Bioactive Compounds of Opuntia spp. Acid Fruits: Micro and Nano-Emulsified Extracts and Applications in Nutraceutical Foods
Abstract
1. Introduction
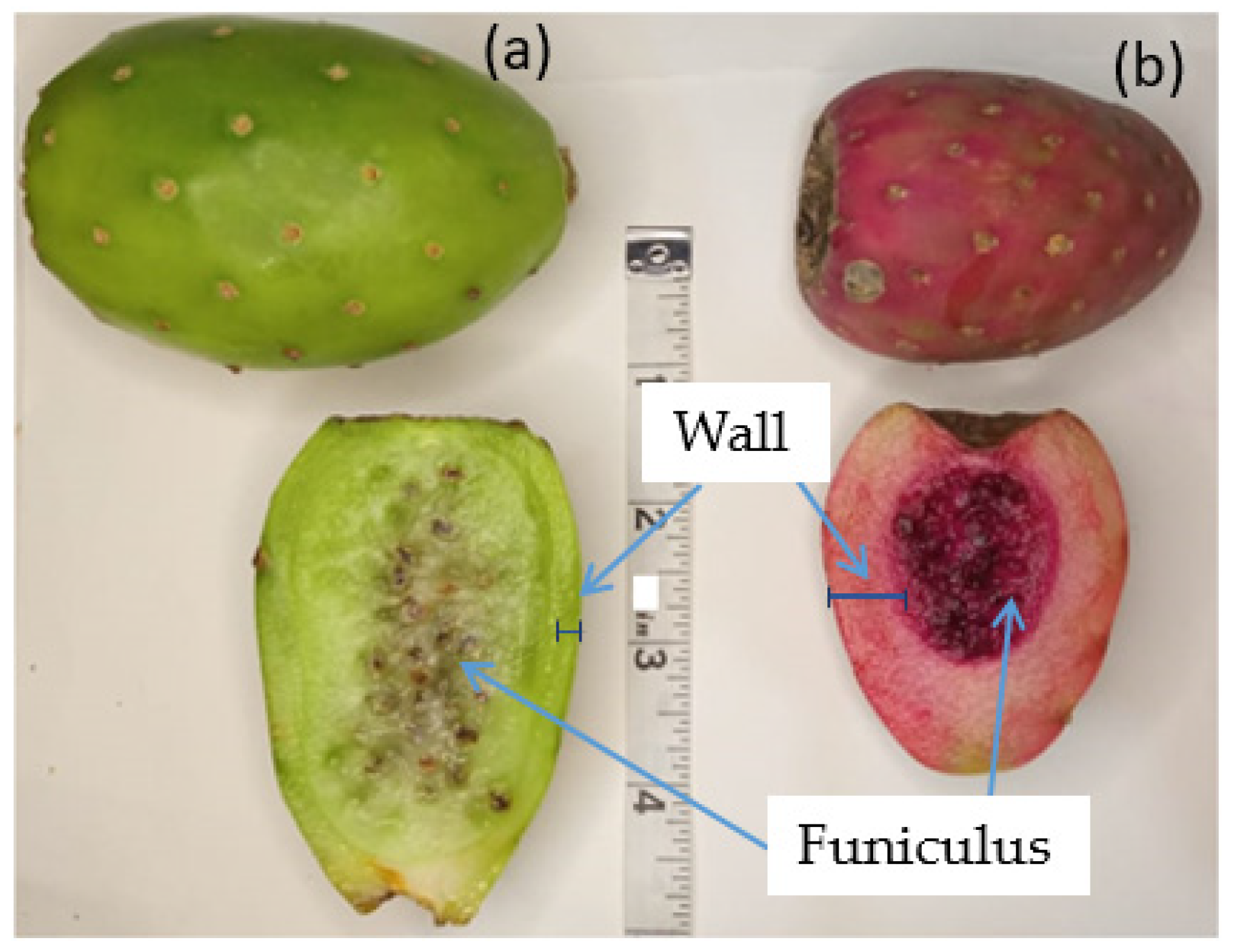
2. Chemical Composition of Xoconostle Fruit
Bioactive Compounds
3. Antioxidant and Antibacterial Activity of Xoconostle Extracts
4. Potential Technological Applications of Xoconostle
5. Potential Nutraceutical Health Effects of Xoconostle Consumption
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morales, P.; Barros, L.; Ramírez-Moreno, E.; Santos-Buelga, C.; Ferreira, I.C. Exploring xoconostle by-products as sources of bioactive compounds. Food Res. Int. 2014, 65, 437–444. [Google Scholar] [CrossRef]
- Hernández-Fuentes, A.D.; Trapala-Islas, A.; Gallegos-Vásquez, C.; Campos-Montiel, R.G.; Pinedo-Espinoza, J.M.; Guzmán-Maldonado, S.H. Physicochemical variability and nutritional and functional characteristics of xoconostles (Opuntia spp.) accessions from Mexico. Fruits 2015, 70, 109–116. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V.; Barragán-Huerta, B.E.; Yáñez-Fernández, J.; Piña-Rosas, J.A.; Mejia, J.A.A. Processing of Xoconostle fruit (Opuntia joconostle ) juice for improving its commercialization using membrane filtration. J. Food Process. Preserv. 2017, 42, 13394. [Google Scholar] [CrossRef]
- Anderson, M.; Carruthers, L.E.; Cromell, C. Cacti and Succulents. Lorenz Books: Trivex, Singapore, 1999. [Google Scholar]
- Gallegos Vázquez, C.; Cervantes Herrera, J.; Corrales García, J.; Medina García, G. La cadena productiva del nopal en Zacatecas: Bases para un desarrollo sostenido; Universidad Autónoma Chapingo: Texcoco, México, 2003. [Google Scholar]
- Scheinvar, L. Biosistemática de los xoconostles mexicanos y su potencial económico. In Memorias del VIII Congreso Nacional y VI Internacional sobre Conocimiento y Aprovechamiento del Nopal; Univ. Autónoma de San Luis Potosí: San Luis, México, 1999. [Google Scholar]
- Martínez-González, C.R.; Luna-Vega, I.; Gallegos-Vázquez, C.; García-Sandoval, R. Opuntia delafuentiana (Cactaceae: Opuntioideae), a new xoconostle from central Mexico. Phytotaxa 2015, 231, 230. [Google Scholar] [CrossRef][Green Version]
- Gallegos-Vazquez, C.; Scheinvar, L.; Núñez-Colín, C.A.; Mondragón-Jacobo, C. Morphological diversity of xoconostles (Opuntia spp.) or acidic cactus pears: A Mexican contribution to functional foods. Fruits 2012, 67, 109–120. [Google Scholar] [CrossRef][Green Version]
- Guzmán-Maldonado, S.H.; Morales-Montelongo, A.L.; Mondragón-Jacobo, C.; Herrera-Hernández, G.; Guevara-Lara, F.; Reynoso-Camacho, R. Physicochemical, nutritional, and functional characterization of fruits xoconostle (Opuntia matudae) pears from Central-Mexico Region. J. Food Sci. 2010, 75, 485–492. [Google Scholar] [CrossRef]
- Osorio-Esquivel, O.; Moreno, A.O.; Álvarez, V.B.; Alvarez, L.D.; Giusti, M.M. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 2011, 44, 2160–2168. [Google Scholar] [CrossRef]
- Pérez-Alonso, C.E.S.A.R.; Campos-Montiel, R.G.; Morales-Luna, E.L.I.Z.A.B.E.T.H.; Reyes-Munguía, A.B.I.G.A.I.L.; Aguirre-Álvarez, G.A.B.R.I.E.L.; Pimentel-González, D.J. Stabilization of phenolic compounds from Opuntia oligacantha Först by microencapsulation with agave SAP (aguamiel). Rev Mex Ing Quim. 2015, 14, 579–588. [Google Scholar]
- Ramírez-Rodríguez, Y.; Martínez-Huélamo, M.; Pedraza-Chaverri, J.; Ramírez, V.; Martínez-Tagüeña, N.; Trujillo-Silva, J. Ethnobotanical, nutritional and medicinal properties of Mexican drylands Cactaceae Fruits: Recent findings and research opportunities. Food Chem. 2019, 312, 126073. [Google Scholar] [CrossRef]
- Valero-Galván, J.; González-Fernández, R.; Sigala-Hernández, A.; Núñez-Gastélum, J.A.; Ruiz-May, E.; Rodrigo-García, J.; Larqué-Saavedra, A.; Martínez-Ruiz, N.D.R. Sensory attributes, physicochemical and antioxidant characteristics, and protein profile of wild prickly pear fruits (O. macrocentra Engelm., O. phaeacantha Engelm., and O. engelmannii Salm-Dyck ex Engelmann.) and commercial prickly pear fruits (O. ficus-indica (L.) Mill.). Food Res. Int. 2021, 40, 109909. [Google Scholar]
- Medina-Pérez, G.; Zaldívar-Ortega, A.K.; Cenobio-Galindo, A.D.J.; Afanador-Barajas, L.N.; Vieyra-Alberto, R.; Estefes-Duarte, J.A.; Campos-Montiel, R.G. Antidiabetic Activity of Cactus Acid Fruit Extracts: Simulated Intestinal Conditions of the Inhibitory Effects on α-amylase and α-glucosidase. Appl. Sci. 2019, 9, 4066. [Google Scholar] [CrossRef]
- Espino-García, J.; Campos-Montiel, R.; González-Lemus, U.; Torres-Cardona, M.G.; Sánchez-Santillán, P.; Almaraz-Buendía, J.J.G.P.Y.I. In vitro fermentation of maize stover mixed with xoconostle (Opuntia matudae Sheinvar) and the effect on gas production. Livest. Res. Rural. Dev. 2020, 32, 229249454. [Google Scholar]
- Espino-Manzano, S.O.; León-López, A.; Aguirre-Álvarez, G.; González-Lemus, U.; Prince, L.; Campos-Montiel, R.G. Application of Nanoemulsions (W/O) of Extract of Opuntia oligacantha C.F. Först and Orange Oil in Gelatine Films. Molecules 2020, 25, 3487. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gastélum, J.A. Morphological characteristics, chemical composition and antioxidant activity of seeds by four wild Opuntia species from North of Mexico. Inst. Cienc. Bioméd. 2018, 20, 23–33. [Google Scholar]
- García Hernández, M. Evaluación del contenido nutrimental, fibra dietética y propiedades antioxidantes de dos variedades de xoconostle (Opuntia, spp.). 2017. Available online: http://dgsa.uaeh.edu.mx:8080/bibliotecadigital/bitstream/handle/231104/2594/Evaluaci%C3%B3n%20del%20contenido%20nutrimental.pdf?sequence=2&isAllowed=y (accessed on 19 September 2021).
- Avello, M.; Suwalsky, M. Radicales libres, antioxidantes naturales y mecanismos de protección. Atenea (Concepción) 2006, 494, 161–172. [Google Scholar] [CrossRef]
- King, A.; Young, G. Characteristics and Occurrence of Phenolic Phytochemicals. J. Am. Diet. Assoc. 1999, 99, 213–218. [Google Scholar] [CrossRef]
- Cartaya, O.; Reynaldo, I. Flavonoides: Características químicas y aplicaciones. Cultivos Tropicales 2001, 22, 5–14. [Google Scholar]
- Niki, E.; Noguchi, N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life 2000, 50, 323–329. [Google Scholar] [CrossRef]
- Sáenz, C.; Sepúlveda, E. Alternativas de industrialización de la tuna (Opuntia ficus-indica). Alimentos 1993, 18, 29–32. [Google Scholar]
- Yahia, E.; Mondragon-Jacobo, C. Nutritional components and anti-oxidant capacity of ten cultivars and lines of cactus pear fruit (Opuntia spp.). Food Res. Int. 2011, 44, 2311–2318. [Google Scholar] [CrossRef]
- Monroy-Gutierrez, T.; Martínez-Damián, M.T.; Barrientos-Priego, A.F.; Gallegos-Vazquez, C.; Cruz-Alvarez, O.; Vargas-Madriz, H. Bioactive compounds and antioxidant capacity in fruits of xocotuna, cactus pear and xoconostle (Opuntia spp.). Chil. J. Agric. Anim. Sci. Ex Agro-Cienc. 2017, 33, 263–272. [Google Scholar]
- Morales Gomez, P.E.; Moreno, R.; Mata, M.S. Nutritional characterization of xoconostle fruits. Ann. Nutr. Metab. 2011, 58, 95–96. [Google Scholar]
- Kumari, P.; Bhargava, B. Phytochemicals from edible flowers: Opening a new arena for healthy lifestyle. J. Funct. Foods 2021, 78, 104375. [Google Scholar] [CrossRef]
- Pensamiento-Niño, C.A.; Campos-Montiel, R.G.; Añorve-Morga, J.; Ramírez-Moreno, E.; Ascacio-Valdés, J.A.; Hernández-Fuentes, A. Nutritional Characterization of the Functional and Antioxidant Activity of Cactus Flowers from Hidalgo, Mexico. Appl. Sci. 2021, 11, 5965. [Google Scholar] [CrossRef]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef]
- González-Aguilar, G.; Robles-Sánchez, R.; Martínez-Téllez, M.; Olivas, G.; Alvarez-Parrilla, E.; De La Rosa, L. Bioactive compounds in fruits: Health benefits and effect of storage conditions. Stewart Postharvest Rev. 2008, 4, 1–10. [Google Scholar] [CrossRef]
- Cortez-García, R.M.; Ortiz-Moreno, A.; Zepeda-Vallejo, L.G.; Necoechea-Mondragón, H. Effects of Cooking Methods on Phenolic Compounds in Xoconostle (Opuntia joconostle). Plant Foods Hum. Nutr. 2015, 70, 85–90. [Google Scholar] [CrossRef]
- Díaz, M.D.S.S.; de la Rosa, A.-P.B.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntiaspp.: Characterization and Benefits in Chronic Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- López, A.L.; Álvarez, A.; Alvarado, R.J.; Montiel, R.G.C.; Munguía, A.R. Películas a base de gelatina adicionadas con compuestos bioactivos. Boletín De Cienc. Agropecu. Del ICAP 2017, 3, 5. [Google Scholar] [CrossRef]
- Solís-Silva, A.; Reyes-Munguía, A.; Madariaga-Navarrete, G.; Medina-Pérez, R.G.; Campos-Montiel, A.J.; Cenobio-Galindo, J. Evaluación de la actividad antifúngica y antioxidante de una nanoemulsión W/O de Opuntia oligacantha y aceite esencial de Citrus X sinensis. Investig. Desarro. Cienc. Tecnol. Aliment. 2018, 3, 182–187. [Google Scholar]
- Espinosa-Muñoz, V.; Roldán-cruz, C.A.; Hernández-Fuentes, A.D.; Quintero-Lira, A.; Almaraz-Buendía, I.; Campos-Montiel, R.G. Ultrasonic-assisted extraction of phenols, flavonoids, and biocompounds with inhibitory effect against Salmonella typhimurium and Staphylococcus aureus from Cactus pear. J. Food Process Eng. 2017, 40, e12358. [Google Scholar] [CrossRef]
- León, D.E.F.; Uribe, J.P.H.; Montiel, R.G.C.; Jiménez-Alvarado, R. Empaques activos en la industria de los alimentos. Boletín De Cienc. Agropecu. Del ICAP 2018, 4, 8. [Google Scholar] [CrossRef]
- Ozdemir, M.; Floros, J.D. Active food packaging technologies. Crit. Rev. Food. Sci. Nutr. 2004, 44, 185–193. [Google Scholar] [CrossRef]
- Parra Huertas, R.A. Food microencapsulation: A review. Rev. Fac. Nac. Agron. Medellin. 2010, 63, 5669–5684. [Google Scholar]
- Reis, L.C.B.; de Souza, C.O.; da Silva, J.; Martins, A.C.; Nunes, I.L.; Druzian, J.I. Active biocomposites of cassava starch: The effect of yerba mate extract and mango pulp as antioxidant additives on the properties and the stability of a packaged product. Food Bioprod. Process. 2015, 94, 382–391. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.D.J.; Díaz-Monroy, G.; Medina-Pérez, G.; Franco-Fernández, M.J.; Ludeña-Urquizo, F.E.; Vieyra-Alberto, R.; Campos-Montiel, R.G. Multiple emulsions with extracts of cactus pear added in a yogurt: Antioxidant activity, in vitro simulated digestion and shelf life. Foods 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, Z.; Hadian, Z.; Mashayekh, M. Nanocomposites in food packaging applications and their risk assessment for health. Electron. Physician 2016, 8, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pérez, G.; Hernández-Uribe, J.P.; Fernández-León, D.; Prince, L.; Fernández-Luqueño, F.; Campos-Montiel, R.G. Application of nanoemulsions (w/o) with active compounds of cactus pear fruit in starch films to improve antioxidant activity and incorporate antibacterial property. J. Food Process. Eng. 2019, 42, e13268. [Google Scholar] [CrossRef]
- Campos-Montiel, R.G.; Santos-Ordoñez, N.; Almaraz-Buendía, I.; Aguirre-Álvarez, G.; Espino-García, J.J.; Ludeña-Urquizo, F.E.; González-Tenorio, R.; Pérez-Soto, E.; Cenobio-Galindo, A.D.J. Impact of incorporating double emulsions with bioactive compounds of acid cactus fruits in emulsified meat products during storage. J. Food Process. Preserv. 2021, 45, 15477. [Google Scholar] [CrossRef]
- Pérez-Soto, E.; Cenobio-Galindo, A.; Espino-Manzano, S.; Franco-Fernández, M.; Ludeña-Urquizo, F.; Jiménez-Alvarado, R.; Zepeda-Velázquez, A.; Campos-Montiel, R. The Addition of Microencapsulated or Nanoemulsified Bioactive Compounds Influences the Antioxidant and Antimicrobial Activities of a Fresh Cheese. Molecules 2021, 26, 2170. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Huezo, M.; Estrada-Fernández, A.; García-Almendárez, B.; Ludeña-Urquizo, F.; Campos-Montiel, R.; Pimentel-González, D. Viability of Lactobacillus plantarum entrapped in double emulsion during Oaxaca cheese manufacture, melting and simulated intestinal conditions. LWT 2014, 59, 768–773. [Google Scholar] [CrossRef]
- Jiménez-Alvarado, R.; Aguirre-Álvarez, G.; Campos-Montiel, R.G.; Contreras-Esquivel, J.C.; Pinedo-Espinoza, J.M.; González-Aguayo, E.; Hernández-Fuentes, A.D. Effect of high-pulsed electric fields on the extraction yield and quality of juices obtained from the endocarp of nine prikly pear (Opuntia spp.) varieties. Jokull 2015, 65, 414–435. [Google Scholar]
- Cenobio-Galindo, A.D.J.; Ocampo-López, J.; Reyes-Munguía, A.; Carrillo-Inungaray, M.L.; Cawood, M.; Medina-Pérez, G.; Campos-Montiel, R.G. Influence of bioactive compounds incorporated in a nanoemulsion as coating on avocado fruits (Persea americana) during postharvest storage: Antioxidant activity, physicochemical changes and structural evaluation. Antioxidants 2019, 8, 500. [Google Scholar] [CrossRef]
- Pimienta-Barrios, E. Effect of Xoconostle (Opuntia joconostle Web.) fruit consumtion of glucose and seric lipids. Agrociencia 2008, 42, 645–653. [Google Scholar]
- Xu, H. Inhibition kinetics of flavonoids on yeast α-glucosidase merged with docking simulations. Protein Pept. Lett. 2010, 17, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Paiz, R.C.; Juárez-Flores, B.I.; Cecilia, J.R.A.R.N.; Ortega, C.; Aguuml, J.A.R.; Chávez, E.G.; Fuentes, G.Á. Glucose-lowering effect of xoconostle (Opuntia joconostle A. Web., Cactaceae) in diabetic rats. J. Med. Plants 2010, 4, 2326–2333. [Google Scholar]
- Osorio-Esquivel, O.; Ortiz-Moreno, A.; Garduño, L.; Álvarez, V.B.; Hernández-Navarro, M.D. Antihyperlipidemic Effect of Methanolic Extract from Opuntia joconostle Seeds in Mice Fed a Hypercholesterolemic Diet. Plant. Foods Hum. Nutr. 2012, 67, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pérez, G.; Peralta-Adauto, L.; Afanador-Barajas, L.; Fernández-Luqueño, F.; Pérez-Soto, E.; Campos-Montiel, R.; Peláez-Acero, A. Inhibition of Urease, Elastase, and β-Glucuronidase Enzymatic Activity by Applying Aqueous Extracts of Opuntia oligacantha CF Först Acid Fruits: In Vitro Essay under Simulated Digestive Conditions. Appl. Sci. 2021, 11, 7705. [Google Scholar] [CrossRef]
| CV. Cuaresmeño | CV. Rosa | Reference | |||
|---|---|---|---|---|---|
| Pulp | Seeds | Pulp | Seeds | [1] | |
| g/100 g | g/100 g | ||||
| Humidity | 93.24 ± 0.02 | 73.95 ± 1.09 | 94.11 ± 0.00 | 60.44 ± 0.66 | |
| Proteins | 0.66 ± 0.01 | 2.12 ± 0.00 | 0.56 ± 0.00 | 3.45 ± 0.02 | |
| Lipids | 0.03 ± 0.0 | 2.45 ± 0.05 | 0.04 ± 0.00 | 3.52 ± 0.12 | |
| Carbohydrates B | 3.69 | 1.71 | 3.93 | 1.56 | |
| Soluble sugars | 2.02 ± 0.09 | 0.95 ± 0.09 | 1.56 ± 0.17 | 1.47 ± 0.19 | |
| Fructose | 1.38 ± 0.03 | 0.71 ± 0.07 | 0.87 ± 0.03 | 0.99 ± 0.12 | |
| Glucose | 0.37 ± 0.05 | 0.15 ± 0.01 | 0.35 ± 0.13 | 0.34 ± 0.05 | |
| Sucrose | 0.27 ± 0.01 | 0.09 ± 0.00 | 0.34 ± 0.01 | 0.14 ± 0.02 | |
| Total dietary fiber | 2.31 ± 0.12 | 19.22 ± 0.15 | 1.74 ± 0.07 | 30.1 ± 0.64 | |
| Insoluble fiber | 1.45 ± 0.07 | 18.85 ± 0.12 | 1.16 ± 0.01 | 29.04 ± 0.57 | |
| Soluble fiber | 0.86 ± 0.05 | 0.36 ± 0.03 | 0.58 ± 0.07 | 1.13 ± 0.07 | |
| Whole fruit | [2] | ||||
| Mineral content | mg 100 g−1 FW | mg 100 g−1 FW | |||
| Ca | 0.143 ± 0.07 | N/D | |||
| Mg | 0.081 ± 0.003 | N/D | |||
| Fe | 0.060 ± 0.006 | N/D | |||
| K | 0.126 ± 0.0027 | N/D | |||
| Zn | 0.0030 ± 0.0001 | N/D | |||
| Acids | mg 100 g−1 FW | mg 100 g−1 FW | [18] | ||
| Malic | 179.2 ± 0.51 | 276 ± 0.5 | |||
| Citric | 2650 ± 2.33 | 1309 ± 0.8 | |||
| Fumaric | 17.67 ± 0.06 | 13.92 ± 0.07 | |||
| Oxalic | 79.54 ± 1.84 | 46.23 ± 0.66 | |||
| Ascorbic | 54.24 ± 0.86 | 16.35 ± 0.26 | |||
| Fruit Color | Cultivar | Structure | Total Phenols | Betalains | Antioxidant Activity | Total Flavonoids | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Betacyanins | Betaxanthins | ABTS | DPPH | Trolox | ||||||
 | Opuntia joconostle F.A.C. Weber ex Diguet. (cv. Cuaresmeño) | Pulp (mesocarp) | 38.57 ± 6.87 mg/100 g (FWB) | ND | ND | ND | 5.14 ± 0.20 mg/mL of extract | ND | 3.93 ± 0.19 mg CE/g of extract | [1] |
 | Opuntia matudae Scheinvar (cv. Rosa) | Pulp (mesocarp) | 33.71 ± 2.09 mg/100 g (FWB) | ND | ND | ND | >16 mg/mL of extract | ND | 0.86 ± 0.09 mg CE/g of extract | [1] |
 | Opuntia joconostle F.A.C. Weber ex Diguet. (cv. Cuaresmeño) | Seeds [10] (Endocarp) | 50.43 ± 4.86 mg/100 g (FWB) | ND | ND | ND | 1.53 ± 0.05 mg/mL of extract | ND | 24.18 ± 1.69 CE/g of extract | [1] |
 | Opuntia matudae Scheinvar (cv. Rosa) | Seeds (Endocarp) | 59.48 ± 0.69 mg/100 g (FWB) | ND | ND | ND | 1.88 ± 0.11 mg/mL of extract | ND | 58.40 ± 0.78 mg CE/g of extract | [1] |
 | Opuntia matudae | Peel (pericarp) | 863 ± 67 mg GAE/100 g (DWB) | 0.59 ± 0.01 mg/100 g (DWB) | 4.10 ± 0.28 mg/100 g (DWB) | ND | ND | 14.5 mmol of Trolox equivalents/100 g (FWB) | ND | [9] |
 | Opuntia matudae | Pulp and seeds (mesocarp and endocarp ) | 128 ± 6 mg GAE/100 g (DWB) | 0.49 ± 0.00 mg 100 g−1 (DWB) | 2.23 ± 0.11 mg 100 g−1 (DWB) | ND | ND | 6.87 mmol of Trolox equivalents/100 g (FWB) | ND | [9] |
 | Opuntia joconostle | Whole fruit | 13.08 ± 0.65 mg GAE/g (DWB) | 27.98 ± 0.64 mg 100 g−1 (DWB) | ND | 32.79 ± 1.42 mmol TE/100 g (DWB) | 4.94 ± 0.64 mmol TE/100 g (DWB) | ND | 1.19 ± 0.03 mg CE/g (DWB) | [31] |
 | Opuntia matudae Scheinvar cv. Blanco” | Whole fruit | 29.61 mg GAE/g (DWB) | ND | 0.95 mg VCEAC g−1 | ND | ND | ND | [25] | |
 | Opuntia matudae Scheinvar cv“Cuaresmeño” | Whole fruit | 44.61 mg GAE/g | 1.18 mg 100 g−1 | 0.34 mg 100 g−1 | 0.96 mg VCEAC g−1 | ND | ND | ND | [25] |
 | Opuntia duranguensis Britton and Rose | Whole fruit | 176.86 ± 3.15 mg GAE 100 g−1 (FWB) | 26.05 ± 0.06 mg 100 g−1 | 9.01 ± 0.06 mg 100 g−1 | ND | 290.52 ± 3.07 mg QE 100 g−1 (FWB) | ND | 1.98 mg QE 100 g−1 (FWB) | [7] |
 | O. oligacantha Föster cv Borrego | Whole fruit | 196.62 ± 2.94 mg GAE 100 g−1 (FWB) | 8.67 0.13 ± 0.13 mg 100 g−1 (FWB) | 3.67 ± 0.03 mg 100 g−1 (FWB) | ND | 255.65 ± 2.35 mg QE 100 g−1 (FWB) | ND | 4.77 ± 0.10 mg QE 100 g−1 (FWB) | [2] |
 | O. oligacantha (Förster) Ulapa | Whole fruit | 278 ± 2.2 | 0.76 ± 0.36 mg 100 g−1 | 4.50 ± 0.36 mg 100 g−1 | ND | ND | 9.80 ± 0.22 (mmol TE 100 g−1) | ND | [2] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Luqueño, F.; Medina-Pérez, G.; Pérez-Soto, E.; Espino-Manzano, S.; Peralta-Adauto, L.; Pérez-Ríos, S.; Campos-Montiel, R. Bioactive Compounds of Opuntia spp. Acid Fruits: Micro and Nano-Emulsified Extracts and Applications in Nutraceutical Foods. Molecules 2021, 26, 6429. https://doi.org/10.3390/molecules26216429
Fernández-Luqueño F, Medina-Pérez G, Pérez-Soto E, Espino-Manzano S, Peralta-Adauto L, Pérez-Ríos S, Campos-Montiel R. Bioactive Compounds of Opuntia spp. Acid Fruits: Micro and Nano-Emulsified Extracts and Applications in Nutraceutical Foods. Molecules. 2021; 26(21):6429. https://doi.org/10.3390/molecules26216429
Chicago/Turabian StyleFernández-Luqueño, Fabián, Gabriela Medina-Pérez, Elizabeth Pérez-Soto, Salvador Espino-Manzano, Laura Peralta-Adauto, Sergio Pérez-Ríos, and Rafael Campos-Montiel. 2021. "Bioactive Compounds of Opuntia spp. Acid Fruits: Micro and Nano-Emulsified Extracts and Applications in Nutraceutical Foods" Molecules 26, no. 21: 6429. https://doi.org/10.3390/molecules26216429
APA StyleFernández-Luqueño, F., Medina-Pérez, G., Pérez-Soto, E., Espino-Manzano, S., Peralta-Adauto, L., Pérez-Ríos, S., & Campos-Montiel, R. (2021). Bioactive Compounds of Opuntia spp. Acid Fruits: Micro and Nano-Emulsified Extracts and Applications in Nutraceutical Foods. Molecules, 26(21), 6429. https://doi.org/10.3390/molecules26216429








