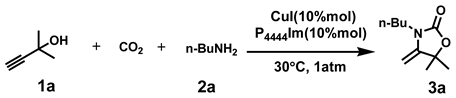
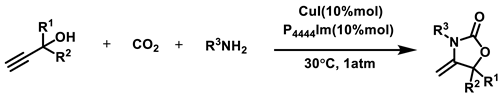
Abstract
Recently, the efficient chemical fixation of carbon dioxide (CO2) into high value chemicals without using noble metal catalysts has become extremely appealing from the viewpoint of sustainable chemistry. In this work, a one-pot three component reaction of propargylic alcohols, anines and CO2 that can proceed in an atom economy and environmentally benign manner by combination of CuI and tetrabutylphosphonium imidazol ([P4444][Im]) as a catalyst was described. Catalysis studies indicate that this catalytic system is an effective catalyst for the conversion of CO2 into oxazolidinones at room temperature and ambient pressure without any solvent. The results provide a useful way to design novel noble metal-free catalyst systems for the transformation of CO2 into other valuable compounds.
1. Introduction
With the global consumption of fossil fuels, the increasing concentration of CO2 in the atmosphere could have a significant impact on the global climate [1,2]. Therefore, the development of efficient strategies to reduce CO2 emissions has becoms an imperative task for scientific researchers [3,4,5]. The chemical fixation and transformation of CO2 into valuable chemicals is an ideal pathway for reducing CO2 emissions and fully utilize this cheap C1 source [6,7,8,9,10]. Thus, considerable efforts have been devoted to the exploration of efficient strategy for chemical utilization of CO2 to produce high-value chemical commodities [11,12,13,14,15,16].
Oxazolidinones are a class of nitrogen-containing heterocyclic compounds which have been used as organic intermediates, chiral additives, antibacterial drugs and muscle relaxants, etc. [17,18,19,20]. The synthesis of oxazolidinones between propargyl alcohol, CO2 and an amine is an important and atom-economic reaction for chemical utilization of CO2 [12,21]. However, the biggest obstacle is the lack of an effective catalyst to facilitate its activation and conversion, since CO2 is a highly oxidized and thermodynamically stable molecule [22]. In recent years, various metal catalysts [23,24,25,26,27,28] and metal-free catalysts [29,30] have been verified to be efficient for the reaction. Nevertheless, most of the catalytic systems usually required high temperatures, high pressures, toxic organic solvents and noble metals. Therefore, a cheap, green and highly active catalyst is urgently needed in order to make the reaction go smoothly at room temperature and atmospheric pressure.
Our previous work has revealed that metal salts combined with ionic liquid (IL) represent a new hybrid catalyst that can be regarded as one of the most promising and efficient catalysts for chemical transformations of CO2 because of their remarkable synergistic catalysis mechanism [31]. For example, a Cu(I) salt/protic IL catalytic system can efficiently catalyze the cycloaddition of CO2 with propargylic alcohols to produce α-alkylidene cyclic carbonates under mild conditions. Inspired by these works, we now report that Cu(I) salt and the IL tetrabutylphosphonium imidazol([P4444][Im]) act as a catalyst for the three component reaction of terminal propargyl alcohols, CO2 and anines (Scheme 1). The results show that the CuI/[P4444][Im] catalyst system exhibits excellent catalytic performance for the reaction with a wide range of substrates under atmospheric pressure and room temperature conditions. In addition, the ionic liquid could be recovered and reused at least five times without an obvious loss of catalytic activity and selectivity.
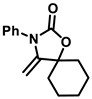
Scheme 1.
Cycloaddition reaction of propargyl alcohol and amines with CO2.
2. Result and Discussion
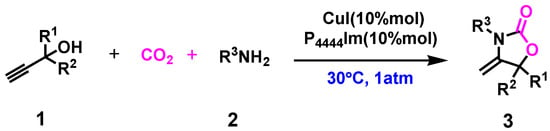
Owing to the unique catalytic performance of ILs in CO2 conversion [32,33,34], several quaternary phosphonium-based ILs and various copper (I) salts were selected to catalyze the reaction. Figure 1 shows the structures of the ILs.
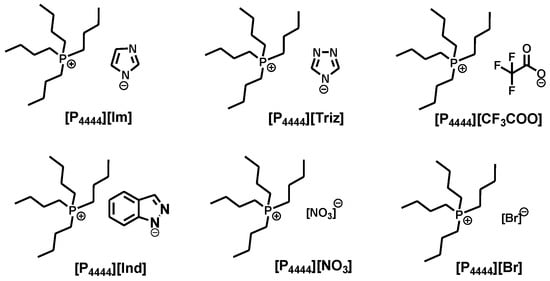
Figure 1.
The structure and abbreviations of CO2-reactive ILs employed in this work.
The synthesis of 1,3-oxazolidin-2-one 3a was carried out to systematically investigate the effect of various parameters on the reaction. The reaction was performed at 30 oC, 1 atm CO2, and 24 h, and the results are shown in Table 1. As expected, the reaction did not occur in the absence of any catalysts. The individual IL [P4444][Im] and CuI as a catalyst were ineffective for the reaction, giving a low yield of product (Table 1, entries 2–3). To our delight, the yield of the product reached rose to 91% in the presence of CuI and [P4444][Im] (Table 1, entry 4). Encouraged by the result, we investigated the effect of the type of anion on the yield of 3a in the reaction. The yield of 3a was found to be 70%, 41%, 35%, 30%, and 28%, respectively, when CuI/[P4444][Triz], CuI/[P4444][Ind], CuI/[P4444][CF3COO], CuI/[P4444][NO3] and [P4444][Br] were used as catalysts (Table 1, entries 5-9). The significant differences in their catalytic activity may be ascribed to the different nucleophilicity of these ILs as a result of their different basicity (Table S1). A larger IL pKa resulted in an improved absorption capacity of CO2 in the reaction system, which led to stronger reactivity between CO2 with propargylic alcohol, amine and the catalyst, to afford a higher product yield [35]. The result indicated that the ionic liquid anions play an important role in this conversion. Next, a variety of copper (I) salts with [P4444][Im] were used for this reaction. The activity of these copper salts followed the order: CuI > CuBr > CuCl (Table 1, entries 4, 10–11). Owing to the fact that among the halogenated metal salts the iodide anion had a stronger dissociation capacity, the metal cation and iodide anion could efficiently activate the substrates and promote the reaction in high yields [36]. The result suggested that the dissociation capacity of the anions acted a significant role in determining the catalytic activity of the halogenated copper salts. In addition, Cu2O and CuCN could also catalyze the reaction, affording moderate yields (65% and 32%, respectively) (Table 1, entries 12,13). Thus, CuI/[P4444][Im] was the best catalyst to further investigate this reaction.

Table 1.
Reaction of 2-methyl-3-butyn-2-ol (1a), n-butylamine (2a) and CO2 in various catalyst systems a.
Subsequently, the influence of catalyst amount on the yield of product was studied at 30 oC and 1 atm CO2 with a reaction time of 24 h. As seen in Figure S1, the yield of 3a was strongly dependent on the catalyst amount, and a maximum yield of 90% was obtained in the presence of 10 mol% CuI. It was shown that the yield of 3a will slowly decrease with further increase of the catalyst loading. Similar results were also obtained for the effect of IL amount in this reaction (Figure S2). Also, the dependence of reaction time on the yield of 3a was studied and the results are shown in Figure 2. The reaction was carried out at 30 °C, 1atm CO2 using CuI (10 mol%) and [P4444][Im] (10mol%) as catalyst. It is shown that a yield of 92% could be obtained after 24h, and the yield of 3a does not improve with further prolonged reaction time. Moreover, the effect of CO2 pressure on the reaction was studied. As shown in Table S2, the yield of 3a increased significantly with increasing pressure. More CO2 could be dissolved in the liquid phase with further increasing pressure, which led to more contact between CO2 with the substrates and the catalyst, and thus a higher yield of product was obtained. From the above findings, the optimal reaction conditions for the reaction were: CuI/[P4444][Im] as the catalyst, 30 °C, 24 h, and 1 atm CO2.
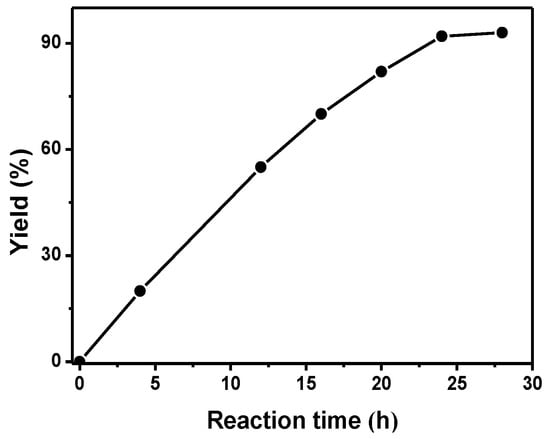
Figure 2.
Effect of reaction time on the product yield over CuI and [P4444][Im]; Reaction conditions: 1a, 1.0 mmol, 2a, 1.0 mmol, CuI, 0. mmo, [P4444][Im], 0.1 mmol, CO2 (1 atm), 30 °C. The yield was isolated yield.
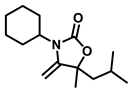
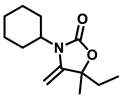
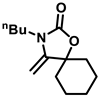
The reactions of CO2 with different kinds of propargylic alcohols and amines were evaluated under the optimized reaction conditions, and the results are listed in Table 2. It was shown that the coupling reaction of different alkyl substituted propargylic alcohols with CO2 and anines proceeded smoothly, producing the corresponding oxazolidinones in good yields under mild conditions. It was noted that a moderate yield of product was obtained for the propargylic alcohol with a cyclohexyl group (3i), indicating the steric hindrance of the substituent seemed to hamper the reaction. Furthermore, we also investigate the effect of amines with various substituents in this transformation. It was clearly shown that most of alkyl-substituted amines were efficient substrates to produce the corresponding oxazolidinones. However, when the R3 group of amines was a phenyl group, no product was obtained (compound 3k), even after a prolonged reaction time of 36 h. This phenomenon can be attributed to both the weak N-nucleophilicity and steric hindrance of the substrates, which further hamper the reaction [37]. From the above analysis, the catalyst system reported here is more favorable for alkyl amines than aromatic amines.

Table 2.
Three-component reactions of propargylic alcohols (1), amines (2) and CO2 under optimized conditions a.
To gain insight into the reaction mechanism, the catalytic role of [P4444][Im]/CuI with CO2 and the substrates were elucidated by 1H-NMR and 13C-NMR spectroscopy. In the 1H-NMR spectrum of the mixture of [P4444][Im]/CuI with 1a and n-BuNH2 (Figure 3), the OH signal became broad and shifted from 5.28 to 5.57, which indicated the formation of a hydrogen bond between [P4444][Im] and 1a [35]. In addition, the 13C-NMR signal assigned to the C1, C2, and C3 sites could be attributed to the interaction between CuI with the C≡C bond, leading to the activation of propargylic alcohol (Figure 4). These analysis suggested that the -OH and C≡C bond on the substrate 1a were synergistically activated by [P4444][Im] and CuI. Moreover, several additional control experiments were performed and the results are shown in Scheme 2. It was shown that the CuI/[P4444][Im] catalyst could efficiently catalyze the reaction of 1a with CO2 to form cyclic carbonate 4a with a yield of 70%. In addition, 4a reacted with n-butylamine to afford 3a smoothly without any catalyst. Therefore, the reaction of propargylic alcohols, anines and CO2 can be assumed to go through the cyclic carbonate pathway [37].
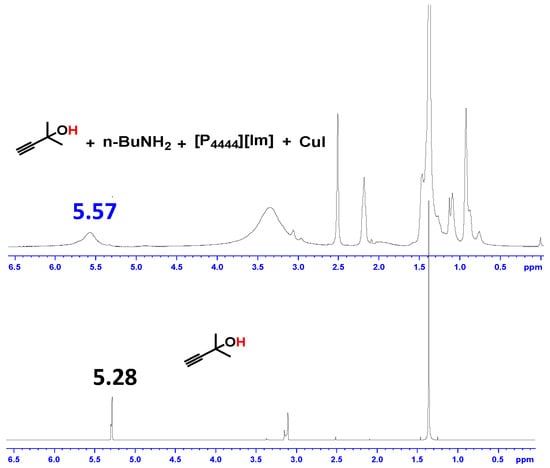
Figure 3.
1H-NMR spectra of propargylic alcohol, n-BuNH2 with and without CuI/[P4444][Im] catalyst.
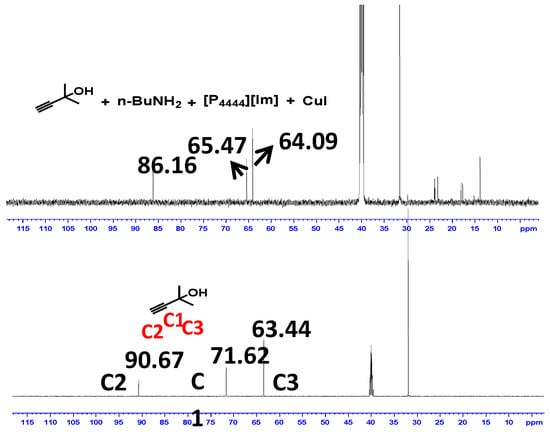
Figure 4.
13C-NMR spectra of propargylic alcohol, n-BuNH2 with and without CuI/[P4444][Im] catalyst.
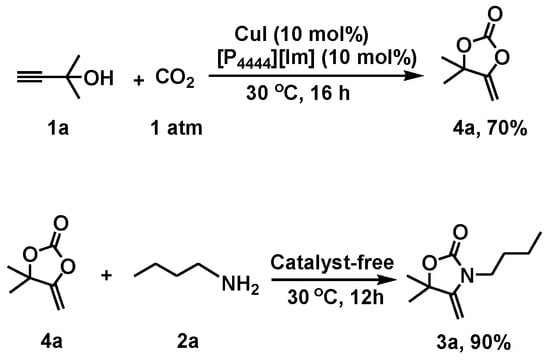
Scheme 2.
Control experiments.
Based on the above results, a plausible mechanism for the reaction catalyzed by CuI/[P4444][Im] as depicted in Scheme 3 is proposed. Firstly, the hydroxyl group of the propargyl alcohol is activated by IL [P4444][Im], then it reacts with CO2 to generate a zwitterionic carbamate species 1. Meanwhile, the C≡C triple bond of the propargyl alcohol was activated by CuI, and then the intermediate 2 was obtained. Then the intermediate cyclic carbonate 3 was formed by an intramolecular ring-closing reaction with the release of the catalyst. Finally, the cyclic carbonate undergoes a nucleophilic attack by a primary amine to generate the product oxazolidinone 5.
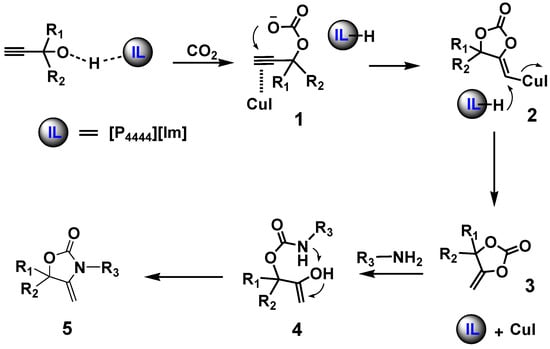
Scheme 3.
The proposed mechanism for the reaction of propargyl alcohol, CO2 and an amine catalyzed by [CuI]/[P4444][Im].
3. Experimental Section
3.1. Chemicals
Propargylic alcohols, amines, CuI, Cu2O, CuCl, CuBr, CuCN were obtained from J&K Scientific Company Limited (Beijing, China). The ILs [P4444][CF3COO], [P4444][NO3] and [P4444][Br] were purchased from Lanzhou Greenchem ILs (Lanzhou, Gansu, China). Other ILs such as [P4444][Triz], [P4444][Ind] and [P4444][Im] were synthesized by the ion exchange method [38].
3.2. General Procedures for the Synthesis of Oxazolidinones
Typically, propargylic alcohol 1a (1.0 mmol), n-butylamine (2a, 1.0 mmol) and CuI (0.1 mmol)/[P4444][Im] (0.1 mmol) were mixed in a 10 mL Schlenk flask. Then the reaction mixture was stirred at 30 °C for 24 h in the pressure of 0.1 MPa CO2. After the reaction was completed, water (5 mL) was added into this mixture, and the organic phase was purified through column chromatography. The ionic liquid was collected and reused without further treatment by removing any water under vacuum.
3.3. Characterization of the Products
The products were confirmed by using 1H-NMR analysis on Avance NEO 400 spectrometer (Bruker, Beijing, China) and Avance III HD 600 spectrometer (Bruker, Beijing, China). All of the products matched well with the previously reported experimental results [26].
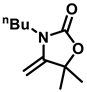
3-butyl-5,5-dimethyl-4-methyleneoxazolidin-2-one (3a), orange oil; 1H-NMR (CDCl3, 400 MHz does not match above) δ: 4.07 (d, J = 4.0 Hz, 1 H), 3.98 (d, J = 4.0 Hz, 1 H), 3.44 (t, J = 8.0 Hz, 2 H), 1.61–1.52 (m, 2 H), 1.49 (s, 6 H), 1.37–1.29 (m, 2 H), 0.95 (t, J = 7.2 Hz, 3 H) ppm.
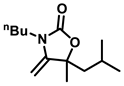
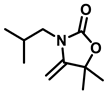
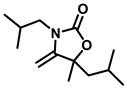
3-butyl-5-isobutyl-5-methyl-4-methyleneoxazolidin-2-one (3b), orange solid; 1H-NMR (CDCl3, 600 MHz) δ: 4.09 (s, 1 H), 3.93 (s, 1 H), 3.51-3.38 (m, 2 H), 1.80–1.43 (m, 8 H), 1.40–1.31 (m, 2 H), 1.02–0.89 (m, 9 H) ppm.
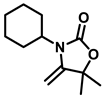
3-cyclohexyl-5,5-dimethyl-4-methyleneoxazolidin-2-one (3c), orange solid; 1H-NMR (CDCl3, 600 MHz) δ: 4.19 (d, J = 3.0 Hz, 1 H), 3.97 (d, J = 3.0 Hz, 1 H), 3.55 (t, J = 12.0 Hz, 1 H), 1.86-1.66 (m, 13 H), 1.25–1.14 (m, 4 H) ppm.
3-isobutyl-5,5-dimethyl-4-methyleneoxazolidin-2-one (3d), colorless oil; 1H-NMR (CDCl3, 600 MHz) δ: 4.27 (s, 1 H), 3.11–3.04 (m, 1 H), 2.96–2.89 (m, 1 H), 1.99-1.92 (m, 1 H), 1.51 (s, 3 H), 1.43. (s, 2 H), 1.34 (d, J = 12 Hz, 2 H), 0.92–0.87 (m, 6 H) ppm.
3-hexyl-5,5-dimethyl-4-methyleneoxazolidin-2-one (3e), colorless oil; 1H-NMR (CDCl3, 600 MHz) δ: 4.07 (d, J = 2.4 Hz, 1 H), 3.97 (d, J =3 Hz, 1 H), 3.43 (t, J = 7.8 Hz, 2 H), 1.63–1.58 (m, 2 H), 1.49 (s, 1 H), 1.32–1.30 (m, 6H), 0.89–0.88 (m, 3H) ppm.
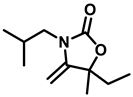
5-ethyl-3-isobutyl-5-methyl-4-methyleneoxazolidin-2-one (3f), colorless oil; 1H-NMR (CDCl3, 600 MHz) δ: 4.10 (d, J = 3 Hz, 1 H), 3.92 (d, J = 3 Hz, 1 H), 3.30–3.19 (m, 2 H), 1.85–1.80 (m, 1 H), 1.48–1.45 (d, J = 7.2 Hz, 5 H), 1.02 (t, J = 7.8 Hz, 2 H), 0.93–0.90 (m, 6 H), 0.89 (s, 1 H)ppm.
3,5-diisobutyl-5-methyl-4-methyleneoxazolidin-2-one (3g), orange solid; 1H-NMR (CDCl3, 600 MHz) δ: 4.09 (s, 1 H), 3.93 (s, 1 H), 3.28–3.23 (m, 2 H), 2.14–2.04 (m, 1 H), 1.51 (s, 1 H), 1.04–0.98 (m, 2 H), 0.97–0.91 (m, 12 H) ppm.
3-cyclohexyl-5-isobutyl-5-methyl-4-methyleneoxazolidin-2-one (3h), orange solid; 1H-NMR (CDCl3, 600 MHz) δ: 4.24 (s, 1 H), 3.93 (s, 1 H), 3.64–3.53 (m, 1 H), 2.15–1.45 (m, 12 H), 1.34–1.15 (m, 4 H), 0.94–0.91 (m, 6 H) ppm.
3-cyclohexyl-5-ethyl-5-methyl-4-methyleneoxazolidin-2-one (3i), orange solid; 1H-NMR (CDCl3, 600 MHz) δ: 4.25 (d, J = 3.0 Hz, 1 H), 3.93 (d, J = 3.0 Hz, 1 H), 3.62–3.55 (m, 1 H), 1.66–1.47 (m, 11 H), 1.35–1.18 (m, 4 H), 0.88 (t, J = 7.8 Hz, 3 H) ppm.
3-Butyl-4-methylene-1-oxa-3-azaspiro[4.5]decan-2-one (3j), orange oil; 1H-NMR (CDCl3, 600 MHz) δ: 4.07 (d, J = 3.0 Hz, 1 H), 3.95 (d, J = 2.4 Hz, 1 H), 3.43 (t, J = 7.8 Hz, 2 H), 1.76–1.65 (m, 5 H), 1.61–1.50 (m, 7 H), 1.40–1.31 (m, 2 H), 0.94 (t, J = 7.2 Hz, 3 H) ppm.
4. Conclusions
In conclusion, we have developed an excellent and cost-competitive catalytic process catalyzed by the CuI/[P4444][Im] system for the transformation of CO2 to form oxazolidinones. The reaction can proceed efficiently under room temperature and atmospheric pressure conditions to give high yields of product. A wide range of propargylic alcohols and amines has been employed, which confirmed the versatility of the catalyst system for the synthesis of oxazolidinones. Preliminary mechanistic studies suggest that the reaction of propargylic alcohols, anines and CO2 could involve the cyclic carbonate pathway. This work reveals the great potential of ionic liquids combined with metal salts as an efficient type of catalysts for CO2 conversion under mild conditions.
Supplementary Materials
The following are available online.
Author Contributions
Organic synthesis and compound characterization, J.Q. and Y.Z (Yue Zhao); NMR spectroscopy, Y.Z. (Yue Zhao); writing—original draft preparation, J.Q.; writing-review and editing, Y.Z. (Yuling Zhao), J.W., Z.L., H.W.; project administration, Z.L. and H.W.; formal analysis, T.J.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 21773058, U1704251, 21733011), National Key Research and Development Program of China (2017YFA0403101), Project funded by China Postdoctoral Science Foundation (No. 2017M622349), and the 111 Project (No. D17007). This work was also supported by the High Performance Computing Center of Henan Normal University.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| [P4444][Im] | Tetrabutylphosphonium imidazole |
| [P4444][CF3COO] | Tetrabutylphosphonium 2,2,2-trifluoroacetic acid |
| [P4444][Triz] | Tetrabutylphosphonium 1,2,4-triazole |
| [P4444][Ind] | Tetrabutylphosphonium indazole |
| [P4444][Br] | Tetrabutylphosphonium bromide |
| [P4444][NO3] | Tetrabutylphosphonium nitrate |
References
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef]
- Das, S.; Bobbink, F.D.; Laurenczy, G.; Dyson, P.J. Metal-free catalyst for the chemoselective methylation of amines using carbon dioxide as a carbon source. Angew. Chem. Int. Ed. 2014, 53, 12876–12879. [Google Scholar] [CrossRef]
- Alvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the greener production of formates/formic Acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Yoshida, S.; Sugawara, Y.; Yamada, W.; Cheng, H.-M.; Fukui, K.; Sekine, K.; Iwakura, I.; Ikeno, T.; Yamada, T. Silver-catalyzed carbon dioxide incorporation and rearrangement on propargylic derivatives. Bull. Chem. Soc. Jpn. 2011, 84, 698–717. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Li, Z.; Wang, H.; Shi, Y.; Wang, J. Imidazolium salts functionalized covalent organic frameworks for highly efficient catalysis of CO2 conversion. ChemSusChem 2019. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Qi, C.; Wu, W.; Jiang, H. Recent advances in organic synthesis with CO2 as C1 synthon. Curr. Opin. Green Sustain. Chem. 2017, 3, 22–27. [Google Scholar] [CrossRef]
- Yoo, W.J.; Li, C.J. Copper catalyzed four component coupling between aldehydes, amines, alkynes, and carbon dioxide. Adv. Synth. Catal. 2008, 350, 1503–1506. [Google Scholar] [CrossRef]
- Yu, B.; Yang, Z.; Zhao, Y.; Hao, L.; Zhang, H.; Gao, X.; Han, B.; Liu, Z. An efficient and general method for formylation of aryl bromides with CO2 and poly(methylhydrosiloxane). Chem. Eur. J. 2016, 22, 1097–1102. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, Y.; Zeng, C.; Song, D.; Chaemchuen, S.; Chen, C.; Verpoort, F. A recyclable AgI/OAc catalytic system for the efficient synthesis of α-alkylidene cyclic carbonates: Carbon dioxide conversion at atmospheric pressure. Green Chem. 2017, 19, 2936–2940. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.-X.; Zhang, W.-Z.; Lu, X.-B. CO2 adducts of phosphorus ylides: Highly active organocatalysts for carbon dioxide transformation. ACS Catal. 2015, 5, 6773–6779. [Google Scholar] [CrossRef]
- Maeda, C.; Miyazaki, Y.; Ema, T. Recent progress in catalytic conversions of carbon dioxide. Catal. Sci. Technol. 2014, 4, 1482–1497. [Google Scholar] [CrossRef]
- Riduan, S.N.; Zhang, Y. Recent developments in carbon dioxide utilization under mild conditions. Dalton Trans. 2010, 39, 3347–3357. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; He, L.-N.; Gao, J.; Liu, A.-H.; Yu, B. Carbon dioxide utilization with C–N bond formation: Carbon dioxide capture and subsequent conversion. Energy Environ. Sci. 2012, 5, 6602–6639. [Google Scholar] [CrossRef]
- Hao, L.; Zhao, Y.; Yu, B.; Yang, Z.; Zhang, H.; Han, B.; Gao, X.; Liu, Z. Imidazolium-based ionic liquids catalyzed formylation of amines using carbon dioxide and phenylsilane at room temperature. ACS Catal. 2015, 5, 4989–4993. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, L.; Liu, Q.; Yang, X.; Ye, X.; Hu, Y.; Huang, Y. A schiff base-modified silver catalyst for efficient fixation of CO2 as carboxylic acid at ambient pressure. Green Chem. 2017, 19, 2080–2085. [Google Scholar] [CrossRef]
- Fujii, A.; Matsuo, H.; Choi, J.-C.; Fujitani, T.; Fujita, K. Efficient synthesis of 2-oxazolidinones and quinazoline-2,4(1 H, 3 H)-diones from CO2 catalyzed by tetrabutylammonium fluoride. Tetrahedron 2018, 74, 2914–2920. [Google Scholar] [CrossRef]
- Wright, T. Streptogramins, oxazolidinones, and other Inhibitors of bacterial protein synthesis. Chem. Rev. 2005, 105, 529–542. [Google Scholar]
- Smith, C.J.; Ali, A.; Hammond, M.L.; Li, H.; Lu, Z.; Napolitano, J.; Taylor, G.E.; Thompson, C.F.; Anderson, M.S.; Chen, Y.; et al. Biphenyl-substituted oxazolidinones as cholesteryl ester transfer protein inhibitors: Modifications of the oxazolidinone ring leading to the discovery of anacetrapib. J. Med. Chem. 2011, 54, 4880–4895. [Google Scholar] [CrossRef]
- Remarchuk, L. Catalytic asymmetric intramolecular aminopalladation: Enantioselective synthesis of vinyl-substituted 2-oxazolidinones, 2-imidazolidinones, and 2-pyrrolidinones. J. Am. Chem. Soc. 2002, 124, 12–13. [Google Scholar]
- Mancuso, R.; Maner, A.; Ziccarelli, I.; Pomelli, C.; Chiappe, C.; Della Ca, N.; Veltri, L.; Gabriele, B. Auto-tandem catalysis in ionic liquids: Synthesis of 2-oxazolidinones by palladium-catalyzed oxidative carbonylation of propargylic amines in EmimEtSO4. Molecules 2016, 21, 897. [Google Scholar] [CrossRef]
- Arshadi, S.; Vessally, E.; Hosseinian, A.; Soleimani-amiri, S.; Edjlali, L. Three-component coupling of CO2, propargyl alcohols, and amines: An environmentally benign access to cyclic and acyclic carbamates (a review). J. CO2 Util. 2017, 21, 108–118. [Google Scholar] [CrossRef]
- Song, Q.-W.; He, L.-N. Robust silver(I) catalyst for the carboxylative cyclization of propargylic alcohols with carbon dioxide under ambient conditions. Adv. Synth. Catal. 2016, 358, 1251–1258. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, J.; Wang, A. An efficient and eco-friendly process for the conversion of carbon dioxide into oxazolones and oxazolidinones under supercritical conditions. Synthesis 2008, 2008, 763–769. [Google Scholar] [CrossRef]
- Li, X.D.; Song, Q.W.; Lang, X.D.; Chang, Y.; He, L.N. Ag(I)/TMG-promoted cascade reaction of propargyl alcohols, carbon dioxide, and 2-aminoethanols to 2-oxazolidinones. Chemphyschem 2017, 18, 3182–3188. [Google Scholar] [CrossRef]
- Song, Q.-W.; Liu, P.; Han, L.-H.; Zhang, K.; He, L.-N. Upgrading CO2 by incorporation into urethanes through silver-catalyzed one-pot stepwise amidation reaction. Chin. J. Chem. 2018, 36, 147–152. [Google Scholar] [CrossRef]
- Song, Q.-W.; Yu, B.; Li, X.-D.; Ma, R.; Diao, Z.-F.; Li, R.-G.; Li, W.; He, L.-N. Efficient chemical fixation of CO2 promoted by a bifunctional Ag2WO4/Ph3P system. Green Chem. 2014, 16, 1633–1638. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Jia, Z.; Zhang, J. Facile and mild process for chemical fixation of CO2 to 4-methylene-1,3-oxazolidin-2-ones under solvent-free conditions. Synth. Commun. 2011, 41, 858–863. [Google Scholar] [CrossRef]
- Gu, Y.; Duan, Z.; Zhang, J.; Zhang, S.; Deng, Y. Ionic liquid as an efficient promoting medium for fixation of carbon dioxide: A clean method for the synthesis of 5-methylene-1,3-oxazolidin-2-ones from propargylic alcohols, amines, and carbon dioxide catalyzed by Cu(I) under mild conditions. J. Org. Chem. 2005, 70, 7376–7380. [Google Scholar] [CrossRef]
- Ca, N.D.; Gabriele, B.; Ruffolo, G.; Veltri, L.; Zanetta, T.; Costa, M. Effective Guanidine-catalyzed synthesis of carbonate and carbamate derivatives from propargyl alcohols in supercritical carbon dioxide. Adv. Synth. Catal. 2011, 353, 133–146. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, F.; Gu, Y.; Yang, J.; Deng, Y. Efficient and eco-friendly process for the synthesis of N-substituted 4-methylene-2-oxazolidinones in ionic liquids. Tetrahedron Lett. 2005, 46, 5907–5911. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, L.; Qiu, J.; Li, Z.; Wang, H.; Cui, G.; Zhang, S.; Wang, J. Remarkable synergistic effect between copper(I) and ionic liquids for promoting chemical fixation of CO2. J. CO2 Utili. 2017, 22, 374–381. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, B.; Yang, Z.; Zhang, H.; Hao, L.; Gao, X.; Liu, Z. A protic ionic liquid catalyzes CO2 conversion at atmospheric pressure and room temperature: Synthesis of quinazoline-2,4(1H,3H)-diones. Angew. Chem. Int. Ed. 2014, 53, 5922–5925. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wabg, X. Imidazolium ionic liquids, imidazolylidene heterocyclic carbenes, and zeolitic imidazolate frameworks for CO2 capture and photochemical reduction. Angew. Chem. Int. Ed. 2016, 55, 2308–2320. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shi, G.; Dao, R.; Mei, K.; Zhou, X.; Li, H.; Wang, C. Tuning the basicity of ionic liquids for efficient synthesis of alkylidene carbonates from CO2 at atmospheric pressure. Chem. Commun. 2016, 52, 7830–7833. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhao, Y.; Li, Z.; Wang, H.; Fan, M.; Wang, J. Efficient ionic-liquid-promoted chemical fixation of CO2 into a-alkylidene cyclic carbonates. ChemSusChem 2017, 10, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ma, J.; Zhu, Q.; Qian, Q.; Han, H.; Mei, Q.; Han, B. Zinc(II)-catalyzed reactions of carbon dioxide and propargylic alcohols to carbonates at room temperature. Green Chem. 2016, 18, 382–385. [Google Scholar] [CrossRef]
- Song, Q.W.; Chen, W.Q.; Ma, R.; Yu, A.; Li, Q.Y.; Chang, Y.; He, L.N. Bifunctional silver(I) complex-catalyzed CO2 conversion at ambient conditions: Synthesis of alpha-methylene cyclic carbonates and derivatives. ChemSusChem 2015, 8, 821–827. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds of 3a–3j are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).