Lignans and Their Derivatives from Plants as Antivirals
Abstract
1. Introduction
2. Antiviral Effect and MOA
2.1. Classical Lignans
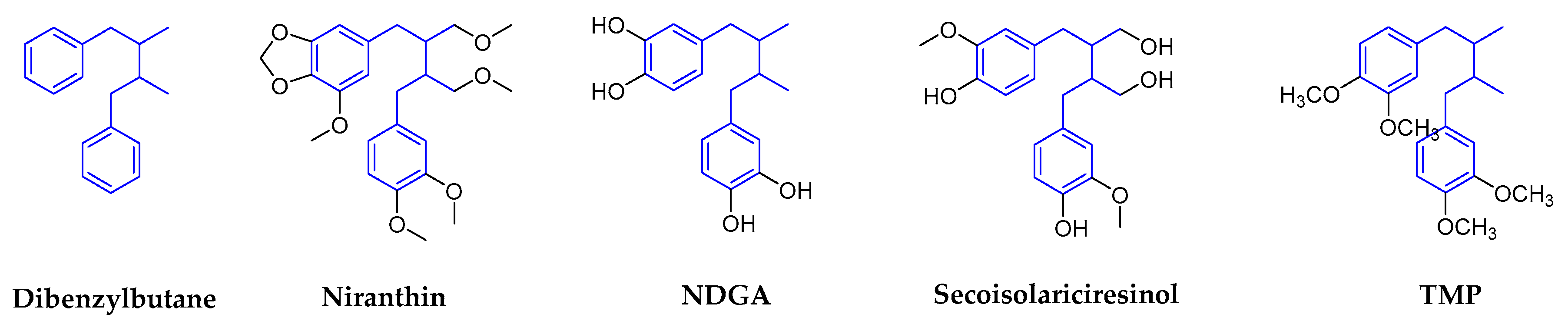
2.1.1. Dibenzylbutanes
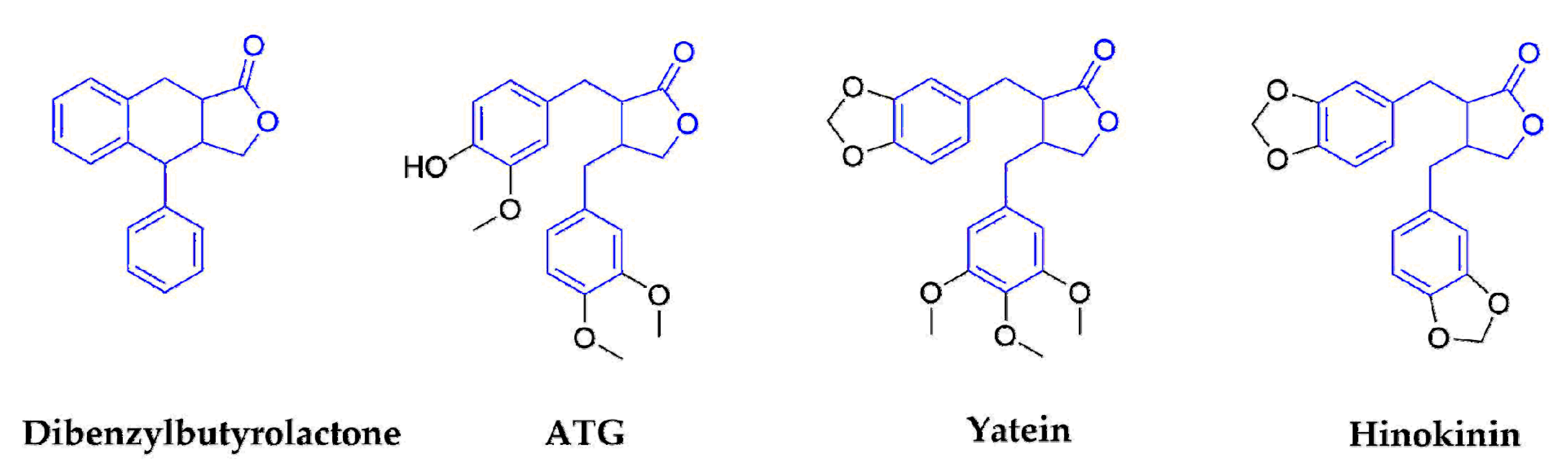
2.1.2. Dibenzylbutyrolactones
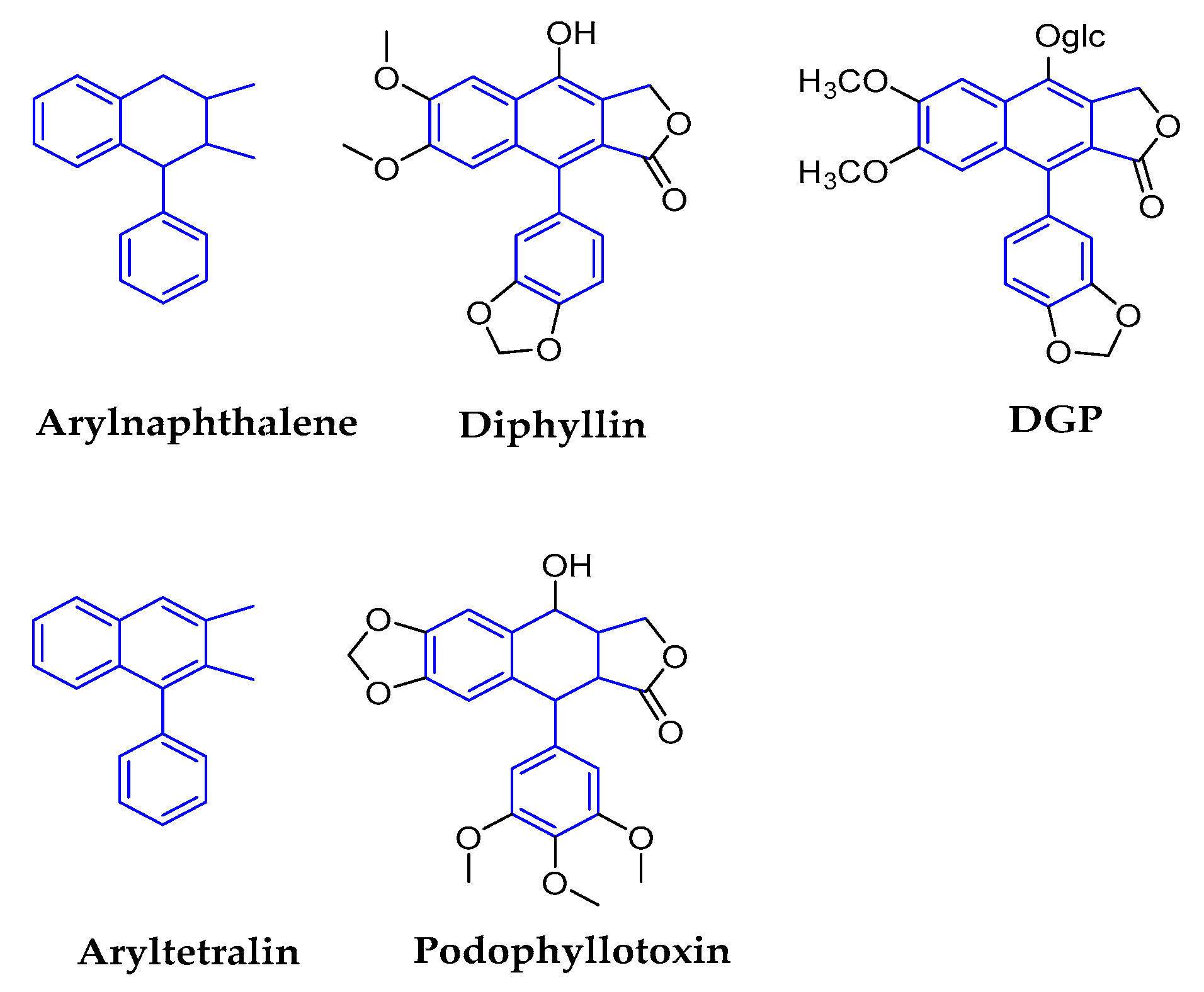
2.1.3. Arylnaphthalenes/Aryltetralins
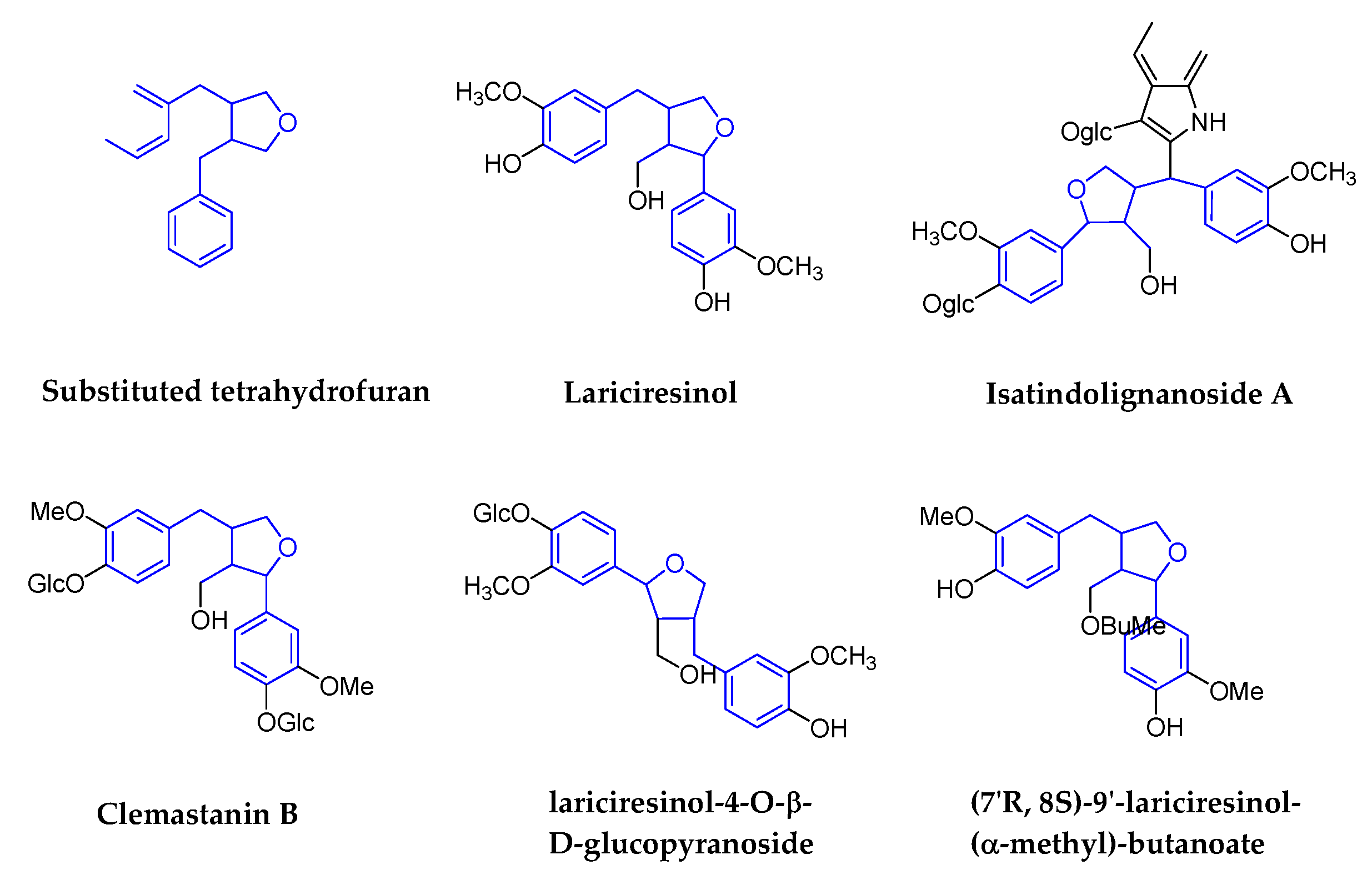
2.1.4. Substituted Tetrahydrofurans
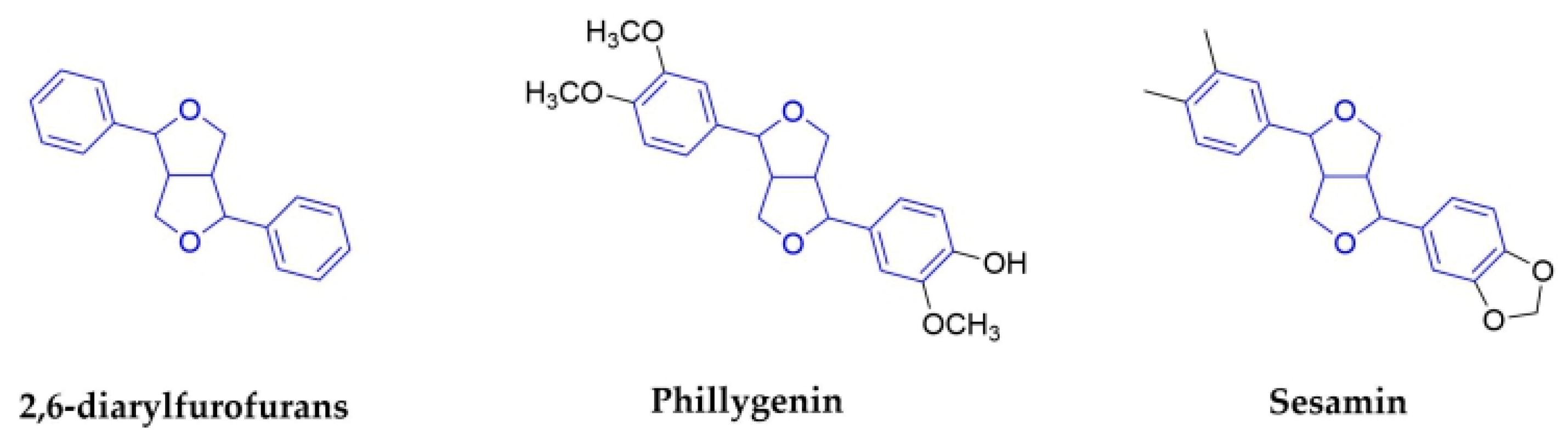
2.1.5. 2,6-Diarylfurofurans
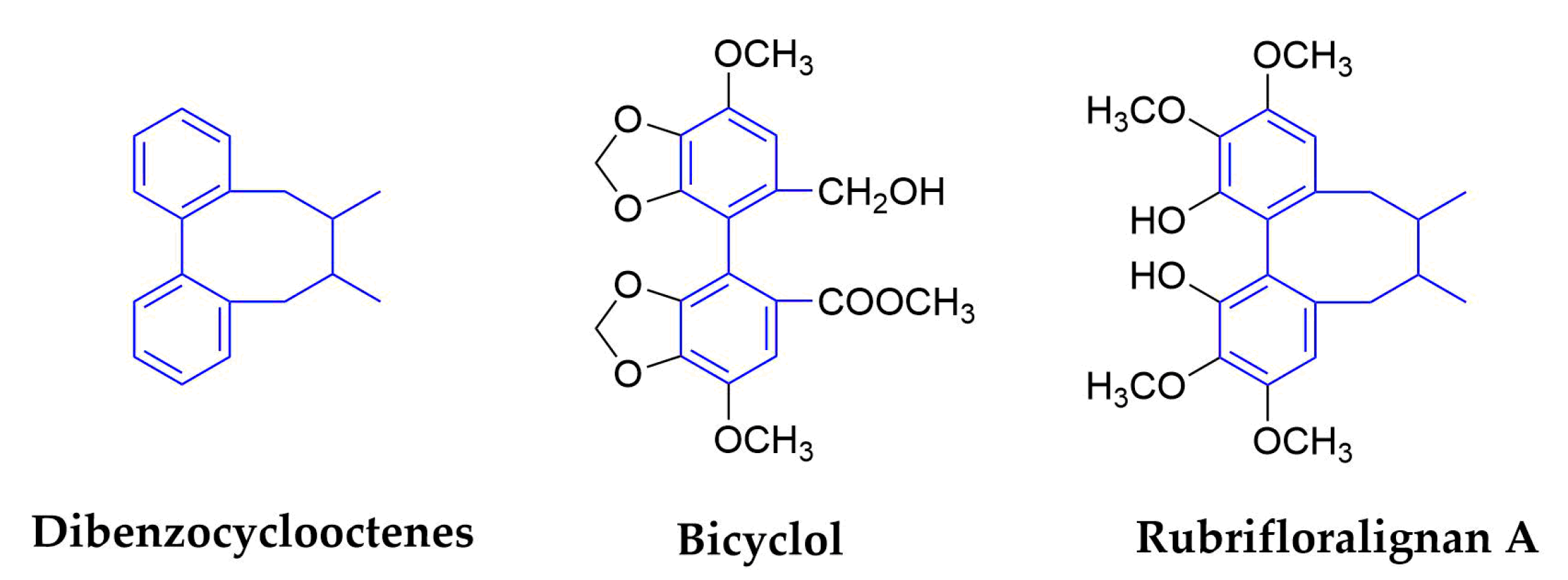
2.1.6. Dibenzocyclooctenes
2.2. Neolignans
3. Prospects of Lignans and Their Derivatives in Antiviral Development
Author Contributions
Funding
Conflicts of Interest
References
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- Ayres, D.C.; Loike, J.D. Lignans. Chemical, Biological and Clinical Properties. Lignans Chem. Biol. Clin. Prop. 1991, 100, 1. [Google Scholar]
- Kaplan, I.W. Condylomata acuminate. New Orleans Med. Surg. J. 1942, 94, 388–390. [Google Scholar]
- Wu, X.Q.; Li, W.; Chen, J.X.; Zhai, J.W.; Xu, H.Y.; Ni, L.; Wu, S.S. Chemical Constituents and Biological Activity Profiles on Pleione (Orchidaceae). Molecules 2019, 24, 3195. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Liu, G.T. Antioxidant activity of dibenzocyclooctene lignans isolated from Schisandraceae. Planta Med. 1992, 58, 4. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.Y.; Chen, S.L.; Yang, M.H.; Wu, J.; Sinkkonen, J.; Zou, K. An update on lignans: Natural products and synthesis. Nat. Prod. Rep. 2009, 26, 1251–1292. [Google Scholar] [CrossRef]
- Capilla, A.S.; Sánchez, I.; Caignard, D.H.; Renard, P.; Pujol, M.D. Antitumor agents. Synthesis and biological evaluation of new compounds related to podophyllotoxin, containing the 2,3-dihydro-1,4-benzodioxin system. Eur. J. Med. Chem. 2001, 36, 11. [Google Scholar] [CrossRef]
- Kawazoe, K.; Yutani, A.; Tamemoto, K.; Yuasa, S.; Shibata, H.; Higuti, T.; Takaishi, Y. Phenylnaphthalene Compounds from the Subterranean Part of Vitex rotundifolia and Their Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus. J. Nat. Prod. 2001, 64, 588–591. [Google Scholar] [CrossRef]
- Hirano, T.; Wakasugi, A.; Oohara, M.; Oka, K.; Sashida, Y. Suppression of mitogen-induced proliferation of human peripheral blood lymphocytes by plant lignans. Planta Med. 1991, 57, 4. [Google Scholar] [CrossRef]
- Iwasaki, T.; Kondo, K.; Kuroda, T.; Moritani, Y.; Yamagata, S.; Sugiura, M.; Kikkawa, H.; Kaminuma, O.; Ikezawa, K. Novel selective PDE IV inhibitors as antiasthmatic agents. synthesis and biological activities of a series of 1-aryl-2,3-bis(hydroxymethyl)naphthalene lignans. J. Med. Chem. 1996, 39, 9. [Google Scholar] [CrossRef]
- Charlton James, L. Antiviral Activity of Lignans. J. Nat. Prod. 1998, 61, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Huang, Y.L.; Ou, J.C.; Chen, C.C.; Hsu, F.L.; Chang, C. Screening of 25 compounds isolated from Phyllanthus species for anti-human hepatitis B virus in vitro. Phytother Res. 2003, 17, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, L.I. Lignans: A Chemometric Analysis. Molecules 2018, 23, 1666. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, L.M.; Lampe, J.W.; Campbell, D.R.; Martini, M.C.; Slavin, J.L. Urinary Lignan and isoflavonoid excretion in men and women consuming vegetable and soy diets. Nutr. Cancer 1995, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, D.E.; Lindsley, C.W. Asymmetric Synthesis of Natural and Unnatural Dibenzylbutane Lignans from a Common Intermediate. J. Org. Chem. 2019, 84, 5974–5979. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, W.; Shi, K.; Cao, X.; Zhou, M.; Liu, Z. In Vitro and in vivo anti-hepatitis B virus activities of the lignan niranthin isolated from Phyllanthus niruri L. J. Ethnopharmacol. 2014, 155, 1061–1067. [Google Scholar] [CrossRef]
- Hernandez, D.J.; Anderica, R.A.C.; Pedraza, C.J. Paradoxical cellular effects and biological role of the multifaceted compound nordihydroguaiaretic acid. Arch. Pharm. 2014, 347, 685–697. [Google Scholar] [CrossRef]
- Zúñiga-Toalá, A.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Negrette-Guzmán, M.; Huerta-Yepez, S.; Torres, I.; Pinzón, E.; Tapia, E.; Pedraza-Chaverri, J. Nordihydroguaiaretic acid induces Nrf2 nuclear translocation in vivo and attenuates renal damage and apoptosis in the ischemia and reperfusion model. Phytomedicine 2013, 20, 775–779. [Google Scholar] [CrossRef]
- Tong, W.; Ding, X.; Adrian, T. The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem. Biophys. Res. Commun. 2002, 296, 942–948. [Google Scholar] [CrossRef]
- Manzanero, S.; Santro, T.; Arumugam, T.V. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem. Int. 2013, 62, 712–718. [Google Scholar] [CrossRef]
- Floriano-Sanchez, E.; Villanueva, C.; Medina-Campos, O.N.; Rocha, D.; Sánchez-González, D.J.; Cárdenas-Rodríguez, N.; Pedraza-Chaverrí, J. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic Res. 2006, 40, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.R.; Bautista, C.P.; Syed, G.H.; Siddiqui, A.; Del Angel, R.M. Nordihydroguaiaretic acid (NDGA) inhibits replication and viral morphogenesis of dengue virus. Antivir. Res. 2014, 109, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Syed, G.H.; Siddiqui, A. Effects of hypolipidemic agent nordihydroguaiaretic acid on lipid droplets and hepatitis C virus. Hepatology 2011, 54, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Merino-Ramos, T.; de Oya, N.J.; Saiz, J.-C.; Martín-Acebes, M.A. Antiviral Activity of Nordihydroguaiaretic Acid and Its Derivative Tetra-O-Methyl Nordihydroguaiaretic Acid against West Nile Virus and Zika Virus. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Wang, S.; Le, T.Q.; Kurihara, N.; Chida, J.; Cisse, Y.; Yano, M.; Kido, H. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J. Infect. Dis. 2010, 202, 991–1001. [Google Scholar] [CrossRef]
- Oyegunwa, A.O.; Sikes, M.L.; Wilson, J.R.; Scholle, F.; Laster, S.M. Tetra-O-methyl nordihydroguaiaretic acid (Terameprocol) inhibits the NF-kappaB-dependent transcription of TNF-alpha and MCP-1/CCL2 genes by preventing RelA from binding its cognate sites on DNA. J. Inflamm. 2010, 7, 59. [Google Scholar] [CrossRef]
- Pollara, J.J.; Laster, S.M.; Petty, I.T. Inhibition of poxvirus growth by Terameprocol, a methylated derivative of nordihydroguaiaretic acid. Antivir. Res. 2010, 88, 287–295. [Google Scholar] [CrossRef]
- Chen, H.; Teng, L.; Li, J.N.; Park, R.; Mold, D.E.; Gnabre, J.; Hwu, J.R.; Tseng, W.N.; Huang, R.C.C. Antiviral Activities of Methylated Nordihydroguaiaretic Acids. 2. Targeting Herpes Simplex Virus Replication by the Mutation Insensitive Transcription Inhibitor Tetra-O-methyl-NDGA. J. Med. Chem. 1998, 41, 3001–3007. [Google Scholar] [CrossRef]
- Gnabre, J.N.; Brady, J.N.; Clanton, D.J.; Ito, Y.; Dittmer, J.; Bates, R.B.; Huang, R.C. Inhibition of human immunodeficiency virus type 1 transcription and replication by DNA sequence-selective plant lignans. Proc. Natl. Acad. Sci. USA 1995, 92, 11239–11243. [Google Scholar] [CrossRef]
- Khanna, N.; Dalby, R.; Tan, M.; Arnold, S.; Stern, J.; Frazer, N. Phase I/II clinical safety studies of terameprocol vaginal ointment. Gynecol. Oncol. 2007, 107, 554–562. [Google Scholar] [CrossRef]
- Xu, X.; Wang, D.; Ku, C.; Zhao, Y.; Cheng, H.; Liu, K.-L.; Rong, L.-J.; Zhang, H.-J. Anti-HIV lignans from Justicia procumbens. Chin. J. Nat. Med. 2019, 17, 945–952. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, X.; Kang, T.G. Activity of in vitro anti-influenza virus of arctigenin. Chin. Herb. Med. 2002, 33, 724–725. [Google Scholar]
- Fu, L.; Xu, P.; Liu, N.; Yang, Z.; Zhang, F.; Hu, Y. Antiviral effect of Arctigenin Compound on Influenza Virus. Tradit. Chin. Drug Res. Clin. Pharmacol. 2008, 19, 4. [Google Scholar]
- Hayashi, K.; Narutaki, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Therapeutic Effect of Arctiin and Arctigenin in Immunocompetent and Immunocompromised Mice Infected with Influenza A Virus. Biol. Pharm. Bull. 2010, 33, 1199–1205. [Google Scholar] [CrossRef]
- Schröder, H.C.; Merz, H.; Steffen, R.; Müller, W.E.; Sarin, P.S.; Trumm, S.; Schulz, J.; Eich, E. Differential in vitro anti-HIV activity of natural lignans. Zeitschrift für Naturforschung C 1990, 45, 1215–1221. [Google Scholar]
- Eich, E.; Pertz, H.; Kaloga, M.; Schulz, J.; Pertz, H.; Eich, E.; Pommier, Y. (−)-Arctigenin as a Lead Structure for Inhibitors of Human Immunodeficiency Virus Type-1 Integrase. J. Med. Chem. 1996, 39, 86–95. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Kuo, Y.H.; Lin, Y.L.; Tsai, W.J. Yatein from Chamaecyparis obtusa suppresses herpes simplex virus type 1 replication in HeLa cells by interruption the immediate-early gene expression. Antivir. Res. 2006, 70, 112–120. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xiong, Y.; Kaushik, A.C.; Muhammad, J.; Khan, A.; Dai, H.; Wei, D.-Q. New strategy for identifying potential natural HIV-1 non-nucleoside reverse transcriptase inhibitors against drug-resistance: An in silico study. J. Biomol. Struct. Dyn. 2019, 1–15. [Google Scholar] [CrossRef]
- Chang, C.; Lien, Y.; Liu, K.C.S.C.; Li, S.-S. Lignans from Phyllanthus urinaria. Phytochemistry 2003, 63, 825–833. [Google Scholar] [CrossRef]
- Kuo, P.; Li, Y.; Wu, T. Chemical Constituents and Pharmacology of the Aristolochia species. J. Tradit. Complement. Med. 2012, 2, 249–266. [Google Scholar] [CrossRef]
- Gangan, V.; Hussain, S.S. Alkaloids from Piper hookeri: Revision of NMR assignments by the application of 2D NMR spectroscopy. J. Pharm. Res. 2011, 4, 3. [Google Scholar]
- Nunomura, S.; Yoshida, M. Lignans and benzoic acid derivatives from pericarps of Virola multinervia (Myristicaceae). Biochem. Syst. Ecol. 2002, 30, 3. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Hemmati, S.; Klaes, M.; Konuklugil, B.; Mohagheghzadeh, A.; Ionkova, I.; Fuss, E.; Alfermann, A.W. Lignans in flowering aerial parts of Linum species—chemodiversity in the light of systematics and phylogeny. Phytochemistry 2010, 71, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Marcotullio, M.C.; Pelosi, A.; Curini, M. Hinokinin, an emerging bioactive lignan. Molecules 2014, 19, 14862–14878. [Google Scholar] [CrossRef]
- Cheng, M.-J.; Lee, K.-H.; Tsai, I.-L.; Chen, I.-S. Two new sesquiterpenoids and anti-HIV principles from the root bark of Zanthoxylum ailanthoides. Bioorg. Med. Chem. 2005, 13, 5915–5920. [Google Scholar] [CrossRef]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Li, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S.; et al. Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef]
- Rozália, P.; Abrantes, M.; Serly, J.; Duarte, N.; Molnar, J.; Ferreira, M.-J.U. Antitumor-promoting Activity of Lignans: Inhibition of Human Cytomegalovirus IE Gene Expression. Anticancer Res. 2010, 30, 451–454. [Google Scholar]
- Chen, H.; Liu, P.; Zhang, T.; Gao, Y.; Zhang, Y.; Shen, X.; Li, X.; Shen, W. Effects of diphyllin as a novel V-ATPase inhibitor on TE-1 and ECA-109 cells. Oncol. Rep. 2018, 39, 921–928. [Google Scholar] [CrossRef]
- Nesmelova, E.F.; Razakova, D.M.; Akhmedzhanova, V.I.; Bessonova, I.A. Diphyllin from Haplophyllum alberti-regelii, H. bucharicum, and H. perforatum. Chem. Nat. Compd. 1983, 19, 608. [Google Scholar] [CrossRef]
- Sørensen, M.G.; Henriksen, K.; Neutzsky-Wulff, A.V.; Dziegiel, M.H.; Karsdal, M.A. Diphyllin, a Novel and Naturally Potent V-ATPase Inhibitor, Abrogates Acidification of the Osteoclastic Resorption Lacunae and Bone Resorption. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22, 9. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Persaud, M.; Chavez, M.P.; Zhang, H.; Rong, L.; Liu, S.; Wang, T.T.; Sarafianos, S.G.; Diaz-Griffero, F. Glycosylated diphyllin as a broad-spectrum antiviral agent against Zika virus. EBioMedicine 2019, 47, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Cheng, J.X.; Liu, M.-T.; King, K.; Peng, J.Y.; Zhang, X.-Q.; Wang, C.-H.; Shresta, S.; Schooley, R.T.; Liu, Y.-T. Inhibitory and combinatorial effect of diphyllin, a v-ATPase blocker, on influenza viruses. Antivir. Res. 2013, 99, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Susplugas, S.; Hung, N.; Bignon, J.; Thoison, O.; Kruczynski, A.; Sévenet, T.; Guéritte, F. Cytotoxic Arylnaphthalene Lignans from a Vietnamese Acanthaceae. Justicia patentiflora. J. Nat. Prod. 2005, 68, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Liu, K.-L.; Wang, D.-Y.; Li, W.-F.; Nguyen, V.H.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.S. Anti-HIV diphyllin glycosides from Justicia gendarussa. Phytochemistry 2017, 136, 94–100. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Wang, D.-Y.; Liu, K.-L.; Li, W.-F.; Nguyen, V.H.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.S. Potent Inhibitor of Drug-Resistant HIV-1 Strains Identified from the Medicinal Plant Justicia gendarussa. J. Nat. Prod. 2017, 80, 1798–1807. [Google Scholar] [CrossRef]
- Zalesak, F.; Bon, D.J.D.; Pospisil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharm. Res. 2019, 146, 104284. [Google Scholar] [CrossRef]
- Alsdorf, W.; Seidel, C.; Bokemeyer, C.; Oing, C. Current pharmacotherapy for testicular germ cell cancer. Expert Opin. Pharm. 2019, 20, 837–850. [Google Scholar] [CrossRef]
- Komericki, P.; Akkilic-Materna, M.; Strimitzer, T.; Aberer, W. Efficacy and Safety of Imiquimod Versus Podophyllotoxin in the Treatment of Anogenital Warts. Sex. Transm. Dis. 2011, 38, 3. [Google Scholar] [CrossRef]
- Ma, Z.-J.; Lu, L.; Yang, J.-J.; Wang, X.-X.; Su, G.; Wang, Z.; Chen, G.; Sun, H.; Wang, M.; Yang, Y. Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway. Eur. J. Pharm. 2018, 821, 1–10. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, T.Y.; Guan, Y.Q.; Ma, G.X.; Zhang, J.; Shi, L.L. Chemical constituents from roots of Stelleropsis tianschanica. China J. Chin. Mater. Med. 2017, 42, 3379–3384. [Google Scholar]
- Bajpai, V.K.; Shukla, S.; Paek, W.K.; Lim, J.; Kumar, P.; Kumar, P.; Na, M.K. Efficacy of (+)-Lariciresinol to Control Bacterial Growth of Staphylococcus aureus and Escherichia coli O157:H7. Front. Microbiol. 2017, 8, 804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, B.; Li, C.; Chen, Q.Y.; Wang, Y.; Li, Z.; Chen, T.; Yang, C.; Jiang, B.; Zhong, Z. Lariciresinol-4-O-beta-d-glucopyranoside from the root of Isatis indigotica inhibits influenza A virus-induced pro-inflammatory response. J. Ethnopharmacol. 2015, 174, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, J.; Liang, X.; Yang, Z.; Jiang, Z. Transcriptome profiling of influenza A virus-infected lung epithelial (A549) cells with lariciresinol-4-beta-D-glucopyranoside treatment. PLoS ONE 2017, 12, e0173058. [Google Scholar]
- Liu, Z.L.; Liu, Y.Q.; Zhao, L.; Xu, J.; Tian, X. The phenylpropanoids of Aster flaccidus. Fitoterapia 2010, 81, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Guo, Q.; Chen, M.; Jiang, J.; Li, Y.; Shi, J. Isatindolignanoside A, a glucosidic indole-lignan conjugate from an aqueous extract of the Isatis indigotica roots. Chin. Chem. Lett. 2018, 29, 1257–1260. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zheng, Z.; Zhao, S.; Zhao, J.; Lin, Q.; Li, C.; Zhu, Q.; Zhong, N. Antiviral activity of Isatis indigotica root-derived clemastanin B against human and avian influenza A and B viruses in vitro. Int. J. Mol. Med. 2013, 31, 867–873. [Google Scholar] [CrossRef]
- Fanhchaksai, K.; Kodchakorn, K.; Pothacharoen, P.; Kongtawelert, P. Effect of sesamin against cytokine production from influenza type A H1N1-induced peripheral blood mononuclear cells: Computational and experimental studies. Vitr. Cell Dev. Biol. Anim. 2016, 52, 107–119. [Google Scholar] [CrossRef]
- Ren, R.; Ci, X.-X.; Li, H.-Z.; Luo, G.-J.; Li, R.-T.; Deng, X.-M. New Dibenzocyclooctadiene Lignans from Schisandra sphenanthera and Their Proinflammatory Cytokine Inhibitory Activities. Z. Für Nat. B 2014, 65, 8. [Google Scholar] [CrossRef]
- Szopa, A.; Barnaś, M.; Ekiert, H. Phytochemical studies and biological activity of three Chinese Schisandra species (Schisandra sphenanthera, Schisandra henryi and Schisandra rubriflora): Current findings and future applications. Phytochem. Rev. 2018, 18, 109–128. [Google Scholar] [CrossRef]
- Checker, R.; Patwardhan, R.; Sharma, D.; Menon, J.; Thoh, M.; Bhilwade, H.N.; Konishi, T.; Sandur, S.K. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-kappaB. Free Radic. Biol. Med. 2012, 53, 1421–1430. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, S.J.; Park, T.G.; Rajasekar, S.; Lee, S.-J.; Choi, Y.W. Schizandrin C exerts anti-neuroinflammatory effects by upregulating phase II detoxifying/antioxidant enzymes in microglia. Int. Immunopharmacol. 2013, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lai, H.; Jia, X.; Liu, J.; Zhang, Z.; Qi, Y.; Zhang, J.; Song, J.; Wu, C.; Zhang, B.; et al. Comprehensive chemical analysis of Schisandra chinensis by HPLC-DAD-MS combined with chemometrics. Phytomed. Int. J. Phytother. Phytopharm. 2013, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-S.; Wu, Y.-H.; Tseng, C.-K.; Lin, C.-K.; Hsu, Y.-C.; Chen, Y.-H.; Lee, J.-C. Schisandrin A inhibits dengue viral replication via upregulating antiviral interferon responses through STAT signaling pathway. Sci. Rep. 2017, 7, 45171. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Grandi, N.; Del Vecchio, C.; Mandas, D.; Corona, A.; Piano, D.; Esposito, F.; Parolin, C.; Tramontano, E. From the traditional Chinese medicine plant Schisandra chinensis new scaffolds effective on HIV-1 reverse transcriptase resistant to non-nucleoside inhibitors. J. Microbiol. 2015, 53, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, G. Bicyclol: A Novel Drug for Treating Chronic Viral Hepatitis B and C. Med. Chem. 2009, 5, 29–43. [Google Scholar] [CrossRef]
- Zhang, T. New drugs derived from medicinal plants. Thérapie 2016, 57, 14. [Google Scholar]
- Ruan, B.; Wang, J.; Bai, X. Comparison of bicyclol therapy for patients with genotype B and C of hepatitis B virus. Chin. J. Exp. Clin. Virol. 2007, 21, 3. [Google Scholar]
- Huang, M.-H.; Li, H.; Xue, R.; Li, J.; Wang, L.; Cheng, J.; Wu, Z.; Li, W.; Chen, J.; Lv, X.; et al. Up-regulation of glycolipid transfer protein by bicyclol causes spontaneous restriction of hepatitis C virus replication. Acta Pharm. Sin. B 2019, 9, 769–781. [Google Scholar] [CrossRef]
- Zhou, Y.; Chai, X. Protective effect of bicyclol against pulmonary fibrosis via regulation of microRNA5 in rats. J. Cell. Biochem. 2019, 121, 651–660. [Google Scholar] [CrossRef]
- Tian, R.R.; Xiao, W.L.; Yang, L.M.; Wang, R.R.; Sun, H.D.; Liu, N.F.; Zheng, Y.T. The Isolation of Rubrifloralignan A and Its Anti-HIV-1 Activities. Chin. J. Nat. Med. 2006, 4, 40–44. [Google Scholar]
- Chen, M.; Kilgore, N.; Lee, K.-H.; Chen, D.-F. Rubrisandrins A and B, Lignans and Related Anti-HIV Compounds from Schisandra rubriflora. J. Nat. Prod. 2006, 69, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Fujihashi, T.; Hara, H.; Sakata, T.; Mori, K.; Higuchi, H.; Tanaka, A.; Kaji, H.; Kaji, A. Anti-human immunodeficiency virus (HIV) activities of halogenated gomisin J derivatives, new nonnucleoside inhibitors of HIV type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1995, 39, 2000–2007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Strader, D.B.; Bacon, B.R.; Lindsay, K.L.; La Brecque, D.R.; Morgan, T.; Wright, E.C.; Seeffff, L.B. Use of complementary and alternative medicine in patients with liver disease. Am. J. Gastroenterol. 2002, 97, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Jassey, A.; Hsu, H.-Y.; Lin, L.-Z. Antiviral Activities of Silymarin and Derivatives. Molecules 2019, 24, 1552. [Google Scholar] [CrossRef]
- Wagoner, J.; Negash, A.; Kane, O.J.; Martinez, L.E.; Nahmias, Y.; Bourne, N.; Owen, D.M.; Grove, J.; Brimacombe, C.; McKeating, J.A.; et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology 2010, 51, 1912–1921. [Google Scholar] [CrossRef]
- DebRoy, S.; Hiraga, N.; Imamura, M.; Hayes, C.N.; Akamatsu, S.; Canini, L.; Perelson, A.S.; Pohl, R.T.; Persiani, S.; Uprichard, S.L. Hepatitis C virus dynamics and cellular gene expression in uPA-SCID chimeric mice with humanized livers during intravenous silibinin monotherapy. J. Viral Hepat. 2016, 23, 708–717. [Google Scholar] [CrossRef]
- Song, J.H.; Choi, H.J. Silymarin efficacy against influenza a virus replication. Phytomedicine 2011, 18, 832–835. [Google Scholar] [CrossRef]
- Li, J.; Meng, A.-P.; Guan, X.-L.; Li, J.; Wu, Q.; Deng, S.-P.; Su, X.-J.; Yang, R.-Y. Anti-hepatitis B virus lignans from the root of Streblus asper. Bioorg. Med. Chem. Lett. 2013, 23, 2238–2244. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Cheng, X.-H.; Wang, H.-G.; Yu, D.-Y.; Feng, B.-M. Advances on the secolignans compounds in natural products. J. Shenyang Pharm. Univ. 2014, 11, 922. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-SYYD201411019.htm.
- Su, X.; Na, L.; Ning, M.-M.; Zhou, C.-H.; Yang, Q.-R.; Wang, M.W. Bioactive Compounds from Peperomia pellucida. J. Nat. Prod. 2006, 69, 247–250. [Google Scholar]
- Feng, B.M.; Qin, H.H.; Wang, H.G.; Shi, L.Y.; Yu, D.Y.; Ji, B.Q.; Zhao, Q.; Wang, Y.Q. Three new secolignan glycosides from Urtica fissa E. Pritz. J. Nat. Med. 2012, 66, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.-S.; Chen, H.; Zheng, X.-K.; Wang, Y.-Z.; Chen, H.; Li, Z. Two new secolignans from Selaginella sinensis (Desv.) Spring. J. Asian Nat. Prod. Res. 2009, 11, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-J.; Lee, S.-J.; Chang, Y.-Y.; Wu, S.-H.; Tsai, I.-L.; Jayaprakasam, B.; Chen, I.-S. Chemical and cytotoxic constituents from Peperomia sui. Phytochemistry 2003, 63, 603–608. [Google Scholar] [CrossRef]
- Tsutsui, C.; Yamada, Y.; Ando, M.; Toyama, D.; Wu, J.L.; Wang, L.; Taketani, S.; Kataoka, T. Peperomins as anti-inflammatory agents that inhibit the NF-kappaB signaling pathway. Bioorg. Med. Chem. Lett. 2009, 19, 4084–4087. [Google Scholar] [CrossRef] [PubMed]
- Govindachari, T.R.; Kumari, G.N.K.; Partho, P.D. Two secolignans from Peperomia dindigulensis. Phytochemistry 1998, 49, 2129–2131. [Google Scholar] [CrossRef]
- Lin, M.; Yu, D.; Wang, Q. Secolignans with Antiangiogenic Activities from Peperomia dindygulensis. Chem. Biodivers. 2011, 8, 862–870. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Li, N.; Wang, Y.-H.; Zheng, Y.-T.; Zhang, Z.; Wang, M.-W. Bioactive lignans from Peperomia heyneana. J. Nat. Prod. 2007, 70, 662–664. [Google Scholar] [CrossRef]
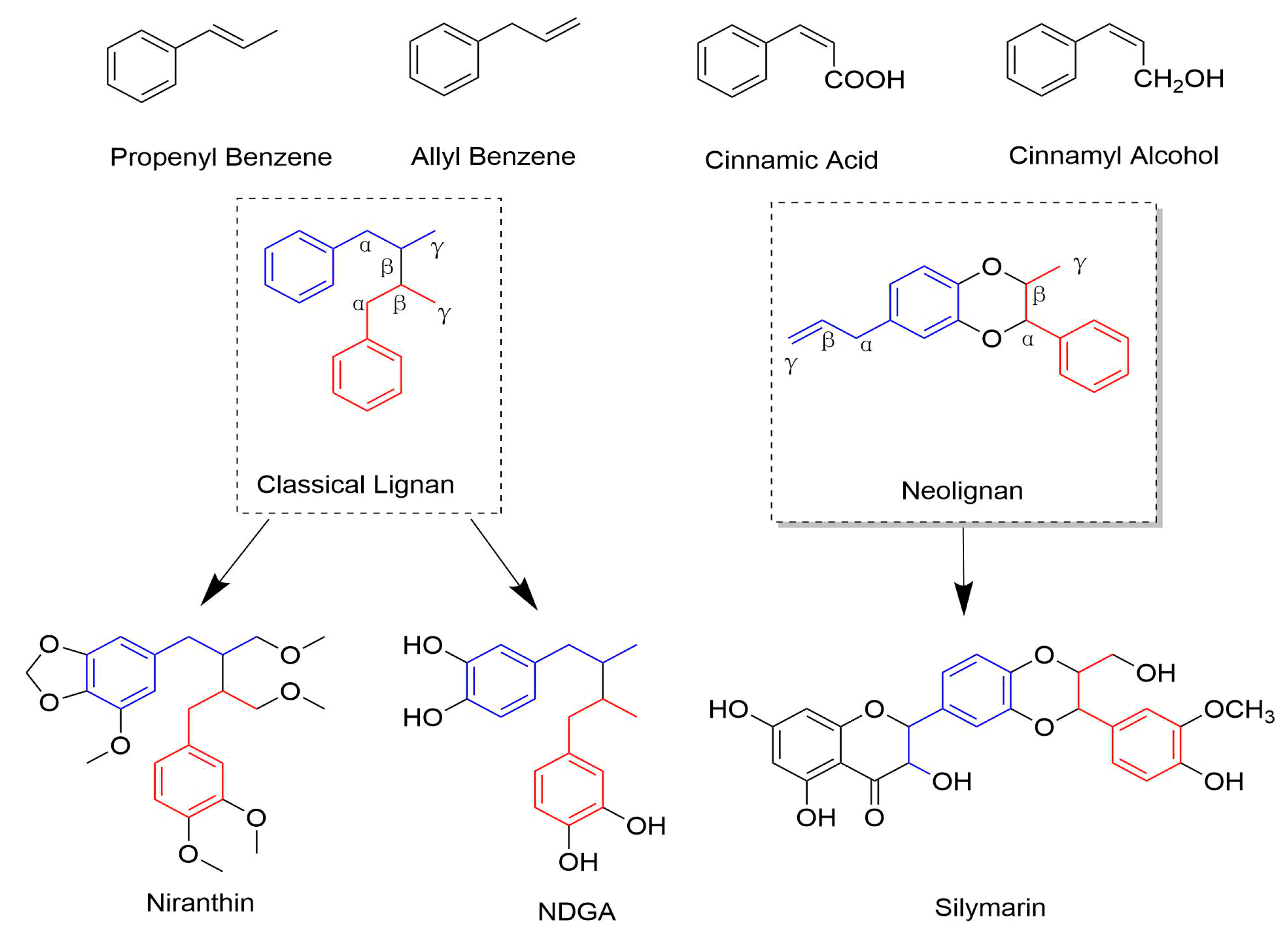


| Subclass | Cpd | From Plants | Organs | Virus(es) | IC50 (μM) | CC50 (μM) | Status | MOA/Targets | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Dibenzylbutanes | Niranthin | Phyllanthus niruri L. (Euphorbiaceae) | Whole plants | HBV | 15.6~25.1 | 369.9 In HepG 2.2.15 | In Vitro In Vivo | inhibits DHBV DNA replication and HBV antigen expression. | [12,16] |
| NDGA | Larrea tridentate (Zygophyllaceae) | Leaves (resin) | DENV | No data | No data | In Vitro | targets genome replication and viral assembly | [22,23,24,25] | |
| HCV | 30 | 70 in Huh7 | NDGA-mediated alterations of host lipid metabolism, LD morphology, and VLDL transport affect HCV proliferation | ||||||
| WNV/ZIKV | 7.9/9.1 | 162.1 in Vero | WNV: disturb the lipid metabolism probably by interfering with the sterol regulatory element binding proteins (SREBP) pathway | ||||||
| IAV | In Vivo | suppresses replication of IAV and induction of cytokines, trypsin, and MMP-9, with improved animal survival | |||||||
| TMP | Larrea tridentata (Zygophyllaceae) | Leaves (resin) | WNV/ZIKV | 9.3/5.7 | 1071.0 in Vero | In Vitro | impaires viral replication | [24,26,27,28,29,30] | |
| poxvirus | No data | No data | In Vitro | prevents the efficient spread of virus particles from cell to cell | |||||
| HSV | 43.5 | 160 in Vero | In Vitro | TMP inhibits both these viruses replication by blocking the binding of the host cell transcription factor, Sp1, to viral promoters. | |||||
| HIV | 25 | No data | In Vitro | ||||||
| HPV | In Clinical | selectively interferes with HPV viral genes E6/E7 with Sp1dependent promoters, and induces apoptosis by inactivation of the CDC2/cyclin B complex (maturation promoting factor) and production and phosphorylation of survivin | |||||||
| Secoisolariciresinol dimethyleTher acetate | Justicia procumbens (Acanthaceae) | Air-dried aerial parts | HIV-1 | 5.27 | 11.6 | In Vitro | waiting for the deeper research | [31] | |
| Dibenzyltyrolactones | ATG | Arctium lappa L. (Compositae) | Whole plants | IAV | No data | No data | In Vitro In Vivo | induce the production of interferon | [32,33,34,35,36] |
| HIV-1 | No data | No data | In Vitro | inhibit the expression of protein P17 and P24 of the HIV-1 virus | |||||
| Yatein | Chamaecyparis obtuse (Cupressaceae) | Dried leaves | HSV-1 | 30.6 ± 5.5 | >100 | In Vitro | inhibiting HSV-1 alpha gene expression, including expression of the ICP0 and ICP4 genes, and by arresting HSV-1 DNA synthesis and structural protein expression in HeLa cells | [37,38] | |
| Hinokinin | Chamecyparis obtusa (Cupressaceae) | Woods | HBV | No data | No data | In Vitro | waiting for the deeper research | [12,45,46,47] | |
| HIV | <28 | 527 in H9 | |||||||
| SARS-CoV | >10 | >750 in Vero | |||||||
| HCMV | No data | 115 in A549 | |||||||
| Arylnaphthalenes | Diphyllin | genus Haplophyllum (Rutaceae) | Epigeal part | ZIKV | 0.06 | 3.48 in MDCK | In Vitro | vacuolar ATPase (V-ATPase) inhibitors | [48,49,50,51,52] |
| IAV | 0.1–0.6 in different strains | 24.1 in A549 | inhibit endosomal acidification, thus interfering with downstream virus replication | ||||||
| DGP | Justicia gendarussa (Acanthaceae) | Stems and leaves | ZIKV | 0.01–0.07 | 15–32 | In Vitro In Vivo | prevented the acidification of endosomal/lysosomal compartments in target cells, thus inhibiting ZIKV fusion with cellular membranes and infection. | [51,53,54,55] | |
| HIV-1 | 15–21 nM | No data | In Vitro | HIV-1 reverse transcription | |||||
| Aryltetralins | Dysosmae Verspiellis & Podophyllum peltatum (Berberidaceae) | Roots and stems | Papilloma virus | Launched in China | waiting for the deeper research | [3,11,56,57,58] | |||
| Substituted tetrahydrofurans | lariciresinol-4-O-β-d-glucopyranoside | Isatis indigotica Fort (Cruciferae) | Roots | IAV | 50 μg/mL | >200 μg/mL | In Vitro | pharmacological actions on the immune system, signal transduction, cell cycle, and metabolism | [62,63] |
| (7′R, 8S)-9′-lariciresinol-(α-methyl)-butanoate | HIV-1 | 0.66 mM | 0.67mM in C8166 | In Vitro | No report | [64] | |||
| Isatindolignanoside A | CVB3 | 25.9 | >100 | In Vitro | waiting for the deeper research | [65] | |||
| Clemastanin B | IAV | 0.087–0.72 mg/mL | 6.2–7.5 mg/mL | In Vitro | targets viral endocytosis, uncoating or RNP export from the nucleus | [66] | |||
| 2,6-diarylfurofurans | Phillygenin | Fructus Forsythiae (Oleaceae) | Fruits | IAV | In Vivo | reduce inflammation caused by IAV. | [57,58] | ||
| Sesamin | Sesamum indicum (Pedaliaceae) | Seeds | inflammatory cytokines induced by H1N1 | No data | No data | In Vitro | anti-inflammatory cytokines in human PBMCs | [67] | |
| Dibenzocyclooctene | Bicyclol | Analogue of schizandrin C from Fructus Schiznadrae | HBV | Launched in China | inhibit virus replication in patients infected with HBV | [76,77,78,79,80] | |||
| HCV | 30 | No data | In vitro/Vivo/Clinical | modulation of cytotoxic T lymphocytes up-regulating the host restrictive factor (GLTP) for HCV replication, and causing spontaneous restriction of HCV replication | |||||
| Rubrifloralignan A | Schisandra rubriflora (Schisandraceae) | Fruits | HIV-1 | 40.46 | 123.35 | In Vitro | inhibit the early stage of HIV-1 replication | [81,82,83] | |
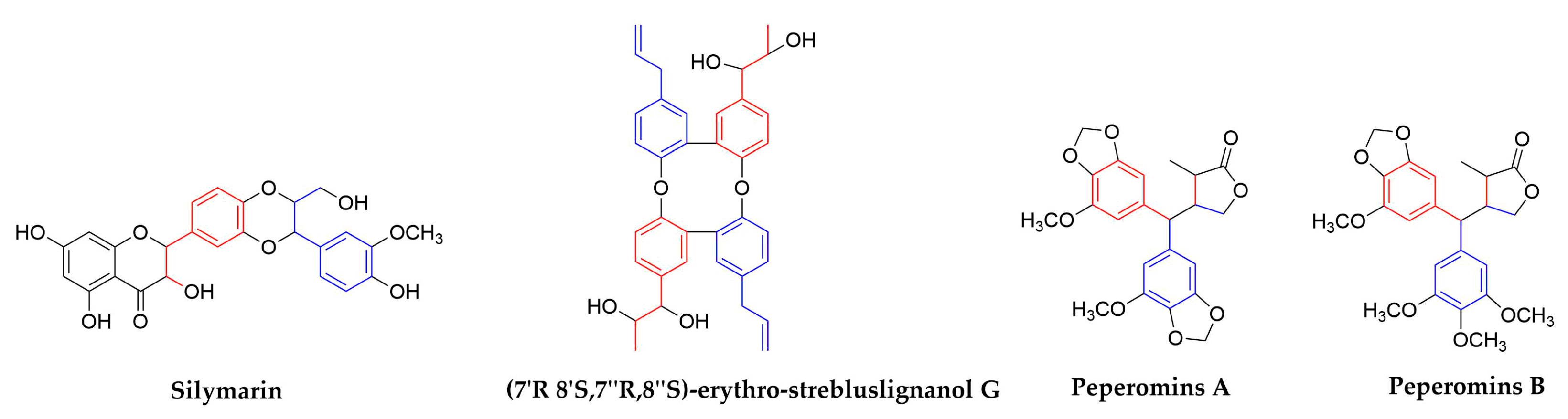
| 1,4-Benzodioxane lignans | Silymarin | Silybum marianum (Compositae) | Seeds | HCV | In Clinical | blocked HCV production, increased anti-inflammatory, anti-proliferative gene expressions without affecting serum albumin levels | [84,85,86,87,88,89] | ||
| IAV | No data | No data | In Vitro | inhibition of late viral RNA synthesis | |||||
| Dimer of strebluslignanols | (7′R,8′S,7″R,8″S)-erythro-strebluslignanolG | Streblus asper (Moraceae) | Roots | HBV | 3.67/HBsAg 14.67/HBeAg | No data | In Vitro | inhibit the secretion of HBsAg and HBeAg | [90] |
| Secolignans | Peperomins A&B | Peperomia pellucida (Piperaceae) | Whole plants | HIV-1 IIIB | 5 | No data | In Vitro | related to the cytotoxicity expressed as CC50 of compounds | [98,99] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and Their Derivatives from Plants as Antivirals. Molecules 2020, 25, 183. https://doi.org/10.3390/molecules25010183
Cui Q, Du R, Liu M, Rong L. Lignans and Their Derivatives from Plants as Antivirals. Molecules. 2020; 25(1):183. https://doi.org/10.3390/molecules25010183
Chicago/Turabian StyleCui, Qinghua, Ruikun Du, Miaomiao Liu, and Lijun Rong. 2020. "Lignans and Their Derivatives from Plants as Antivirals" Molecules 25, no. 1: 183. https://doi.org/10.3390/molecules25010183
APA StyleCui, Q., Du, R., Liu, M., & Rong, L. (2020). Lignans and Their Derivatives from Plants as Antivirals. Molecules, 25(1), 183. https://doi.org/10.3390/molecules25010183







