Inactivation of Venom PLA2 Alleviates Myonecrosis and Facilitates Muscle Regeneration in Envenomed Mice: A Time Course Observation
Abstract
1. Introduction
2. Results
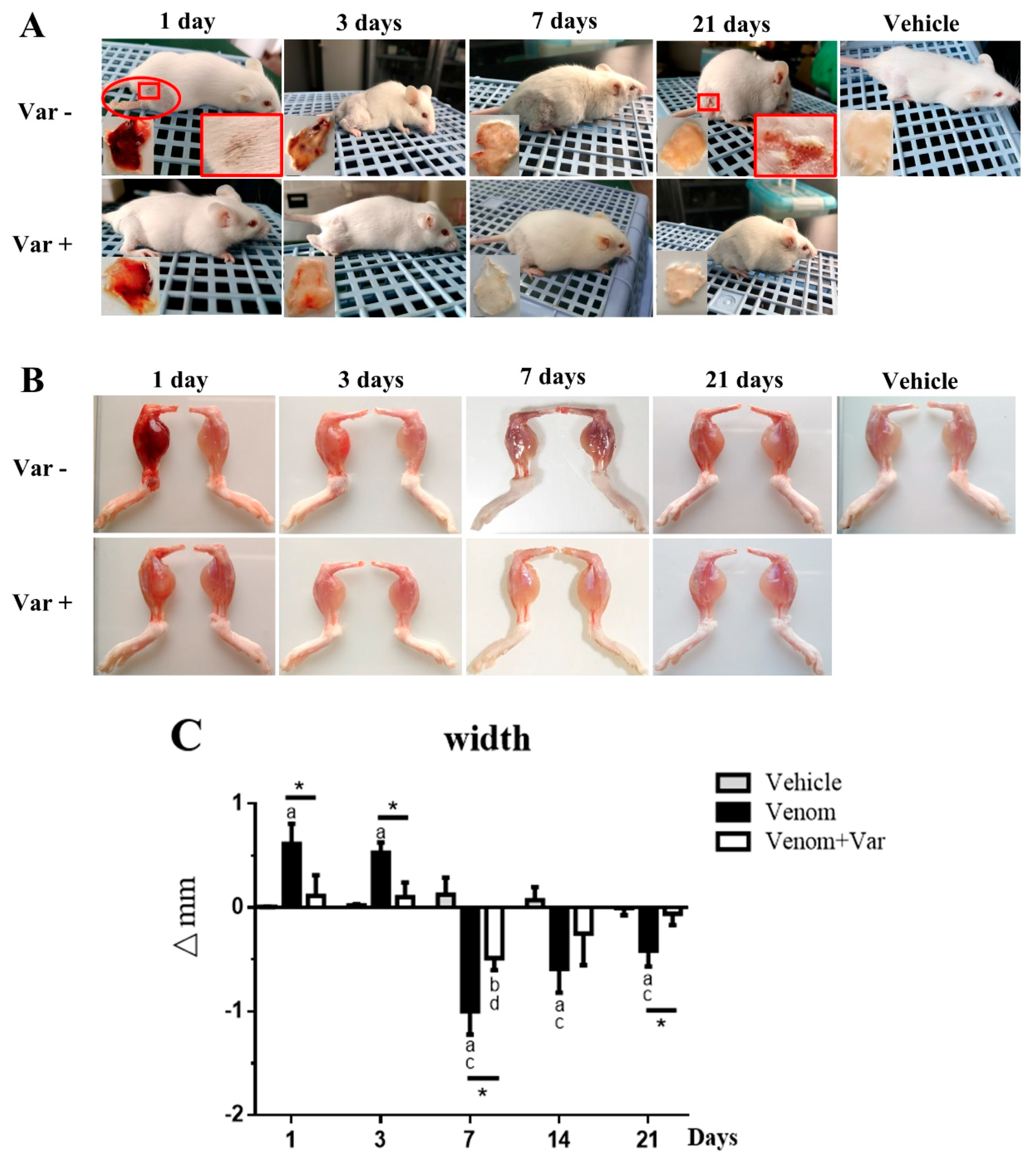
2.1. Signs of Envenomation in Mice
2.2. Edema and Atrophy in Gastrocnemius Muscle
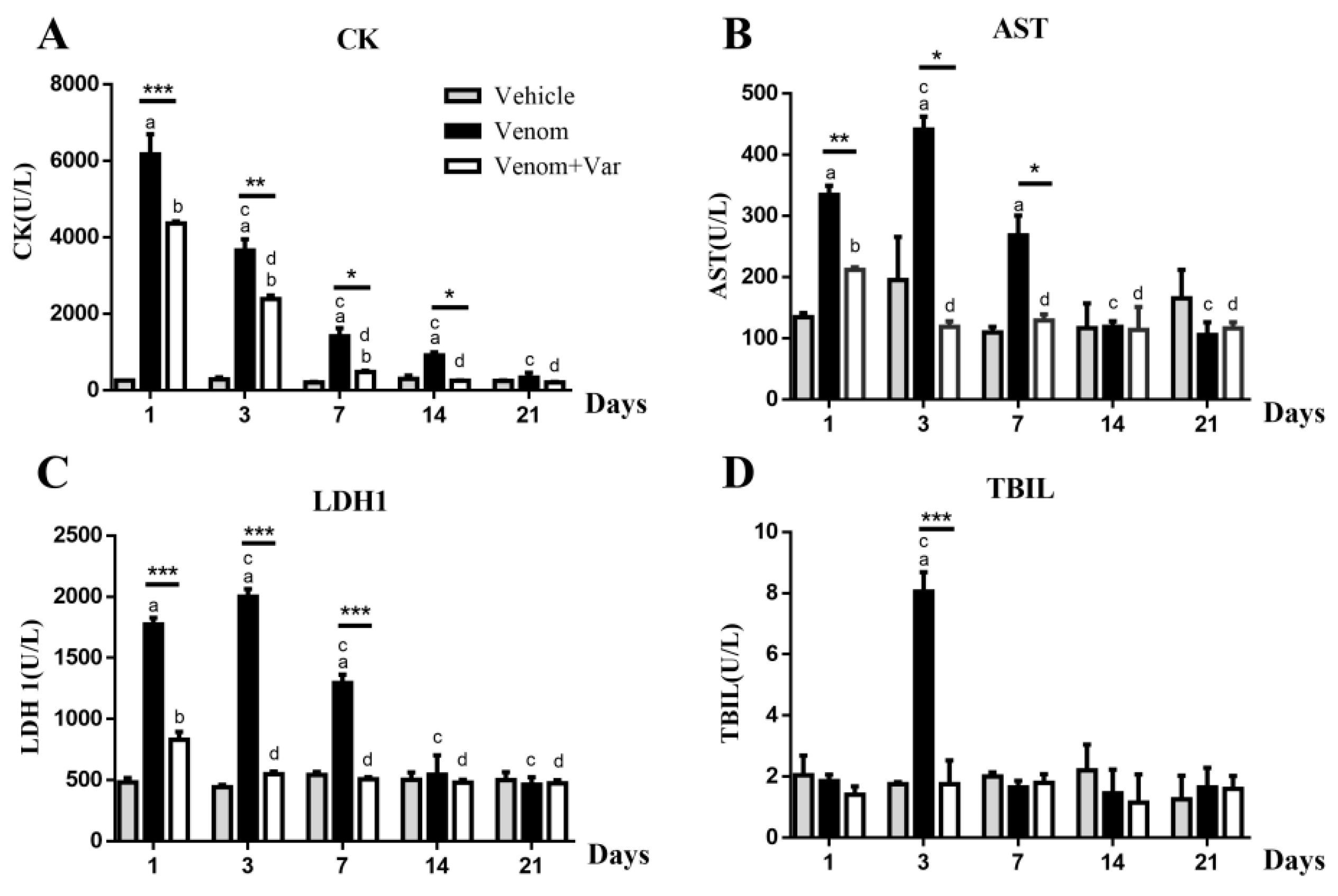
2.3. Plasma Enzyme Activity
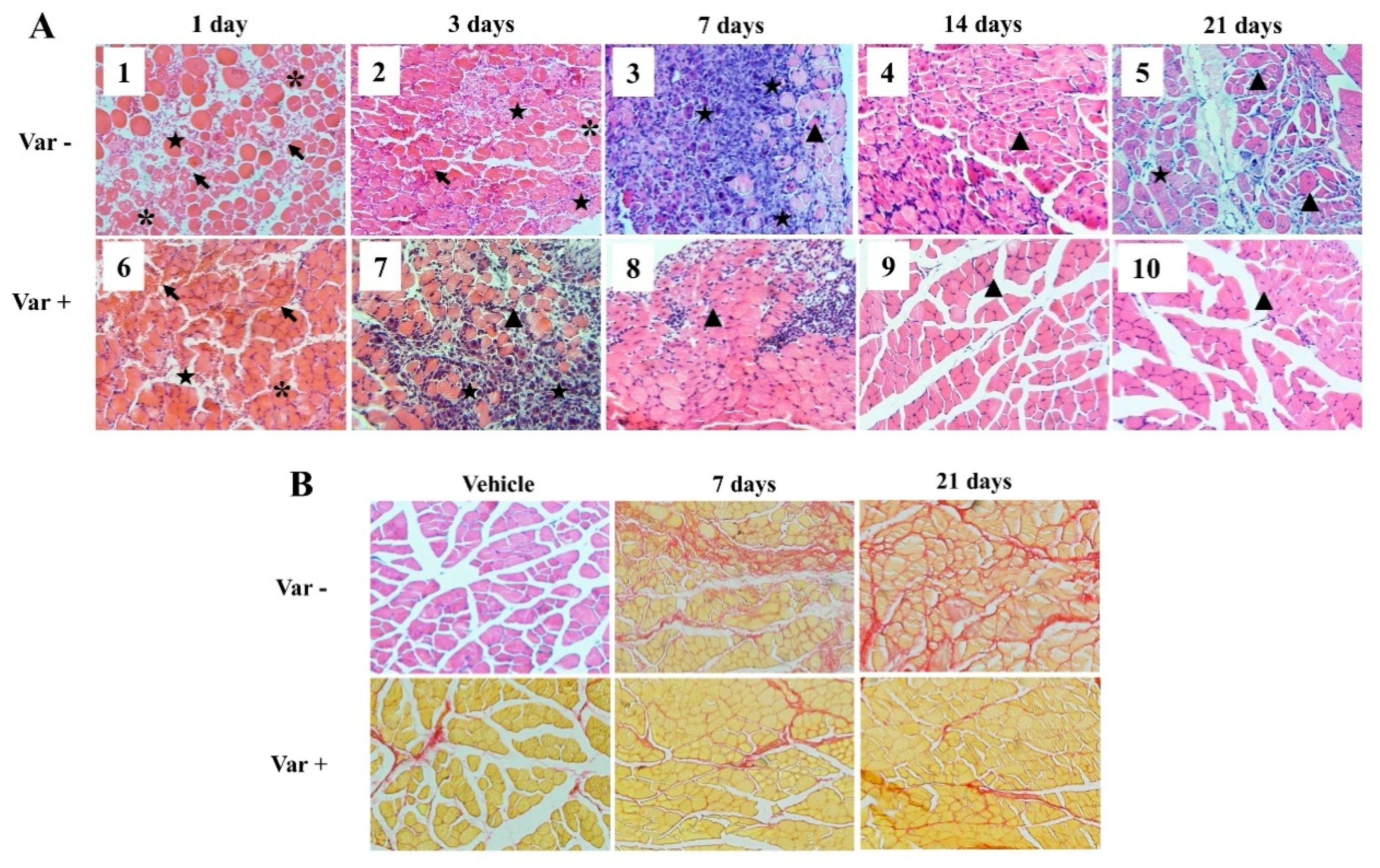
2.4. Histological Analysis
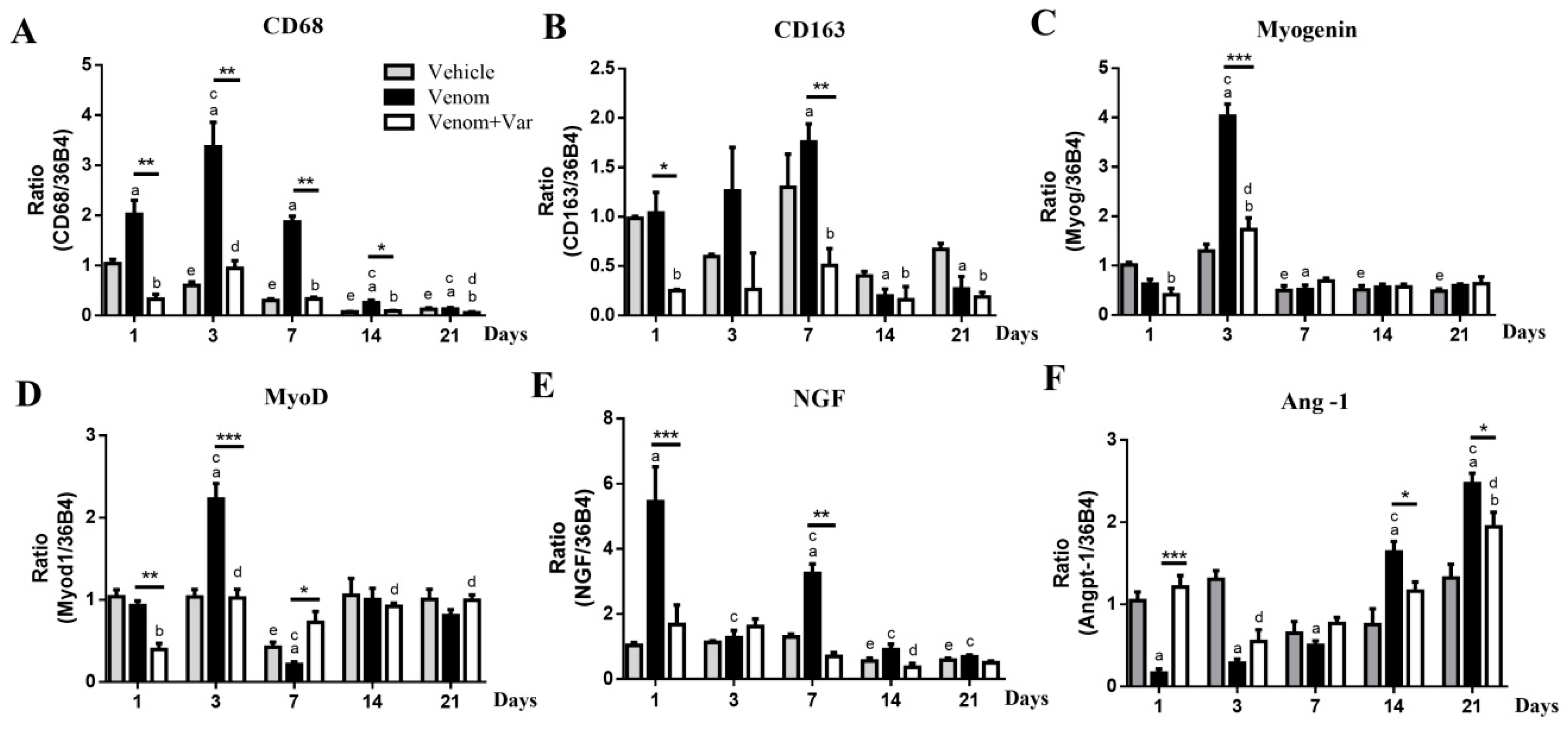
2.5. Inflammation in Gastrocnemius Muscle
2.6. Coordinate Recovery in Muscle Regeneration
3. Discussion
4. Materials and Methods
4.1. Venom and Varespladib
4.2. Animal Models
4.3. Serum CK, AST, LDH1, and TBIL
4.4. Muscle Injury and Regeneration Evaluation
4.5. Quantitative Real-time PCR Analysis
4.6. Histological Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Escalante, T.; Rucavado, A. Experimental pathophysiology of systemic alterations induced by bothrops asper snake venom. Toxicon 2009, 54, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Rucavado, A.; Chaves, F.; Diaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by bothrops asper snake venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Leon, G.; Rojas, G.; Lomonte, B.; Rucavado, A.; Chaves, F. Neutralization of local tissue damage induced by bothrops asper (terciopelo) snake venom. Toxicon 1998, 36, 1529–1538. [Google Scholar] [CrossRef]
- Hernandez, R.; Cabalceta, C.; Saravia-Otten, P.; Chaves, A.; Gutierrez, J.M.; Rucavado, A. Poor regenerative outcome after skeletal muscle necrosis induced by bothrops asper venom: Alterations in microvasculature and nerves. PLoS ONE 2011, 6, e19834. [Google Scholar] [CrossRef]
- Hai Lan, Y.C. Biological characteristics of 80 snakes in china. In Chinese Venomous Snake and Snakebite Treatment, 1st ed.; Hai Lan, Y.C., Ed.; Shanghai Century Publishing Co., Ltd.: Shanghai, China; Shanghai Science and Technology Press: Shanghai, China, 2008; pp. 113–117. ISBN 978-7-5323-9097-7. [Google Scholar]
- Liu, Q.; Zhang, X.; Yin, W.; Li, C.; Huang, Y.; Qiu, P.; Su, X.; Hu, S.; Yan, G. A catalog for transcripts in the venom gland of the agkistrodon acutus: Identification of the toxins potentially involved in coagulopathy. Biochem. Biophys. Res. Commun. 2006, 341, 522–531. [Google Scholar] [CrossRef]
- Mackessy, S.P. The field of reptile toxinology snakes, lizards, and their venoms. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2010; pp. 3–19. ISBN 0849391652. [Google Scholar]
- Xiao, H.; Pan, H.; Liao, K.; Yang, M.; Huang, C. Snake venom PLA2, a promising target for broad-spectrum antivenom drug development. Biomed. Res. Int. 2017, 2017, 6592820. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Zhang, D.; Xiao, H.; Xiong, S.; Huang, C. Exploration of the inhibitory potential of varespladib for snakebite envenomation. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Bustillo, S.; Gay, C.C.; Garcia Denegri, M.E.; Ponce-Soto, L.A.; Bal de Kier Joffe, E.; Acosta, O.; Leiva, L.C. Synergism between baltergin metalloproteinase and ba spii RP4 PLA2 from bothrops alternatus venom on skeletal muscle (C2C12) cells. Toxicon 2012, 59, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.W.; Bach, N.J.; Dillard, R.D.; Draheim, S.E.; Carlson, D.G.; Fox, N.; Roehm, N.W.; Armstrong, C.T.; Chang, C.H.; Hartley, L.W.; et al. Pharmacology of LY315920/S-5920,[[3-(Aminooxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl] oxy] acetate, a Potent and Selective Secretory Phospholipase A2Inhibitor: A New Class of Anti-Inflammatory Drugs, SPI. J. Pharmacol. Exp. Ther. 1999, 288, 1117–1124. [Google Scholar] [PubMed]
- Nicholls, S.J.; Kastelein, J.J.; Schwartz, G.G.; Bash, D.; Rosenson, R.S.; Cavender, M.A.; Brennan, D.M.; Koenig, W.; Jukema, J.W.; Nambi, V.; et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: The vista-16 randomized clinical trial. JAMA 2014, 311, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (ly315920) appears to be a potent, broad-spectrum, inhibitor of snake venom phospholipase a2 and a possible pre-referral treatment for envenomation. Toxins (Basel) 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Laustsen, A.H. Recent advances in next generation snakebite antivenoms. Trop. Med. Infect. Dis. 2018, 3, 42. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Engmark, M.; Milbo, C.; Johannesen, J.; Lomonte, B.; Gutierrez, J.M.; Lohse, B. From fangs to pharmacology: The future of snakebite envenoming therapy. Curr. Pharm. Des. 2016, 22, 5270–5293. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Lomonte, B.; Lohse, B.; Fernández, J.; Gutiérrez, J.M. Unveiling the nature of black mamba (dendroaspis polylepis) venom through venomics and antivenom immunoprofiling: Identification of key toxin targets for antivenom development. J. Proteom. 2015, 119, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Marsh, N.; Gattullo, D.; Pagliaro, P.; Losano, G. The gaboon viper, bitis gabonica: Hemorrhagic, metabolic, cardiovascular and clinical effects of the venom. Life Sci. 1997, 61, 763–769. [Google Scholar] [CrossRef]
- Rahmy, T.R.; Hemmaid, K.Z. Histological and histochemical alterations in the liver following intramuscular injection with a sublethal dose of the egyptian cobra venom. J. Nat. Toxins 2000, 9, 21–32. [Google Scholar] [PubMed]
- Ramprasad, M.P.; Fischer, W.; Witztum, J.L.; Sambrano, G.R.; Quehenberger, O.; Steinberg, D. The 94- to 97-kda mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human cd68. Proc. Natl. Acad. Sci. USA 1995, 92, 9580–9584. [Google Scholar] [CrossRef] [PubMed]
- St Pierre, B.; Tidball, J.G. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J. Appl. Physiol. 1994, 77, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Villalta, S.A.; Nguyen, H.X.; Deng, B.; Gotoh, T.; Tidball, J.G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 2009, 18, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Ciciliot, S.; Schiaffino, S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr. Pharm. Des. 2010, 16, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors myf5, myod, myogenin and mrf4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- McLoon, L.K.; Nguyen, L.T.; Wirtschafter, J. Time course of the regenerative response in bupivacaine injured orbicularis oculi muscle. Cell Tissue Res. 1998, 294, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; von Roth, P.; Matziolis, G.; Schumann, M.R.; Hahn, S.; Strube, P.; Stoltenburg-Didinger, G.; Perka, C.; Duda, G.N.; Tohtz, S.V. Time course of skeletal muscle regeneration after severe trauma. Acta Orthop. 2011, 82, 102–111. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.M.; Reinardy, J.L.; Mueller, S.B.; McCord, T.J.; Kontos, C.D.; Brown, D.A.; Hussain, S.N.; Schmidt, C.A.; Ryan, T.E.; Green, T.D. Muscle cell derived angiopoietin-1 contributes to both myogenesis and angiogenesis in the ischemic environment. Front. Physiol. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Mofarrahi, M.; McClung, J.M.; Kontos, C.D.; Davis, E.C.; Tappuni, B.; Moroz, N.; Pickett, A.E.; Huck, L.; Harel, S.; Danialou, G.; et al. Angiopoietin-1 enhances skeletal muscle regeneration in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R576–R589. [Google Scholar] [CrossRef] [PubMed]
- Emanueli, C.; Salis, M.B.; Pinna, A.; Graiani, G.; Manni, L.; Madeddu, P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 2002, 106, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the snake venom lyophilized powder of D. acutus are available from the authors. |
| Gene | Sequences (5′-3′) |
|---|---|
| 36B4 | F: GCTTCATTGTGGGAGCAGAC |
| R: TTCTCCAGAGCTGGGTTGTT | |
| CD68 | F: CCATCCTTCACGATGACACCT |
| R: GGCAGGGTTATGAGTGACAGTT | |
| CD163 | F: TGTGCAGTAACGGCTGGAG |
| R: ATCATGTTTGCAGTCCCAAAGA | |
| Ang-1 | F: CACATAGGGTGCAGCAACCA |
| R: CGTCGTGTTCTGGAAGAATGA | |
| NGF | F: AGACTCCACTCACCCCGTG |
| R: GGCTGTGGTCTTATCTCCAAC | |
| Myog | F: GAGACATCCCCCTATTTCTACCA |
| R: GCTCAGTCCGCTCATAGCC | |
| MyoD | F: CCACTCCGGGACATAGACTTG |
| R: AAAAGCGCAGGTCTGGTGAG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Li, H.; Zhang, D.; Li, Y.; Sun, S.; Huang, C. Inactivation of Venom PLA2 Alleviates Myonecrosis and Facilitates Muscle Regeneration in Envenomed Mice: A Time Course Observation. Molecules 2018, 23, 1911. https://doi.org/10.3390/molecules23081911
Xiao H, Li H, Zhang D, Li Y, Sun S, Huang C. Inactivation of Venom PLA2 Alleviates Myonecrosis and Facilitates Muscle Regeneration in Envenomed Mice: A Time Course Observation. Molecules. 2018; 23(8):1911. https://doi.org/10.3390/molecules23081911
Chicago/Turabian StyleXiao, Huixiang, Haoran Li, Denghong Zhang, Yuanyuan Li, Shimin Sun, and Chunhong Huang. 2018. "Inactivation of Venom PLA2 Alleviates Myonecrosis and Facilitates Muscle Regeneration in Envenomed Mice: A Time Course Observation" Molecules 23, no. 8: 1911. https://doi.org/10.3390/molecules23081911
APA StyleXiao, H., Li, H., Zhang, D., Li, Y., Sun, S., & Huang, C. (2018). Inactivation of Venom PLA2 Alleviates Myonecrosis and Facilitates Muscle Regeneration in Envenomed Mice: A Time Course Observation. Molecules, 23(8), 1911. https://doi.org/10.3390/molecules23081911





