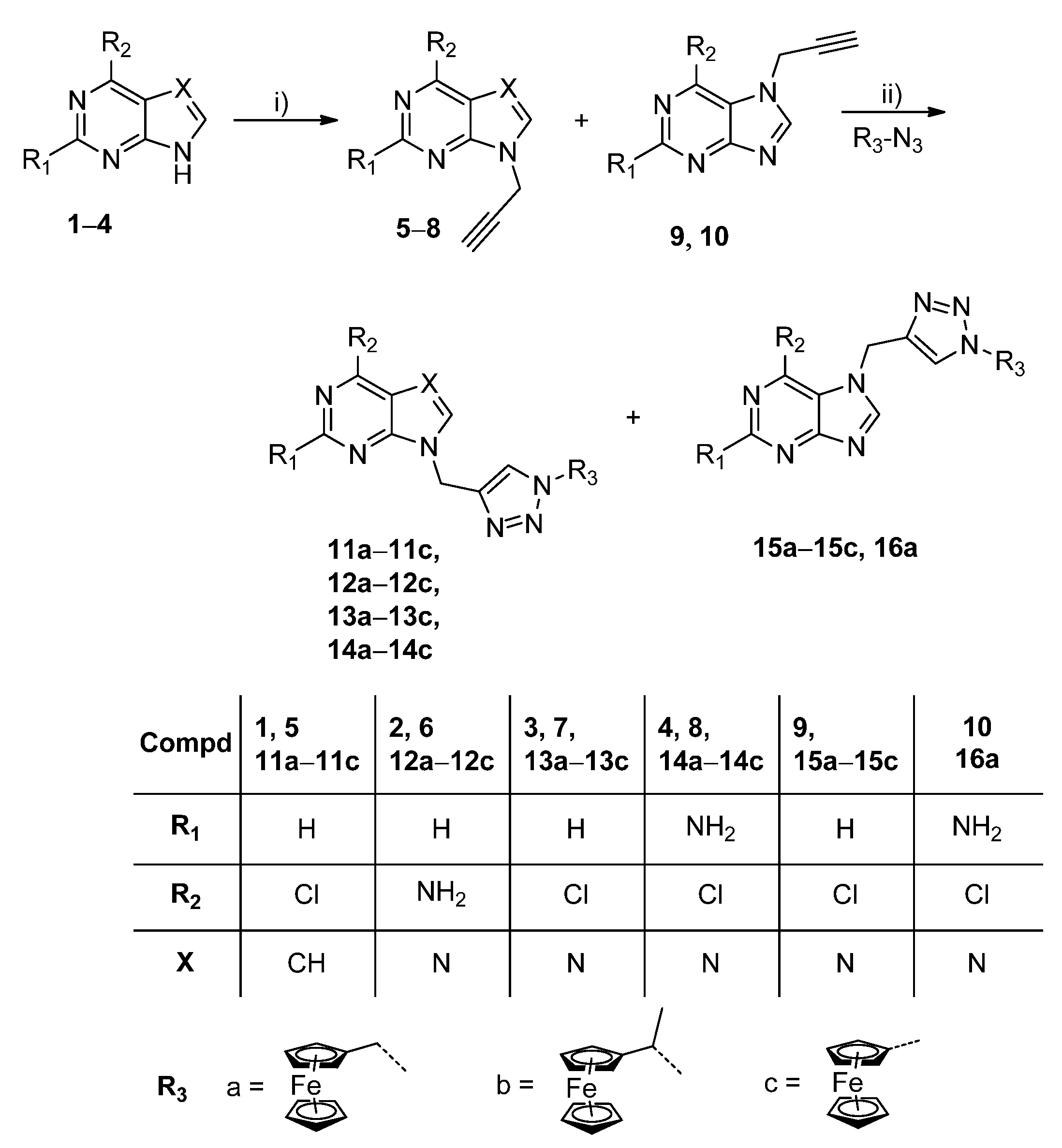
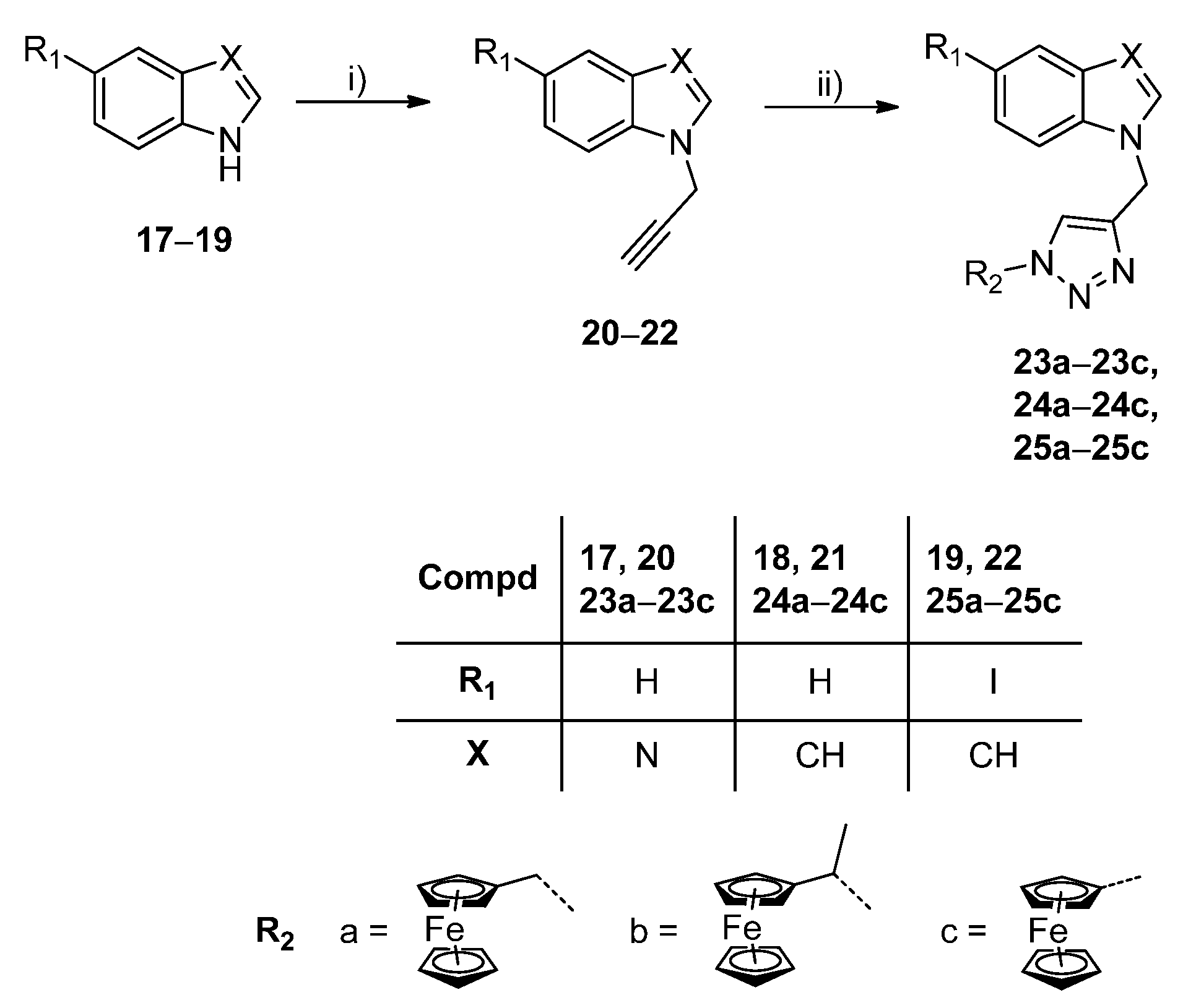
3. Materials and Methods
3.1. General Information
All chemicals and solvents were purchased from Aldrich (St. Louis, MO, USA), Fluorochem (Hadfield, UK) and Acros (Geel, Belgium). Anhydrous dimethyl formamide (DMF) was prepared using CaH
2 and stored over 3Å molecular sieves [
50]. Thin layer chromatography (TLC) was performed on pre-coated silica gel 60F-254 plates (Merck, Kenilworth, NJ, USA), while glass column slurry-packed under gravity with 0.063–0.2 mm silica gel (Fluka, Seelze, Germany) was employed for column chromatography. Melting points were determined using a Kofler micro hot-stage (Reichert, Vienna, Austria).
1H and
13C-NMR spectra were recorded on a Bruker 300 and 600 MHz spectrometers (Bruker, Billerica, MA, USA). All data were recorded in dimethyl sulfoxide (DMSO)-
d6 at 298 K. NMR chemical shifts were referenced to the residual solvent signal of DMSO at δ 2.50 ppm for
1H and δ 39.50 ppm for
13C. Individual resonances were assigned on the basis of their chemical shifts, signal intensities, multiplicity of resonances, and H–H coupling constants.
1H and
13C-NMR spectra of compounds are available in
Supplementary Materials. Microwave-assisted syntheses were carried out in a microwave oven (Milestone Start S, Sorisole, BG, Italy) at 100 °C and pressure of 1 bar.
4-Chloro-7-(prop-2-yn-1-yl)-7
H-pyrrolo[2,3-
d]pyrimidine (
5) [
29], 6-amino-9-(prop-2-yn-1-yl)-9
H-purine (
6) [
30], 6-chloro-9-(prop-2-yn-1-yl)-9
H-purine (
7) [
31], 2-amino-6-chloro-9-(prop-2-yn-1-yl)-9
H-purine (
8) [
32], 6-chloro-9-(prop-2-yn-1-yl)-9
H-purine (
9) [
31], 2-amino-6-chloro-7-(prop-2-yn-1-yl)-7
H-purine (
10) [
32], 1-(prop-2-yn-1-yl)-1
H-benzimidazole (
20) [
34], 1-(prop-2-yn-1-yl)-1
H-indole (21) [
51], 5-iodo-1-(prop-2-yn-1-yl)-1
H-indole (
22) [
34], 6-(pyrrolidin-1-yl)-9
H-purine (
28) [
52], 6-(piperidin-1-yl)-
9H-purine (
29) [
53], and 9-(2-bromoethyl)-6-chloro-
7H-pyrrolo[2,3-
d]pyrimidine (
30a) [
36] were synthesized in accordance with procedures given in the literature.
3.2. General Procedure for the Synthesis of Purine and Purinomimetics with Ferrocene at N-1 of 1,2,3-Triazole
The corresponding N-propargylated heterocyclic base 5−10 (1 eq.) was dissolved in methanol, and the corresponding terminal azide (1.2 eq.) and Cu(OAc)2 (0.05 eq.) were added. The reaction mixture was stirred at room temperature overnight. The solvent was removed under reduced pressure and the residue was purified by column chromatography (CH2Cl2:CH3OH = 60:1).
4-Chloro-7-[1-(1-ferrocenymethyl-1,2,3-triazol-4-yl)methyl]-7H-pyrrolo[2,3-d]pyrimidine (11a) Compound 11a was prepared using the above-mentioned procedure using compound 5 (100 mg, 0.52 mmol) and 1-methylazidoferrocene (150 mg, 0.62 mmol) to obtain 11a as orange oil (60.3 mg, 33 %). 1H-NMR (300 MHz, DMSO-d6) δ 8.65 (1H, s, H2), 8.06 (1H, s, H5′), 7.80 (1H, d, J = 3.6 Hz, H6), 6.67 (1H, d, J = 3.6 Hz, H5), 5.56 (2H, s, CH2), 5.25 (2H, s, CH2), 4.30 (2H, t, J = 1.8 Hz, CH-Fc), 4.18–4.14 2H, (m, CH-Fc), 4.12 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO-d6) δ 151.12 (C4), 150.87 (C2), 150.82 (C7a), 143.04 (C4′), 131.77 (C6), 123.51 (C5′), 117.22 (C4a), 99.30 (C5), 82.83 (Cq-Fc), 69.08 (CH-Fc), 69.04 (Cp-Fc), 68.76 (CH-Fc), 49.35 (CH2), 40.05 (CH2). Anal. calcd. for C20H17ClFeN6: C, 55.52; H, 3.96; N, 19.42. Found: C, 55.46; H, 3.95; N, 19.44.
4-Chloro-7-[1-(1-ferrocenyl-1-methylmethyl-1,2,3-triazol-4-yl)methyl]-7H-pyrrolo[2,3-d] pyrimidine (11b) Compound 11b was prepared using the above-mentioned procedure using compound 5 (100 mg, 0.52 mmol) and 1-azidoethylferrocene (159 mg, 0.62 mmol) to obtain 11b as orange oil (82.6 mg, 35 %). 1H-NMR (300 MHz, DMSO-d6) δ 8.65 (1H, s, H2), 8.09 (1H, s, H5′), 7.80 (1H, d, J = 3.6 Hz, H6), 6.67(1H, d, J = 3.6 Hz, H5), 5.65 (1H, q, J = 7.0 Hz, CH), 5.56 (2H, s, CH2), 4.33–4.28 (1H, m, CH-Fc), 4.17–4.15 (2H, m, CH-Fc), 4.08 (5H, H,s, Cp-Fc), 1.78 (3H, d, J = 7.0 Hz, CH3). 13C-NMR (151 MHz, DMSO-d6) δ 150.60 (C4), 150.36 (C2), 150.30 (C7a), 142.26 (C4′), 131.25 (C6), 121.58 (C5′), 116.70 (C4a), 98.82 (C5), 88.53 (Cq-Fc), 68.61 (Cp-Fc), 68.09 (CH-Fc), 67.62 (CH-Fc), 67.15 (CH-Fc), 66.21 (CH-Fc), 55.60 (CH), 39.54 (CH2), 20.83 (CH3). Anal. calcd. for C21H19ClFeN6: C, 56.46; H, 4.29; N, 18.81. Found: C, 56.35; H, 4.28; N, 18.87.
4-Chloro-7-[1-(1-ferrocenyl-1,2,3-triazol-1-yl)methyl]-7H-pyrrolo[2,3-d]pyrimidine (11c) Compound 11c was prepared using the above-mentioned procedure using compound 5 (100 mg, 0.52 mmol) and 1-azidoferrocene (142 mg, 0.62 mmol) to obtain 11c as orange oil (39.4 mg, 18 %). 1H-NMR (600 MHz, DMSO-d6) δ 8.68 (1H, s, H2), 8.55 (1H, s, H5′), 7.81 (1H, d, J = 3.6 Hz, H6), 6.70 (1H, d, J = 3.6 Hz, H5), 5.64 (2H, s, CH2), 5.00 (2H, t, J = 1.9 Hz, CH-Fc), 4.35–4.31 (2H, m, CH-Fc), 4.18 (5H, s, Cp-Fc). 13C-NMR (151 MHz, DMSO-d6) δ 150.85 (C4), 150.39 (C2), 150.19 (C7a), 142.95 (C4′), 130.83 (C6), 123.33 (C5′), 116.83 (C4a), 99.33 (C5), 93.32 (Cq-Fc), 69.87 (Cp-Fc), 66.57 (CH-Fc), 61.86 (CH-Fc), 34.42 (CH2). Anal. calcd. for C19H15ClFeN6: C, 54.51; H, 3.61; N, 20.07. Found: C, 55.40; H, 3.90; N, 20.03.
6-Amino-9-[1-(1-ferrocenymethyl-1,2,3-triazol-4-yl)methyl]-9H-purine (12a) Compound 12a was prepared using the above-mentioned procedure using compound 6 (100 mg, 0.58 mmol) and 1-methylazidoferrocene (168 mg, 0.70 mmol) to obtain 12a as orange powder (132 mg, 76%, m.p. = 107 °C). 1H-NMR (300 MHz, DMSO-d6) δ 8.18 (1H, s, H8), 8.12 (1H, s, H2), 8.07 (1H, s, H5′), 7.21 (2H, s, NH2), 5.41 (2H, s, CH2), 5.26 (2H, s, CH2), 4.30 (2H, dd, J = 3.4, 1.6 Hz, CH-Fc), 4.174.14 (2H, m, CH-Fc), 4.13 (5H, s, Cp-Fc). 13C-NMR (151 MHz, DMSO-d6) δ 155.91 (C6), 152.50 (C2), 149.21 (C4), 142.54 (C4′), 140.59 (C8), 123.05 (C5′), 118.51 (C5), 82.35 (Cq-Fc), 68.57 (CH-Fc), 68.56 (Cp-Fc), 68.26 (CH-Fc), 48.85 (CH2), 37.96 (CH2). Anal. calcd. for C19H18FeN8: C, 55.09; H, 4.38; N, 27.05. Found: C, 54.98; H, 4.39; N, 27.00.
6-Amino-9-[1-(1-ferrocenyl-1-methylmethyl-1,2,3-triazol-4-yl)methyl]-9H-purine (12b) Compound 12b was prepared using the above-mentioned procedure using compound 6 (100 mg, 0.58 mmol) and 1-azidoethylferrocene (177 mg, 0.70 mmol) to obtain 12b as orange powder (53.7 mg, 31 %, m.p. = 223 °C). 1H-NMR (600 MHz, DMSO) δ 8.57 (1H, s, H8), 8.26 (1H, s, H5′), 8.15 (1H, s, H2), 7.27 (2H, s, NH2), 5.49 (2H, s, CH2), 5.01 (2H, s, CH-Fc), 4.33 (2H, s, CH-Fc), 4.19 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO) δ 156.46 (C6), 153,17 (C8), 153.01 (C2), 149.87 (C4), 143.39 (C4′), 123.94 (C5′), 119.13 (C5), 93.79 (Cq-Fc), 70.38 (Cp-Fc), 67.08 (CH-Fc), 62.38 (CH-Fc), 38.46 (CH2). Anal. calcd. for C20H20FeN8: C, 56.09; H, 4.71; N, 26.16. Found: C, 56.17; H, 4.69; N, 26.11.
6-Amino-9-[1-(1-ferrocenyl-1,2,3-triazol-1-yl)methyl]-9H-purine (12c) Compound 12c was prepared using the above-mentioned procedure using compound 6 (100 mg, 0.58 mmol) and 1-azidoferrocene (160 mg, 0.70 mmol) to obtain 12c as orange powder (13.5 mg, 5.8 %, m.p. > 250 °C). 1H-NMR (600 MHz, DMSO) δ 8.57 (1H, s, H8), 8.26 (1H, s, H5′), 8.15 (1H, s, H2), 7.27 (2H, s, NH2), 5.49 (2H, s, CH2), 5.01 (2H, s, CH-Fc), 4.33 (2H, s, CH-Fc), 4.19 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO) δ 156.46 (C6), 153,17 (C8), 153.01 (C2), 149.87 (C4), 143.39 (C4′), 123.94 (C5′), 119.13 (C5), 93.79 (Cq-Fc), 70.38 (Cp-Fc), 67.08 (CH-Fc), 62.38 (CH-Fc), 38.46 (CH2). Anal. calcd. for C18H16FeN8: C, 54.02; H, 4.03; N, 28.00. Found: C, 53.86; H, 4.04; N, 27.97.
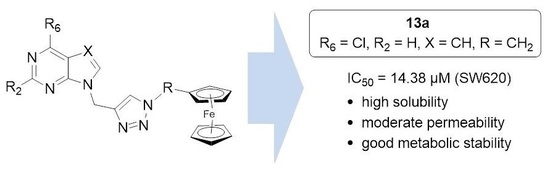
6-Chloro-9-[1-(1-ferrocenymethyl-1,2,3-triazol-4-yl)methyl]-9H-purine (13a) Compound 13a was prepared using the above-mentioned procedure using compound 7 (55 mg, 0.28 mmol) and 1-methylazidoferrocene (81 mg, 0.34 mmol) to obtain 13a as orange powder (53.2 mg, 43 %, m.p. = 156 °C). 1H-NMR (300 MHz, DMSO) δ 8.79 (1H, s, H8), 8.77 (1H, s, H2), 8.15 (1H, s, H5′), 5.60 (2H, s, CH2), 5.26 (2H, s, CH2), 4.30 (2H, t, J = 1.8 Hz, CH-Fc), 4.18–4.15 (2H, m, CH-Fc), 4.13 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO) δ 152.15 (C6), 152.13 (C2), 149.53 (C4), 147.92 (C8), 142.18 (C4′), 131.22 (C5), 123.69 (C5′), 82.75 (Cq-Fc), 69.10 (CH-Fc), 69.05 (Cp-Fc), 68.78 (CH-Fc), 49.43 (CH2), 39.42 (CH2). Anal. calcd. for C19H16ClFeN7: C, 52.62; H, 3.72; N, 22.61. Found: C, 52.46; H, 3.73; N, 22.66.
6-Chloro-9-[1-(1-ferrocenyl-1-methylmethyl-1,2,3-triazol-4-yl)methyl]-9H-purine (13b) Compound 13b was prepared using the above-mentioned procedure using compound 7 (100mg, 0.52 mmol) and 1-azidoethylferrocene (157 mg, 0.62 mmol) to obtain 13b as orange oil (55.5 mg, 23 %). 1H-NMR (600 MHz, DMSO) δ 8.79 (1H, s, H8), 8.78 (1H, s, H2), 8.16 (1H, s, H5′), 5.66 (1H, q, J = 7.0 Hz, CH), 5.60 (2H, s, CH2), 4.31 (1H, s, CH-Fc), 4.17 (2H, d, J = 1.7 Hz, CH-Fc), 4.15 (1H, s, CH-Fc), 4.09 (5H, s, Cp-Fc), 1.78 (3H, d, J = 7.0 Hz, CH3). 13C-NMR (75 MHz, DMSO) δ 152.17 (C6), 152.12 (C2), 149.52 (C4), 141.95 (C4′), 131.21 (C5), 122.23 (C5′), 88.99 (Cq-Fc), 69.13 (Cp-Fc), 68.61 (CH-Fc), 68.15 (CH-Fc), 67.67 (CH-Fc), 66.70 (CH-Fc), 56.19 (CH), 39.42 (CH2), 21.33 (CH3). Anal. calcd. for C20H18ClFeN7: C, 53.65; H, 4.05; N, 21.90. Found: C, 53.54; H, 4.04; N, 21.86.
6-Chloro-9-[1-(1-ferrocenyl-1,2,3-triazol-1-yl)methyl]-9H-purine (13c) Compound 13c was prepared using the above-mentioned procedure using compound 7 (100 mg, 0.52 mmol) and 1-azidoferrocene (138 mg, 0. 62 mmol) to obtain 13c as orange oil (43.2 mg, 19 %). 1H-NMR (600 MHz, DMSO) δ 8.85 (1H, s, H8), 8.81 (1H, s, H2), 8.59 (1H, s, H5′), 5.68 (2H, s, CH2), 4.99 (2H, s, CH-Fc), 4.33 (2H, s, CH-Fc), 4.19 (5H, s, Cp-Fc). 13C-NMR (151 MHz, DMSO) δ 151.77 (C6), 151.65 (C2), 149.05 (C4), 142.18 (C4′), 130.77 (C5), 123.45 (C5′), 93.17 (Cp-Fc), 69.91 (Cp-Fc), 66.61 (CH-Fc), 61.84 (CH-Fc). Anal. calcd. for C18H14ClFeN7: C, 51.52; H, 3.36; N, 23.36. Found: C, 51.57; H, 3.38; N, 23.33.
2-Amino-6-chloro-9-[1-(1-ferrocenymethyl-1,2,3-triazol-4-yl)methyl]-9H-purine (14a) Compound 14a was prepared using the above-mentioned procedure using compound 8 (100 mg, 0.48 mmol) and 1-methylazidoferrocene (139 mg, 0.58 mmol) to obtain 14a as orange powder (123 mg, 57 %; m.p. = 222 °C). 1H-NMR (300 MHz, DMSO) δ 8.17 (1H, s, H8), 8.05 (1H, s, H5′), 6.93 (2H, s, NH2), 5.33 (2H, s, CH2), 5.27 (2H, s, CH2), 4.31 (2H, t, J = 1.8 Hz, CH-Fc), 4.18–4.15 (2H, m, CH-Fc), 4.14 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO) δ 160.32 (C2), 154.33 (C6), 149.85 (C4), 143.46 (C8) 142.74 (C4′), 123.64 (C5), 123.40 (C5′), 82.75 (Cq-Fc), 69.12 (CH-Fc), 69.06 (Cp-Fc), 68.80 (CH-Fc), 49.45 (CH2), 38.71 (CH2). Anal. calcd. for C19H17ClFeN8: C, 50.86; H, 3.82; N, 24.97. Found: C, 50.91; H, 3.80; N, 24.92.
2-Amino-6-chloro-9-[1-(1-ferrocenyl-1-methylmethyl-1,2,3-triazol-4-yl)methyl]-9H-purine (14b) Compound 14b was prepared using the above-mentioned procedure using compound 8 (100 mg, 0.48 mmol) and 1-azidoethylferrocene (147 mg, 0.58 mmol) to obtain 14b as orange powder (55 mg, 24 %, m.p. = 214 °C). 1H-NMR (300 MHz, DMSO) δ 8.17 (1H, s, H8), 8.07 (1H, s, H5′), 6.93 (2H, s, NH2), 5.66 (1H, q, J = 7.0 Hz, CH), 5.33 (2H, s, CH2), 4.32 (2H, d, J = 2.1 Hz, CH-Fc), 4.23–4.13 (3H, m, CH-Fc), 4.10 (5H, s, Cp-Fc), 1.80 (3H, d, J = 7.0 Hz, CH3). 13C-NMR (151 MHz, DMSO) δ 159.82 (C2), 153.84 (C6), 149.34 (C4), 142.96 (C8), 141.95 (C4′), 123.15 (C5), 121.47 (C5′), 88.49 (Cq-Fc), 68.64 (Cp-Fc), 68.12 (CH-Fc), 67.66 (CH-Fc), 67.23 (CH-Fc), 66.17 (CH-Fc), 55.70 (CH), 38.24 (CH2), 20.77 (CH3). Anal. calcd. for C20H19ClFeN8: C, 51.91; H, 4.14; N, 24.22. Found: C, 51.75; H, 4.13; N, 24.24.
2-Amino-6-chloro-9-[1-(1-ferrocenyl-1,2,3-triazol-1-yl)methyl]-9H-purine (14c) Compound 14c was prepared using the above-mentioned procedure using compound 8 (100 mg, 0.48 mmol) and 1-azidoferrocene (131 mg, 0.58 mmol) to obtain 14c as orange powder (44 mg, 21 %, m.p. > 250 °C). 1H-NMR (300 MHz, DMSO) δ 8.50 (1H, s, H8), 8.24 (1H, s, H5′), 6.96 (2H, s, NH2), 5.41 (2H, s, CH2), 4.99 (2H, t, J = 1.9 Hz, CH-Fc), 4.384.30 (2H, m, CH-Fc), 4.19 (5H, s, Cp-Fc). 13C-NMR (151 MHz, DMSO) δ 159.83 (C2), 153.93 (C6), 149.38 (C4), 142.63 (C4′), 123.28 (C5′), 123.23 (C5), 93.34 (Cq-Fc), 69.89 (Cp-Fc), 66.58 (CH-Fc), 61.86 (CH-Fc), 38.24 (CH2). Anal. calcd. for C18H15ClFeN8: C, 49.74; H, 3.48; N, 25.78. Found: C, 49.78; H, 3.47; N, 25,74.
6-Chloro-7-[1-(1-ferrocenymethyl-1,2,3-triazol-4-yl)methyl]-7H-purine (15a) Compound 15a was prepared using the above-mentioned procedure using compound 9 (50 mg, 0.26 mmol) and 1-methylazidoferrocene (75 mg, 0.31 mmol) to obtain 15a as orange powder (56.5 mg, 50 %, m.p. = 96 °C). 1H-NMR (300 MHz, DMSO) δ 8.94 (1H, s, H8), 8.80 (1H, s, H2), 8.16 (1H, s, H5′), 5.80 (2H, s, CH2), 5.26 (2H, s, CH2), 4.30 (2H, t, J = 1.8 Hz, CH-Fc), 4.204.14 (2H, m, CH-Fc), 4.12 (5H, s, Cp-Fc). 13C-NMR (151 MHz, DMSO) δ 161.57 (C4), 151.74 (C2), 151.17 (C8), 142.54 (C4′), 142.29 (C6), 122.82 (C5′), 121.98 (C5), 82.36 (Cq-Fc), 68.53 (Cp-Fc), 68.51 (CH-Fc), 68.26 (CH-Fc), 48.93 (CH2), 41.89 (CH2). Anal. calcd. for C19H16ClFeN7: C, 52.62; H, 3.72; N, 22.61. Found: C, 52.56; H, 3.71; N, 22.57.
6-Chloro-7-[1-(1-ferrocenyl-1-methylmethyl-1,2,3-triazol-4-yl)methyl]-7H-purine (15b) Compound 15b was prepared using the above-mentioned procedure using compound 9 (90 mg, 0.47 mmol) and 1-azidoethylferrocene (145 mg, 0.56 mmol) to obtain 15b as orange powder (56.5 mg, 27 %, m.p. = 68 °C). 1H-NMR (300 MHz, DMSO) δ 8.94 (1H, s, H8), 8.80 (1H, s, H2), 8.19 (1H, s, H5′), 5.80 (2H, s, CH2), 5.66 (1H, q, J = 7.0 Hz, CH), 4.334.27 (1H, m, CH-Fc), 4.16 (3H, m, CH-Fc), 4.07 (5H, s, Cp-Fc), 1.78 (3H, d, J = 7.0 Hz, CH3). 13C-NMR (151 MHz, DMSO) δ 161.57 (C4), 151.72 (C2), 142.34 (C4′), 142.30 (C6), 122.02 (C5), 121.37 (C5′), 88.62 (Cq-Fc), 68.59 (Cp-Fc), 68.08 (CH-Fc), 67.61 (CH-Fc), 67.01 (CH-Fc), 66.23 (CH-Fc), 55.70 (CH), 41.97 (CH2), 20.94 (CH3). Anal. calcd. for C20H18ClFeN7: C, 53.65; H, 4.05; N, 21.90. Found: C, 53.68; H, 4.03; N, 21.86.
6-Chloro-7-[1-(1-ferrocenyl-1,2,3-triazol-1-yl)methyl]-7H-purine (15c) Compound 15c was prepared using the above-mentioned procedure using compound 9 (90 mg, 0.47 mmol) and 1-azidoferrocene (129 mg, 0.56 mmol) to obtain 15c as orange powder (44.7 mg, 22 %, m.p. = 76 °C). 1H-NMR (600 MHz, DMSO) δ 9.00 (1H, s, H8), 8.83 (1H, s, H2), 8.58 (1H, s, H5′), 5.87 (2H, s, CH2), 4.99 (2H, s, CH-Fc), 4.33 (2H, s, CH-Fc), 4.17 (5H, s, Cp-Fc). 13C-NMR (151 MHz, DMSO) δ 161.71 (C4), 151.77 (C2), 151.24 (C8), 143.23 (C6), 142.29 (C4′), 122.75 (C5′), 122.13 (C5), 93.24 (Cq-Fc), 69.89 (Cp-Fc), 66.61 (CH-Fc), 61.74 (CH-Fc), 42.00 (CH2). Anal. calcd. for C18H14ClFeN7: C, 51.52; H, 3.36; N, 23.36. Found: C, 51.55; H, 3.34; N, 23.39.
2-Amino-6-chloro-7-[1-(1-ferrocenymethyl-1,2,3-triazol-4-yl)methyl]-7H-purine (16a) Compound 16a was prepared using the above-mentioned procedure using compound 10 (70 mg, 0.34 mmol) and 1-methylazidoferrocene (98 mg, 0.41 mmol) to obtain 16a as orange powder (76 mg, 50 %, m.p. >250 °C). 1H-NMR (300 MHz, DMSO) δ 8.49 (1H, s, H8), 8.09 (1H, s, H5′), 6.64 (2H, s, NH2), 5.60 (2H, s, CH2), 5.26 (2H, s, CH2), 4.29 (2H, t, J = 1.8 Hz, CH-Fc), 4.174.14 (2H, m, CH-Fc), 4.12 (5H, s, Cp-Fc). 13C-NMR (151 MHz, DMSO) δ 164.27 (C4), 159.98 (C2), 142.82 (C6), 142.33 (C4′), 122.74 (C5′), 82.48 (Cq-Fc), 68.54 (CH-Fc), 68.46 (CH-Fc), 68.22 (CH-Fc), 48.89 (CH2), 41.56 (CH2). Anal. calcd. for C19H17ClFeN8: C, 50.86; H, 3.82; N, 24.97. Found: C, 50.80; H, 3.83; N, 24.91.
3.3. General Procedure for the Synthesis of Purinomimetics with Ferrocene at N-1 of 1,2,3-Triazole
The corresponding N-propargylated heterocyclic base 20−22 (1 eq.) was dissolved in methanol, and the corresponding terminal azide (1.2 eq.) and Cu(OAc)2 (0.05 eq.) were added. The reaction mixture was stirred at room temperature overnight. The solvent was removed under reduced pressure and the residue was purified by column chromatography (CH2Cl2:CH3OH = 60:1).
1-[1-(1-Ferrocenylmethyl-1,2,3-triazol-4-yl)methyl]-1H-benzimidazole (23a) Compound 23a was prepared using the above-mentioned procedure using compound 20 (70 mg, 0.45 mmol) and 1-methylazidoferrocene (130.2 mg, 0.54 mmol) to obtain 23a as orange powder (113 mg, 63 %, m.p. = 163 °C). 1H-NMR (300 MHz, DMSO) δ 8.32 (1H, s, H2), 8.14 (1H, s, H5′), 7.65 (2H, dd, J = 7.1, 1.9 Hz, H4/7), 7.287.16 (2H,m, H5/6), 5.56 (2H, s, CH2), 5.28 (2H, s, CH2), 4.354.28 (2H, m, CH-Fc), 4.194.17 (2H, m, CH-Fc), 4.15 (5H, s, Cp-Fc). 13C-NMR (151 MHz, DMSO) δ 143.87 (C2), 143.40 (C3a), 142.54 (C4′), 133.45 (C7a), 123.09 (C5′), 122.28 (C6), 121.54 (C4), 119.37 (C5), 110.66 (C7), 82.24 (Cq-Fc), 68.56 (Cp-Fc), 68.29 (CH-Fc), 48.88 (CH2). Anal. calcd. for C21H18FeN5: C, 63.65; H, 4.58; N, 17.67. Found: C, 63.71; H, 4.59; N, 17.69.
1-[1-(1-Ferrocenyl-1-methylmethyl-1,2,3-triazol-4-yl)methyl]-1H-benzimidazole (23b) Compound 23b was prepared using the above-mentioned procedure using compound 20 (82 mg, 0.53 mmol) and 1-azidoethylferrocene (163.2 mg, 0.64 mmol) to obtain 23b as brown powder (37.48 mg, 17 %, m.p. = 140 °C). 1H-NMR (600 MHz, DMSO) δ 8.33 (1H, s, H2), 8.15 (1H, s, H5′), 7.64 (2H, dd, J = 7.4, 4.7 Hz, H4/), 7.23 (1H, t, J = 7.4 Hz, H6), 7.19 (1H, t, J = 7.5 Hz, H5), 5.66 (1H, q, J = 7.0 Hz, CH), 5.53 (2H, s, CH2), 4.31 (1H, s, CH-Fc), 4.214.13 (3H, m, CH-Fc), 4.09 (5H, s, Cp-Fc), 1.78 (3H, d, J = 7.0 Hz, CH3). 13C-NMR (75 MHz, DMSO) δ 143.94 (C3a), 142.73 (C4′), 133.99 (C7a), 122.77 (C6), 122.19 (C5′), 122.03 (C4), 119.88 (C5), 111.15 (C7), 88.96 (Cq-Fc), 69.13 (Cp-Fc), 68.61 (CH-Fc), 68.15 (CH-Fc), 67.65 (CH-Fc), 66.64 (CH-Fc), 56.12 (CH), 39.86 (CH2), 21.37 (CH3). Anal. calcd. for C22H20FeN5: C, 64.40; H, 4.91; N, 17.07. Found: C, 64.14; H, 4.92; N, 17.10.
1-[1-(1-Ferrocenyl-1,2,3-triazol-4-yl)methyl]-1H-benzimidazole (23c) Compound 23c was prepared using the above-mentioned procedure using compound 20 (83 mg, 0.53 mmol) and 1-azidoferrocene (144.7 mg, 0.64 mmol) to obtain 23c as orange powder (25 mg, 12 %, m.p. > 250 °C). 1H-NMR (600 MHz, DMSO) δ 8.64 (1H, s, H5′), 8.39 (1H, s, H2), 7.66 (2H, s, H4/7), 7.24 (1H, t, J = 7.4 Hz, H6), 7.20 (1H, t, J = 7.2 Hz, H5), 5.62 (2H, s, CH2), 4.99 (2H, s, CH-Fc), 4.33 (2H, s, CH-Fc), 4.17 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO) δ 144.01 (C3a), 143.39 (C4′), 134.00 (C7a), 124.01 (C5’), 122.84 (C6), 122.12 (C4), 119.96 (C5), 111.15 (C7), 93.78 (Cq-Fc), 70.37 (Cp-Fc), 67.11 (CH-Fc), 62.41 (CH-Fc). Anal. calcd. for C20H17FeN5: C, 62.68; H, 4.47; N, 18.27. Found: C, 62.74; H, 4.46; N, 18.29.
1-[1-(1-Ferrocenylmethyl-1,2,3-triazol-4-yl)methyl]-1H-indole (24a) Compound 24a was prepared using the above-mentioned procedure using compound 21 (70 mg, 0.45 mmol) and 1-methylazidoferrocene (130.2 mg, 0.54 mmol) to obtain 24a as orange powder (25 mg, 14 %, m.p. = 135 °C). 1H-NMR (600 MHz, DMSO) δ 7.99 (1H, s, H5′), 7.56 (1H, d, J = 7.9 Hz, H7), 7.51 (1H, d, J = 7.9 Hz, H4), 7.42 (1H, d, J = 3.1 Hz, H2), 7.11 (1H, t, J = 7.2 Hz, H6), 7.00 (1H, t, J = 7.0 Hz,H5), 6.42 (1H, d, J = 2.7 Hz, H3), 5.42 (2H, s, CH2), 5.24 (2H, s, CH2), 4.28 (2H, t, J = 1.8 Hz, CH-Fc), 4.15 (2H, t, J = 1.8 Hz, CH-Fc), 4.13 (5H, s, Cp-Fc). 13C-NMR (101 MHz, DMSO) δ 144.07 (C4′), 135.98 (C7a), 129.04 (C2), 128.68 (C3a), 123.18 (C5′), 121.53 (C6), 120.85 (C4), 119.56 (C5), 110.55 (C7), 101.40 (C3), 82.83 (Cq-Fc), 69.07 (Cp-Fc), 68.77 (CH-Fc), 49.32 (CH2), 41.25 (CH2). Anal. calcd. for C22H20FeN4: C, 66.68; H, 5.09; N, 14.14. Found: C, 66.48; H, 5.08; N, 14.18.
1-[1-(1-Ferrocenyl-1-methylmethyl-1,2,3-triazol-4-yl)methyl]-1H-indole (24b) Compound 24b was prepared using the above-mentioned procedure using compound 21 (75 mg, 0.48 mmol) and 1-azidoethylferrocene (147.9 mg, 0.58 mmol) to obtain 24b as brown oil (40 mg, 20 %). 1H-NMR (300 MHz, DMSO) δ 8.02 (1H, s, H5′), 7.57 (1H, d, J = 7.6 Hz, H7), 7.51 (1H, d, J = 7.8 Hz, H4), 7.41 (1H, d, J = 3.1 Hz, H2), 7.11 (1H, t, J = 7.0 Hz, H6), 7.00 (1H, t, J = 7.0 Hz, H5), 6.42 (1H, dd, J = 3.1, 0.6 Hz, H3), 5.64 (1H, q, J = 7.0 Hz, CH), 5.41 (2H, s, CH2), 4.314.27 (1H, m, CH-Fc), 4.174.12 (3H, m, CH-Fc), 4.08 (5H, s, Cp-Fc), 1.77 (3H, d, J = 7.0 Hz, CH3). 13C-NMR (75 MHz, DMSO) δ 143.75 (C4′), 135.98 (C7a), 128.99 (C2), 128.65 (C3a), 121.79 (C5′), 121.52 (C6), 120.84 (C4), 119.55 (C5), 110.53 (C7), 101.39 (C3), 89.00 (Cq-Fc), 69.14 (Cp-Fc), 68.59 (CH-Fc), 68.13 (CH-Fc), 67.66 (CH-Fc), 66.62 (CH-Fc), 56.02 (CH), 41.25 (CH2), 21.37 (CH3). Anal. calcd. for C23H22FeN4: C, 67.33; H, 5.40; N, 13.66. Found: C, 67.19; H, 5.41; N, 13.63.
1-[1-(1-Ferrocenyl-1,2,3-triazol-4-yl)methyl]-1H-indole (24c) Compound 24c was prepared using the above-mentioned procedure using compound 21 (132 mg, 0. 85 mmol) and 1-azidoferrocene (230.6 mg, 1.02 mmol) to obtain 24c as brown crystals (32 mg, 9.8 %, m.p. = 120 °C). 1H-NMR (300 MHz, DMSO) δ 8.53 (1H, s, H5′), 7.61 (1H, d, J = 8.2 Hz, H7), 7.55 (1H, d, J = 7.8 Hz, H4), 7.48 (1H, d, J = 3.2 Hz, H2), 7.14 (1H, t, J = 7.6 Hz, H6), 7.02 (1H, t, J = 7.0 Hz, H5), 6.47 (1H, d, J = 3.1 Hz, H3), 5.50 (2H, s, CH2), 4.97 (2H, t, J = 1.9 Hz, CH-Fc), 4.354.27 (2H, m, CH-Fc), 4.17 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO) δ 144.37 (C4′), 136.01 (C7a), 129.14 (C2), 128.77 (C3a), 123.71 (C5′), 121.58 (C6), 120.91 (C4), 119.63 (C5), 110.57 (C7), 101.50 (C3), 93.86 (Cq-Fc), 70.35 (Cp-Fc), 67.06 (CH-Fc), 62.40 (CH-Fc), 41.23 (CH2). Anal. calcd. for C21H18FeN4: C, 65.99; H, 4.75; N, 14.66. Found: C, 66.06; H, 4.76; N, 14.63.
5-Iodo-1-[1-(1-ferrocenylmethyl-1,2,3-triazol-4-yl)methyl]-1H-indole (25a) Compound 25a was prepared using the above-mentioned procedure using compound 22 (70 mg, 0.25 mmol) and 1-methylazidoferrocene (72.3 mg, 0.30 mmol) to obtain 25a as brown crystals (79 mg, 15 %, m.p. = 109 °C). 1H-NMR (300 MHz, DMSO) δ 7.97 (1H, s, H5′), 7.89 (1H, s, H4), 7.44 (2H, m, H2/7), 7.37 (1H, dd, J = 8.6, 1.5 Hz, H6), 6.39 (1H, d, J = 3.0 Hz, H3), 5.42 (2H, s, CH2), 5.24 (2H, s, CH2), 4.304.24 (2H, m, CH-Fc), 4.164.14 (2H, m, CH-Fc), 4.12 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO) δ 143.77 (C4′), 135.10 (C7a), 131.44 (C3a), 130.17 (C2), 129.43 (C6), 129.25 (C4), 123.19 (C5′), 113.17 (C7), 100.84 (C3), 83.49 (C5), 82.79 (Cq-Fc), 69.06 (Cp-Fc), 68.77 (CH-Fc), 49.32 (CH2), 41.35 (CH2). Anal. calcd. for C22H19FeIN4: C, 50.60; H, 3.67; N, 10.73. Found: C, 50.65; H, 3.68; N, 10.76.
5-Iodo-1-[1-(1-ferrocenyl-1-methylmethyl-1,2,3-triazol-4-yl)methyl]-1H-indole (25b) Compound 25b was prepared using the above-mentioned procedure using compound 22 (223 mg, 0.79 mmol) and 1-azidoethylferrocene (242.3 mg, 0.95 mmol) to obtain 25b as orange powder (125 mg, 29 %, m.p. = 118 °C). 1H-NMR (300 MHz, DMSO) δ 7.97 (1H, s, H5′), 7.89 (1H, s, H4), 7.44 (2H, m, H2/7), 7.37 (1H, dd, J = 8.6, 1.5 Hz, H6), 6.39 (1H, d, J = 3.0 Hz, H3), 5.42 (2H, s, CH2), 5.24 (2H, s, CH2), 4.304.24 (2H, m, CH-Fc), 4.164.14 (2H, m, CH-Fc), 4.12 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO) δ 143.77 (C4′), 135.10 (C7a), 131.44 (C3a), 130.17 (C2), 129.43 (C6), 129.25 (C4), 123.19 (C5′), 113.17 (C7), 100.84 (C3), 83.49 (C5), 82.79 (Cq-Fc), 69.06 (Cp-Fc), 68.77 (CH-Fc), 49.32 (CH2), 41.35 (CH2). Anal. calcd. for C23H21FeIN4: C, 51.52; H, 3.95; N, 10.45. Found: C, 51.57; H, 3.96; N, 10.49.
5-Iodo-1-[1-(1-ferrocenyl-1,2,3-triazol-4-yl)methyl]-1H-indole (25c) Compound 25c was prepared using the above-mentioned procedure using compound 22 (345 mg, 1.23 mmol) and 1-azidoferrocene (334.5 mg, 1.48 mmol) to obtain 25c as brown crystal (53 mg, 10 %, m.p. = 169 °C). 1H-NMR (600 MHz, DMSO) δ 8.52 (1H, s, H5′), 7.92 (1H, s, H4), 7.517.46 (2H, m, H2/7), 7.39 (1H, d, J = 8.6 Hz, H6), 6.44 (1H, d, J = 3.0 Hz, H3), 5.49 (2H, s, CH2), 4.97 (2H, s, CH-Fc), 4.32 (2H, s, CH-Fc), 4.17 (5H, s, Cp-Fc). 13C-NMR (151 MHz, DMSO) δ 143.60 (C4′), 134.63 (C7a), 131.01 (C3a), 129.77 (C2), 128.97 (C6), 128.81 (C4), 123.22 (C5′), 112.67 (C7), 100.43 (C3), 93.32 (Cq-Fc), 83.09 (C5), 69.86 (Cp-Fc), 66.58 (CH-Fc), 61.91 (CH-Fc), 40.81 (CH2). Anal. calcd. for C21H17FeIN4: C, 49.64; H, 3.73; N, 11.03. Found: C, 49.54; H, 3.72; N, 10.99.
3.4. General Procedure for N-alkylation of Compounds 26 and 27
To a solution of potassium hydroxide (2 eq.) in water, corresponding heterocyclic base 1, 3 (1 eq.) and corresponding amine (4 eq.) were added. Reaction mixture was stirred for 10 min under microwave irradiation at 100 °C and 400 W. Formed precipitate was filtered off and dried.
4-(Pyrrolidin-1-yl)-7H-pyrrolo[2,3-d]pyrimidine (26) Compound 26 was prepared using the above-mentioned procedure using compound 1 (500 mg; 3.26 mmol) to obtain 26 as white powder (613 mg, 95 %, m.p. > 250 °C). 1H-NMR (300 MHz, DMSO) δ 11.56 (1H, bs, NH), 8.08 (1H, s, H2), 7.09 (1H, d, J = 3.5 Hz, H6), 6.56 (1H, d, J = 3.5 Hz, H5), 3.71 (4H, bs, CH2-pyrrolidine), 1.95 (4H, bs, CH2-pyrrolidine). 13C-NMR (151 MHz, DMSO) δ 154.77 (C4), 151.18 (C2), 150.89 (7a), 120.31 (C6), 102.43 (4a), 100.79 (C5), 47.38 (CH2-pyrrolidine). Anal. calcd. for C10H12N4: C, 63.81; H, 6.43; N, 29.77. Found: C, 63.87; H, 6.44; N, 29.65.
4-(Piperidin-1-yl)-7H-pyrrolo[2,3-d]pyrimidine (27) Compound 27 was prepared using the above-mentioned procedure using compound 1 (500 mg; 3.26 mmol) to obtain 27 as white powder (659 mg, 84 %, m.p. = 189 °C). 1H-NMR (300 MHz, DMSO) δ 11.64 (1H, bs, NH), 8.12 (1H, s, H2), 7.15 (1H, d, J = 3.5 Hz, H6), 6.55 (1H, d, J = 3.5 Hz, H5), 3.96–3.76 (4H, m, CH2-piperidine), 1.73–1.62 (2H, m, CH2-piperidine), 1.62–1.50 (4H, m, CH2-piperidine). 13C-NMR (151 MHz, DMSO) δ 156.27 (C4), 151.90 (C7a), 150.65 (C2), 120.99 (C6), 101.99 (C4a), 100.90 (C5), 46.23 (CH2-piperidine), 25.45 (CH2-piperidine), 24.25 (CH2-piperidine). Anal. calcd. for C11H14N4: C, 65.32; H, 6.98; N, 27.70. Found: C, 65.12; H, 6.99; N, 27.76.
3.5. General Procedure for N-alkylation of Compounds 30b, 30c, 31a–31c
To a solution of the corresponding heterocyclic base 1, 3, 26–29 (1 eq.) in anhydrous DMF, NaH (1.2 eq.) was added and stirred for 30 min under argon atmosphere. 1,2-Dibromoethane (1 eq.) was added and the reaction mixture was stirred at room temperature for 24 h. Solvent was evaporated and the residue was purified by column chromatography (hexane:ethyl acetate = 8:1).
9-(2-Bromoethyl)-6-(pyrrolidin-1-yl)-7H-pyrrolo[2,3-d]pyrimidine (30b) Compound 30b was prepared using the above-mentioned procedure using compound 26 (400 mg; 2.13 mmol) to obtain 30b as yellow powder (106 mg; 16 %, m.p.= 109 °C). 1H-NMR (600 MHz, DMSO) δ 8.11 (1H, s, H2), 7.24 (1H, d, J = 3.5 Hz, H8), 6.61 (1H, d, J = 3.5 Hz, H7), 4.52 (2H, t, J = 6.4 Hz, CH2), 3.85 (2H, t, J = 6.3 Hz, CH2), 3.70 (4H, bs, CH2-pyrrolidine), 1.96 (4H, bs, CH2-pyrrolidine). 13C-NMR (101 MHz, DMSO) δ 155.17 (C6), 151.61 (C2), 150.27 (C4), 124.29 (C8), 103.18 (C5), 101.08 (C7), 47.96 (CH2-pyrrolidine), 45.93 (CH2), 32.35 (CH2). Anal. calcd. for C12H15BrN4: C, 48.83; H, 5.12; N, 18.98. Found: C, 48.73; H, 5.11; N, 18.94.
9-(2-Bromoethyl)-6-(piperidin-1-yl)-7H-pyrrolo[2,3-d]pyrimidine (30c) Compound 30c was prepared using the above-mentioned procedure using compound 27 (400 mg; 1.97 mmol) to obtain 30c as yellow powder (87 mg; 14 %, m.p. = 70 °C). 1H-NMR (600 MHz, DMSO) δ 8.14 (1H, s, H2), 7.29 (1H, d, J = 3.6 Hz, H8), 6.59 (1H, d, J = 3.7 Hz, H7), 4.52 (2H, t, J = 6.4 Hz, CH2), 3.84 (6H, m, CH2, CH2-piperidine), 1.65 (2H, m, CH2-piperidine), 1.59–1.54 (4H, m, CH2-piperidine). 13C-NMR (151 MHz, DMSO) δ 156.20 (C6), 150.73 (C4), 150.67 (C2), 124.32 (C8), 102.21 (C5), 100.65 (C7), 46.21 (CH2-piperidine), 45.50 (CH2), 31.74 (CH2), 25.46 (CH2-piperidine), 24.20 (CH2-piperidine). Anal. calcd. for C13H17BrN4: C, 50.50; H, 5.54; N, 18.12. Found: C, 50.60; H, 5.52; N, 18.06.
9-(2-Bromoethyl)-6-chloro-9H-purine (31a) Compound 31a was prepared using the above-mentioned procedure using compound 3 (500 mg; 3.23 mmol) to obtain 31a as white powder (312 mg; 36 %; m.p.= 111°C). 1H-NMR (400 MHz, DMSO) δ 8.82 (1H, s, H2), 8.77 (1H, s, H8), 4.75 (2H, t, J = 6.1 Hz, CH2), 4.01 (2H, t, J = 6.1 Hz, CH2). 13C-NMR (101 MHz, DMSO) δ 152.42 (C6), 152.14 (C2), 149.59 (C4), 147.99 (C8), 131.26 (C5), 45.82 (CH2), 31.64 (CH2). Anal. calcd. for C7H6BrClN4: C, 32.15; H, 2.31; N, 21.42. Found: C, 32.05; H, 2.32; N, 21.47.
9-(2-Bromoethyl)-6-(pyrrolidin-1-yl)-9H-purine (31b) Compound 31b was prepared using the above-mentioned procedure using compound 28 (500 mg; 2.65 mmol) to obtain 31b as white powder (208 mg; 27 %; m.p.= 163 °C). 1H-NMR (400 MHz, DMSO) δ 8.22 (1H, s, H2), 8.17 (1H, s, H8), 4.58 (2H, t, J = 6.1 Hz, CH2), 4.07 (2H, bs, CH2-pyrrolidine), 3.95 (2H, t, J = 6.1 Hz, CH2), 3.64 (2H, s, CH2-pyrrolidine), 1.95 (4H, s, CH2-pyrrolidine). 13C-NMR (101 MHz, DMSO) δ 152.95 (C4), 152.70 (C2), 150.37 (C6), 140.71 (C8), 119.81 (C5), 45.07 (CH2), 32.03 (CH2). Anal. calcd. for C11H14BrN5: C, 44.61; H, 4.76; N, 23.65. Found: C, 44.65; H, 4.75; N, 23.60.
9-(2-Bromoethyl)-6-(piperidin-1-yl)-9H-purine (31c) Compound 31c was prepared using the above-mentioned procedure using compound 29 (500 mg; 2.46 mmol) to obtain 31c as white powder (200 mg; 27 %; m.p. = 149 °C. 1H-NMR (400 MHz, DMSO) δ 8.23 (1H, s, H2), 8.20 (1H, s, H8), 4.58 (2H, t, J = 6.1 Hz, CH2), 4.20 (4H, bs, CH2-piperidine), 3.94 (2H, t, J = 6.1 Hz, CH2), 1.73–1.63 (2H, m, CH2-piperidine), 1.63–1.55 (4H, m, CH2-piperidine). 13C-NMR (101 MHz, DMSO) δ 153.58 (C4), 152.39 (C2), 150.95 (C6), 140.18 (C8), 119.34 (C5), 46.08 (CH2-piperidine), 45.13 (CH2), 31.94 (CH2), 26.15 (CH2-piperidine), 24.74 (CH2-piperidine). Anal. calcd. for C16H16BrN5: C, 46.46; H, 5.20; N, 22.58. Found: C, 46.36; H, 5.19; N, 22.62.
3.6. General Procedure for the Synthesis of Azidoethyl Derivatives of Purinomimetics
The corresponding N-alkylated heterocyclic base 30a–30c, 31a–31c (1 eq.) was dissolved in acetone. To suspension was added dropwise solution of sodium azide (4 eq.) in water. The reaction mixture was stirred at 60 °C overnight. Solvent was evaporated and crude product was used as is in next step.
3.7. General Procedure for the Synthesis of Purinomimetics and Purines with Ferrocene at C-4 of 1,2,3-Triazole
The corresponding terminal azide 32a–32c, 33a–33c (1 eq.) was dissolved in methanol, Cu(OAc)2 (0.05 eq), and the ethynylferrocene (1.2 eq.) were added. The reaction mixture was stirred at room temperature overnight. Solvent was evaporated and the residue was purified by column chromatography (CH2Cl2:CH3OH = 60:1).
4-Chloro-7-(2-(4-ferrocenyl-1,2,3-triazol-1-yl)ethyl)-7H-pyrrolo[2,3-d]pyrimidine (34a). Compound 34a was prepared using the above-mentioned procedure using compound 32a (0.76 mmol) and ethynylferrocene (193.2 mg, 0.92 mmol) to obtain 34a as orange powder (20.7 mg, 6 %, m.p. = 196 °C). 1H-NMR (600 MHz, DMSO) δ 8.61 (1H, s, H2), 7.93 (1H, s, H5′), 7.62 (1H, d, J = 3.6 Hz, H6), 6.63 (1H, d, J = 3.6 Hz, H5), 4.88 (2H, dd, J = 6.7, 4.5 Hz, CH2), 4.81 (2H, dd, J = 6.7, 4.6 Hz, CH2), 4.59 (2H, t, J = 1.8 Hz, CH-Fc), 4.27–4.21 (2H, m, CH-Fc), 3.91 (5H, s,Cp-Fc). 13C-NMR (151 MHz, DMSO) δ 150.70 (C4), 150.59 (C7a), 150.27 (C2), 145.16 (C4′), 131.09 (C6), 120.58 (C5′), 116.69 (C4a), 98.76 (C5), 75.60 (Cq-Fc), 69.12 (Cp-Fc), 68.18 (CH-Fc), 66.13 (CH-Fc), 48.80 (CH2), 44.54 (CH2). Anal. calcd. for C20H17ClFeN6: C, 55.52; H, 3.96; N, 19.42. Found: C, 55.56; H, 3.95; N, 19.37.
4-(Pyrrolidin-1-yl)-7-[2-(4-ferrocenyl-1,2,3-triazol-1-yl)ethyl]-7H-pyrrolo[2,3-d]pyrimidine (34b) Compound 34b was prepared using the above-mentioned procedure using compound 32b (0.68 mmol) and ethynylferrocene (172.2 mg, 0.82 mmol) to obtain 34b as orange powder (122 mg, 38 %, m.p. = 200 °C). 1H-NMR (300 MHz, DMSO) δ 8.12 (1H, s, H2), 7.89 (1H, s, H5′), 6.96 (1H, d, J = 3.6 Hz, H6), 6.53 (1H, d, J = 3.6 Hz, H5), 4.80 (2H, t, J = 5.5 Hz, CH2), 4.73–4.64 (2H, m, CH2), 4.63–4.59 (2H, m, CH-Fc), 4.29–4.24 (2H, m, CH-Fc), 3.94 (5H, s, Cp-Fc), 3.66 (4H, s, CH2-pyrrolidine), 1.91 (4H, s, CH2-pyrrolidine). 13C-NMR (75 MHz, DMSO) δ 155.19 (C4), 151.71 (C2), 150.31 (C7a), 145.56 (C4′), 123.87 (C6), 121.03 (C5′), 103.11 (C4a), 101.23 (C5), 76.26 (Cq-Fc), 69.64 (Cp-Fc), 68.63 (CH-Fc), 66.63 (CH-Fc), 49.46 (CH2), 47.90 (CH2-pyrrolidine), 44.27 (CH2). Anal. calcd. for C24H25FeN7: C, 61.68; H, 5.39; N, 20.98. Found: C, 61.63; H, 5.38; N, 21.04.
4-(Piperidin-1-yl)-7-[2-(4-ferrocenyl-1,2,3-triazol-1-yl)ethyl]-7H-pyrrolo[2,3-d]pyrimidine (34c) Compound 34c was prepared using the above-mentioned procedure using compound 32c (0.64 mmol) and ethynylferrocene (162.8 mg, 0.77 mmol) to obtain 34c as orange powder (55 mg, 17 %, m.p. = 171 °C). 1H-NMR (600 MHz, DMSO) δ 8.16 (1H, s, H2), 7.89 (1H, s, H5′), 7.03 (1H, d, J = 3.6 Hz, H6), 6.54 (1H, d, J = 3.6 Hz, H5), 4.81 (2H, t, J = 5.8 Hz, CH2), 4.67 (2H, t, J = 5.7 Hz, CH2), 4.61 (2H, d, J = 1.5 Hz, CH-Fc), 4.28–4.24 (2H, m, CH-Fc), 3.93 (5H, s, Cp-Fc), 3.82–3.79 (4H, m, CH2-pipridin), 1.64–1.60 (2H, m, CH2-pipridin), 1.52 (4H, d, J = 3.7 Hz, CH2-pipridin). 13C-NMR (75 MHz, DMSO) δ 156.63 (C4), 151.27 (C7a), 151.20 (C2), 145.60 (C4′), 124.33 (C6), 121.05 (C5′), 102.59 (C4a), 101.40 (C5), 76.22 (Cq-Fc), 69.65 (Cp-Fc), 68.63 (CH-Fc), 66.63 (CH-Fc), 49.41 (CH2), 46.63 (CH2-piperidine), 44.30 (CH2), 25.87 (CH2-piperidine), 24.65 (CH2-piperidine). Anal. calcd. for C25H27FeN7: C, 62.38; H, 5.65; N, 20.37. Found: C, 62.42; H, 5.64; N, 20.31.
6-Chloro-9-[2-(4-ferrocenyl-1,2,3-triazol-1-yl)ethyl]-9H-purine (35a) Compound 35a was prepared using the above-mentioned procedure using compound 33a (0.76 mmol) and ethynylferrocene (193.2 mg, 0.92 mmol) to obtain 35a as orange powder (123 mg, 37 %, m.p. = 240 °C).1H-NMR (300 MHz, DMSO) δ 8.76 (1H, s, H2), 8.50 (1H, s, H5′), 8.02 (1H, s, H8), 4.94–4.90 (2H, m, CH2), 4.84 (2H, d, J = 6.1 Hz, CH2), 4.60 (2H, d, J = 1.6 Hz, CH-Fc), 4.28–4.25 (2H, m, CH-Fc), 3.94 (5H, s, Cp-Fc). 13C-NMR (75 MHz, DMSO) δ 152.43 (C6), 152.06 (C2), 149.52 (C4), 147.73 (C8), 145.87 (C4′), 131.16 (C5), 121.23 (C5′), 76.02 (Cq-Fc), 69.64 (Cp-Fc), 68.70 (CH-Fc), 66.67 (CH-Fc), 48.88 (CH2), 44.37 (CH2). Anal. calcd. for C19H16ClFeN7: C, 52.62; H, 3.72; N, 22.61. Found: C, 52.56; H, 3.71; N, 22.66.
6-(Pyrrolidin-1-yl)-9-[2-(4-ferrocenyl-1,2,3-triazol-1-yl)ethyl]-9H-purine (35b) Compound 35b was prepared using the above-mentioned procedure using compound 33b (0.68 mmol) and ethynylferrocene (172.2 mg, 0.82 mmol) to obtain 35b as orange powder (124 mg, 39 %, m.p. = 230 °C). 1H-NMR (300 MHz, DMSO) δ 8.21 (1H, s, H2), 7.96 (1H, s, H5′), 7.84 (1H, s, H8), 4.86 (2H, t, J = 5.6 Hz, CH2), 4.70 (2H, t, J = 5.6 Hz, CH2), 4.62 (2H, t, J = 1.8 Hz, CH-Fc), 4.31–4.21 (2H, m, CF-Fc), 3.95 (7H, s, Cp-Fc), 3.59 (2H, s, CH2-pyrrolidine), 1.89 (4H, s, CH2-pyrrolidine). 13C-NMR (75 MHz, DMSO) δ 152.90 (C6), 152.72 (C2), 150.36 (C4), 145.73 (C4’), 140.32 (C8), 121.12 (C5’), 119.71 (C5), 76.13 (Cq-Fc), 69.64 (Cp-Fc), 68.65 (CH-Fc), 66.63 (CH-Fc), 48.94 (CH2-pyrrolidine), 43.45 (CH2-pyrrolidine). Anal. calcd. for C23H24FeN8: C, 58.98; H, 5.17; N, 23.93. Found: C, 58.92; H, 5.16; N, 23.96.
6-(Piperidin-1-yl)-9-[2-(4-ferrocenyl-1,2,3-triazol-1-yl)ethyl]-9H-purine (35c) Compound 35c was prepared using the above-mentioned procedure using compound 33c (0.32 mmol) and ethynylferrocene (162.8 mg, 0.77 mmol) to obtain 35c as orange powder (47 mg, 15 %, m.p. = 233 °C). 1H-NMR (300 MHz, DMSO) δ 8.22 (1H, s, H2), 7.97 (1H, s, H5′), 7.88 (1H, s, H8), 4.87 (2H, t, J = 5.5 Hz, CH2), 4.70 (2H, t, J = 5.7 Hz, CH2), 4.66–4.55 (2H, m, CH-Fc), 4.30–4.22 (2H, m, CH-Fc), 4.14 (4H, bs, CH2-piperidin), 3.94 (5H, s, Cp-Fc), 1.62 (2H, s, CH2-piperidine), 1.52 (4H, s, CH2-piperidine). 13C-NMR (75 MHz, DMSO) δ 153.52 (C6), 152.39 (C2), 150.95 (C4), 145.75 (C4′), 121.14 (C5′), 119.22 (C5), 76.10 (Cq-Fc), 69.65 (Cp-Fc), 68.66 (CH-Fc), 66.64 (CH-Fc), 48.93 (CH2), 43.48 (CH2), 26.04 (CH2-piperidine), 24.68 (CH2-piperidine). Anal. calcd. for C24H26FeN8: C, 59.76; H, 5.43; N, 23.23. Found: C, 59.70; H, 5.42; N, 23.28.
3.8. Kinetic Solubility Assay
The compounds’ DMSO stock solutions in concentration of 10 mM were serially diluted by factor 3.3×, 3×, 3.3× and 3× in DMSO. Prepared solutions were spiked into phosphate-buffered saline (PBS, 100 mM) to obtain final compounds’ concentrations of 100, 30, 10, 3 and 1 µM (3 µL of prepared dilutions were spiked to 297 µL of PBS, with final DMSO content of 1%, v/v). All compounds were tested in two replicas per concentration. Prepared solutions were incubated at 37 °C for 2 h with gentle shaking. Blank solutions were also prepared by spiking DMSO in PBS buffer (1% of DMSO, v/v). After the incubation, absorbance of suspensions was measured by Microplate reader Infinite F500 (Tecan, Männedorf, Switzerland) at 620 nm and compared to a blank control to obtain the tested solutions’ absorbance, which is proportionally increased with concentration of insoluble particles. Standard compounds α-naphtoflavone (low solubility) and sulfaphenazole (high solubility) were used as controls. Compound/control samples are compared to the blank solution. Precipitation occurred if significant increase of sample absorbance is observed, i.e., when its absorbance is 3-fold standard deviation of average blank absorbance. Results were expressed as an estimated solubility range (lower and upper bound).
3.9. Chrom logD Determination
The distribution coefficient, Chrom logD, was determined from compounds’ gradient retention times by employing a high-performance liquid chromatography method with fast acetonitrile gradient. Compounds were prepared for analysis in final concentration of 1.25 mM by dilution of 10 mM stock solutions in DMSO with acetonitrile. Aqueous mobile phase was 50 mM ammonium acetate adjusted with ammonia solution to pH 7.4 and the organic mobile phase was acetonitrile. A reversed phase HPLC column Luna C18, 50 × 3 mm i.d., 5 µm particle size (Phenomenex, Torrance, CA, USA) was used for analysis. Sample injection volume was 2 µL and the flow rate was 1 mL min
−1. All compounds were analysed in duplicates. Fast-gradient elution was as follows: from 0 to 100% of acetonitrile (0−3 min), 100% of acetonitrile (3.0−3.5 min), from 100 to 0% of acetonitrile (3.5−3.7 min), and re-equilibration with 100% of aqueous mobile phase (3.7−5.0 min). All measurements were performed on an HPLC-DAD instrument Agilent 1100 Series (Agilent Technologies, Santa Clara, CA, USA) coupled with a mass spectrometer Micromass Quattro API (Waters, MA, USA). MassLynx software, version 4.1 (Waters, Manchester, UK) was used for data acquisition and processing. Before compound analysis, a calibration step was performed. A set of standard compounds with literature CHI values [
36] were plotted against gradient retention times to obtain a calibration equation, which was used to determine CHI value from the compounds’ retention times. Chrom logD values were calculated from the CHI value using the equation Chrom logD = 0.0857 × CHI − 2. [
38]
3.10. ADME Properties
3.10.1. Permeability and P-glicoprotein Substrate Assessment
MDCKII-hMDR1 cell monolayer was used for determination of permeability and P-gp substrate assessment. MDCKII-hMDR1 cells (Solvo Biotechnology, Szeged, Hungary) were grown in the controlled atmosphere (37 °C, 95% humidity, 5% CO2) in MDCK cell culture medium containing Dulbecco’s Modified Eagle Medium (DMEM) + 10% Fetal bovine serum (FBS, inactivated) + 1% glutamax-100 + 1% antibiotic/antimycotic + 1% of Non-essential amino acids Minimum Essential Medium (MEM NEAA). Cultures were split every 3–4 days using 0.05% Trypsin-EDTA. Cells were seeded 4 days prior to the experiment in concentration of 0.3 × 106 cells mL−1 and cultured in CO2 incubator. On the experiment day, cell monolayers were washed with Dulbecco’s phosphate buffer saline (D-PBS) and equilibrated for 45 min in CO2 incubator in D-PBS containing 1% DMSO (1% v/v) with or without Elacridar (2 µM). Compounds, in final concentration of 10 µM, were prepared in transport medium containing Lucifer yellow (100 µM), with DMSO contentment of 1% (v/v) and in the presence and without Elacridar. Lucifer yellow calibration curve was prepared by serial dilution (12 points, with 100 µM as the highest point). Monolayer integrity was examined by fluorescent measurement on a Microplate reader Infinite F500 (Tecan) by using an excitation of 485 nm and emission of 530 nm. Experiment was started by applying the solutions containing test compounds to apical and basolateral side of the cell monolayer. Starting compound concentration (C0) was sampled at the start of the experiment and apical and basolateral compartments were sampled after incubation at 37 °C with gentle shaking for 60 min. Compound were tested in duplicate. Amprenavir was used as a control with low permeability and as a P-gp substrate without inhibitor presence, turning into a highly permeable compound with the inhibitor present. Diclofenac was used as a control with high permeability and no interaction with P-gp.
Samples were analyzed on an ABSciex API 4000 Triple Quadrupole Mass Spectrometer (Sciex, Division of MDS Inc., Toronto, ON, Canada) coupled to a UHPLC Nexera X2 (Shimadzu, Kyoto, Japan). Samples (1 µL) were injected onto a reversed phase HPLC column Luna Omega Polar C18, 30 × 2.1 mm i.d., 1.6 µm particle size (Phenomenex) which was kept at 50 °C. Aqueous solution was 0.1% formic acid in deionized water and the organic mobile phase was water/acetonitrile/formic acid mixture (90/10/0.1,
v/
v/
v). Flow rate was kept at 0.7 mL min
−1 for all measurements. Fast-gradient elution was as follows: 2% of organic mobile phase through 0.15 min, from 2 to 95% of organic mobile phase (0.15−0.7 min), 95% of organic mobile phase (0.7−1.1 min), from 95 to 2% of organic mobile phase (1.1−1.11 min), and re-equilibration with 98% of aqueous mobile phase (1.11−1.5 min). Positive ion mode with turbo spray, an ion source temperature of 550 °C and a dwell time of 75 ms were utilized for mass spectrometric detection. Quantitation was performed using multiple reaction monitoring (MRM) at the specific transition corresponding to the compound of interest. Warfarin was used as an internal standard. The ratios between compound and internal standard peak areas were used instead of real compound concentration. Apparent permeability coefficient, P
app (nm s
−1) values were calculated by using the following Equation (1):
where: dQ/dT = permeability rate; C
0 = initial concentration in donor compartment; A = surface area of the cell monolayer (0.7 cm
2).
The efflux ratio in the presence or absence of the P-gp inhibitor was calculated from P
app values, using following Equation (2):
3.10.2. Metabolic Stability in Liver Microsomes
Metabolic stability of compounds was assessed in human and mouse liver microsomes (Corning, Tewksbury, MA, USA). Compounds, in final concentration of 1 µM, were incubated in phosphate buffer (50 mM, pH 7.4) for 60 min at 37 °C together with liver microsomes and NADPH generating system (nicotinamide adenine dinucleotide phosphate (NADP, 0.5 mM), glucose-6-phosphate (G6P, 5 mM), glucose-6-phosphate dehydrogenase (1.5 U mL
−1) and magnesium chloride (0.5 mM)). Also, compounds were incubated without the presence of NADPH cofactor, as a buffer stability control. Metabolic activity of liver microsomes was verified by including testosterone and propranolol as positive controls, as well as caffeine as a negative control. Sampling was performed at six time points (0, 10, 20, 30, 45 and 60 min), followed by reaction termination by addition of acetonitrile/methanol mixture (2:1,
v/
v) containing dicofenac as internal standard. Samples were analyzed as previously described for samples from permeability assay. The in vitro half-life (t
1/2) was calculated from the slope of the linear regression by plotting ln % remaining of parent compound against incubation time. In vitro intrinsic clearance, CL
int was calculated from half-life using following Equation (3):
where 52.5 mg protein/g liver is used as a constant.
Predicted in vivo hepatic clearance, in vivo CL
h was calculated as follows:
where: LW/BW = liver weight/body weight [g kg
−1]; 25.7 (human), 87.5 (mouse); Q = LBF = liver blood flow [mL min
−1 kg
−1]; 21/1.26 (human), 131/7.86 (mouse).
Predicted in vivo hepatic clearance can be expressed as %LBF and calculated as follows:
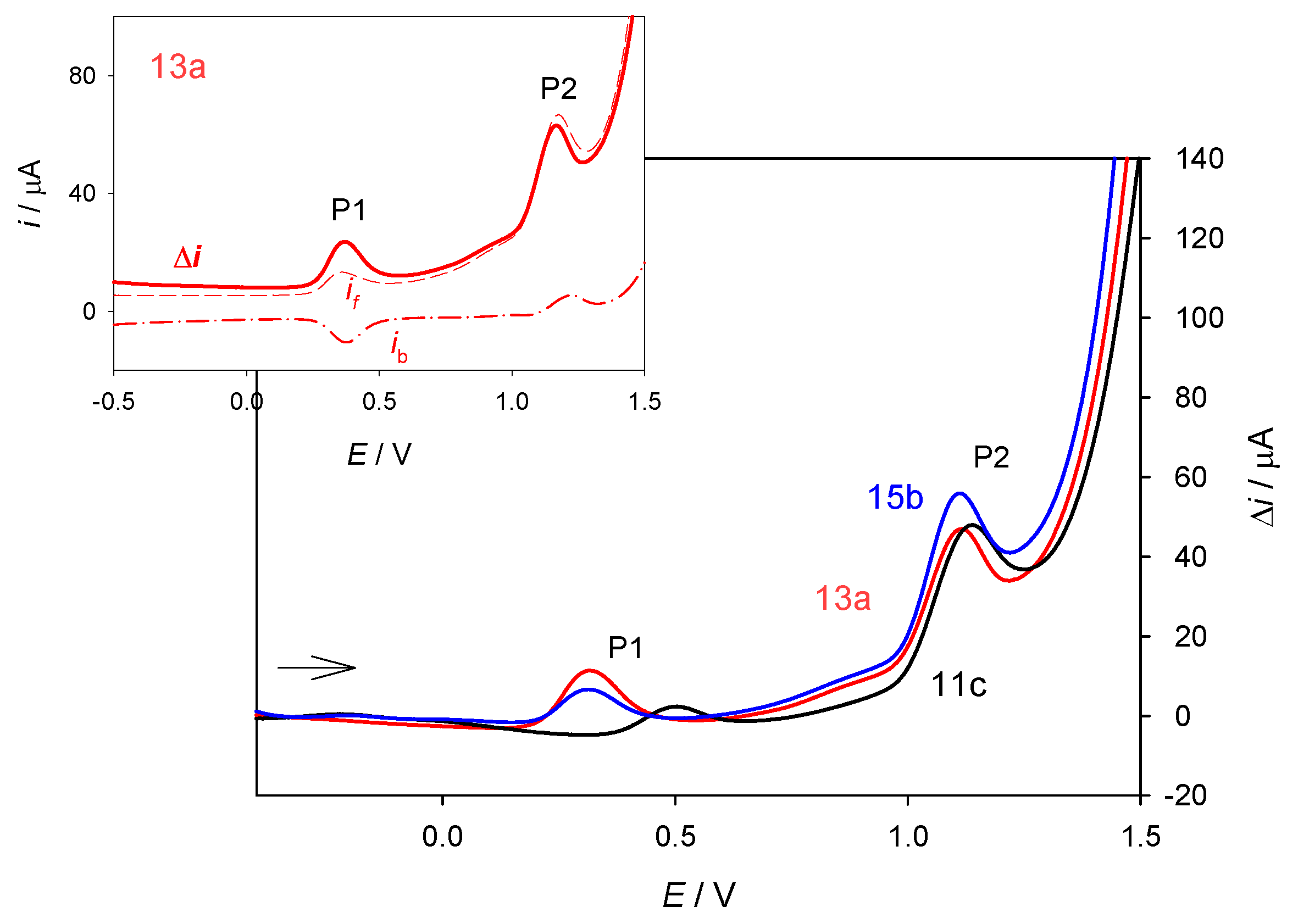
3.11. Voltammetric Measurements
All chemicals used in the experiments were of the best grade commercially available (Sigma-Aldrich) and were used without further purification. Stock standard solutions of compounds 11c, 13a, 15b (c = 2.0 × 10−3 mol/L) and 6-chloropurine (c = 1.2 × 10−2 mol/L) were prepared from dry pure substances in dimethyl sulfoxide (DMSO, p.a.), purchased from Kemika (Zagreb, Croatia). Stock standard solution of ethynyl ferrocene (c = 1.1 × 10−2 mol/L) was prepared from dry pure substance in ethanol (Kemika). For the supporting electrolyte, analytical grade NaClO4 (Kemika) was used. Water was deionized by the Millipore Milli-Q system to the resistivity ≥ 18 MΩcm. Voltammetric measurements were carried out using the computer-controlled electrochemical system „PGSTAT 101“ (Eco-Chemie, Utrecht, The Netherlands), controlled by the electrochemical software “NOVA 1.5”. A three-electrode system (BioLogic, Claix, France) with glassy carbon electrode (GCE) of 3 mm in diameter as a working electrode, Ag/AgCl (3 mol/L NaCl) as a reference electrode and a platinum wire as a counter electrode were used. All potentials were expressed versus Ag/AgCl (3 mol/L NaCl) reference electrode. The supporting electrolyte (0.5 mol/L NaClO4), adjusted to the desired pH value, was placed in the electrochemical cell and the required aliquot of the standard analyte solution was added. The solution in the electrochemical cell was degassed with high purity nitrogen for 10 min before measurement, and the nitrogen blanket was maintained thereafter. Before each run, the glassy carbon working electrode was polished with diamond suspension in spray (grain size 6 µm) and rinsed with ethanol and deionized water. All experiments were performed at room temperature. The presented results are reported as the mean value of three independent measurements.