Abstract
The discovery of environmentally friendly and inexpensive plant growth regulators (PGRs) for agronomically important crops is a necessity and must be considered a priority worldwide. This study provides the synthesis, structure determination and the biological evaluation of two binary organic salts as potential PGRs. New compounds have dual biological activity and are based on natural metabolite p-aminobenzoic acid (pABAH) and different alkanolamines. Studied compounds exhibit hydrogen-bonded 3D supramolecular architectures with different crystal packing due to the formation of one homosynthon and various heterosynthons. The biological profile of new compounds was investigated in laboratory and greenhouse on Solanum lycopersicum L., revealing the efficiency in promoting plant rooting and plant productivity. The results may have a positive impact on agricultural economics, developing new sustainable PGRs for tomatoes.
1. Introduction
Growing populations have imposed the use of plant growth regulators (PGRs), which have become an integral part of agricultural and horticultural practices, maximizing crop production. To mitigate the harmful effects of conventional synthetic PGRs, such as 2,4-dichlorophenoxyacetic acid-2,4-D, on the environment and plant health [1], extensive efforts have been devoted to discovering sustainable alternatives. Since natural compounds extracted from plants or produced by bacteria rapidly degrade, limiting the applicability of the product [2], the use of PGRs based on synthetic analogues of natural products or active pharmaceutical ingredients commercially available are considered to be more effective [3]. Therefore, developing innovative, environmentally friendly and cost competitive PGRs remains an important task for researchers and a necessity at the global level for nutrition security. Understanding of chemical and crystal structure, inter- and intramolecular interactions as well as the structure–biological activity relationship is essential for investigation of new PGRs. Extensive studies show the importance of the carboxyl group and the planar aromatic ring in the structure of auxin-like PGRs, essential molecules that control almost every aspect of dormancy, seed germination and plant development [4].
Thus, benzoic acids, well-known for their importance as building blocks in drug development, gained increasing attention in plant science, being involved in various physiological processes from the regulation of seed germination [5,6,7] to disease resistance and stress tolerance in plants [8,9]. Among these compounds, p-aminobenzoic acid (pABAH) is a well-known natural metabolite present in plant and animal tissues, widely described in literature as a precursor of folic acid [10] and recently of coenzyme Q [11]. It possesses numerous biological activities in medicine such as antioxidant [12], antibacterial [13], antimutagenic [14], anticoagulant [15], fibrinolytic and immunomodulating agent [16], protective drug against UV-irradiation [17] and also in agriculture as a chemical inducer associated with thermotolerance [18] and pathogens resistance [19] in plants. Moreover, an examination of the Cambridge Structural Database (CSD) has confirmed the pABAH versatility as a fundamental building block in the design of soluble forms of organic multicomponent crystals and coordination compounds with various supramolecular architectures [20,21]. Besides being frequently used as co-formers, such as aliphatic and heterocyclic amines, alkanolamines are very few despite their lower toxicity [22], and their use as intermediates for the production of active pharmaceutical and cosmetic ingredients.
Our previous studies show the huge potential of alkanolamine-substituted benzoic acid systems which can generate supramolecular architectures with different topologies (1D, 2D and 3D) guided by different non-covalent interactions [23,24,25], have low toxicity [25,26], thermal stability [27] and promising applicability as auxin-like PGRs on the model plant Arabidopsis thaliana Col 0 [6]. The aim of this work was to develop new alkanolammonium p-aminobenzoates with characteristics of ideal PGRs: low toxicity, easily synthesized in laboratories, non-expensive, soluble in water and to investigate their role in growth and development of the most important vegetable cultivated in Romania and European Union in the last years, Solanum lycopersicum L. The discovery of new PGRs for commercial vegetables is essential, because vegetables play a vital role in food front, being the cheapest natural sources. Herein, two new alkanolammonium p-aminobenzoates were synthesized, structurally and physicochemically characterized and evaluated for their PGRs’ activity on tomatoes (S. lycopersicum L).
2. Results and Discussion
The new compounds were prepared by proton exchange reaction of pABAH with different alkanolamines (HEEA-pABA—ethylethanolammonium p-aminobenzoate; HDEEA-pABA—diethylethanolammonium p-aminobenzoate) and isolated in excellent yields (>95%). The pKa rule [28] predicted the salts formation, because ΔpKa values pKa(alkanolamine)-pKa(pABAH) were greater than three. Physicochemical and structural characterizations of new compounds are presented below.
2.1. Crystallographic Study
The single crystal X-ray diffraction data indicated that HEEA-pABA crystallizes in the monoclinic centrosymmetric P21/c space group, while HDEEA-pABA crystallizes in the orthorhombic asymmetric Pna21 space group (Table 1).

Table 1.
Crystallographic data and structure refinement details for new compounds.
The analysis of both crystal structures revealed the formation of 1:1 organic salts HEEA-pABA and HDEEA-pABA with proton transfer from carboxylic group of pABAH to nitrogen atoms of alkanolamine molecules (Figure 1). The (pABA)‒ anion formed non-planar system in HEEA-pABA and a practically planar system in HDEEA-pABA, since the dihedral angle between the least squares plane of the phenyl ring C6 and the COO‒ group was equal to 20.1° and 5.6°, respectively.
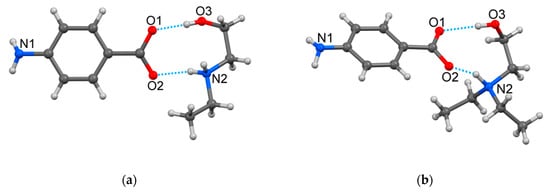
Figure 1.
View of the HEEA-pABA (a) and the HDEEA-pABA (b) with partial atomic labelling and charge-assisted hydrogen bonds.
The nitrogen atoms in the amino group in both structures had almost a pyramidal configuration, its valence angles being equal to 108.07, 109.00 and 111.22° in (HEEA)+ of HEEA-pABA, and 108.80, 109.83 and 110.01° in (HDEEA)+ of HDEEA-pABA. The (HEEA)+ and (HDEEA)+ cations adopt the Syn-Clinal conformation with the N(2)CCO(3) torsion angles equal to −66.90 and −66.65°, respectively. The alkanolamine cations and (pABA)‒ anions were held together by two charge-assisted O‒H∙∙∙O‒ and N+‒H∙∙∙O hydrogen bonds in HEEA-pABA as distances C‒O(1) and C‒O(2) are equal to 1.270(2) and 1.254(3) Å and one charge-assisted N+‒H∙∙∙O‒ and one classic O‒H∙∙∙O hydrogen bonds in HDEEA-pABA with C‒O(1) and C‒O(2) distances equal 1.254(8) and 1.276(8) Å. As a result, in both compounds, a similar R22(9) graph set was formed. In both structures, the NH2 group of (pABA)‒ anion participated to charge-assisted N‒H∙∙∙O‒ and classic N‒H∙∙∙O hydrogen bonds with carboxylic oxygen atoms of adjacent pABA anions (Table 2).

Table 2.
Hydrogen bond distances (Å) and angles (°) in HEEA-pABA and HDEEA-pABA.
Cations and anions in the HEEA-pABA structure were held together in a supramolecular network (Figure 2a) obtained from R22(9) heterosynthons (Figure 2b) and R44(22) homosynthons formed by four anions (Figure 2c) with the involvement of fine C‒H∙∙∙O hydrogen bond between amine and carboxylic groups (Table 2), in addition to the mentioned N(O)‒H∙∙∙O hydrogen interaction.
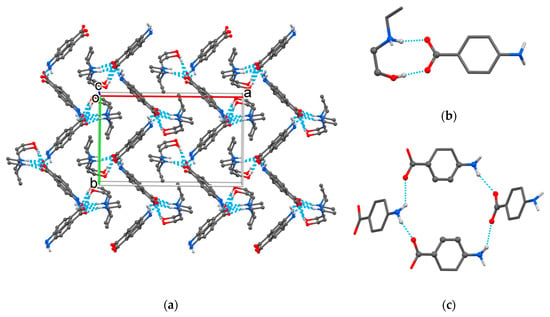
Figure 2.
The crystal packing of HEEA-pABA (a) with representation of R22(9) heterosynthon (b) and R44(22) homosynthon in crystal (c).
The crystal packing of HDEEA-pABA (Figure 3a) was based on seven heterosynthons between HDEEA cations and pABA anions: bicomponent R22(9) heterosynthon discussed above, three tetracomponent heterosynthons formed by two cations and two anions (Figure 3b–d) and three hexacomponent heterosynthons assembled by two cations and four anions (Figure 3e–g). The crystalline structure was additionally stabilized by fine C‒H∙∙∙O hydrogen bonds with the involvement of C(8) and C(13) as donors of H (Table 2).
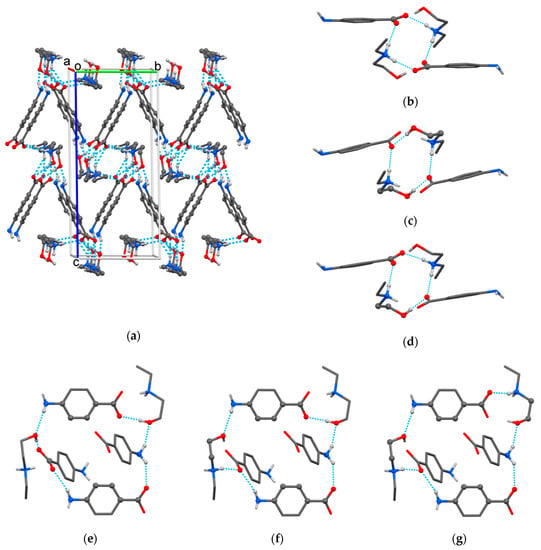
Figure 3.
The crystal packing (a) and multicomponent heterosynthons found in HDEEA-pABA crystal: R44(12) (b), R24(14) (c), R34(13) (d), R66(26) (e), R56(27) (f) and R56(30) (g).
2.2. FTIR Spectroscopy
The FTIR spectroscopic investigations (Figure 4) provide a supplementary insight into alkanolammonium salts formation by exhibiting the differences in the intensities and wavenumbers of C=O, N‒H and O‒H stretching modes in new compounds compared to the corresponding acid.
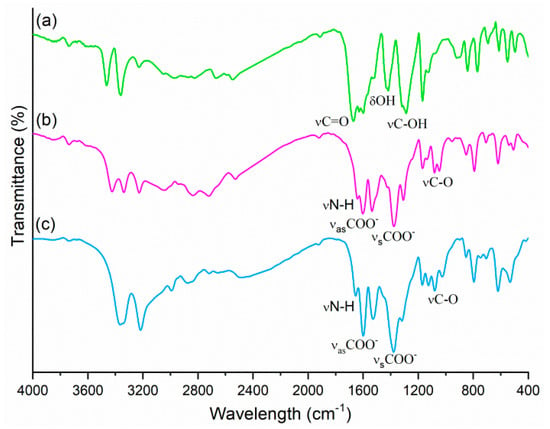
Figure 4.
FTIR spectra of pABAH (a), HEEA-pABA (b) and HDEEA-pABA (c).
The HEEA-pABA and HDEEA-pABA spectra had certain similarities: the presence of strong bands responsible for the symmetric (1378 cm‒1; 1381 cm‒1) and asymmetric (1602 cm‒1; 1599 cm‒1) stretching vibrations of carboxylate group which do not exist in the spectra of pABAH, as well as the absence of νC=O (1671 cm‒1), νC‒OH (1287 cm‒1) and δOH (1417 cm‒1) bands characteristic of the carboxylic group [29]. The lower values for symmetric and asymmetric νCOO‒ stretching vibrations compared to the free acid indicate that carbonyl was H-bonded in the anion–cation and anion–anion systems. Furthermore, the absence of bands characteristic of the –COOH group confirmed the formation of HEEA-pABA and HDEEA-pABA salts.
The strong and narrow absorption bands (3464 cm‒1 and 3360 cm‒1) assigned for asymmetric and symmetric NH2 stretching vibrations in pABAH spectrum decreased in intensity, and wavenumbers in the case of two alkanolammonium salts, becoming 3425 cm‒1 and 3341 cm‒1 for HEEA-pABA and 3367 cm‒1 for HDEEA-pABA. These indicate N‒H∙∙∙O‒ and N‒H∙∙∙O intermolecular interactions between anion–anion in HEEA-pABA and anion–anion/anion–cation in HDEEA-pABA. The broad bands in the range 3200–2700 cm‒1 are attributed to O‒H stretching vibrations and overlap with NH2+ vibration band (3043 cm‒1) in the case of secondary alkanolamine. Weak bands of N‒H deformation vibrations were observed at 1640 cm‒1 and 1655 cm‒1, respectively, in salt spectra. Supplementary proof of the salt formation was confirmed by the appearance in HEEA-pABA and HDEEA-pABA spectra of C‒O stretching vibrations at 1100–1000 cm−1, belonging to alkanolamines. The differences between two new alkanolammonium salts spectra also illustrate the diversity of the synthons in crystals.
2.3. Thermal Analysis
The salts were characterized by thermal analysis (thermogravimetric analysis—TGA; derivative thermogravimetry—DTG; and heat flow—HF) in order to determine thermal stability (Figure 5). The TGA and DTG curves show that HEEA-pABA and HDEEA-pABA are thermally stable up to 124 °C and 90 °C, respectively when they begin to decompose into two steps with a total mass loss of over 99.4%.
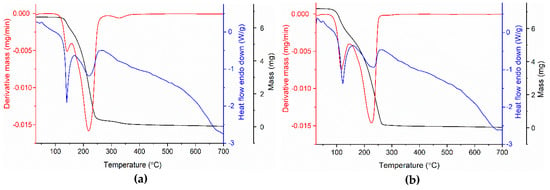
Figure 5.
Thermo-analytical curves of HEEA-pABA (a) and HDEEA-pABA (b).
Therefore, HEEA-pABA had a first mass loss of 13.1% in the range 124.77 °C–160.15 °C, probably due to the removal of the ethyl group from the cation. This decomposition process was accompanied by melting at 138.52 °C. The second step up to 700 °C corresponded to 81.68% mass loss and complete decomposition of the compound. In the case of HDEEA-pABA, the decomposition process, up to 700 °C, began with a 22.59% mass loss due to the elimination of diethyl groups from the cation and continued with the second step which involved a final 76.25% mass loss ascribed to the C9H12N2O3 molecule. The DTG curve accompanied the decomposition process by one sharp peak at 115.87 °C and another broad peak at 226.13 °C. HF curve showed a first strong and sharp endothermic peak at 120.85 °C, attributed to the melting point of the compound. Thermogravimetric studies indicate that the studied compounds were anhydrous and HEEA-pABA was more thermally stable than HDEEA-pABA.
2.4. Biological Studies
The auxin-like PGRs’ effect on new compounds was investigated in laboratory and greenhouse conditions, on tomato, the main commercial vegetable cultivated in Romania and Europe in the last years. Commercial indole-3-acetic acid (IAA), the most abundant and studied natural auxin in plants, was used as the reference compound. The results of experiment 1 (Table 3) showed that new compounds (HEEA-pABA, HDEEA-pABA) and IAA inhibited germination at all tested concentrations or even blocking it (e.g., IAA, at concentrations above 0.5 mM) especially in the first interval of treatment (after 7 days), compared with the control. The results obtained show that increasing the concentration leads to inhibition of germination. Thus, in the case of HEEA-pABA application, increasing concentration from 0.1 mM to 0.5 mM and then to 1 mM, reduced significantly seed germination by about 20% and 9%, respectively. Instead, HDEEA-pABA decreased seed germination by approximately 24.5% only from 0.5 mM to 1 mM.

Table 3.
The influence of treatments on tomato seed germination (tomato cultivar (cv.) Tomtim).
A tendency towards uniformization of values was revealed ten days after the induction of germination. An increase of germination with approximately 22% was observed only at the concentration of 0.1 mM, for HDEEA-pABA treatment compared to IAA. In the case of 0.5 and 1 mM concentrations, HEEA-pABA and HDEEA-pABA treatments had similar effects; the increase of the concentration did not significantly influence seed germination. Analysis of the effects of the three treatments at the same concentration level shows that the treatments with HEEA-pABA and HDEEA-pABA had significantly higher effects than IAA on tomato seed germination.
Recent studies have characterized auxins as regulators that act positively by maintaining dormancy [30] and negatively by inhibiting seed germination [31]. The hormonal signalling of natural auxin IAA has often been associated with physiological processes manifested during plant growth and development, including seed germination [32]. Some previous studies have shown that exogenous application of IAA inhibits and delays seed germination [33,34]. The mechanism underlying germination blockade is not yet fully elucidated, but it seems to be due to the fact that auxin induces seminal break by enhancing signal transduction of abscisic acid, which associates auxin with maintaining seminal dormancy [30].
Therefore, the results of the tested compounds on tomato seeds germination confirm that IAA shows clear germination blocking effects, and the new compounds, even if they belong to the same category of compounds, have inhibitory effects only in the first part of the interval, the differences from the control being insignificant after a 10 day treatment.
Otherwise there are some studies that prove that besides phytohormones, there are chemical compounds that have the ability to regulate plant growth and development, such as nitrogen oxide and ROS (superoxide; hydrogen peroxide; hydroxyl radical; hydroxyl ion; and nitric oxide), both types being proven to regulate seed dormancy and germination [35,36,37,38].
Regarding experiment 2 (Table 4), the chlorophyll content of tomato seedlings was influenced by HEEA-pABA and HDEEA-pABA treatments, showing a significant increase compared to the control and IAA. The effect of new treatments on morphological characters in tomato seedlings indicated that plant height and primary root length presented variations comparatively with control and IAA, while maximum length of secondary roots had similar values to control and significantly smaller effect to IAA. The number of secondary roots (SR) registered the highest values under the effect of IAA treatment compared to the other treatments. The HEEA and HDEEA generated a significant increase (more than 86% of the number of SR) compared to the control.

Table 4.
The influence of treatments on tomato seedlings (cv. Tomtim).
It is known that auxin plays an important role in controlling the roots growth and plant development. The total amount of this major phytohormone is generated by the local biosynthesis and transport. The natural auxin IAA reveals an inhibitory effect on primary root (PR) growth. SR are essential components of the root system which contribute to maximizing the absorption capacity of water and nutrients, facilitating rapid adaptation to environmental changes [39,40]. It is well known that IAA is involved in each stage of SR′s formation, the disorders in auxin biosynthesis and transport resulting in a reduced number of SR [41,42,43,44,45].
Therefore, our results attest that HEEA-pABA and HDEEA-pABA treatment solutions stimulate the plants height and the length growth of PR in contrast to IAA, which has inhibitory effects. Also, new compounds promote the formation of a large number of SR and support their growth, compared to control. Contrary, exogenous IAA generates the formation of a large number of SR (104.5 ± 18.42), probably due to the ability of acropetal migration within the root [46], but without ensuring their length growth. These results can also be correlated with the higher capacity of chlorophyll biosynthesis in variants treated with 0.5mM HEEA-pABA and HDEEA-pABA exogenous solutions.
The dynamics of the plants height (Table 5) shows that the foliar treatments with HDEEA-pABA and IAA have a similar and significantly superior influences to the treatments with HEEA-pABA and control, in all the stages of development, from the beginning of flowering until the first stage of fruit emergence.

Table 5.
The treatments effect on height (cm) of tomato plants (cv. Tomtim) in different stages of development.
The three treatments and control did not differ significantly in their effect on the number of leaves at the beginning of flowering (Table 6). Significantly higher number of leaves compared to control has been stimulated by IAA during the flowering period and by HDEEA-pABA treatment at the emergence of first fruiting floor of tomato plants. All treatments presented a significant increase in the number of leaves from the beginning of flowering to full flowering, with differences between 3.33 in HEEA-pABA and 5.33 in HDEEA-pABA. In the period from flowering to the emergence of first fruiting floor, the number of leaves per plant showed little and no significant variations in all cases.

Table 6.
The influence of treatments on leaves number of tomato plants (cv. Tomtim) in different stages of development.
It is known that auxins can stimulate plant growth by enhancing photosynthesis due to the fact of chlorophyll increases [47]. The chlorophyll pigments are probably the most relevant natural biomolecules due to the fact of their importance in photosynthesis. Therefore, a direct correlation between their quantity and gross primary productivity has been demonstrated [48]. Our results present a significant increase in chlorophyll content induced by HDEEA-pABA (41.43 ± 0.69) and a significantly lower one for HEEA-pABA (37.07 ± 1.74) compared to IAA treatment and control, at the beginning of flowering (Table 7). In the flowering and emergence of first fruiting floor periods, the highest chlorophyll content was determined in the tomato plants treated with HDEEA-pABA and IAA, respectively, while in the variants HEEA-pABA and control, the chlorophyll content was lower. Control plants recorded a significant increase of chlorophyll content from the beginning of flowering to the emergence of first fruiting floor, in contrast to treated plants, which showed a notable increase only between the first two phenophases.

Table 7.
Effect of treatments on chlorophyll content (SPAD) of tomato plants’ foliar apparatus (cv. Tomtim) in different stages of development.
These results support the hypothesis that the initiation of the flowering and fruiting processes, which means the passage of plants from the vegetative to the generative stage, diminishes or stagnates the dynamics of chlorophyll biosynthesis, the plant concentrating the metabolic activity towards the biosynthesis of photosynthetic compounds such as carbohydrates. Therefore, the cellular metabolism was modified by reducing the biosynthesis of functional molecules, with nitrogen, and stimulating the synthesis of structural biomolecules, with carbon [49].
There are studies which show that foliar application of auxinic compounds, at different concentrations, induced increases in plant height, fresh and dried mass, number of shoots and leaves per plant, as well as productivity-related components [50,51]. Other research, on the contrary, concluded that the application of low or moderate doses of exogenous auxins did not generate significant changes on the plant growth parameters and the high doses even had effects of reducing the values of these parameters compared to the untreated variants [52,53,54,55].
The foliar apparatus, or the number of leaves per plant, is the essential component of the photosynthetic process. The leaves are considered the primary photosynthetic organs, and their number, along with their surface, determines the amplitude of the assimilation process. Previous studies have shown that phytohormones, especially auxin (IAA) and gibberellins (GA), play a key role in the emergence and development of foliar apparatus [56]. The results prove the stimulatory effect of foliar auxin application in tomatoes plant growth, the best results being obtained by using HDEEA-pABA and IAA, respectively, at a 0.5 mM concentration. Plants with a large number of leaves show an increased photosynthetic potential, which allows the synthesis, transport, and accumulation of larger amounts of valuable bioactive compounds [57,58].
3. Materials and Methods
3.1. Materials and Physical Measurements
Reagents pABAH, EEA (ethylethanolamine) and DEEA (diethylethanolamine) were purchased from Fluka Chemie AG (Buchs SG, Switzerland)) in analytical purity and were used without further purification. Melting points of recrystallized compounds were determined on a Boetius melting point apparatus and were uncorrected. The FTIR spectra (KBr pellet) were recorded on a JASCO-FTIR-4200 spectrometer (Easton, MD, USA), in the range 4000–400 cm−1 with a resolution of 4.0 cm−1 and a scanning speed of 16 mm s−1. Thermal analysis was performed using a TGA/SDTA 851-LF 1100 Mettler apparatus (Columbus, OH, USA) The sample weight of about 7 mg was used for the test. The measurements were carried out in a dynamic air atmosphere, in the temperature range of 25–700 °C with a heating rate of 10 °C min−1.
3.2. Synthesis Procedure of HEEA-pABA and HDEEA-pABA
New compounds were prepared in a 1:1 molar ratio, adding dropwise EEA (0.71 mL, 7.28 mmol) and DEEA (0.97 mL, 7.3 mmol) respectively into a solution of pABA (1 g, 7.29 mmol) in acetone (20 mL), under constant stirring at room temperature. White microcrystalline precipitates in high yields (>95 %) were formed after the addition of alkanolamines. The compounds were collected by filtration, washed with acetone, and dried in air. Colourless crystals suitable for single-crystal X-ray diffraction analysis were obtained after few days by slow evaporation in acetone. Melting points (Boetius) were: HEEA-pABA m.p. = 140–141 °C and HDEEA-pABA m.p. = 122–123 °C.
3.3. Single Crystal X-ray Diffraction Study
Diffraction measurements for compounds HEEA-pABA and HDEEA-pABA were carried out at room temperature on an Xcalibur E diffractometer (Abingdon, Oxfordshire, United Kingdom) equipped with a CCD area detector and a graphite monochromator utilizing MoKα radiation. Final unit cell dimensions were obtained and refined on an entire data set. All calculations to solve the structures and to refine the proposed models were carried out with the SHELXL2014 program package [59]. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms attached to carbon, nitrogen and oxygen atoms were positioned geometrically and treated as riding atoms. The X–ray data and the details of the refinement for both compounds are summarized in Table 1, and selected geometric parameters are given in Table 2. Figure 1, Figure 2 and Figure 3 were produced using the Mercury program (Cambridge, United Kingdom) [60]. Crystallographic data of the new compounds reported herein were deposited with the Cambridge Crystallographic Data Centre (Cambridge, United Kingdom) and allocated under the deposition numbers CCDC 1989303–1989304 (Supplementary Materials).
3.4. Biological Assays
Seeds of tomato cultivar named Tomtim created by BUASVM Timisoara, included in the CPVO (Community Plant Variety Office) test protocols, UPOV (The International Union for the Protection of New Varieties of Plants) and National Tests Guidelines were used in this study.
3.4.1. Germination Tests (Experiment 1)
Tomatoes’ mature seeds were surface sterilized by keeping them in a commercial 50% (v/v) sodium hypochlorite solution for 10 min and rinsed three times with distilled water before transferring them to Petri dishes. Twenty-five sterilized seeds were each placed into a 9 cm Petri dish containing two Whatman filter papers moistened with 5 mL solution of MES (2-(N-morpholino)ethanesulfonic acid) 5 mM and CaSO4 0.5 mM (pH 6) for control and different concentrations (0.1, 0.5 and 1 mM) of HEEA-pABA, HDEEA-pABA or IAA solutions for treated samples. Each experiment was repeated three times. Petri dishes of all variants (control and treated) were placed in a growth chamber at 25 ± 2 °C, 70%–75% relative humidity, and 16/8 light/dark conditions. The seeds with emerged radicle were counted at 7 and 10 days and percentage were calculated.
3.4.2. Seedling Growth Tests (Experiment 2)
Germinated seeds of each control and 0.5 mM treated samples were transplanted into pots (200 mL) with peat. Two germinated seeds/pot were placed in 15 replications for each experimental variant as follows: control watered with distilled water and other variants treated with 0.5 mM IAA, HEEA-pABA and HDEEA-pABA solutions. The treatments were performed by applying the solutions in pots, at roots, 25 mL at intervals of 7 days. The determinations were made after 3 applications, after 21 days of growing plants in the greenhouse (25/18 °C, day/night; 65% rh, and 12/12 light/dark conditions). Parameters included seedling height (cm), chlorophyll content (SPAD), main root length (cm), number and maximum length of secondary roots (cm) were determined.
3.4.3. Tests on Tomato Plants (Experiment 3)
After 56 days, 10 seedlings from each experimental variant were planted in the greenhouse, on a fertile soil substrate with fermented manure (5 kg/sqm). The same experimental variants were maintained as in the case of seedling tests, being carried out three treatments with foliar application, in doses correlated with the plant sizes of 8 mL/plant (the first treatment), 12 mL/plant (2nd) and 16 mL/plant (third) at 7 day intervals.
3.4.4. Data and Statistical Analysis
In this study, ten replicates of each moment of measurement were carried out for each sample, regarding the effect of different plant growth regulator substances and concentrations. All data were expressed as means ± standard error of the mean (SEM). Means were compared using least significant difference (LSD) test. The significant differences between the sample means (p < 0.05) were expressed by different letters.
4. Conclusions
Two new binary organic salts of pABA with different alkanolamines were successfully synthesized and characterized, and their auxin-like PGRs’ properties investigated in laboratory and greenhouse on Solanum lycopersicum L. by comparison with the most abundant and studied natural auxin in plants, IAA. The single crystal structures’ interpretation was correlated with the FTIR spectral data showing the intermolecular interactions established within the salts. Both new structures exhibited hydrogen-bonded supramolecular network architectures obtained from: (i) R22(9) heterosynthons and R44(22) homosynthons in HEEA-pABA; and (ii) R22(9), R44(12), R24(14) and R34(13), R66(26), R56(27) and R56(30) heterosynthons in HDEEA-pABA. The presence of R22(9) graph set in both structures indicates the relevance of charge-assisted N+‒H∙∙∙O‒ hydrogen bond in the formation of salts. The results obtained by investigating the biological profile of new alkanolammonium p-aminobenzoates as potential PGRs reveal the possibility of their use in stimulating the rooting and growth processes of tomato seedlings, as well as the positive involvement in plant growth and chlorophyll pigment biosynthesis. Considering the distinct supramolecular synthons in the design of molecular crystals and the promising biological results, this study sheds light on the alkanolammonium p-aminobenzoates as potential alternatives in the search of sustainable PGRs for tomatoes.
Supplementary Materials
Cifs for title compounds are available online.
Author Contributions
M.C. and M.P. carried out synthesis, crystallization and physicochemical characterization of new compounds; L.C. and P.B. performed the diffraction measurements and the structure refinement of single crystals; R.-L.S. and I.R. carried out the biological activity; R.-M.S. performed the statistical analysis. All authors discussed and approved the final manuscript.
Funding
This work was supported by project “Ensuring Excellence in RDI Activities within USAMVBT”, code 35PFE/2018, financed by the Ministry of Research and Innovation (MCI) through Program 1-Development of the national research and development system, Subprogram 1.2-Institutional Performance, Institutional Development Projects-Projects to Fund Excellence in RDI.
Acknowledgments
The authors thank for the support of the “Coriolan Drăgulescu” Institute of Chemistry - Program 2, Project 2.1 and of the Institute of Applied Physics - project ANCD 20.80009.5007.15.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, Y. Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J. Integr. Plant Biol. 2014, 56, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Small, C.C.; Degenhardt, D. Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G. Plant growth regulators I: Introduction; auxins, their analogues and inhibitors. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., Klerk, G.J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 175–204. [Google Scholar] [CrossRef]
- Paque, S.; Weijers, D. Q&A: Auxin: The plant molecule that influences almost anything. BMC Biol. 2016, 14, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.L.L.; Ferrarese, M.L.L.; Huber, D.A.; Ravagnani, A.L.S.; Ferrarese-Filho, O. Canola (Brassica napus L.) seed germination influenced by cinnamic and benzoic acids and derivatives: Effects on peroxidase. Seed Sci. Technol. 2003, 31, 39–46. [Google Scholar] [CrossRef]
- Crisan, M.E.; Bourosh, P.; Maffei, M.E.; Forni, A.; Pieraccini, S.; Sironi, M.; Chumakov, Y.M. Synthesis, crystal structure and biological activity of 2-hydroxyethylammonium salt of p-aminobenzoic acid. PLoS ONE 2014, 9, e101892. [Google Scholar] [CrossRef]
- Grozav, M.; Neamtiu, I.; Dorosencu, M.; Laichici, M.; Mercea, M. The synthesis of some ammonium salts of benzoic acids with etanolamine, possible plant growth stimulators. Rev. Chim. 2003, 54, 287–288. [Google Scholar]
- Dempsey, D.A.; Klessig, D.F. How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol. 2017, 15, 23–33. [Google Scholar] [CrossRef]
- Senaratna, T.; Merritt, D.; Dixon, K.; Bunn, E.; Touchell, D.; Sivasithamparam, K. Benzoic acid may act as the functional group in salicylic acid and derivatives in the induction of multiple stress tolerance in plants. Plant Growth Regul. 2003, 39, 77–81. [Google Scholar] [CrossRef]
- Basset, G.J.C.; Quinlivan, E.P.; Ravanel, S.; Rebeille, F.; Nichols, B.P.; Shinozaki, K.; Seki, M.; Adams-Phillips, L.C.; Giovannoni, J.J.; Gregory, J.F.; et al. Folate synthesis in plants: The p-aminobenzoate branch is initiated by a bifunctional PabA-PabB protein that is targeted to plastids. Proc. Natl. Acad. Sci. USA 2004, 101, 1496–1501. [Google Scholar] [CrossRef]
- Marbois, B.; Xie, L.X.; Choi, S.; Hirano, K.; Hyman, K.; Clarke, C.F. para-Aminobenzoic acid is a precursor in Coenzyme Q6 biosynthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2010, 285, 27827–27838. [Google Scholar] [CrossRef]
- Galbinur, T.; Obolensky, A.; Berenshtein, E.; Vinokur, V.; Chowers, I.; Chevion, M.; Banin, E. Effect of para-aminobenzoic acid on the course of retinal degeneration in the rd10 mouse. J. Ocul. Pharmacol. Ther. 2009, 25, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.M.R.; Xing, D.K.L.; King, T.P. Activity of p-aminobenzoic acid compared with other organic acids against selected bacteria. J. Appl. Bacteriol. 1995, 78, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Vasilieva, S. Para-aminobenzoic acid inhibits a set of SOS functions in Escherichia coli K12. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2001, 496, 89–95. [Google Scholar] [CrossRef]
- Drozd, N.N.; Makarov, V.A.; Miftakhova, N.T.; Kalugin, S.A.; Stroeva, O.G.; Akberova, S.I. Antithrombotic activity of para-aminobenzoic acid. Eksp. Klin. Farmakol. 2000, 63, 40–44. [Google Scholar] [PubMed]
- Andreenko, G.V.; Karabasova, M.A.; Liutova, L.A.; Podorolskaia, L.V.; Serebriakova, T.N.; Sologub, A.A.; Akberova, S.I.; Stroeva, O.G. Effect of para-aminobenzoic acid on the fibrinolytic activity of blood. Dokl. Akad. Nauk 1996, 346, 268–270. [Google Scholar] [PubMed]
- Hanson, K.M.; Gratton, E.; Bardeen, C.J. Sunscreen enhancement of UV-induced reactive oxygen species in the skin. Free Radic. Biol. Med. 2006, 41, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Kong, X.; Lu, Z.; Xiao, M.; Chen, M.; Zhu, L.; Shen, Y.; Hu, X.; Song, S. para-Aminobenzoic acid (PABA) synthase enhances thermotolerance of mushroom Agaricus bisporus. PLoS ONE 2014, 9, e91298. [Google Scholar] [CrossRef]
- Song, G.C.; Choi, H.K.; Ryu, C.M. The folate precursor para-aminobenzoic acid elicits induced resistance against Cucumber mosaic virus and Xanthomonas axonopodis. Ann. Bot. 2013, 111, 925–934. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Crisan, M.E.; Gorobet, A.; Siminel, A.V.; Bourosh, P.N.; Croitor, L. A new supramolecular isomer of p-aminobenzoate Zn (II) coordination polymer: Structure and photoluminescent property. Polyhedron 2019, 171, 502–507. [Google Scholar] [CrossRef]
- Chicu, S.A.; Herrmann, K.; Berking, S. An approach to calculate the toxicity of simple organic molecules on the basis of QSAR analysis in Hydractinia echinata (Hydrozoa, Cnidaria). Quant. Struct. Act. Relat. 2000, 19, 227–236. [Google Scholar] [CrossRef]
- Croitor, L.; Petric, M.F.; Szerb, E.I.; Vlase, G.; Bourosh, P.N.; Chumakov, Y.; Crisan, M.E. Role of 4-nitrobenzoic acid polymorphs in the crystallization process of the organic acid-base multicomponent system. CrystEngComm 2019, 21, 6038–6047. [Google Scholar] [CrossRef]
- Crisan, M.; Vlase, G.; Plesu, N.; Petric, M.; Croitor, L.; Kravtsov, V.; Chumakov, Y.; Bouros, P.; Vlase, T. Ethylethanolammonium 4-nitrobenzoate: Synthesis, structural characterization, thermal analysis, non-isothermal kinetic investigations and corrosion inhibitor efficiency. J. Therm. Anal. Calorim. 2018, 134, 343–352. [Google Scholar] [CrossRef]
- Crisan, M.; Halip, L.; Bourosh, P.; Chicu, S.A.; Chumakov, Y. Synthesis, structure and toxicity evaluation of ethanolamine nitro/chloronitrobenzoates: A combined experimental and theoretical study. Chem. Cent. J. 2017, 11, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Chicu, S.A.; Grozav, M.; Kurunczi, L.; Crisan, M. SAR for amine salts of carboxylic acids to Hydractinia echinata. Rev. Chim. 2008, 59, 582–587. [Google Scholar] [CrossRef]
- Crisan, M.; Vlase, G.; Szerb, E.I.; Vlase, T. Thermal and kinetics studies of primary, secondary and tertiary alkanolammonium salts of 4-nitrobenzoic acid. J. Therm. Anal. Calorim. 2018, 132, 1409–1418. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 4th ed.; Brooks/Cole, Cengage Learning: Boston, MA, USA; Belmont, CA, USA, 2009; p. 80. [Google Scholar]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Zhang, A.; Li, Y.; Liu, R.; Shi, L.; Jia, L. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by Abscisic acid in rice seeds. J. Exp. Bot. 2012, 63, 1809–1822. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Zhou, W.; Dai, Y.; Qi, Y.; Du, J.; Yang, F.; Liu, J.; et al. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tsygankova, V.; Andrusevich, Y.; Shtompel, O.; Myroljubov, O.; Hurenko, A.; Solomyanny, R.; Mrug, G.; Frasinyuk, M.; Shablyn, O.; Brovarets, V. Study of auxin, cytokinetin and gibberellins -like activity of heterocyclic compounds derivatives of pyrimidine, pyridine, pyrazole and isoflavones. Eur. J. Biotechnol. Biosci. 2016, 4, 29–44. [Google Scholar]
- Bethke, P.C.; Libourel, I.G.; Jones, R.L. Nitric oxide reduces seed dormancy in Arabidopsis. J. Exp. Bot. 2006, 57, 517–526. [Google Scholar] [CrossRef]
- Oracz, K.; Karpiński, S. Phytohormones signaling pathways and ROS involvement in seed germination. Front. Plant Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2017, 80, 3–12. [Google Scholar] [CrossRef]
- Dash, M.; Yordanov, Y.S.; Georgieva, T.; Tschaplinski, T.J.; Yordanova, E.; Busov, V. Poplar PtabZIP1-like enhances lateral root formation and biomass growth under drought stress. Plant J. 2017, 89, 692–705. [Google Scholar] [CrossRef]
- Qin, H.; Huang, R. Auxin controlled by ethylene steers root development. Int. J. Mol. Sci. 2018, 19, 3656. [Google Scholar] [CrossRef]
- Du, Y.; Scheres, B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot. 2018, 69, 155–167. [Google Scholar] [CrossRef]
- Cai, X.T.; Xu, P.; Zhao, P.X.; Liu, R.; Yu, L.H.; Xiang, C.B. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat. Commun. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, N.; Chen, H.; Wang, G.; Huang, J. OsMADS25 regulates root system development via auxin signalling in rice. Plant J. 2018, 95, 1004–1022. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Yang, X.; Zhang, J.; Liu, X.; Zheng, H.; Dong, G.; Nian, J.; Feng, J.; Xia, B.; Qian, Q.; et al. Peptidyl-prolyl isomerization targets rice Aux/IAAs for proteasomal degradation during auxin signalling. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C.; Brady, S.R.; Muday, G.K. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998, 118, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Dao, G.H.; Wu, G.X.; Wang, X.X.; Zhuang, L.L.; Zhang, T.Y.; Hu, H.Y. Enhanced growth and fatty acid accumulation of microalgae Scenedesmus sp. LX1 by two types of auxin. Bioresour. Technol. 2018, 247, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Beinsan, C.; Camen, D.; Sumalan, R.; Babau, M. Study concerning salt stress effect on leaf area dynamics and chlorophyll content in four bean local landraces from Banat area. In Proceedings of the 44th Croatian and 4th International Symposium on Agriculture, Opatija, Croatia, 16–20 February 2009; pp. 416–419. [Google Scholar]
- El-Saeid, H.M.; Abou-Hussein, S.D.; El-Tohamy, W.A. Growth characters, yield and endogenous hormones of cowpea plants in response to IAA application. Res. J. Agric. Biol. Sci. 2010, 6, 27–31. [Google Scholar]
- Finet, C.; Jaillais, Y. Auxology: When auxin meets plant evo-devo. Dev. Biol. 2012, 369, 19–31. [Google Scholar] [CrossRef]
- Khalil, S.; Mandurah, H.M. Growth and metabolic changes of cowpeas plants as affected by water deficiency and indole acetic acid. J. Agron. Crop Sci. 1989, 16, 160–166. [Google Scholar] [CrossRef]
- El-Mergawi, R.A. Sensitivity of faba bean cultivars to low glyphosate doses and the efficiency of IAA as indicator to glyphosate effects. Egypt J. Hort. 2003, 30, 197–214. [Google Scholar]
- Gaspar, T.H.; Kevers, C.; Faivre-Rampant, O.; Crèvecour, M.; Penel, C.L.; Greppin, H.; Dommes, J. Changing concepts in plant hormone action. Vitro Cell. Dev. Biol. Plant 2003, 39, 85–106. [Google Scholar] [CrossRef]
- Alarcón, M.V.; Salguero, J.; Lloret, P.G. Auxin modulated initiation of lateral roots is linked to pericycle cell length in maize. Front. Plant Sci. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Jia, B.; Liu, H.; Kan, X.; Zhang, Y.; Zhou, R.; Li, Z.; Yang, L.; Deng, D.; Yin, Z. Genetic mapping of the leaf number above the primary ear and its relationship with plant height and flowering time in maize. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shaver, G.R. Mineral nutrition and leaf longevity in Ledum palustre: The role of individual nutrients and the timing of leaf mortality. Oecologia 1983, 56, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Mauro, P.R.; Agnello, M.; Distefano, M.; Sabatino, L.; san Bautista, P.A.; Leonardi, C.; Giuffrida, F. Chlorophyll fluorescence, photosynthesis and growth of tomato plants as affected by long-term oxygen root zone deprivation and grafting. Agronomy 2020, 10, 137. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).