumour cell lines and preliminary evaluation of its toxicity in vivo. Pharmacol. Res. 2005, 52, 271–282. [Google Scholar] [CrossRef]
- Novichikhina, N.P.; Shestakov, A.S.; Potapov, A.Y.; Kosheleva, E.A.; Shatalov, G.V.; Verezhnikov, V.N.; Shikhaliev, K.S. Synthesis of 4H-pyrrolo[3,2,1-ij]quinoline-1,2-diones containing a piperazine fragment and study of their inhibitory properties against protein kinases. Russ. Chem. Bull. 2020, 69, 787–792. [Google Scholar] [CrossRef]
- Stolpovskaya, N.V.; Kruzhilin, A.A.; Zorina, A.V.; Shikhaliev, K.S.; Ledeneva, I.V.; Kosheleva, E.A.; Vandyshev, D.Y. Synthesis of Substituted Aminopyrimidines as Novel Promising Tyrosine Kinase Inhibitors. Russ. J. Org. Chem. 2019, 55, 1322–1328. [Google Scholar] [CrossRef]
- Viale, M.; Cordazzo, C.; Cosimelli, B.; De Totero, D.; Castagnola, P.; Aiello, C.; Severi, E.; Spinelli, D. Inhibition of MDR1 activity in vitro by a novel class of diltiazem analogues: Towards new candidates. J. Med. Chem. 2009, 52, 259–266. [Google Scholar] [CrossRef]
- Ilin, I.; Lipets, E.; Sulimov, A.; Kutov, D.; Shikhaliev, K.; Potapov, A.; Sulimov, V. New factor Xa inhibitors based on 1,2,3,4-tetrahydroquinoline developed by molecular modelling. J. Mol. Graph. Model. 2019, 89, 215–224. [Google Scholar] [CrossRef]
- Mohlala, R.L.; Coyanis, E.M.; Fernandes, M.A.; Bode, M.L. Synthesis of highly functionalised 5-membered ring fused pyrimidine derivatives using an isocyanide-based one-pot, three component reaction. Tetrahedron Lett. 2020, 61, 151796. [Google Scholar] [CrossRef]
- Reimlinger, H.; Jacquier, R.; Daunis, J. Weiteresynthesen von 7-oxo-7,8-dihydro-s-triazolo[4,3-a]pyrimidinen. Chem. Ber. 1971, 104, 2702–2708. [Google Scholar] [CrossRef]
- Abdel-Aziem, A.; El Gendy, M.S.; Abdelhamid, A.O. Synthesis and antimicrobial activities of pyrido[2,3-d]pyrimidine, pyridotriazolopyrimidine, triazolopyrimidine, and pyrido[2,3-d:6,5d’]dipyrimidine derivatives. Euro. J. Chem. 2012, 3, 455–460. [Google Scholar] [CrossRef]
- Khera, M.K.; Cliffe, I.A.; Mathur, T.; Prakash, O. Synthesis and in vitro activity of novel 1,2,4-triazolo[4,3-a]pyrimidine oxazolidinone antibacterial agents. Bioorg. Med. Chem. Lett. 2011, 21, 2887–2889. [Google Scholar] [CrossRef]
- Aouali, M.; Allouche, F.; Zouari, I.; Mhalla, D.; Trigui, M.; Chabchoub, F. Synthesis, Antibacterial, and Antifungal Activities of Imidazo[2,1-c][1,2,4]triazoles and 1,2,4-Triazolo[4,3-a]pyrimidinones. Synth. Commum. 2014, 44, 748–756. [Google Scholar] [CrossRef]
- Hassanabadi, A. PPh3-Mediated One-Pot Synthesis of Functionalised 4-oxo-4H-benzo[4,5]imidazo[1,2-a]pyrimidines. J. Chem. Res. 2013, 37, 340–341. [Google Scholar] [CrossRef]
- Nair, M.D.; Sudarsanam, V.; Desai, J.A. Nitroimidazoles: Part XIV—Synthesis of 2-Nitroimidazo[1,2-a]pyrimidin-5-ones. Ind. J. Chem. Sect. B Org. Med. Chem. 1983, 21, 1030–1032. [Google Scholar] [CrossRef]
- Reimlinger, H.; Peiren, M.A.; Merenyi, R. Reaktionen des 3(5)-amino-pyrazolsmit α,β-ungesattigtenestern. Derstellung und charakterisierungisomerer oxo-dihydro-pyrazolo-pyrimidine. Chem. Ber. 1970, 103, 3252–3265. [Google Scholar] [CrossRef]
- Meyer, M.; Guyot, M. Synthesis of 2-substituted indolopyridine-4-ones. Tetrahedron Lett. 1996, 37, 4931–4932. [Google Scholar] [CrossRef]
- Ahdenov, R.; Amini, S.K.; Azarlak, R.; Halvagar, M.R.; Mohammadi, A.A.; Taheri, S. A straightforward approach for the synthesis of novel fused thiopyrano [2,3-b]indole derivatives from the Intramolecular Friedel-Crafts acylation. J. Mol. Struct. 2020, 1208, 127854. [Google Scholar] [CrossRef]
- Da Settimo, F.; Primofiore, G.; Da Settimo, A.; La Motta, C.; Taliani, S.; Simorini, F.; Novellino, E.; Greco, G.; Lavecchia, A.; Boldrini, E. [1,2,4]Triazino[4,3-a]benzimidazole Acetic Acid Derivatives: A New Class of Selective Aldose Reductase Inhibitors. J. Med. Chem. 2001, 44, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.S.; Bennett, G.A.; Radov, L.A.; Kamp, D.K.; Trusso, L.A. 2-Substituted 2,3-dihydro-5H-thiazolo[2,3-b]quinazoline derivatives. J. Heterocycl. Chem. 1986, 23, 1359–1362. [Google Scholar] [CrossRef]
- Jazinizadeh, T.; Yazdani-Elah-Abadi, A.; Maghsoodlou, M.T.; Heydari, R. CeCl3-Catalyzed a Highly Efficient and Eco-friendly Synthesis of New and Densely Functionalized Thiazolo[3,2-a]Pyrimidins via Biginelli-type Reaction. Polycycl. Aromat. Compd. 2018, 40, 732–742. [Google Scholar] [CrossRef]
- Darehkordi, A.; Ghazi, S. An efficient ultrasonic-assisted synthesis of ethyl-5-(aryl)-2-(2-alkokxy-2-oxoethylidene)-7-methyl-3-oxo-3, 5-dihydro-2H-thiazolo[3,2-a]pyrimidine-6-carboxylate derivatives. Arab. J. Chem. 2015, 38, 2175–2182. [Google Scholar] [CrossRef]
- Ueda, T.; Kawabata, Y.; Murakami, N.; Nagai, S.-I.; Sakakibara, J.; Goto, M. Novel annelations of xanthines by the reaction of 8-aminoxanthines with dimethyl acetylenedicarboxylate. Chem. Pharm. Bull. 1991, 39, 270–276. [Google Scholar] [CrossRef][Green Version]
- Tominaga, Y.; Yoshioka, N.; Kataoka, S.; Aoyama, N.; Masunari, T.; Miike, A. Synthesis and chemiluminescence of 1,3-disubstituted pyrazolo[4’,3’:5,6]pyrido[2,3-d]pyridazine-5,8(6H,7H)-diones and related compounds. Tetrahedron Lett. 1995, 36, 8641–8644. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Yen, W.-P.; Tsai, S.-E.; Hu, Y.-T.; Takayama, H.; Kuo, Y.-H.; Wong, F.F. ZnCl2-Catalyzed aza-Diels-Alder reaction for the synthesis of 1H-oyrazolo[3,4-b]pyridine-4,5-dicarboxylate derivatives. Eur. J. Org. Chem. 2018, 2018, 1567–1571. [Google Scholar] [CrossRef]
- Bouvier, M.; Marinier, A.; Ruel, R.; René, P.; Chantigny, Y.; Dagneau, P.; Gingras, S. Pyrazolopyridine and Pyrazolopyrimidine Derivatives as Melanocortin-4 Receptor Modulators. WO2012/100342 A1. U.S. Patent US9018395B2, 26 January 2012. [Google Scholar]
- Connor, D.T.; Young, P.A.; Strandtman, M. Synthesis of 4,10-dihydro-4,10-dioxo-1H-[1]-benzopyrano[3,2-b]pyridine, 4,5-dihydro-4,5-dioxo-1H-[1]-benzopyrano[2,3-b]pyridine and 1,5-dihydro-1,5-dioxo-4H-[1]-benzopyrano[3,4]pyridine derivatives from aminobenzopyrones. J. Heterocycl. Chem. 1981, 18, 697–702. [Google Scholar] [CrossRef]
- Merck Serono, S.A.; Montagne, C.; Bombrun, A.; Desforges, G.; Quattropani, A.; Gaillard, P. 4-Morpholino-pyrido[3,2-d]pyrimidines. WO2010/91996 A1. U.S. Patent US20110257170A1, 20 October 2010. [Google Scholar]
- Srinivasan, A.; Broom, A.D. Pyridopyrimidines. 10. Nucleophilic substitutions in the pyrido[3,2-d]pyrimidine series. J. Org. Chem. 1979, 44, 435–440. [Google Scholar] [CrossRef]
- Miyamoto, Y. Synthesis of Nitrogen-Containing Heterocycles. 12. Reactions of 2-Amino-1-benzylideneamino-1H-imidazoles with Dimethyl Acetylenedicarboxylate. Heterocycles 2009, 78, 691–698. [Google Scholar] [CrossRef]
- Kruzhilin, A.A.; Kosheleva, E.A.; Shikhaliev, K.S.; Denisov, G.L.; Vandyshev, D.Y. Regioselective Synthesis of Imidazo[1,5-b]pyridazines by Cascade Cyclizations of 1,2-Diamino-4H-phenylimidazole with 1,3-Diketones, Acetoacetic Ester and Their Derivatives. Chem. Select. 2021, 6, 5801–5806. [Google Scholar] [CrossRef]
- Vandyshev, D.Y.; Shikhaliev, K.S.; Potapov, A.Y.; Krysin, M.Y.; Zubkov, F.I.; Sapronova, L.V. A novel synthetic approach to hydroimidazo[1,5-b]pyridazines by the recyclization of itaconimides and HPLC–HRMS monitoring of the reaction pathway. Beilstein J. Org. Chem. 2017, 13, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Vandyshev, D.Y.; Kosheleva, E.A.; Polikarchuk, V.A.; Mangusheva, D.A.; Denisovb, G.L.; Shikhalieva, H.S. Regioselective synthesis of novel imidazo[1,5-b]pyridazine derivatives from diaminoimidazoles and α-acylacrylonitriles. Mendeleev Commun. 2021, 31, 821–823. [Google Scholar] [CrossRef]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, USA, 2006; p. 1104. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 15, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Peng, Y.-J.; Jiang, Y.-X.; Peng, X.; Liu, J.-Y.; Lai, W.-P. Reaction mechanism of 3,4-dinitrofuroxan formation from glyoxime: Dehydrogenation and cyclization of oxime. ChemPhysChem 2016, 17, 541–547. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gausian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Peng, C.; Schlegel, H.B. Combining synchronous transit and quasi-Newton methods to find transition states. Isr. J. Chem. 1993, 33, 449–454. [Google Scholar] [CrossRef]
- Peng, C.; Ayala, P.Y.; Schlegel, H.B.; Frisch, M.J. Using redundant internal co-ordinates to optimize equilibrium geometries and transition states. J. Comput. Chem. 1996, 17, 49–56. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).