Dual Catalytic Hairpin Assembly-Based Automatic Molecule Machine for Amplified Detection of Auxin Response Factor-Targeted MicroRNA-160
Abstract
:1. Introduction
2. Results and Discussion
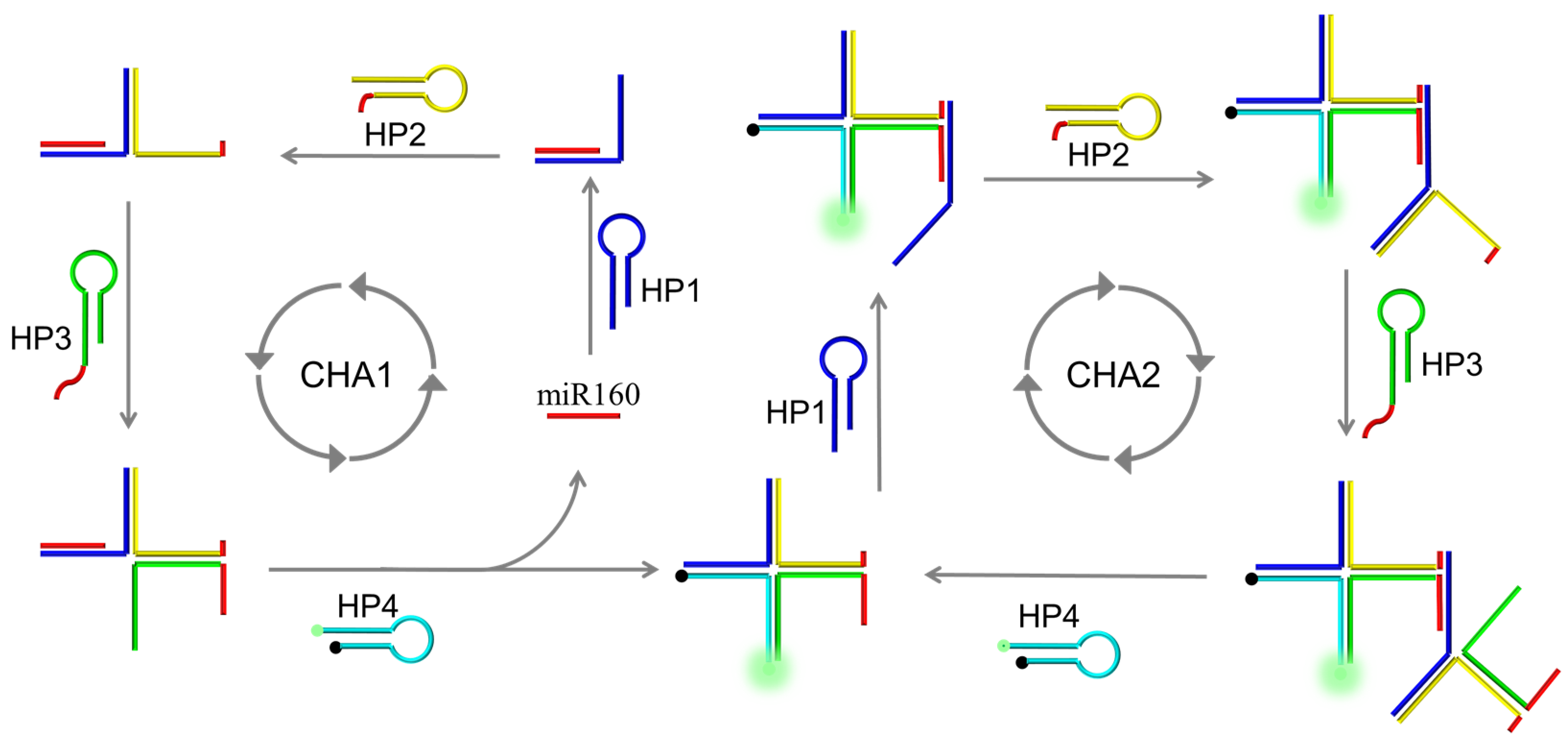
2.1. Design of AMM Machine and Its Dual Catalytic Hairpin Assembly
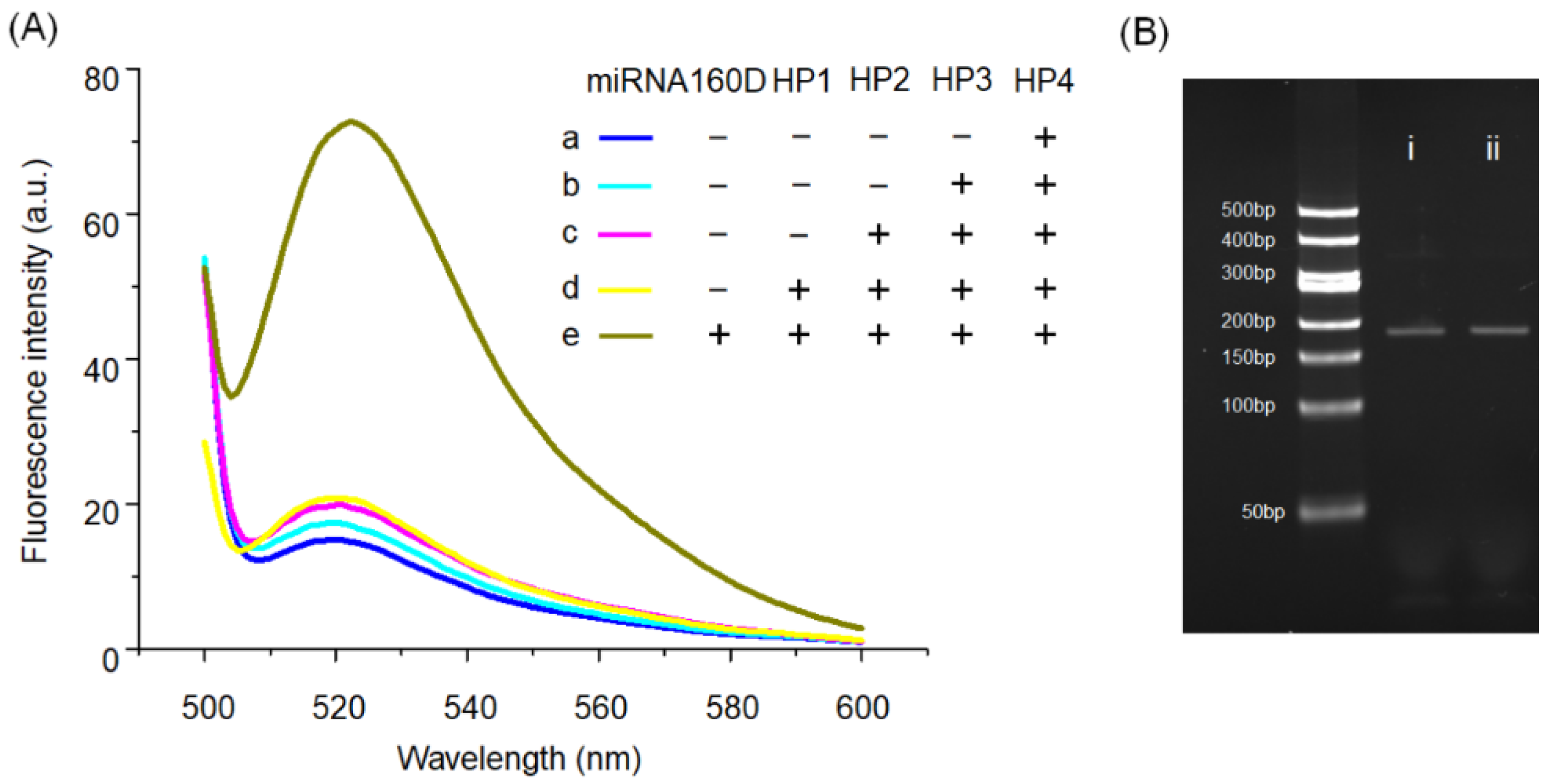
2.2. Feasibility of AMM for miRNA160 Detection
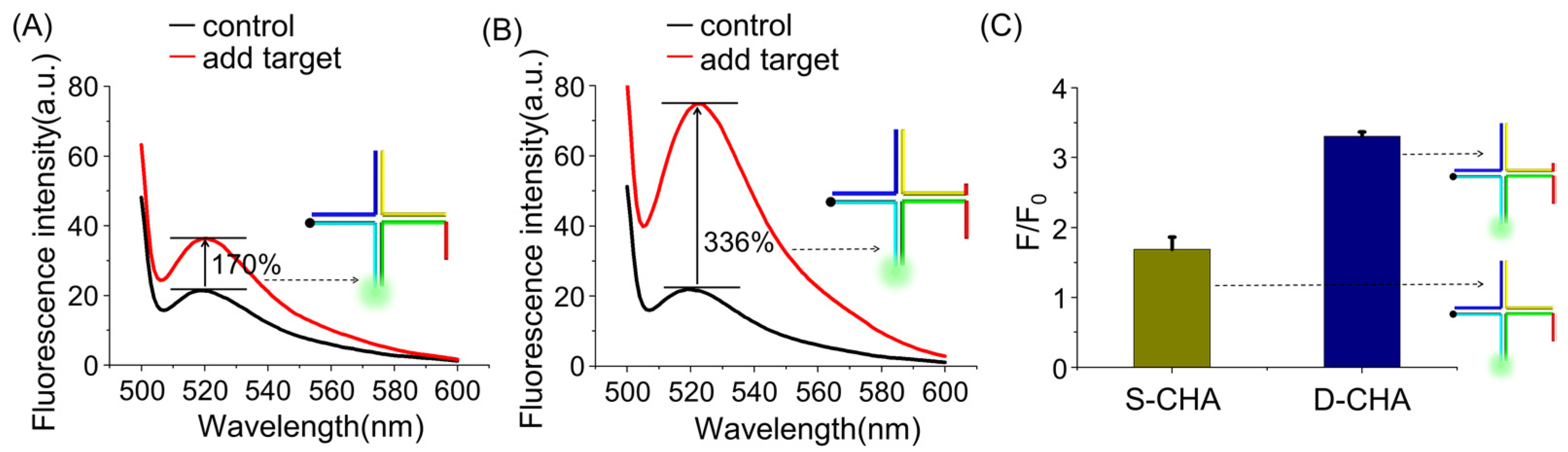
2.3. D-CHA-Based Signal Amplification
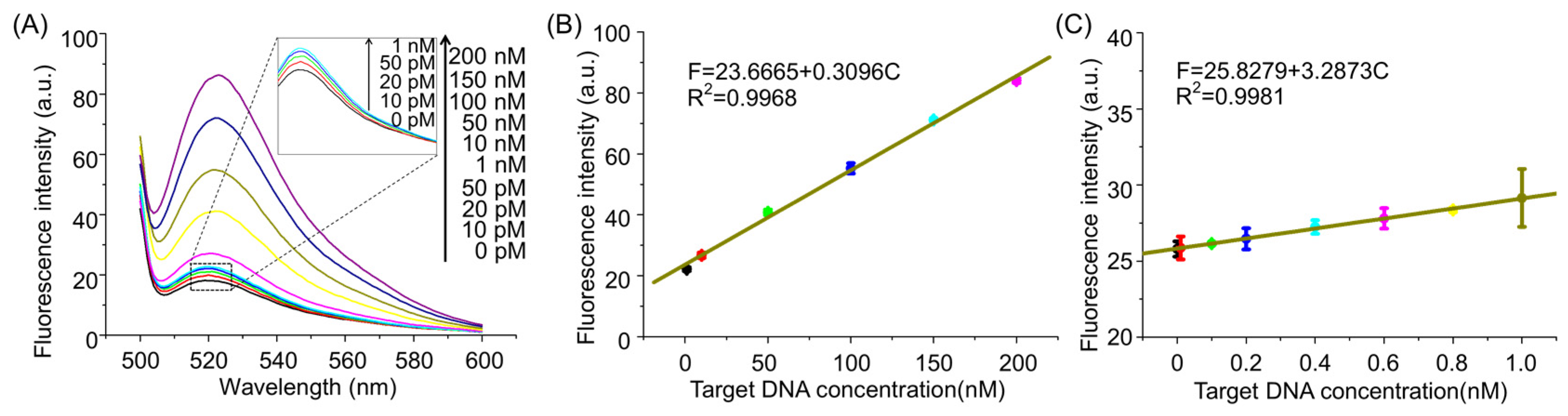
2.4. Assay Performance of the AMM System for miRNA160D Detection
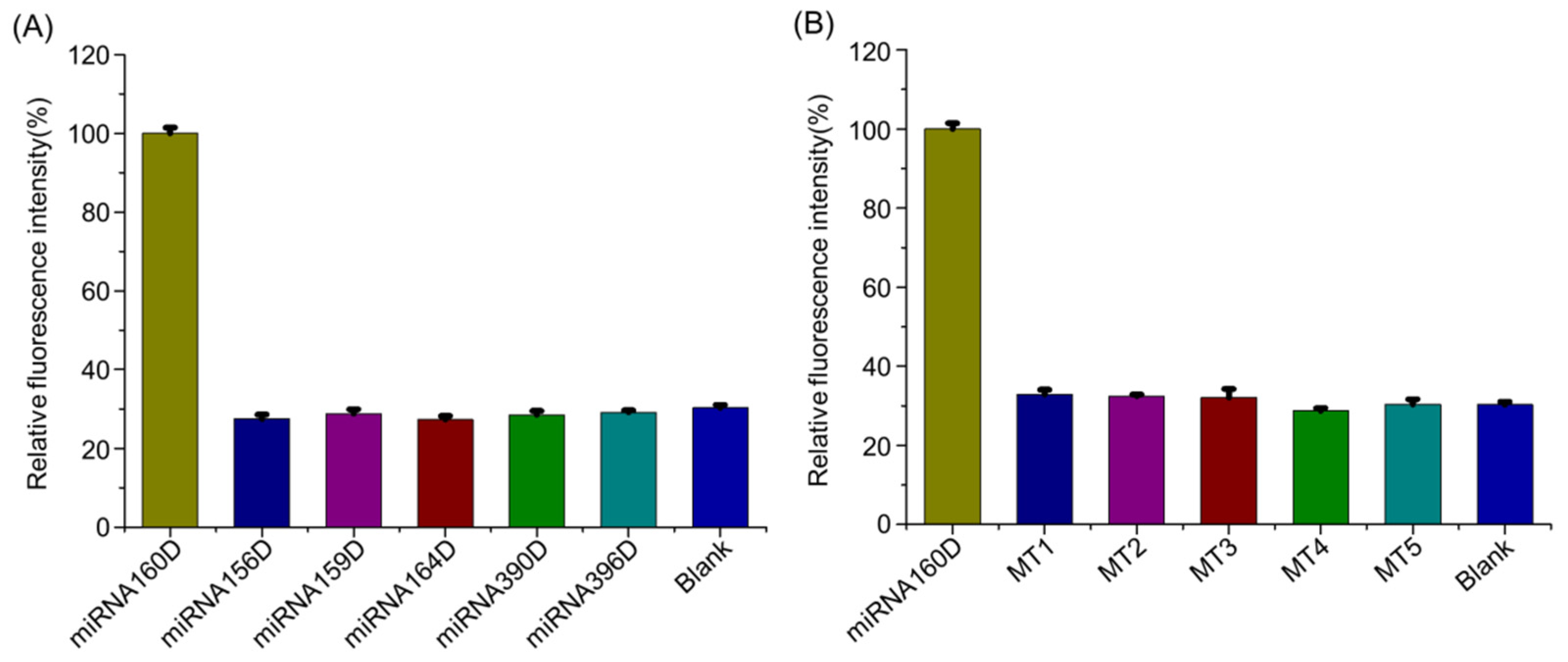
2.5. Detection Specificity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of AMM System
4.3. RNA Extracted from Peach
4.4. Fluorescence Measurement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sun, G. MicroRNAs and their diverse functions in plants. Plant Mol. Biol. 2012, 80, 17–36. [Google Scholar] [CrossRef]
- Li, M.; Yu, B. Recent advances in the regulation of plant miRNA biogenesis. RNA Biol. 2021, 1–10. [Google Scholar] [CrossRef]
- Djami-Tchatchou, A.T.; Neeti, S.M.; Khayalethu, N.; Dubery, I.A. Functional Roles of microRNAs in Agronomically Important Plants—Potential as Targets for Crop Improvement and Protection. Front. Plant Sci. 2017, 8, 378. [Google Scholar] [CrossRef] [Green Version]
- Kar, M.M.; Raichaudhuri, A. Role of microRNAs in mediating biotic and abiotic stress in plants. Plant Gene 2021, 26, 100277. [Google Scholar] [CrossRef]
- Xu, S. Research Progress in Plant MiRNA Functions and the Roles in Stresses. Biotechnol. Bull. 2012, 4, 1–7. [Google Scholar]
- Pagano, L.; Rossi, R.; Paesano, L.; Marmiroli, N.; Marmiroli, M. miRNA regulation and stress adaptation in plants. Environ. Exp. Bot. 2021, 184, 104369. [Google Scholar] [CrossRef]
- Xin, L.; Dong, X.; Liu, Z.; Shi, Z.; Li, T. Repression of ARF10 by microRNA160 plays an important role in the mediation of leaf water loss. Plant Mol. Biol. 2016, 92, 313–336. [Google Scholar]
- Liu, P.P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Carrington, J.C. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef]
- Liu, N.; Wu, S.; Li, Z.; Khan, A.Q.; Tu, L. Repression of microRNA 160 results in retarded seed integument growth and smaller final seed size in cotton. Crop J. 2020, 8, 602–612. [Google Scholar] [CrossRef]
- Natarajan, B.; Kalsi, H.S.; Godbole, P.; Malankar, N.; Thiagarayaselvam, A.; Siddappa, S.; Thulasiram, H.V.; Chakrabarti, S.K.; Banerjee, A.K. MiRNA160 is associated with local defense and systemic acquired resistance against Phytophthora infestans infection in potato. J. Exp. Bot. 2018, 69, 2023–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcik, A.; Nodine, M.; Gaj, M. miR160 and miR166/165 Contribute to the LEC2-Mediated Auxin Response Involved in the Somatic Embryogenesis Induction in Arabidopsis. Front. Plant Sci. 2017, 8, 2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Wang, Y.; Teotia, S.; Wang, Z.; Shi, C.; Sun, H.; Gu, Y.; Zhang, Z.; Tang, G. The interaction between miR160 and miR165/166 in the control of leaf development and drought tolerance in Arabidopsis. Sci. Rep. 2019, 9, 2832. [Google Scholar] [CrossRef]
- Lin, J.S.; Chia-Chia, K.; Yang, I.C.; Wei-An, T.; Shen, Y.H.; Lin, C.; Liang, Y.C.; Li, Y.C.; Yun-Wei, K.; Yu-Chi, K. MicroRNA160 Modulates Plant Development and Heat Shock Protein Gene Expression to Mediate Heat Tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xue, J.S.; Yu, Y.H.; Liu, S.Q.; Yang, Z.N. Fine regulation of ARF17 for anther development and pollen formation. BMC Plant Biol. 2017, 17, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Dong, L.; Zhang, J.; Zhao, Y.; Li, Z. Recent advances in microRNA detection. Analyst 2018, 143, 1758–1774. [Google Scholar]
- Wee, E.J.H.; Trau, M. Simple Isothermal Strategy for Multiplexed, Rapid, Sensitive, and Accurate miRNA Detection. ACS Sens. 2016, 1, 670–675. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.S.; Wang, Z.; Le, J.; Zheng, T.; Jia, L. Autonomous assembly of ordered metastable DNA nanoarchitecture and in situ visualizing of intracellular microRNAs. Biomaterials 2017, 120, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, Y.; Zhao, W.; Wu, Z.; Wang, S.; Yu, R. Palindromic molecular beacon-based intramolecular strand-displacement amplification strategy for ultrasensitive detection of K-ras gene. Anal. Chim. Acta 2019, 1065, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.S.; Zhu, L.; Tan, D.A.; Qiu, X.Y.; Zhu, L.Y. Avenues toward microRNA detection: A review of technical advances and challenges. Comput. Struct. Biotechnol. J. 2019, 17, 904–916. [Google Scholar] [CrossRef]
- Wei, L.; Ruan, K. MicroRNA detection by microarray. Anal. Bioanal. Chem. 2009, 394, 1117–1124. [Google Scholar]
- Wang, X.; Tong, Y.; Wang, S. Rapid and Accurate Detection of Plant miRNAs by Liquid Northern Hybridization. Int. J. Mol. Sci. 2010, 11, 3138–3148. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.Z.; Kiselev, E.; Lountos, G.T.; Wang, W.; Tropea, J.E.; Needle, D.; Hilimire, T.A.; Schneekloth, J.S., Jr.; Waugh, D.S.; Pommier, Y.; et al. Small molecule microarray identifies inhibitors of tyrosyl-DNA phosphodiesterase 1 that simultaneously access the catalytic pocket and two substrate binding sites. Chem. Sci. 2021, 12, 3876–3884. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Zhang, X.; Luo, S.; Li, L. Research advances for exosomal miRNAs detection in biosensing: From the massive study to the individual study. Biosens. Bioelectron. 2020, 177, 112962. [Google Scholar] [CrossRef] [PubMed]
- Jebelli, A.; Oroojalian, F.; Fathi, F.; Mokhtarzadeh, A.; Guardia, M. Recent advances in surface plasmon resonance biosensors for microRNAs detection. Biosens. Bioelectron. 2020, 169, 112599. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.P.; Ngo, T.A.; Pernestig, A.K.; Tilevik, D.; Kant, K.; Nguyen, T.; Wolff, A.; Dang, D.B. MicroRNA amplification and detection technologies: Opportunities and challenges for point of care diagnostics. Lab. Investig. 2019, 99, 452–469. [Google Scholar] [CrossRef]
- Tian, T.; Wang, J.; Xiang, Z. A review: MicroRNA detection methods. Org. Biomol. Chem. 2015, 13, 2226–2238. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.D.; Carletti, M.Z.; Christenson, L.K. Quantitative RT-PCR methods for mature microRNA expression analysis. Methods Mol. Biol. 2010, 630, 49–64. [Google Scholar] [PubMed]
- Ren, K.; Liu, Y.; Wu, J.; Zhang, Y.; Zhu, J.; Yang, M.; Ju, H. A DNA dual lock-and-key strategy for cell-subtype-specific siRNA delivery. Nat. Commun. 2016, 7, 13580. [Google Scholar] [CrossRef] [PubMed]
- Bossert, N.; de Bruin, D.; Götz, M.; Bouwmeester, D.; Heinrich, D. Fluorescence-tunable Ag-DNA biosensor with tailored cytotoxicity for live-cell applications. Sci. Rep. 2016, 6, 37897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Luo, M.; Wang, J.; Niu, H.; Shen, Z.; Wu, Z.S. Rigidified DNA Triangle-Protected Molecular Beacon from Endogenous Nuclease Digestion for Monitoring microRNA Expression in Living Cells. ACS Sens. 2020, 5, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhang, S.; Yu, X.; Hu, S.; Lu, Y.; Wu, Z.S. Periodically Ordered, Nuclease-Resistant DNA Nanowires Decorated with Cell-Specific Aptamers as Selective Theranostic Agents. Angew. Chem. Int. Ed. 2020, 59, 17540–17547. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qiu, X.; Hildebrandt, N. When Nanoworlds Collide: Implementing DNA Amplification, Nanoparticles, Molecules, and FRET into a Single MicroRNA Biosensor. Nano Lett. 2021, 21, 4802–4808. [Google Scholar] [CrossRef]
- Soomro, S.; Venkateswaran, S.; Vanarsa, K.; Kharboutli, M.; Nidhi, M.; Susarla, R.; Zhang, T.; Sasidharan, P.; Lee, K.H.; Rosh, J.; et al. Predicting disease course in ulcerative colitis using stool proteins identified through an aptamer-based screen. Nat. Commun. 2021, 12, 3989. [Google Scholar] [CrossRef]
- Xu, W.; Tian, J.; Luo, Y.; Zhu, L.; Huang, K. A rapid and visual turn-off sensor for detecting copper (II) ion based on DNAzyme coupled with HCR-based HRP concatemers. Sci. Rep. 2017, 7, 43362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Zhang, S.; Zhao, H.; Niu, H.; Wu, Z.S.; Chang, H.T. Branched DNA Junction-Enhanced Isothermal Circular Strand Displacement Polymerization for Intracellular Imaging of MicroRNAs. Anal. Chem. 2018, 90, 13891–13899. [Google Scholar] [CrossRef]
- Shen, Z.F.; Li, F.; Jiang, Y.F.; Chen, C.; Xu, H.; Li, C.C.; Yang, Z.; Wu, Z.S. Palindromic Molecule Beacon-Based Cascade Amplification for Colorimetric Detection of Cancer Genes. Anal. Chem. 2018, 90, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, H.; Wang, Z.Y.; Wu, Z.S.; Yang, Z.; Li, C.C.; Xu, H.; Lyu, J.X.; Shen, Z.F. Single palindromic molecular beacon-based amplification for genetic analysis of cancers. Biosens. Bioelectron. 2017, 91, 692–698. [Google Scholar] [CrossRef]
- Deng, R.; Tang, L.; Tian, Q.; Wang, Y.; Lin, L.; Li, J. Toehold-initiated rolling circle amplification for visualizing individual microRNAs in situ in single cells. Angew. Chem. Int. Ed. 2014, 53, 2389–2393. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Lv, M.; Li, J.; Yu, R.; Jiang, J. A ligation-based loop-mediated isothermal amplification (ligation-LAMP) strategy for highly selective microRNA detection. Chem. Commun. 2016, 52, 12721–12724. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Ahn, J.K.; Lee, C.Y.; Park, H.G. A hairpin probe-mediated isothermal amplification method to detect target nucleic acid. Anal. Chim. Acta 2020, 1114, 7–14. [Google Scholar] [CrossRef]
- Liao, R.; He, K.; Chen, C.; Cai, C.; Chen, X. Double-strand Displacement Biosensor and Quencher-free Fluorescence Strategy for Rapid Detection of microRNA. Anal. Chem. 2016, 88, 4254–4258. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, Z.; Deng, Z.; Wang, Y.; Guo, Y. An enzyme free electrochemical biosensor for sensitive detection of miRNA with a high discrimination factor by coupling the strand displacement reaction and catalytic hairpin assembly recycling. Analyst 2017, 142, 4116–4123. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, J.; Jiang, X.; Gan, H.; Wang, L. SPR/SERS dual-mode plasmonic biosensor via catalytic hairpin assembly-induced AuNP network. Biosens. Bioelectron. 2021, 190, 113376. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Wang, J.; Guo, J.; Gao, H.; Peng, M. A plasmonic colorimetric strategy for visual miRNA detection based on hybridization chain reaction. Sci. Rep. 2016, 6, 32219. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Guo, M.M.; Tang, H.; Wu, Z.; Tang, L.J.; Yu, R.Q.; Jiang, J.H. Nucleic acid amplification-based methods for microRNA detection. Anal. Methods 2015, 7, 2258–2263. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Xie, H.; Zhao, L.; Zheng, L.; Ye, H. Applications of Catalytic Hairpin Assembly Reaction in Biosensing. Small 2019, 15, e1902989. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Xiao, S.; Ouyang, C.H.; Li, C.C.; Gao, Z.H.; Shen, Z.F.; Wu, Z.S. Inverted mirror image molecular beacon-based three concatenated logic gates to detect p53 tumor suppressor gene. Anal. Chim. Acta 2019, 1051, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Ellington, A.D. Diagnostic Applications Applications of Nucleic Acid Circuits. Acc. Chem. Res. 2014, 47, 1825–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.P.; Ma, P.Q.; Liu, H.; Guo, X.G.; Yin, B.C.; Ye, B.C. Rational Engineering of a Dynamic, Entropy-Driven DNA Nanomachine for Intracellular MicroRNA Imaging. Angew. Chem. Int. Ed. 2017, 56, 9077–9081. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhang, S.; Li, C.; Yu, X.; Ouyang, C.; Lu, Y.; Wu, Z.S. Y-Shaped Backbone-Rigidified Triangular DNA Scaffold-Directed Stepwise Movement of a DNAzyme Walker for Sensitive MicroRNA Imaging within Living Cells. Anal. Chem. 2019, 91, 15678–15685. [Google Scholar] [CrossRef]
- Li, D.; Cheng, W.; Li, Y.; Xu, Y.J.; Li, X.; Yin, Y.; Ju, H.; Ding, S. Catalytic Hairpin Assembly Actuated DNA Nanotweezer for Logic Gate Building and Sensitive Enzyme-Free Biosensing of MicroRNAs. Anal. Chem. 2016, 88, 7500–7506. [Google Scholar] [CrossRef] [PubMed]
- Tafese, T. Enzyme-free signal amplified Au nanoparticle fluorescence detection of thrombin via target-triggered catalytic hairpin assembly. Microchem. J. 2020, 160, 105649. [Google Scholar]
- Wang, J.; Sun, Y.; Lau, C.; Lu, J. Target-fueled catalytic hairpin assembly for sensitive and multiplex microRNA detection. Anal. Bioanal. Chem. 2020, 412, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.N.; Wu, Q.; Zhou, Y.J.; Gong, X.; Liu, X.Q.; Wang, F. A DNAzyme-powered cross-catalytic circuit for amplified intracellular imaging. Chem. Commun. 2019, 55, 6519–6522. [Google Scholar] [CrossRef]
- Park, C.; Park, H.; Lee, H.J.; Lee, H.S.; Park, K.H.; Choi, C.H.; Na, S. Double amplified colorimetric detection of DNA using gold nanoparticles, enzymes and a catalytic hairpin assembly. Microchim. Acta. 2018, 186, 34. [Google Scholar] [CrossRef]
- Chen, R.P.; Blackstock, D.; Sun, Q.; Chen, W. Dynamic protein assembly by programmable DNA strand displacement. Nat. Chem. 2018, 10, 474–481. [Google Scholar] [CrossRef]
- Li, J.B.; Lei, P.H.; Ding, S.J.; Zhang, Y.; Yang, J.R.; Cheng, Q.; Yan, Y.R. An enzyme-free surface plasmon resonance biosensor for real-time detecting microRNA based on allosteric effect of mismatched catalytic hairpin assembly. Biosens. Bioelectron. 2016, 77, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.N.; Feng, Z.; Zhao, Y.N.; Jia, L.P.; Ma, R.N.; Zhang, W.; Shang, L.; Xue, Q.W.; Wang, H.S. A sensitive electrochemical aptasensor for Mucin 1 detection based on catalytic hairpin assembly coupled with PtPdNPs peroxidase-like activity. Talanta 2019, 200, 503–510. [Google Scholar] [CrossRef]
- Ouyang, W.; Liu, Z.; Zhang, G.; Chen, Z.; Guo, L.; Lin, Z.; Qiu, B.; Chen, G. Enzyme-free fluorescent biosensor for miRNA-21 detection based on MnO2 nanosheets and catalytic hairpin assembly amplification. Anal. Methods 2016, 8, 8492–8497. [Google Scholar] [CrossRef]
- Dai, J.Y.; He, H.F.; Duan, Z.J.; Zhou, C.S.; Long, Y.Y.; Zheng, B.Z.; Du, J.; Guo, Y.; Xiao, D. Target-triggered autonomous assembly of DNA polymer chains and its application in colorimetric nucleic acid detection. J. Mater. Chem. B 2016, 4, 3191–3194. [Google Scholar] [CrossRef]
- Wu, J.; Tian, Y.H.; He, L.; Zhang, J.; Huang, Z.J.; Luo, Z.W.; Duan, Y.X. An efficient localized catalytic hairpin assembly-based DNA nanomachine for miRNA-21 imaging in living cells dagger. Analyst 2021, 146, 3041–3051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Wang, K.Y.; Bu, S.J.; Li, Z.Y.; Ju, C.J.; Wan, J.Y. Colorimetric detection of microRNA based on DNAzyme and nuclease-assisted catalytic hairpin assembly signal amplification. Mol. Cell. Probes 2018, 38, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Li, N.X.; Du, M.Y.; Liu, Y.C.; Ji, X.H.; He, Z.K. Multipedal DNA Walker Biosensors Based on Catalyzed Hairpin Assembly and Isothermal Strand-Displacement Polymerase Reaction for the Chemiluminescent Detection of Proteins. ACS Sens. 2018, 3, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Dai, X.; Feng, Y.; Zhao, Q.; Liu, L.; Xue, C.; Xiao, L.; Wang, R. Dual Catalytic Hairpin Assembly-Based Automatic Molecule Machine for Amplified Detection of Auxin Response Factor-Targeted MicroRNA-160. Molecules 2021, 26, 6432. https://doi.org/10.3390/molecules26216432
Wang L, Dai X, Feng Y, Zhao Q, Liu L, Xue C, Xiao L, Wang R. Dual Catalytic Hairpin Assembly-Based Automatic Molecule Machine for Amplified Detection of Auxin Response Factor-Targeted MicroRNA-160. Molecules. 2021; 26(21):6432. https://doi.org/10.3390/molecules26216432
Chicago/Turabian StyleWang, Lei, Xing Dai, Yujian Feng, Qiyang Zhao, Lin Liu, Chang Xue, Langtao Xiao, and Ruozhong Wang. 2021. "Dual Catalytic Hairpin Assembly-Based Automatic Molecule Machine for Amplified Detection of Auxin Response Factor-Targeted MicroRNA-160" Molecules 26, no. 21: 6432. https://doi.org/10.3390/molecules26216432
APA StyleWang, L., Dai, X., Feng, Y., Zhao, Q., Liu, L., Xue, C., Xiao, L., & Wang, R. (2021). Dual Catalytic Hairpin Assembly-Based Automatic Molecule Machine for Amplified Detection of Auxin Response Factor-Targeted MicroRNA-160. Molecules, 26(21), 6432. https://doi.org/10.3390/molecules26216432





