Abstract
l-Carnitine is an amino acid derivative widely known for its involvement in the transport of long-chain fatty acids into the mitochondrial matrix, where fatty acid oxidation occurs. Moreover, l-Carnitine protects the cell from acyl-CoA accretion through the generation of acylcarnitines. Circulating carnitine is mainly supplied by animal-based food products and to a lesser extent by endogenous biosynthesis in the liver and kidney. Human muscle contains high amounts of carnitine but it depends on the uptake of this compound from the bloodstream, due to muscle inability to synthesize carnitine. Mitochondrial fatty acid oxidation represents an important energy source for muscle metabolism particularly during physical exercise. However, especially during high-intensity exercise, this process seems to be limited by the mitochondrial availability of free l-carnitine. Hence, fatty acid oxidation rapidly declines, increasing exercise intensity from moderate to high. Considering the important role of fatty acids in muscle bioenergetics, and the limiting effect of free carnitine in fatty acid oxidation during endurance exercise, l-carnitine supplementation has been hypothesized to improve exercise performance. So far, the question of the role of l-carnitine supplementation on muscle performance has not definitively been clarified. Differences in exercise intensity, training or conditioning of the subjects, amount of l-carnitine administered, route and timing of administration relative to the exercise led to different experimental results. In this review, we will describe the role of l-carnitine in muscle energetics and the main causes that led to conflicting data on the use of l-carnitine as a supplement.
1. Introduction
Carnitine (3-hydroxy-4-N-trimethylaminobutyrate) represents an amino acid derivative and a micronutrient that plays a key role in intermediary metabolism with the main function being the transport of long-chain fatty acids from the cytosol to the mitochondrial matrix where fatty acid β-oxidation occurs. Other established functions of carnitine are the preservation of membrane integrity [1], the stabilization of a physiologic coenzyme A (CoASH)/acetyl-CoA ratio in mitochondria, and the reduction of lactate production [2,3].
Carnitine is present in most, if not all, animal species and in several micro-organisms and plants. In the human body, carnitine is mainly found in a free form (free carnitine) and in the form of acylcarnitine esters, a pool of carnitine bounded to various acyl groups that are delivered throughout the body for a wide range of functions [4]. At rest, the skeletal muscle carnitine pool is distributed as 80–90% free carnitine, 10–20% short-chain acylcarnitines (with a number of carbon atoms <10), and <5% long-chain acylcarnitines (with a number of carbon atoms >10) [5].
It has been estimated that the total carnitine content in the human body is about 300 mg/kg, with about 95% stored intracellularly in the heart and skeletal muscle, and the remaining part in the liver, kidney, and plasma [6]. The amount of circulating plasma carnitine accounts for only 0.5% of total body carnitine [7].
Carnitine does not undergo metabolic changes and, therefore, is eliminated as free carnitine in urine. However, a part of carnitine that is not absorbed at the level of the small intestine is completely degraded by bacteria in the large intestine to produce trimethylamine, a quaternary amine that, after enterocyte absorption, is oxidized in the liver by flavin-containing monooxygenase 3 to form trimethylamine-N-oxide (TMAO) [8].
In light of the fundamental role of carnitine in fatty acids β-oxidation for energy production, studies have been conducted to understand whether carnitine supplementation can affect skeletal muscle function and athletic performance in healthy individuals [2,9]. There is controversy as to whether or not carnitine administration can improve physical performance. The difference in exercise intensity, the training or conditioning of the subjects, the amount of carnitine administered, the route of administration and the timing of administration relative to the exercise led to different experimental results [10]. In this review, we will summarize the main roles of carnitine in the skeletal muscle energetics and the principal pharmacokinetic characteristics of carnitine in order to highlight the main critical points for carnitine supplementation during exercise.
2. Endogenous Synthesis and Cell Transport of l-Carnitine
Humans obtain carnitine mainly from the diet, predominantly from animal-based food products such as red meat, chicken, fish and dairy. Only 25% of carnitine comes from endogenous synthesis [2,11]. Carnitine biosynthesis requires two essential amino acids: l-lysine, which provides the carbon backbone and l-methionine that furnishes the N-methyl group [12]. The pathway of carnitine synthesis requires vitamin C, vitamin B6, niacin, and reduced iron as cofactors [13,14]. Lysine residues in some proteins undergo N-methylation using S-adenosylmethionine as a methyl donor, forming 6-N-trimethyl-lysine residues which are converted in carnitine in four enzymatic steps, namely hydroxylation at carbon 3, aldol cleavage, oxidation of the aldehyde to 4-butyrobetaine and hydroxylation of 4-butyrobetaine at carbon 3 [15]. The last enzymatic step is catalyzed by the enzyme 4-butyrobetaine dioxygenase to yield carnitine. It is generally recognized that the first three reactions of carnitine synthesis are widely distributed in the body but the final reaction is only present in the liver, kidney, and brain [7]. Thus, other tissues depend on carnitine uptake from the circulation.
Carnitine homeostasis is guaranteed by intestinal absorption from the diet, modest endogenous synthesis and efficient renal reabsorption. Intestinal absorption of carnitine occurs by both passive (small intestine and colon) and active (duodenum and ileum) mechanisms [16]. The renal process ensures tubular reabsorption of about 98–99% of filtered carnitine thus conserving a normal carnitine level also in strict vegetarians (vegans) and lacto-ovo-vegetarians [17]. The renal threshold for carnitine excretion is about 50 µmol/L that is about the same value of the normal carnitine plasma concentration [18]. Thus, when plasma carnitine concentration raises, the renal excretion increases whereas the reabsorption decreases, thus maintaining baseline carnitine plasma level [18]. The opposite occurs when the plasma carnitine level is decreased, as in the case of low dietary intake of carnitine [18]. Neither renal reabsorption nor changes in dietary carnitine intake appear to affect the rate of endogenous carnitine synthesis [19,20].
The plasma membrane transport of carnitine is made by the carnitine/organic cation transporters (OCTN), a family of transporters that consists of three isoforms, i.e., OCTN1 (Scarnitine22A4) and OCTN2 (Scarnitine22A5) in humans and animals [21], and Octn3 (Slc22a21) in mice and humans [22]. Among them, OCTN2 is a high-affinity plasma membrane protein that can transport carnitine in a sodium-dependent manner and whose functional defect causes primary systemic carnitine deficiency [23]. OCTN2 is mainly involved in maintaining carnitine homeostasis that results from intestinal absorption, distribution to tissues, and renal excretion/reabsorption. OCTN2 has a high affinity for carnitine and its derivatives and it is present not only at the level of polarized cells of intestine, kidney, placenta and mammary gland but also in other tissues such as liver, heart, testis, skeletal muscle and brain [24,25]. OCTN1, highly expressed at the renal epithelium level and, to a lesser extent, in other tissues, is also involved in carnitine transport but with a lower affinity with respect to OCTN2 [23,24]. Moreover, other transporters such as ATB0,+ [26] and carnitine transporter-2 [27] can also carry carnitine.
Among different peripheral tissues, muscle is probably the main target of carnitine transport. About 90–95% of total carnitine is concentrated in muscle, and the ratio between muscle carnitine concentration and plasma concentration is around 50:1 [28].
Carnitine transport defect, associated with mutations in the OCTN2 transporter, causes profound intracellular and plasma carnitine depletion and very high renal carnitine excretion [29]. The consequence of such a defect is the inability of carnitine to perform its functions, particularly in β-oxidation. Metabolic and clinical aberrations associated with the defect in carnitine transport can be prevented by oral supplementation of pharmacological carnitine dosages (100–400 mg/kg/day). Due to the defective OCTN2, this treatment can increase the plasma carnitine level nearly to normal values but does not bring muscle carnitine concentration to normal values [29].
3. Role of Carnitine in Mitochondrial Fatty Acid Transport and β-Oxidation
Fatty acids from different sources (diet, endogenous de novo synthesis and adipose tissue hydrolysis) can cross the plasma membrane and enter the muscle cell by fatty acid transport proteins (FATPs), fatty acid translocase (FAT/CD36), caveolins and plasma membrane fatty acid-binding proteins (FABPpm) [30]. Once in the cell, long-chain fatty acids are activated to fatty acyl-CoAs by a family of acyl-CoA synthetases (ACS) identified at the plasma membrane, mitochondria, and lipid droplets [31]. FATPs have also ACS activity [32]. The activation reaction, that requires ATP and CoASH, traps long-chain fatty acyl-CoA thioesters inside the cell and, maintaining intracellular free long-chain fatty acids to low concentrations, allows further fatty acid uptake.
As long-chain acyl-CoA derivatives cannot directly cross the mitochondrial inner membrane, the entry of acyl-CoAs into the mitochondrial matrix for β-oxidation is guaranteed, under normal conditions, by carnitine palmitoyltransferase-1 (CPT-1), located on the external surface of the outer mitochondrial membrane [33]. CPT-1 catalyzes the transfer of acyl groups from acyl-CoAs to carnitine to produce acylcarnitines and free CoASH (Figure 1). The reaction catalyzed by CPT-1 is tightly regulated to control both fatty acid β-oxidation and ketone body formation [34,35]. There are three different isoforms of CPT-1: CPT-1A, CPT-1B, and CPT-1C [35]. CPT-1 A is expressed in the liver, brain, kidney, lung, spleen, intestine, pancreas, ovary and fibroblasts [36]. CPT-1B is the muscle isoform and is highly expressed in skeletal muscle, heart and testis [36]. CPT1-C is the neuron-specific isoform, but its function in neural metabolism remains controversial [37]. CPT-1 is sensitive to inhibition by malonyl-CoA [38]. Since malonyl-CoA represents the product of acetyl-CoA carboxylase, a key enzyme of the cytosolic fatty acid synthesis pathway, malonyl-CoA can reciprocally regulate fatty acid synthesis and oxidation [38]. Thus, a high rate of fatty acid synthesis results in a low rate of fatty acid oxidation, and vice versa.
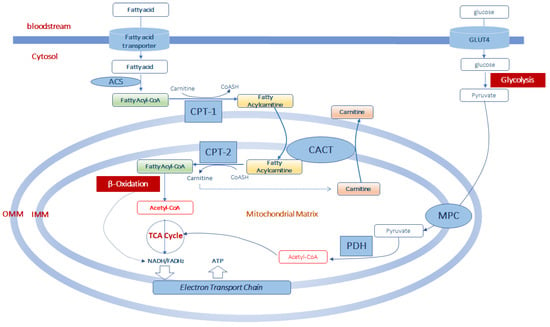
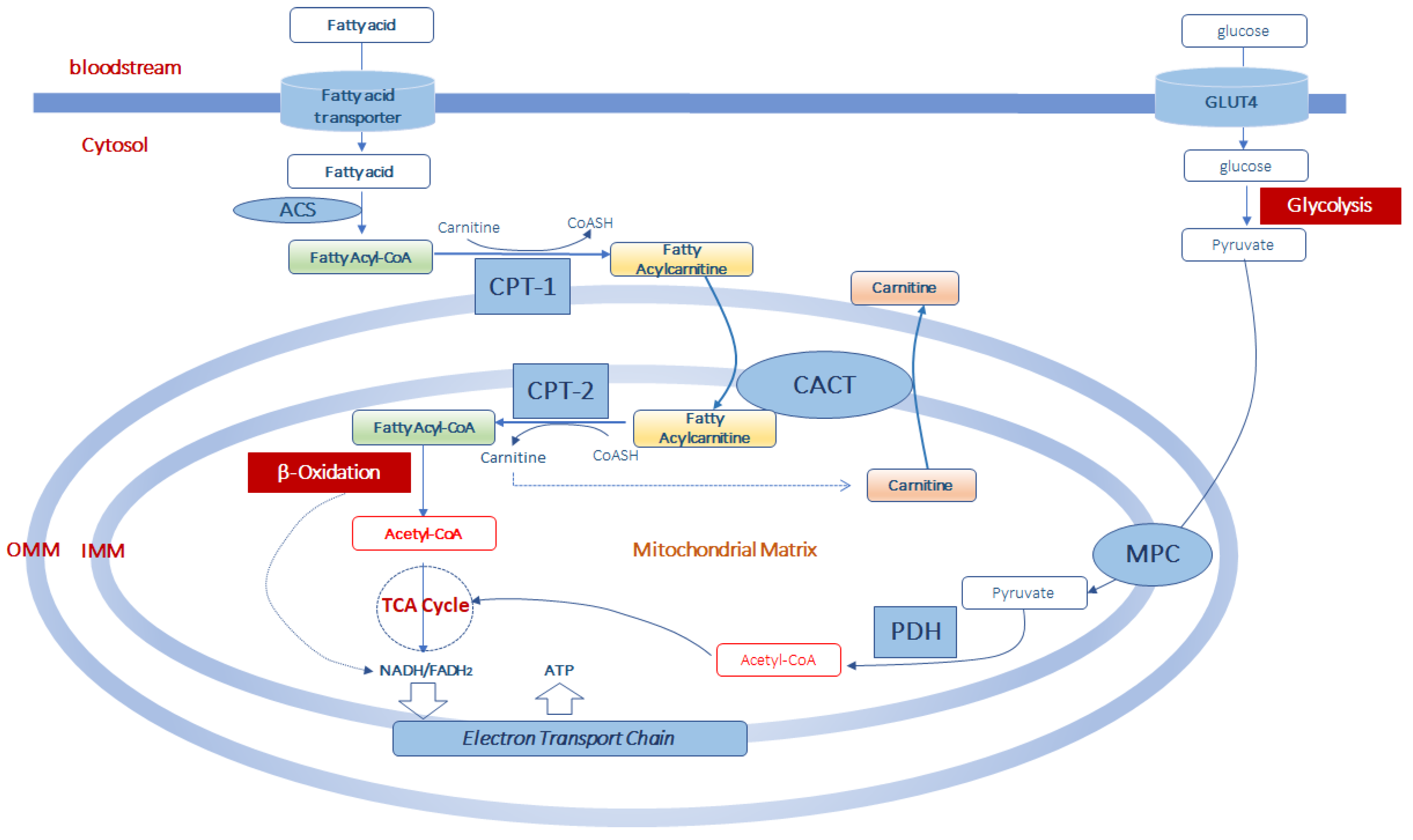
Figure 1.
Acetyl-CoA production from fatty acid and glucose metabolism in muscle mitochondria. Fatty acids enter the cells by fatty acid transporters. Once in the cell they are activated to fatty acyl-CoA by acyl-CoA synthetase (ACS) before entering the mitochondria. At the level of the outer part of the outer mitochondrial membrane (OMM), fatty acyl-CoAs are bind to carnitine, by carnitine palmitoyltransferase-1 (CPT-1) activity, to form fatty acylcarnitine derivatives which diffuse through the outer mitochondrial membrane. Thus, the formed fatty acylcarnitines are transported across the inner mitochondrial membrane (IMM) via carnitine-acylcarnitine translocase (CACT). In the mitochondrial matrix, CPT-2 converts fatty acylcarnitines back to fatty acyl-CoAs, which enters the β-oxidation pathway, and to free carnitine which can exit from the mitochondria in exchange with other acylcarnitines through CACT. Mitochondrial acetyl-CoA is generated from both β-oxidation of fatty acids and from pyruvate. Pyruvate is formed in the glycolytic pathway form glucose which enters the muscle cell via the glucose transporter type 4 (GLUT4). Pyruvate is transported in the mitochondrial matrix by the pyruvate carrier (MPC) of the inner mitochondrial membrane. Once in the mitochondria, pyruvate is converted into acetyl-CoA throughout the complex of the pyruvate dehydrogenase (PDH). This acetyl-CoA, together with that formed in the β-oxidation pathway can enter the tricarboxylic acid cycle (TCA cycle) to produce equivalent donors in the form of NADH (H+) and FADH2 which are oxidized in the mitochondrial electron transport chain to produce ATP.
Once synthesized, acylcarnitines cross the outer mitochondrial membrane, which is permeable to small molecules [39], and translocate into the matrix by the carnitine/acylcarnitine transporter (CACT), a protein of the inner mitochondrial membrane belonging to the mitochondrial carrier protein family [40,41]. CACT transports acylcarnitines in the matrix in exchange for intramitochondrial free carnitine [41,42,43]. Once in the mitochondrial matrix, fatty acyl units are transferred from carnitine to CoASH by carnitine palmitoyltransferase-2 (CPT-2) to form acyl-CoAs that enter the β-oxidation pathway (Figure 1).
The fatty acid β-oxidation, a mitochondrial process regulated by both nutritional and hormonal factors [44,45], involves the repetitive removal of two carbon units, in the form of acetyl-CoA, from the fatty acyl-chain. The enzymes 2-enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase and 3-oxoacyl-CoA thiolase are subsequently involved in the fatty acid β-oxidation process to complete the conversion of the acyl-CoA ester into acetyl-CoAs. The last step releases the two-carbon acetyl-CoA and a ready primed acyl-CoA that takes another turn down the spiral. In total each turn of the β-oxidation spiral produces reduced cofactors in the form of NADH (H+) and FADH2, and one acetyl-CoA. Further oxidation of acetyl-CoAs via the Krebs cycle produces ATP and additional NADH (H+) and FADH2 [46]. Electrons from NADH (H+) and FADH2 pass through the electron transport chain, localized at the inner mitochondrial membrane, to oxygen which is reduced to water. During the passage of electrons throughout the different complexes of the electron transport chain is released energy to generate a transmembrane proton gradient which is used to generate ATP (Figure 1). The complete oxidation of a fatty acid produces numerous ATP molecules.
While the transport of long-chain acyl-CoAs, such as palmitoyl-CoA, oleoyl-CoA and linoleoyl-CoA, requires the presence of both CPT-1 and CPT-2, the transport and oxidation of medium- (C6-C12) and short-chain (C4-C6) fatty acids seems largely independent from the carnitine shuttle [47,48].
The hydroxyl group of carnitine can also form esters with acetate, to generate acetyl-carnitine, or with different carboxylic acids, including fatty acids of all chain lengths, to form a wide array of acylcarnitines [49].
4. Muscle l-Carnitine Selects Fuels during Exercise
During muscle contraction, aerobic and anaerobic metabolic pathways contribute to the energy supply according to the duration and intensity of muscle effort [50]. There is an inverse relationship between the duration and the intensity of muscle effort, i.e., very intense muscle contractions can be maintained only for a short duration, while less intense contractions can be sustained or repeated for longer periods. Exercise can be classified as low-to-moderate intensity (<70% maximal oxygen consumption, VO2max), or high intensity (>75%, VO2max) [51]. At low work rates, muscle aerobic metabolism predominates, lactate does not accumulate, and exercise can be sustained. By contrast, at high work rates, lactate accumulates in muscle and blood, and subjects become rapidly fatigued.
Fats and carbohydrates represent the two major energy sources for physical exercise. Either source can predominate, depending upon the duration and intensity of exercise, degree of prior physical conditioning, and the composition of the diet consumed in the days before the exercise [52]. To guarantee a high delivery of these substrates to the muscle, during the passage from a moderate to maximal exercise intensity a higher blood flow to legs has been demonstrated in steady-state 1-leg kicking performed for several minutes [53].
During an exercise with a VO2max below 50%, fatty acid oxidation is favored as a source of energy. In this condition, the utilization of energy from fat increases endurance, spares the use of muscle glycogen and delays the onset of fatigue [54]. At a moderate intensity of work (between 50% and 70% VO2max), both glucose and fatty acids may be used, with a gradual increase in glucose over fat consumption with incremental exercise intensity. Increasing the intensity of physical activity (above 75%, VO2max), fatty acid utilization begins to decline and, above this intensity exercise, glycogen becomes the major fuel supporting ATP synthesis for muscle activities [55]. Glycogen represents a major source for ATP regeneration during prolonged exercise (>1 h) and high-intensity intermittent exercise [56] so that when glycogen stores are limited, exercise cannot continue [57]. Anyway, due to its limited store, glycogen is rapidly depleted under high-intensity endurance exercise and this phase coincides with fatigue [58,59]. However, it must be considered that an inadequate supply of oxygen to the working muscle has been correlated with rapid glycogen depletion via anaerobic glycolysis [60]. During anaerobic glycolysis, lactic acid is produced which promotes acidosis. A strong connection between pH regulation and muscle work capacity has been shown, suggesting that acidosis strongly contributes to fatigue [61].
5. How l-Carnitine Can Regulate Fatty Acid Oxidation during Physical Exercise
Different studies have been conducted to explain the regulatory points influencing the decline of fatty acid oxidation in skeletal muscle during high-intensity endurance exercise [62,63,64]. Considering that carnitine is necessary for the transmembrane fatty acid transport, changes in the muscle free carnitine availability may contribute to the regulation of fatty acid oxidation. Generally, intramitochondrial acetyl-CoA can be generated by both fatty acid β-oxidation and by the activity of the multi-enzymatic complex of the pyruvate dehydrogenase, from the glycolytic pyruvate. Mitochondrial acetyl-CoA can be buffered by conversion in acetyl-carnitines by the ACS enzyme. When acetyl-CoA is generated over its metabolism in the tricarboxylic acid cycle, high amount of muscle free carnitine can be entrapped in the form of acetyl-carnitine thus decreasing the free carnitine pool and compromising the catalytic rate of CPT-1 [65].
Indeed, when the glycolytic flux is increased, as during the initial phase of a high-intensity exercise, the amount of pyruvate and then of mitochondrial acetyl-CoA is increased. In the mitochondria, by the activity of the pyruvate dehydrogenase, the glycolytic pyruvate is converted in acetyl-CoA which represents a negative modulator of the pyruvate dehydrogenase. Thus, to allow glycolysis to proceed, acetyl-CoA is bound to carnitine and converted into acetyl-carnitine by acetyl-carnitine synthase enzyme. Actually, during the passage from a low to a high-intensity exercise, muscle acetyl-CoA and acetyl-carnitine increase in parallel [55]. Thus, a high glycolytic flux, reducing muscle free carnitine availability, limits the CPT-1 reaction and ultimately mitochondrial fatty acid import and oxidation. When the glycolytic flux is reduced, as in the case of prolonged exercise or at lower exercise intensity, the reduced supply of glycolysis-derived acetyl-CoA allows saving a greater amount of free carnitine, which can then be used for the transport and oxidation of fatty acids into mitochondria. Thus, it appears evident that muscle total free carnitine availability influences fuel selection during exercise.
However, in contrast to the idea that supplementation of carnitine can accelerate fatty acid oxidation, data from animal models demonstrated that carnitine supplementation during high-intensity exercise increases glucose oxidation at the expense of fatty acid oxidation [66]. The mechanism at the basis of this phenomenon is that, by decreasing the mitochondrial glycolytic acetyl-CoA by acetyl-carnitine synthesis, the pyruvate dehydrogenase enzyme can be activated, thus facilitating complete glucose utilization with a reduction in lactate accumulation. Therefore, the raised availability of carbohydrate during exercise increases glycolysis and pyruvate flux through the pyruvate dehydrogenase with a corresponding decrease in the rate of fat oxidation. This observation should lead to paying attention to the use of carnitine as a supplement to increase the burning of fatty acids during high-intensity exercise, in particular when carnitine administration is preceded by a load of carbohydrates before exercise.
Moreover, a defect in β-oxidation of long-chain fatty acids, mainly associated with a deficiency in very-long-chain acyl-CoA dehydrogenase, an enzyme of the β-oxidation pathway, can result in accumulation of C14-C18 acyl-CoAs in mitochondria [67]. These molecules are converted into acylcarnitine esters to leave the mitochondria. As a result of the increased production of acylcarnitines, blood free carnitine may decrease. Physical exercise in these patients results in a further decrease in free carnitine concentration in skeletal muscle and the appearance of clinical symptoms [67]. It has been widely discussed whether supplementation of exogenous carnitine is advisable to recover intracellular carnitine concentrations in these patients. What emerges from studies is that increased supply of carnitine can result in a further increase, in these patients, of long-chain acylcarnitines compounds associated with possibly lethal heart rhythm disturbance [68].
6. Can Carnitine Supplementation Be Useful in Physical Exercise?
Due to the availability of carnitine over-the-counter, the use of carnitine as a supplement is often disproportionate among endurance athletes. Furthermore, since it has been suggested that carnitine saves muscle glycogen and promotes fat oxidation [69], its integration is recommended to lose weight. Carnitine supplementation has been also reported to spare the use of amino acids as energy sources during exercise making them potentially available for new protein synthesis [70]. This notion justifies the use of carnitine to increase muscle mass during endurance exercise. Indeed, a study conducted on dogs demonstrated that supplemented carnitine experienced less protein degradation as a result of exercise [71].
Despite many years of research on the role of carnitine in muscle metabolism, it is yet not completely established whether carnitine supplementation can improve physical performance in healthy subjects. Data on the effect of carnitine supplementation on exercise performance, maximal aerobic capacity, blood lactate response, or substrate utilization during exercise yielded contradictory results (Table 1). The oral administration of 4 g/day of carnitine for 2 weeks was demonstrated to significantly increase VO2max in competitive walkers [72]. The same result was obtained by Dragan and coll. in two different studies, one conducted on 40 and the other on 110 top athletes, both orally supplemented with 3 g carnitine for 3 weeks [73,74]. Dragan and coll. also reported that 1 g of carnitine for 6 weeks or 2 g carnitine supplementation for 10 days induced higher performances in 7 junior athletes [75] and 1 g carnitine intravenously furnished ameliorated physical output and muscle contraction [74]. Two grams of carnitine furnished orally before a high-intensity exercise, in moderately trained males, also demonstrated to increase VO2max [76]. An effect on mitochondrial respiratory chain enzyme activities was also measured after the oral supplementation of 2 g carnitine for 4 weeks in 14 endurance athletes [77]. Vice versa, 6 g carnitine furnished for a couple of weeks in 8 healthy males failed to influence the VO2max and the respiratory exchange ratio [78].

Table 1.
Effect of carnitine supplementation on muscle energetics and exercise performance.
Exercise performance was slightly improved in 9 untrained subjects orally supplemented with 2 g of carnitine for 2 weeks [79]. Besides, Siliprandi and coll. provided evidence that 2 g/day of carnitine furnished 1 h before exercise enhanced high-intensity exercise by increasing pyruvate dehydrogenase activity and preventing lactate accumulation [80]. Instead, 2 g carnitine supplementation 2 h before the start of marathon [81] or 2 g furnished for 7 days [82] did not affect physical performance and recovery after exercise. Similarly, no significant increase in exercise performance was measured after 4g of carnitine supplementation for 3 months to healthy males [83]. The single supplementation of 3 g or 4 g of carnitine to 26 athletes before exercise led to a decrease in lactate and heart rate responses to the running speeds in supplemented groups compared with placebo, suggesting carnitine taken before physical exercise prolonged exhaustion [84].
Contrasting results were obtained on the effect of carnitine supplementation on substrate utilization during exercise. The supplementation of 2 g or 3 g of carnitine for two weeks did not affect substrate utilization during prolonged moderate-intensity exercise or short-duration exercise [85,86]. No effect on substrate utilization was also measured in 10 exercising subjects after oral supplementation of 2 g carnitine for 4 weeks [87] and after 5 g of oral carnitine supplementation for 5 days in 7 moderately trained males [88]. A similar result was obtained by other different studies [81,89,90,91,92]. A certain effect on lipid utilization by muscle during exercise was reported after the oral supplementation of 2 g of carnitine in 10 trained athletes [93]. Interestingly, a recent work conducted on vegetarians demonstrated that 2 g of carnitine supplemented for 12 weeks did not affect muscle functions and energy metabolism of these subjects [94].
The discrepancies in published researches could be related to the features of carnitine’s pharmacokinetic. First of all, it must be considered that, when orally furnished, carnitine bioavailability is only 5–15% [18,95]. Moreover, as the renal threshold for carnitine secretion is near the physiological plasma carnitine concentration, when the plasma concentration of carnitine dominates this threshold, carnitine is promptly eliminated in the urine [18,95]. In fact, after an acute administration of a large amount of carnitine, most of the carnitine is recovered in the urine [91]. Considering the amount of total carnitine content in the body (about 20 g) [9], the low carnitine bioavailability and the large amount of carnitine lost with the urine after integration, a very high dose of carnitine and for long periods should be integrated to obtain a real increase in the amount of muscular carnitine in healthy subjects.
After supplementation, carnitine must be transported from the plasma into tissues. To this respect, it has been reported that muscle, with respect to other tissues, has a much lower net turnover of carnitine [96]; this feature makes muscle, unlike other tissues, particularly refractory to carnitine supplementation. Furthermore, we must consider that, in physiological conditions, carnitine is transported in the muscle against a concentration gradient and that OCTN2 transporter, having a Km value (3–5 µM) below plasma carnitine concentrations (30–50 µM), is saturated at the physiological carnitine concentrations [11,70]. Thus, it is unlikely that an increased plasma concentration of carnitine can cause higher transport of carnitine in the muscle.
Based on these considerations, it is conceivable to predict that oral carnitine supplementation would have little if any effect on muscle carnitine content in humans, and thus on muscle metabolism. Indeed, studies have demonstrated that even if long-term carnitine administration in humans increases plasma carnitine concentrations it does not increase muscle carnitine content [18].
Studies have reported that carnitine can have a protective effect against muscle disruption after strenuous exercise, with a significant reduction in the release of cytosolic proteins such as myoglobin and creatine kinase [70]. Moreover, carnitine supplementation, by increasing the muscle androgen receptor, may improve protein signaling, needed for recovery after exercise [70]. In addition, mitigating oxidative stress during exercise, carnitine can facilitate muscle recovery [70,71].
Other studies reported, both in animals and humans, an effect of carnitine supplementation on vascular function through endothelial function modulation. Indeed, muscle contractile force in dogs was significantly increased and accompanied by an elevated blood flow after infusion with carnitine and in the absence of increased muscle carnitine content and the result was due to an effect on the vasculature surrounding of the muscle [97,98]. Thus, these studies pointed out an effect that is independent of muscle carnitine accretion and energy production. A crossover study demonstrated that 3 weeks of carnitine supplementation increased, with respect to placebo in which decreased, the post-prandial brachial artery flow-mediated dilation [99].
It has to be considered that an increase in muscle carnitine content was obtained supplementing carnitine in the presence of high circulating insulin level (>50 mU/L) and the effect was correlated with an increase in Na+/K+ pump activity [100,101]. The same result has been also demonstrated with alternative oral nutrients that stimulate insulin secretion such as whey proteins [102].
Finally, it must be considered that carnitine metabolism by gut microbiota produces trimethylamine which is then converted into TMAO in the liver [8]. Indeed, dietary carnitine supplementation can significantly increase serum TMAO levels in both humans and rodents [103]. Painfully, TMAO has been demonstrated to promote atherosclerosis and to increase cardiovascular risk in animals [102]. In human studies, a significant positive correlation has been found between fasting plasma TMAO levels and major cardiovascular events [8,104,105,106,107,108]. Thus, due to the production of TMAO, the beneficial effect of carnitine is controversial and deserves more attention.
Moreover, oral supplementation of carnitine in animals with defects in the very-long-chain acyl-CoA dehydrogenase enzyme can induce significant accumulation in skeletal muscle of acylcarnitine compounds associated with possibly lethal heart rhythm disturbance [68].
7. Conclusions
Carnitine is a compound with well-established functions in cellular metabolism, in particular for energetics purposes, as it supports fatty acid transport into mitochondria for β-oxidation and consequent ATP production. It appears that skeletal muscle carnitine availability influences fuel selection during exercise. Indeed, during high-intensity exercise, carnitine availability limits CPT-1 reaction thus reducing fatty acid oxidation. Theoretically, carnitine supplementation should increase carnitine muscle content thus improving fatty acid oxidation and exercise function in healthy humans. However, so far, no scientific basis supports improvement in exercise performance for healthy individuals or athletes after carnitine supplementation. Moreover, considering that carnitine metabolism produces TMAO which has been recently recognized as a novel risk factor for cardiovascular diseases [104,105,106,107,108], the use of uncontrolled amounts of carnitine as supplements must be carefully reviewed.
Since carnitine is often used by athletes with no clear understanding of its effects and risks, it becomes critical to provide information about the characteristics of this compound and its probable harmful effects on health. With the adoption of educational approaches, it will be possible to reduce the risk associated with dietary and nutritional carnitine supplementation, in particular among athletes.
Author Contributions
Conceptualization: A.M.G.; Writing—original draft preparation: A.M.G. and S.L.; Supervision: G.V.G.; Writing—review: A.G.; The authors declare that the content of this paper has not been published or submitted for publication elsewhere. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siliprandi, N.; Di Lisa, F.; Menabo, R. Clinical use of carnitine. Past, present and future. Adv. Exp. Med. Biol. 1990, 272, 175. [Google Scholar] [PubMed]
- Karlic, H.; Lohninger, A. Supplementation of l-carnitine in athletes: Does it make sense? Nutrition 2004, 20, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Friolet, R.; Hoppeler, H.; Krähenbühl, S. Relationship between the coenzyme A and the carnitine pools in human skeletal muscle at rest and after exhaustive exercise under normoxic and acutely hypoxic conditions. J. Clin. Investig. 1994, 94, 1490–1495. [Google Scholar] [CrossRef]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Regensteiner, J.G.; Wolfel, E.E.; Ruff, L.; Brass, E.P. Carnitine and acylcarnitine metabolism during exercise in humans. Dependence on skeletal muscle metabolic state. J. Clin. Investig. 1989, 84, 1167–1173. [Google Scholar] [CrossRef]
- Brass, E.P. Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin. Ther. 1995, 17, 176–185. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Brass, E.P. Carnitine and sports medicine: Use or abuse? Ann. N. Y. Acad. Sci. 2004, 1033, 67–78. [Google Scholar] [CrossRef]
- Heinonen, O.J. Carnitine and physical exercise. Sports Med. 1996, 22, 109–132. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Tanphaichitr, V.; Broquist, H.P. Role of lysine and e-N-trimethyllysine in carnitine biosynthesis. Ii. Studies in the rat. J. Biol. Chem. 1973, 248, 2176–2181. [Google Scholar]
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef]
- Borum, P.R. Carnitine. Annu. Rev. Nutr. 1983, 3, 233–259. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Calvo-Castro, I.; Fernández-Fernández, C.; Donapetry-García, C.; Pedre-Piñeiro, A.M. Significance of l-carnitine for human health. IUBMB Life 2017, 69, 578–594. [Google Scholar] [CrossRef]
- Hamilton, J.W.; Li, B.U.; Shug, A.L.; Olsen, W.A. Carnitine transport in human intestinal biopsy specimens. Demonstration of an active transport system. Gastroenterology 1986, 91, 10–16. [Google Scholar] [CrossRef]
- Rebouche, C.J. Kinetics, pharmacokinetics, and regulation of l-carnitine and acetyl-l-carnitine metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 30–41. [Google Scholar] [CrossRef]
- Evans, A.M.; Fornasini, G. Pharmacokinetics of L-carnitine. Clin. Pharmacokinet. 2003, 42, 941–967. [Google Scholar] [CrossRef]
- Rebouche, C. Carnitine: Modern Nutrition in Health and Disease; Shils, M., Shike, M., Ross, A., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2006; pp. 537–544. [Google Scholar]
- Mohammed, A.; Majid, A.; Ayman, W.E. Carnitine inborn errors of metabolism. Molecules 2019, 24, 3251. [Google Scholar]
- Tamai, I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: Scarnitine22A4, Scarnitine22A5 and Slc22a21). Biopharm. Drug Dispos. 2013, 34, 29–44. [Google Scholar] [CrossRef]
- Lamhonwah, A.M.; Ackerley, C.A.; Tilups, A.; Edwards, V.D.; Wanders, R.J.; Tein, I. OCTN3 is a mammalian peroxisomal membrane carnitine transporter. Biochem. Biophys. Res. Commun. 2005, 338, 1966–1972. [Google Scholar] [CrossRef]
- Stanley, C.A.; DeLeeuw, S.; Coates, P.M.; Vianey-Liaud, C.; Divry, P.; Bonnefont, J.P.; Saudubray, J.M.; Haymond, M.; Trefz, F.K.; Breningstall, G.N.; et al. Chronic cardiomyopathy and weakness or acute coma in children with a defect in carnitine uptake. Ann. Neurol. 1991, 30, 709–716. [Google Scholar] [CrossRef]
- Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; Barilli, A.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional activity of l-carnitine transporters in human airway epithelial cells. Biochim. Biophys. Acta 2016, 1858, 210–219. [Google Scholar] [CrossRef]
- Tein, I.; Bukovac, S.W.; Xie, Z.W. Characterization of the human plasmalemmal carnitine transporter in cultured skin fibroblasts. Arch. Biochem. Biophys. 1996, 329, 145–155. [Google Scholar] [CrossRef]
- Nakanishi, T.; Hatanaka, T.; Huang, W.; Prasad, P.D.; Leibach, F.H.; Ganapathy, M.E.; Ganapathy, V. Na+- and Cl- coupled active transport of carnitine by the amino acid transporter ATB(0,1) from mouse colon expressed in HRPE cells and Xenopus oocytes. J. Physiol. 2001, 532, 297–304. [Google Scholar] [CrossRef]
- Enomoto, A.; Wempe, M.F.; Tsuchida, H.; Shin, H.J.; Cha, S.H.; Anzai, N.; Goto, A.; Sakamoto, A.; Niwa, T.; Kanai, Y.; et al. Molecular identification of a novel carnitine transporter specific to human testis. Insights into the mechanism of carnitine recognition. J. Biol. Chem. 2002, 277, 36262–36271. [Google Scholar] [CrossRef]
- Georges, B.; Le Borgne, F.; Galland, S.; Isoir, M.; Ecosse, D.; Grand-Jean, F.; Demarquoy, J. Carnitine transport into muscular cells. Inhibition of transport and cell growth by mildronate. Biochem. Pharmacol. 2000, 59, 1357–1363. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Scaglia, F. Disorders of carnitine biosynthesis and transport. Mol. Genet. Metab. 2015, 116, 107–112. [Google Scholar] [CrossRef]
- Jain, S.S.; Luiken, J.J.; Snook, L.A.; Han, X.X.; Holloway, G.P.; Glatz, J.F.; Bonen, A. Fatty acid transport and transporters in muscle are critically regulated by Akt2. FEBS Lett. 2015, 589, 2769–2775. [Google Scholar] [CrossRef]
- Radif, Y.; Ndiaye, H.; Kalantzi, V.; Jacobs, R.; Hall, A.; Minogue, S.; Waugh, M.G. The endogenous subcellular localisations of the long chain fatty acid-activatingenzymes ACSL3 and ACSL4 in sarcoma and breast cancer cells. Mol. Cell. Biochem. 2018, 448, 275–286. [Google Scholar] [CrossRef]
- Jia, Z.; Pei, Z.; Maiguel, D.; Toomer, C.J.; Watkins, P.A. The fatty acid transport protein (FATP) family: Very long chain acyl-CoA synthetases or solute carriers? J. Mol. Neurosci. 2007, 33, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, C.L. Carnitine and carnitine palmitoyltransferase in fatty acid oxidation and ketosis. Fed. Proc. 1982, 41, 2853–2857. [Google Scholar] [PubMed]
- Rufer, A.C.; Thoma, R.; Hennig, M. Structural insight into function and regulation of carnitine palmitoyltransferase. Cell. Mol. Life Sci. 2009, 66, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Giudetti, A.M.; Bellanti, F.; Priore, P.; Rollo, T.; Tamborra, R.; Siculella, L.; Vendemiale, G.; Altomare, E.; Gnoni, G.V. Oxidation of hepatic carnitine palmitoyl transferase-I (CPT-I) impairs fatty acid beta-oxidation in rats fed a methionine-choline deficient diet. PLoS ONE 2011, 6, e24084. [Google Scholar] [CrossRef] [PubMed]
- Woldegiorgis, G.; Shi, J.; Zhu, H.; Arvidson, D.N. Functional characterization of mammalian mitochondrial carnitine palmitoyltransferases I and II expressed in the yeast Pichia pastoris. J. Nutr. 2000, 130, 310S–314S. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Wolfgang, M.J. Metabolomic profiling reveals a role for CPT1c in neuronal oxidative metabolism. BMC Biochem. 2012, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.W. Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J. Clin. Investig. 2012, 122, 1958–1959. [Google Scholar] [CrossRef]
- Zeth, K.; Thein, M. Porins in prokaryotes and eukaryotes: Common themes and variations. Biochem. J. 2010, 431, 13–22. [Google Scholar] [CrossRef]
- Indiveri, C.; Iacobazzi, V.; Tonazzi, A.; Giangregorio, N.; Infantino, V.; Convertini, P.; Console, L.; Palmieri, F. The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Mol. Asp. Med. 2011, 32, 223–233. [Google Scholar] [CrossRef]
- Tonazzi, A.; Galluccio, M.; Oppedisano, F.; Indiveri, C. Functional reconstitution into liposomes and characterization of the carnitine transporter from rat liver microsomes. Biochim. Biophys. Acta 2006, 1758, 124–131. [Google Scholar] [CrossRef][Green Version]
- Giudetti, A.M.; Stanca, E.; Siculella, L.; Gnoni, G.V.; Damiano, F. Nutritional and hormonal regulation of citrate and carnitine/acylcarnitine transporters: Two mitochondrial carriers involved in fatty acid metabolism. Int. J. Mol. Sci. 2016, 25, 817. [Google Scholar] [CrossRef] [PubMed]
- Priore, P.; Stanca, E.; Gnoni, G.V.; Siculella, L. Dietary fat types differently modulate the activity and expression of mitochondrial carnitine/acylcarnitine translocase in rat liver. Biochim. Biophys. Acta 2012, 1821, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Priore, P.; Gnoni, G.V.; Papa, S.; Zanotti, F.; Gnoni, A. 3,5-Diiodo-L-thyronine administration to hypothyroid rats rapidly enhances fatty acid oxidation rate and bioenergetic parameters in liver cells. PLoS ONE 2013, 8, e52328. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Taurino, F.; Damiano, F.; Siculella, L.; Sardanelli, A.M.; Gnoni, A. Acute administration of 3,5-diiodo-L-thyronine to hypothyroid rats stimulates bioenergetic parameters in liver mitochondria. J. Bioenerg. Biomembr. 2016, 48, 521–529. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar]
- Violante, S.; Ijlst, L.; Te Brinke, H.; Koster, J.; Tavares de Almeida, I.; Wanders, R.J.; Ventura, F.V.; Houten, S.M. Peroxisomes contribute to the acylcarnitine production when the carnitine shuttle is deficient. Biochim. Biophys. Acta 2013, 1831, 1467–1474. [Google Scholar] [CrossRef]
- Schrader, M.; Costello, J.; Godinho, L.F.; Islinger, M. Peroxisome-mitochondria interplay and disease. J. Inherit. Metab. Dis. 2015, 38, 681–702. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Westerblad, H.; Bruton, J.D.; Katz, A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 2010, 316, 3093–3099. [Google Scholar] [CrossRef]
- Scribbans, T.D.; Vecsey, S.; Hankinson, P.B.; Foster, W.S.; Gurd, B.J. The Effect of Training Intensity on VO2max in Young Healthy Adults: A Meta-Regression and Meta-Analysis. Int. J. Exerc. Sci. 2016, 9, 230–247. [Google Scholar]
- Askew, E.W. Role of fat metabolism in exercise. Clin. Sports Med. 1984, 3, 605–621. [Google Scholar] [PubMed]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [PubMed]
- Ranallo, R.F.; Rhodes, E.C. Lipid metabolism during exercise. Sports Med. 1998, 26, 29–42. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef]
- Hargreaves, M.; McConell, G.; Proietto, J. Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise in humans. J. Appl. Physiol. 1995, 78, 288–292. [Google Scholar] [CrossRef]
- Hermansen, L.; Hultman, E.; Saltin, B. Muscle glycogen during prolonged severe exercise. Acta Physiol. Scand. 1967, 71, 129–139. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef]
- Wan, J.J.; Qin, Z.; Wang, P.Y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef]
- Beneke, R.; Leithauser, R.M.; Ochentel, O. Blood lactate diagnostics in exercise testing and training. Int. J. Sports Physiol. Perform. 2011, 6, 8–24. [Google Scholar] [CrossRef]
- Messonnier, L.; Kristensen, M.; Juel, C.; Denis, C. Importance of pH regulation and lactate/H+ transport capacity for work production during supramaximal exercise in humans. J. Appl. Physiol. 2007, 102, 1936–1944. [Google Scholar] [CrossRef]
- van Hall, G. The physiological regulation of skeletal muscle fatty acid supply and oxidation during moderate-intensity exercise. Sports Med. 2015, 45, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Kiens, B. Regulation and limitations to fatty acid oxidation during exercise. J. Physiol. 2012, 590, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the factors that affect maximal fat oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. Molecular regulation of fatty acid oxidation in skeletal muscle during aerobic exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Broderick, T.L.; Quinney, H.A.; Lopaschuk, G.D. Carnitine stimulation of glucose oxidation in the fatty acid perfused isolated working rat heart. J. Biol. Chem. 1992, 267, 3758–3763. [Google Scholar] [PubMed]
- Primassin, S.; Ter Veld, F.; Mayatepek, E.; Spiekerkoetter, U. Carnitine supplementation induces acylcarnitine production in tissues of very long-chain acyl-CoA dehydrogenase-deficient mice, without replenishing low free carnitine. Pediatr. Res. 2008, 63, 632–637. [Google Scholar] [CrossRef]
- Bonnet, D.; Martin, D.; Pascale, D.L.; Villain, E.; Jouvet, P.; Rabier, D.; Brivet, m.; Saudubray, J.M. Arrhythmias and conduction defects as presenting symptoms of fatty acidoxidation disorders in children. Circulation 1999, 100, 2248–2253. [Google Scholar] [CrossRef]
- Pooyandjoo, M.; Nouhi, M.; Shab-Bidar, S.; Djafarian, K.; Olyaeemanesh, A. The effect of l-carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 970–976. [Google Scholar] [CrossRef]
- Fielding, R.; Riede, L.; Lugo, J.P.; Bellamine, A. l-Carnitine supplementation in recovery after exercise. Nutrients 2018, 10, 349. [Google Scholar] [CrossRef]
- Varney, J.L.; Fowler, J.W.; Gilbert, W.C.; Coon, C.N. Utilisation of supplemented l-carnitine for fuel efficiency, as an antioxidant, and for muscle recovery in Labrador retrievers. J. Nutr. Sci. 2017, 6. [Google Scholar] [CrossRef]
- Marconi, C.; Sassi, G.; Carpinelli, A.; Cerretelli, P. Effects of l-carnitine loading on the aerobic and anaerobic performance of endurance athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 54, 131–135. [Google Scholar] [CrossRef]
- Drăgan, G.I.; Vasiliu, A.; Georgescu, E.; Dumas, I. Studies concerning chronic and acute effects of l-carnitine on some biological parameters in elite athletes. Physiologie 1987, 24, 23–28. [Google Scholar] [PubMed]
- Drăgan, G.I.; Wagner, W.; Ploeşteanu, E. Studies concerning the ergogenic value of protein supply and l-carnitine in elite junior cyclists. Physiologie 1988, 25, 129–132. [Google Scholar] [PubMed]
- Drăgan, I.G.; Vasiliu, A.; Georgescu, E.; Eremia, N. Studies concerning chronic and acute effects of l-carnitina in elite athletes. Physiologie 1989, 26, 111–129. [Google Scholar] [PubMed]
- Vecchiet, L.; Di Lisa, F.; Pieralisi, G.; Ripari, P.; Menabò, R.; Giamberardino, M.A.; Siliprandi, N. Influence of l-carnitine administration on maximal physical exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Huertas, R.; Campos, Y.; Díaz, E.; Esteban, J.; Vechietti, L.; Montanari, G.; D’Iddio, S.; Corsi, M.; Arenas, J. Respiratory chain enzymes in muscle of endurance athletes: Effect of L-carnitine. Biochem. Biophys. Res. Commun. 1992, 188, 102–107. [Google Scholar] [CrossRef]
- Vukovich, M.D.; Costill, D.L.; Fink, W.J. Carnitine supplementation: Effect on muscle carnitine and glycogen content during exercise. Med. Sci. Sports Exerc. 1994, 26, 1122–1129. [Google Scholar] [CrossRef][Green Version]
- Greig, C.; Finch, K.M.; Jones, D.A.; Cooper, M.; Sargeant, A.J.; Forte, C.A. The effect of oral supplementation with l-carnitine on maximum and submaximum exercise capacity. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 457–460. [Google Scholar] [CrossRef]
- Siliprandi, N.; Di Lisa, F.; Pieralisi, G.; Ripari, P.; Maccari, F.; Menabo, R.; Giamberardino, M.A.; Vecchiet, L. Metabolic changes induced by maximal exercise in human subjects following l-carnitine administration. Biochim. Biophys. Acta 1990, 1034, 17–21. [Google Scholar] [CrossRef]
- Colombani, P.; Wenk, C.; Kunz, I.; Krähenbühl, S.; Kuhnt, M.; Arnold, M.; Frey-Rindova, P.; Frey, W.; Langhans, W. Effects of l-carnitine supplementation on physical performance and energy metabolism of endurance-trained athletes: A double-blind crossover field study. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 434–439. [Google Scholar] [CrossRef]
- Trappe, S.W.; Costill, D.L.; Goodpaster, B.; Vukovich, M.D.; Fink, W.J. The effects of l-carnitine supplementation on performance during interval swimming. Int. J. Sports Med. 1994, 15, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Wächter, S.; Vogt, M.; Kreis, R.; Boesch, C.; Bigler, P.; Hoppeler, H.; Krähenbühl, S. Long-term administration of l-carnitine to humans: Effect on skeletal muscle carnitine content and physical performance. Clin. Chim. Acta 2002, 318, 51–61. [Google Scholar] [CrossRef]
- Orer, G.E.; Guzel, N.A. The effects of acute l-carnitine supplementation on endurance performance of athletes. J. Strength Cond. Res. 2014, 28, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Broad, E.M.; Maughan, R.J.; Galloway, S.D. Carbohydrate, protein, and fat metabolism during exercise after oral carnitine supplementation in humans. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Broad, E.M.; Maughan, R.J.; Galloway, S.D.R. Effects of exercise intensity and altered substrate availability on cardiovascular and metabolic responses to exercise after oral carnitine supplementation in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 385–397. [Google Scholar] [CrossRef][Green Version]
- Oyono-Enguelle, S.; Freund, H.; Ott, C.; Gartner, M.; Heitz, A.; Marbach, J.; Maccari, F.; Frey, A.; Bigot, H.; Bach, A.C. Prolonged submaximal exercise and l-carnitine in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 58, 53–61. [Google Scholar] [CrossRef]
- Soop, M.; Björkman, O.; Cederblad, G.; Hagenfeldt, L.; Wahren, J. Influence of carnitine supplementation on muscle substrate and carnitine metabolism during exercise. J. Appl. Physiol. 1988, 64, 2394–2399. [Google Scholar] [CrossRef]
- Wyss, V.; Ganzit, G.P.; Rienzi, A. Effects of l-carnitine administration on VO2max and the aerobic-anaerobic threshold in normoxia and acute hypoxia. Eur. J. Appl Physiol. Occup. Physiol. 1990, 60, 1–6. [Google Scholar] [CrossRef]
- Decombaz, J.; Deriaz, O.; Acheson, K.; Gmuender, B.; Jequier, E. Effect of l-carnitine on submaximal exercise metabolism after depletion of muscle glycogen. Med. Sci. Sports Exerc. 1993, 25, 733–740. [Google Scholar] [CrossRef]
- Brass, E.P.; Hoppel, C.L.; Hiatt, W.R. Effect of intravenous l-carnitine on carnitine homeostasis and fuel metabolism during exercise in humans. Clin. Pharmacol. Ther. 1994, 55, 681–692. [Google Scholar] [CrossRef]
- Broad, E.M.; Maughan, R.J.; Galloway, S.D. Effects of four weeks l-carnitine L-tartrate ingestion on substrate utilization during prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Gorostiaga, E.M.; Maurer, C.A.; Eclache, J.P. Decrease in respiratory quotient during exercise following l-carnitine supplementation. Int. J. Sports Med. 1989, 10, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Novakova, K.; Kummer, O.; Bouitbir, J.; Stoffel, S.D.; Hoerler-Koerner, U.; Bodmer, M.; Roberts, P.; Urwyler, A.; Ehrsam, R.; Krähenbühl, S. Effect of l-carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetarians. Eur. J. Nutr. 2016, 55, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Harper, P.; Elwin, C.E.; Cederblad, G. Pharmacokinetics of intravenous and oral bolus doses of l-carnitine in healthy subjects. Eur. J. Clin. Pharmacol. 1988, 35, 555–562. [Google Scholar] [CrossRef]
- Stephens, F.B.; Constantin-Teodosiu, D.; Laithwaite, D.; Simpson, E.J.; Greenhaff, P.L. Insulin stimulates l-carnitine accumulation in human skeletal muscle. FASEB J. 2006, 20, 377–379. [Google Scholar] [CrossRef]
- Dubelaar, M.L.; Lucas, C.M.; Hulsmann, W.C. The effect of l-carnitine on force development of the latissimus dorsi muscle in dogs. J. Card. Surg. 1991, 6, 270–275. [Google Scholar] [CrossRef]
- Hulsmann, W.C.; Dubelaar, M.L. Carnitine requirement of vascular endothelial and smooth muscle cells in imminent ischemia. Mol. Cell. Biochem. 1992, 116, 125–129. [Google Scholar] [CrossRef]
- Volek, J.S.; Judelson, D.A.; Silvestre, R.; Yamamoto, L.M.; Spiering, B.A.; Hatfield, D.L.; Vingren, J.L.; Quann, E.E.; Anderson, J.M.; Maresh, C.M.; et al. Effects of carnitine supplementation on flow-mediated dilation and vascular inflammatory responses to a high-fat meal in healthy young adults. Am. J. Cardiol. 2008, 102, 1413–1417. [Google Scholar] [CrossRef]
- Stephens, F.B.; Constantin-Teodosiu, D.; Laithwaite, D.; Simpson, E.J.; Greenhaff, P.L. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J. Clin. Endocrinol. Metab. 2006, 91, 5013–5018. [Google Scholar] [CrossRef]
- Steenge, G.R.; Simpson, E.J.; Greenhaff, P.L. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J. Appl. Physiol. 2000, 89, 1165–1171. [Google Scholar] [CrossRef]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2019, 129, 373–387. [Google Scholar] [CrossRef]
- Wu, W.K.; Chen, C.C.; Liu, P.Y.; Panyod, S.; Liao, B.Y.; Chen, P.C.; Kao, H.L.; Kuo, H.C.; Kuo, C.H.; Chiu, T.H.T.; et al. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut 2019, 68, 1439–1449. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of serum Trimethylamine N-Oxide (TMAO) levels with early atherosclerosis in humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Hazen, S.L. Microbiome, Trimethylamine N-Oxide (TMAO), and cardiometabolic disease. Transl. Res. 2017, 179, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chang, M.; Guo, Y.; Zhang, L.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018, 17, 286. [Google Scholar] [CrossRef] [PubMed]
- Arsenian, M.A. Carnitine and its derivatives in cardiovascular disease. Prog. Cardiovasc. Dis. 1997, 40, 265–286. [Google Scholar] [CrossRef]
- Gao, X.; Tian, Y.; Randell, E.; Zhou, H.; Sun, G. Unfavorable associations between serum trimethylamine N-oxide and l-carnitine levels with components of metabolic syndrome in the Newfoundland population. Front. Endocrinol. 2019, 10, 168. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).