Abstract
The extract from Cnidium officinale rhizomes was shown in a prior experiment to markedly recover otic hair cells in zebrafish damaged by neomycin. The current study was brought about to identify the principal metabolite. Column chromatography using octadecyl SiO2 and SiO2 was performed to isolate the major metabolites from the active fraction. The chemical structures were resolved on the basis of spectroscopic data, including NMR, IR, MS, and circular dichroism (CD) data. The isolated phthalide glycosides were assessed for their recovery effect on damaged otic hair cells in neomycin-treated zebrafish. Three new phthalide glycosides were isolated, and their chemical structures, including stereochemical characteristics, were determined. Two glycosides (0.1 μM) showed a recovery effect (p < 0.01) on otic hair cells in zebrafish affected by neomycin ototoxicity. Repeated column chromatography led to the isolation of three new phthalide glycosides, named ligusticosides C (1), D (2), and E (3). Ligusticoside C and ligusticoside E recovered damaged otic hair cells in zebrafish.
1. Introduction
Cnidium officinale Makino (Apiaceae) is a perennial flowering plant extensively cultivated in East Asian countries [1]. The rhizomes of C. officinale have been used traditionally in Korea, China, and Japan to treat female sexual disorders such as oligomenorrhea, hypomenorrhea, and amenorrhea by improving blood circulation, but also to relieve pain and inflammation [2,3]. Extracts of Cnidium officinale rhizome are reported to have antioxidant [4], anti-inflammatory [5,6], anticancer [5], blood circulation improvement [7], analgesic [6], anticonvulsive [8], and sedative [9] effects. A variety of components were identified as the major metabolites of Cnidium officinale rhizome, such as phthalides, phenyl alkanoids, triterpenoids, and polyacetylenes, etc. Among them, phthalide derivatives are the most important components and are reported to be key materials participating in several pharmacological activities of C. officinale rhizome [10]. Our advance experiment showed that the n-BuOH fraction from C. officinale rhizome recovered otic hair cells in zebrafish damaged by neomycin treatment. In a subsequent study presented herein, repeated column chromatography (CC) for the n-BuOH fraction yielded three new phthalide glycosides, the chemical structures of which, including stereostructures, were determined without ambiguity based on several spectroscopic data, that is, NMR, IR, MS, and circular dichroism spectroscopy (CD). Only a few glycosides have been isolated from plants. However, the phthalide glycosides with a double bond at C-6 and C-7 in the aglycone moiety have never been reported, to date. The phthalide glycosides were also evaluated for their recovery effect on damaged otic hair cells.
2. Results and Discussion
2.1. Structure Determination of Three New Phthalide Glycosides
Aqueous EtOH was used to obtain an extract of the rhizomes of Cnidium officinale, and the concentrate was fractionated using EtOAc, n-BuOH, and H2O. The n-BuOH fraction (Fr) was treated using Sephadex LH-20, silica gel (SiO2), and octadecyl SiO2 (ODS) as the stationary phase for column chromatography, resulting in the isolation of three new phthalide glycosides. Their chemical structures, including their absolute stereochemistry, were revealed based on MS, IR, NMR, and CD data.
Compound 1, a white powder, showed UV absorption at 254 and 365 nm and a deep brown color on TLC upon spraying with 10% sulfuric acid and heating. The molecular formula (MF) was determined to be C18H28O8 (calcd for C18H29O8, 373.1858) from a protonated molecular (PM) at m/z 373.1861 [M + H]+ in the high-resolution ESI-MS (HR-ESI-MS). The IR bands showed absorbance bands at 3367 (hydroxyl), 1742 (ester), and 1606 and 1514 cm−1 (double bond). In the 13C NMR (CMR) spectrum (Table 1), 18 carbon signals, including 6 due to one hexose, were observed. The above data assuredly suggest compound 1 to be a phthalide monoglycoside. The sugar was identified to be a β-glucopyranose from the chemical shifts of the carbon signals due to one hemiacetal (δC 102.76), four oxygenated methine (δC 78.06, 78.01, 75.03, 71.85), and one oxygenated methylene (δC 63.08) moieties. The carbon signals of the aglycone included one ester (δC 179.04), two olefin methines (δC 127.66, 122.29), two oxygenated methines (δC 83.20, 73.19), two methines (δC 43.90, 42.67), four methylenes (δC 36.06, 28.77, 27.75, 23.51), and one methyl (δC 14.31) observed, indicating that the aglycone had a hexahydro-butylphthalide moiety with one hydroxyl group and one double bond. The 1H NMR (PMR) spectrum (Table 2; δH, coupling pattern, J in Hz) showed two olefin methines (5.72, br. ddd, 4.2, 6.6, 10.2; 5.71, ddd, 2.4, 4.2, 10.2), two oxygenated methines (4.55, ddd, 5.4, 5.4, 9.6; 4.12, ddd, 3.6, 4.8, 6.6), three methylenes with germinal coupling (2.21, overlapped; 2.19, overlapped; 1.83–1.77, m; 1.60–1.54, m; 1.46–1.39, m; 1.27–1.21, overlapped), one methylene (1.27, overlapped), and one terminal methyl (0.84, t, 7.2), the same as those of the aglycone moiety. The signals of the β-glucopyranose were the same as those of a hemiacetal (4.22, d, 7.8), four oxygenated methines (δH 3.30 to 3.31), and one oxygenated methylene (3.79, dd, 2.8, 12.0; 3.53, dd, 4.2, 12.0). The 1H-1H COSY spectrum suggested the connection of carbons with protons shown in Figure 1 The positions of two oxygenated methine carbons (C-3 and C-4) were confirmed from the cross peaks in the HMBC spectrum, that is, H-3 (δH 4.55)/C-11 (δC 28.77), C-8 (δC 42.67), C-4 (δC 73.19), and C-1 (δC 179.04); H-4 (δH 4.12)/C-8, C-3 (δC 83.20), C-1′ (δC 102.76), and C-6 (δC 127.66), respectively. Additionally, the HMBC spectrum confirmed the position of two olefin methines (C-6 and C-7), which showed cross peaks such as H-6 (δH 5.72)/C-5 (δC 27.75), C-8, C-4, C-7 (δC 122.29), and C-1; H-7 (δH 5.71)/C-5, C-8, C-9 (δC 43.90), C-6, and C-1, respectively. The sugar was revealed to be linked to the hydroxyl group at C-4 from the cross peaks in the HMBC spectrum as H-1′(δH 4.22)/C-4 and H-4/C-1′. Consequently, the planar structure was determined as shown in Figure 2. In the NOESY spectrum (Figure 1), H-3 (δH 4.55) showed a correlation with H-4 (δH 4.12) and H-8 (δH 3.27), while H-9 (δH 2.71) showed a correlation with H-10 (δH 1.83, 1.60), proving the relative stereostructure as shown in Figure 1 and 2. Compound 1 was hydrolyzed using 1N HCl to give the aglycone, 1a, which exhibited the positive Cotton effect at 255 nm (Δε 3.22) and the negative Cotton effect at 212 nm (Δε -3.26), indicating the hydroxyl group at C-4 to have an α-position [11,12]. Therefore, the chemical structure, including the absolute stereochemistry, of compound 1 was determined as shown in Figure 2, and it was named ligusticoside C.

Table 1.
13C-NMR data on the phthalide glycosides from the rhizome of Cnidium officinale Makino (125 MHz, CD3OD, δC).

Table 2.
1H-NMR data on the phthalide glycosides from the rhizome of Cnidium officinale Makino (600 MHz, CD3OD, δH, coupling pattern, J in Hz).
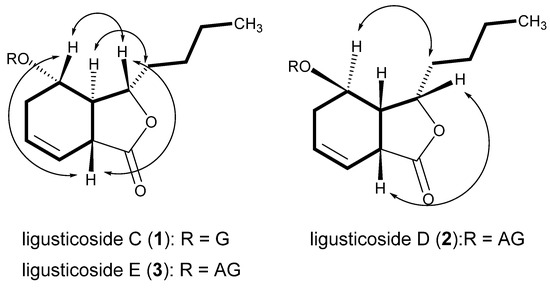
Figure 1.
Key correlations in the 1H-1H COSY (—) and NOESY (  ) spectra. G: β-D-glucopyranosyl; AG: β-D-apiofuranosyl-(1→6)-β-D-glucopyranosyl.
) spectra. G: β-D-glucopyranosyl; AG: β-D-apiofuranosyl-(1→6)-β-D-glucopyranosyl.
 ) spectra. G: β-D-glucopyranosyl; AG: β-D-apiofuranosyl-(1→6)-β-D-glucopyranosyl.
) spectra. G: β-D-glucopyranosyl; AG: β-D-apiofuranosyl-(1→6)-β-D-glucopyranosyl.
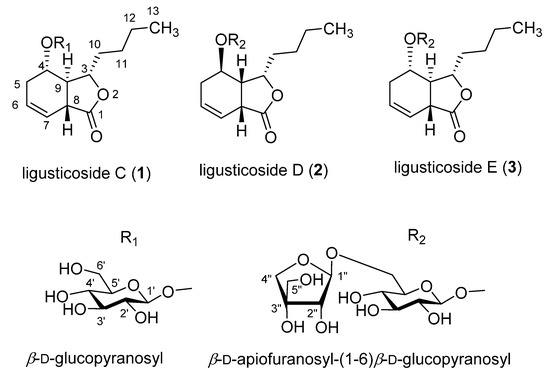
Figure 2.
Chemical structures of phthalide glycosides from the rhizome of Cnidium officinale Makino.
Compound 3, a white powder, showed UV absorption at 254 and 365 nm and a deep brown color on TLC upon spraying with 10% sulfuric acid and heating. MF was determined to be C23H36O12 (calcd for C23H37O12, 505.2281) from a PM at m/z 505.2283 [M + H]+ in HR-ESI-MS. The IR bands showed absorbance bands at 3357 (hydroxyl), 1745 (ester), and 1685 and 1458 cm−1 (double bond). CMR and PMR (Table 1 and Table 2) were almost the same as those of compound 1 with the exception of the additional signals due to one pentose. The signals of the pentose included those of one hemiacetal (δH 5.04, d, 2.4, H1”; δC 111.19, C-1”), one oxygenated quaternary carbon (δC 80.62, C-3”), one oxygenated methine (δH 3.89, d, 2.4, H-2”; δC 78.16, C-2”), and two oxygenated methylene carbons (δH 3.96, d, 9.6 and δH 3.76, d, 9.6, H-4”; δH 3.57, s, H-5”; δC 75.09, C-4”; δC 65.57, C-5”). The oxygenated quaternary carbon signal and the singlet oxygenated methylene proton signals suggested the sugar to be a branched-chain aldopentose, which was identified to be an apiofuranose from the chemical shift in CMR. The characteristics of the stereostructure were revealed to be the same as those of compound 1 via the same methods as used previously. Therefore, the chemical structure, including the absolute stereochemistry, of compound 3 was determined as shown in Figure 2, and it was named ligusticoside E.
Compound 2, a white powder, showed UV absorption at 254 and 365 nm and a deep brown color on TLC upon spraying with 10% sulfuric acid and heating. MF was determined to be C23H36O12 (calcd for C23H37O12, 505.2281) from a PM at m/z 505.2285 [M + H]+ in HR-ESI-MS. The IR bands showed absorbance bands at 3356 (hydroxyl), 1747 (ester), and 1600 and 1456 cm−1 (double bond). From PMR and CMR, we concluded that the planar structure was the same as that of 3. In the NOESY spectrum of compound 2 (Figure 1), H-3 (δH 4.57) showed a correlation with H-8 (δH 3.60) and H-9 (δH 2.78–2.74), while H-10 (δH 2.18–2.12 and δH 2.05) showed a correlation with not H-4 (δH 4.15) but H-3, proving the relative stereostructure. The aglycone of compound 2, 2a, obtained by acid hydrolysis, exhibited the negative Cotton effect at 250 nm (Δε −1.66) and the positive Cotton effect at 218 nm (Δε 1.29), indicating the n-butyl group at C-3, the hydrogen at C-9, and the hydroxyl group at C-4 to have α-, β-, and β-positions, respectively [12,13]. Therefore, the chemical structure, including the absolute stereochemistry, of compound 2 was determined as shown in Figure 2, and it was named ligusticoside D.
To date, lots of phthalides have been isolated from Cnidium officinale or Angelica gigas. However, less than 10 phthalide glycoside have been reported. Among them, most glycosides have a double bond at C-7 and C-8 or at C-8 and C-9. Ligusticosides C–E isolated in this study are the first occurrence for phthalide glycosides to have a double bond at C-6 and C-7 in the aglycone moiety.
2.2. Recovery Effects for the Extract, Solvent Fractions, and Compounds 1–3 on Otic Hair Cells in Zebrafish Damaged by Neomycin Treatment
The recovery effects of the phthalide glycosides 1–3 on otic neuromast hair cells exposed to neomycin were evaluated. Treatment with the ototoxic drug severely damaged the hair cells, which significantly decreased in number (p < 0.001). Ligusticoside C (1) and ligusticoside E (3) significantly recovered the damaged hair cells (p < 0.01), while ligusticoside D (2) exhibited an effect without statistical significance (Figure 3). Therefore, a phthalide glycoside-enriched fraction can be a promising source to prevent or treat the auditory pathological symptoms.
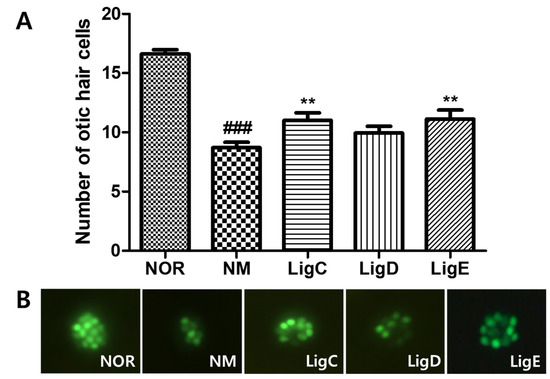
Figure 3.
Recovery of otic hair cells after neomycin-induced hair cell damage. (A) The numbers of otic hair cells in the untreated group (NOR), the neomycin treatment group (NM), and the phthalide glycoside treatment groups (LigC, LigD, LigE; 0.1 µM). (B) Fluorescence images of the zebrafish otic hair cells. Hair cells were stained with 0.1% YO-PRO-1. Data are presented as means ± SEM. ** p < 0.01 (control versus treated groups). ### p < 0.001 (normal group versus control group). LigC, ligusticoside C (1); LigD, ligusticoside D (2); LigE, ligusticoside E (3).
3. Materials and Methods
3.1. Plant Materials
The Department of Herbal Crop Research, RDA, Eumseong, Korea, supplied the rhizomes of Cnidium officinale, and Dr. J.T. Jeong, Department of Herbal Crop Research, identified them. A voucher specimen (NPCL-20200023) was stored at the Natural Products Chemistry Laboratory of Kyung Hee University, Yongin, Korea.
3.2. General Experimental Procedures
The equipment and chemicals used for the isolation of the phthalide glycosides and structure determination of the isolated metabolites were selected by referring to the literature [14]. For the breeding and maintenance of the zebrafish to test the recovery effect on otic hair cells damaged by neomycin treatment, we followed the methods previously reported in the literature [15].
3.3. Isolation of Phthalide Glycosides from the Rhizomes of Cnidium officinale
Ten kilograms of the dried rhizomes of Cnidium officinale Makino (10 kg) were soaked in aqueous ethanol (70% EtOH, 54 L × 2) at room temperature for 12 h. The concentrated brownish residue (2.1 kg) was poured into H2O (4 L) and extracted with EtOAc (4 L × 3) and n-BuOH (3.2 L × 3), successively, to yield EtOAc (COE, 280 g), n-BuOH (COB, 125 g), and H2O (COW, 1.695 kg) Fr. The CC for COB (120 g) was carried out using SiO2 resin (Figure 3) to give 17 fractions. The subsequent repeated CC using SiO2 and ODS as packing materials yielded three new phthalide glycosides, compounds 1–3 (Figure 4).
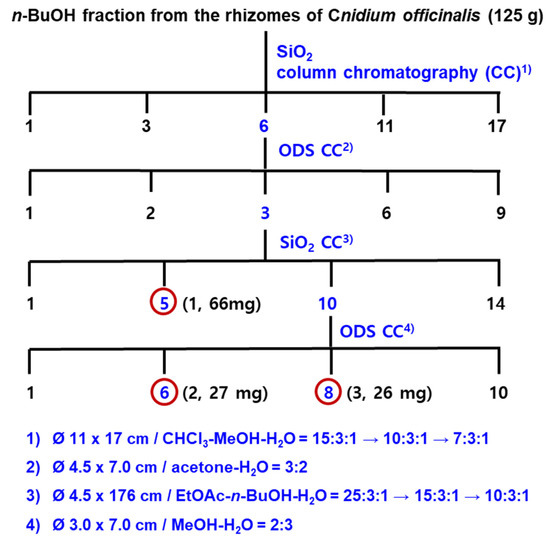
Figure 4.
Isolation of phthalide glycosides from the n-BuOH fraction of Cnidium officinale rhizomes.
Ligusticoside C (1)
White powder; TLC (SiO2) Rf 0.28, EtOAc-n-BuOH-H2O (15:3:1), (ODS) Rf 0.31, MeOH–H2O (2:3); [α]D −7.0 (c 0.01, CH3OH); IR (LiF plates) νmax 3367, 2928, 1742, 1606, 1514, 1455 cm−1; CMR and PMR: Table 1 and Table 2. ESI-MS: m/z 373.1861 [M + H]+ (calcd for C18H29O8, 373.1858).
Ligusticoside D (2)
White powder; TLC (SiO2) Rf 0.44, CHCl3-MeOH-H2O (7:3:1), (ODS) Rf 0.30, MeOH–H2O (2:3); [α]D −47.0 (c 0.01, CH3OH); IR (LiF plates) νmax 3356, 2930, 1747, 1600, 1456 cm−1; CMR and PMR: Table 1 and Table 2; ESI-MS: m/z 505.2285 [M + H]+ (calcd for C23H37O12, 505.2281).
Ligusticoside E (3)
3.4. Acid Hydrolysis of Phthalide Glycosides 1–3
A quantity of 10 mg of each glycoside was dissolved in 1N HCl (5 mL) and refluxed for 3 hrs. H2O (15 mL) was added to the reaction mixture followed by being extracted with EtOAc (20 mL × 2). The EtOAc phase was concentrated in vacuo and purified through open SiO2 CC (2 × 8 cm) using CHCl3–MeOH (10:1) to give each aglycone (1a, 3 mg; 2a, 4 mg; 3a, 3 mg).
1a: Colorless oil; CD (CH3OH) λ nm (Δε) 255 (3.22), 212 (–3.26); PMR (600 MHz, CDCl3, δH, coupling pattern, J in Hz) 5.84 (1H, br. dd, 1.8, 10.2, H-6), 5.73–5.70 (1H, m, H-7), 4.48 (1H, ddd, 4.4, 4.8, 9.6, H-3), 3.84 (1H, ddd, 3.6, 4.8, 7.4, H-4), 3.34–3.31 (1H, m, H-8), 2.49 (1H, m, H-5a), 2.28 (1H, ddd, 4.9, 4.3, 9.5, H-9), 2.16–2.13 (1H, m, H-10a), 1.97 (1H, overlapped, H-10b), 1.95 (1H, overlapped, H-5b), 1.33–1.28 (2H, overlapped, H-11), 1.21 (2H, overlapped, H-12), 0.81 (3H, t, 7.8, H-13).
2a: Colorless oil; CD (CH3OH) λ nm (Δε) 250 (–1.66), 218 (1.29); PMR (600 MHz, DMSO-d6, δH, coupling pattern, J in Hz) 5.75 (1H, ddd, 1.2, 6.0, 6.0, H-6), 5.71 (1H, dd, 9.6, 3.0, H-7), 4.48 (1H, ddd, 5.4, 5.4, 8.4, H-3), 3.93 (1H, ddd, 5.4, 8.4, 10.8, H-4), 3.61–3.59 (1H, m, H-8), 2.61, (1H, ddd, 5.4, 5.4, 10.8, H-5a), 2.59–2.56 (1H, m, H-9), 2.00–1.96 (1H, m, H-10a), 1.91–1.87 (1H, m, H-10b), 1.85–1.81 (1H, m, H-5b), 1.41–1.35 (2H, m, H-11), 1.34–1.29 (2H, m, H-12), 0.88 (3H, t, 6.9, H-13).
3a: Colorless oil; CD (CH3OH) λ nm (Δε) 257 (2.99), 214 (–2.63); PMR: same as 1a.
3.5. Evaluation of the Recovery Effect on Otic Hair Cells in Zebrafish Damaged by Neomycin Treatment
The evaluation of the recovery effect was followed by the methods previously reported in the literature [15].
4. Conclusions
Column chromatography for an active Fr, the n-BuOH Fr obtained from Cnidium officinale rhizomes, yielded three new phthalide glycosides, named ligusticosides C (1), D (2), and E (3). As each aglycone had four chiral carbons, their relative and absolute stereostructures were determined by examining their NOESY and circular dichroism (CD) data. This is the first report of the isolation of phthalide glycosides to have double bonds at C-6 and C-7. Ligusticoside C (1) and ligusticoside E (3) (0.1 μM) recovered the damage (p < 0.01) caused by neomycin treatment in zebrafish otic hair cells.
Supplementary Materials
The following are available online, Figures S1–S3: 13C and 1H NMR spectra of ligusticosides C, D, and E. Figures S4–S6: 2-D NMR spectra of ligusticosides C, D, and E.
Author Contributions
Conceptualization and visualization: H.-G.K. and N.-I.B.; Material preparation: H.-G.K., S.M.O., T.N.N. and N.-I.B.; Isolate compounds: H.-G.K., M.-H.L. and T.N.N.; Identify compounds (UV, IR, NMR, MS): H.-G.K., D.Y.L. and N.-I.B.; Anti-ototoxicity assay: N.W.K., J.H.S., Y.H.N. and T.H.K.; Writing—original draft preparation: H.-G.K. and S.M.O.; Writing—review and editing: H.-G.K., S.M.O. and N.-I.B.; Funding acquisition: D.Y.L. and N.-I.B. All authors have read and agreed to the published version of the manuscript.
Funding
Rural Development Administration (RDA), Republic of Korea (Project no. PJ01420403) funded this study.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Acknowledgments
This work was supported by the “Cooperative Research Program for Agriculture Science & Technology Development” (Project no. PJ01420403), Rural Development Administration, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, W.; Tang, Y.; Chen, Y.; Duan, J.-A. Advances in the Chemical Analysis and Biological Activities of Chuanxiong. Molecules 2012, 17, 10614–10651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qu, F. Treating Gynaecological Disorders with Traditional Chinese Medicine: A Review. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 494–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.Y.; Lee, S.H.; Kim, H.R.; Kim, J.H.; Yang, S.B.; Cho, S.Y.; Park, J.M.; Ko, C.N.; Park, S.U. A Review of Clinical Research Trends in the Treatment of Primary Headache Disorders with Pharmacopuncture. J. Int. Korean Med. 2018, 39, 1191–1205. [Google Scholar] [CrossRef] [Green Version]
- Hiroshi, H.; Tomonori, H.; Ichiro, H.; Sator, S. Skin Care Preparation for External Use/Skin External Preparation Containing Plant Extracts with Moisture-Retaining and Antibacterial Effects. Japan Patent JP2004010526, 15 January 2004. [Google Scholar]
- Lee, K.Y.; Kim, J.H.; Kim, E.Y.; Yeom, M.; Jung, H.S.; Sohn, Y. Water extract of Cnidii Rhizoma suppresses RANKL-induced osteoclastogenesis in RAW 264.7 cell by inhibiting NFATc1/c-Fos signaling and prevents ovariectomized bone loss in SD-rat. BMC Complement. Altern. Med. 2019, 19, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Kwon, O.I.; Kim, C.J. Anti-inflammatory and Analgesic Activities of the Extracts and Fractions of Cnidii Rhizoma. Kor. J. Pharmacogn. 1996, 27, 282–287. [Google Scholar]
- Jeong, J.B.; Ju, S.Y.; Park, J.H.; Lee, J.R.; Yun, K.W.; Kwon, S.T.; Lim, J.-H.; Chung, G.Y.; Jeong, H.J. Antioxidant activity in essential oils of Cnidium officinale makino and Ligusticum chuanxiong hort and their inhibitory effects on DNA damage and apoptosis induced by ultraviolet B in mammalian cell. Cancer Epidemiol. 2009, 33, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.E.; Lee, Y.J.; Choi, Y.A.; Park, J.M.; Lee, S.M.; Jo, N.Y.; Lee, E.Y.; Lee, C.K.; Roh, J.D. Seizure after Subdural Hematoma Treated with Combination Western-Korean Medicine. J. Acupunct. Res. 2021, 38, 72–78. [Google Scholar] [CrossRef]
- Lee, J.T.; Park, J.H.; Lee, K.H. Effect of methanol extract of Cnidii rhizoma on the function of receptors for GABA and glycine. J. Korean Acad. Pediatr. Dent. 2005, 32, 55–66. [Google Scholar]
- Sim, Y.; Shin, S. Antibacterial activities of the essential oil from the leaves and rhizomes of Cnidium officinale Makino. J. Essent. Oil Res. 2014, 26, 452–457. [Google Scholar] [CrossRef]
- Xu, Z.; Bing, H.; Ziming, F.; Jianshuang, J.; Yanan, Y.; Peicheng, Z. Bioactive Thionic Compounds and Aromatic Glycosides from Ligusticum chuanxiong. Acta Pharm. Sin. B 2018, 8, 818–824. [Google Scholar]
- Li, L.-J.; Su, Y.-F.; Yan, S.-L. Three new phthalide glycosides from the rhizomes of Ligusticum chuanxiong. Phytochem. Lett. 2016, 17, 14–17. [Google Scholar] [CrossRef]
- Qian, W.; Jianbo, Y.; Jin, R.; Aiguo, W.; Tengfei, J.; Yalun, S. Bioactive Phthalides from Ligusticum sinense Oliv cv. Chaxiong. Fitoter. 2014, 93, 226–232. [Google Scholar]
- Kim, H.-G.; Jung, Y.S.; Oh, S.M.; Oh, H.-J.; Ko, J.-H.; Kim, D.-O.; Kang, S.C.; Lee, Y.-G.; Lee, D.Y.; Baek, A.N.-I. Coreolanceolins A–E, New Flavanones from the Flowers of Coreopsis lanceolate, and Their Antioxidant and Anti-Inflammatory Effects. Antioxidants 2020, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Chang, J.; Jun, H.J.; Im, G.J.; Chae, S.W.; Lee, S.H.; Kwon, S.-Y.; Jung, H.H.; Chung, A.-Y.; Park, H.-C. Protective role of edaravone against neomycin-induced ototoxicity in zebrafish. J. Appl. Toxicol. 2014, 34, 554–561. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).