Optimization of Regioselective α-Glucosylation of Hesperetin Catalyzed by Cyclodextrin Glucanotransferase
Abstract
1. Introduction
2. Results and Discussion
2.1. Enzymatic Glucosylation of Hesperetin
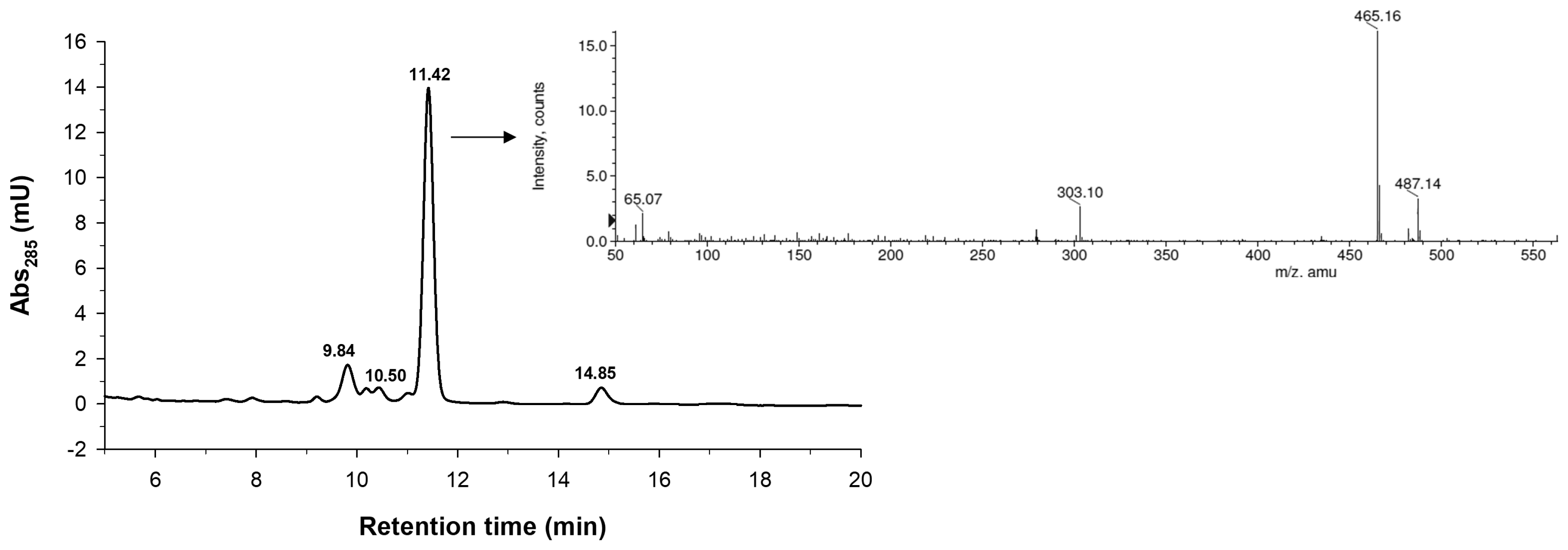
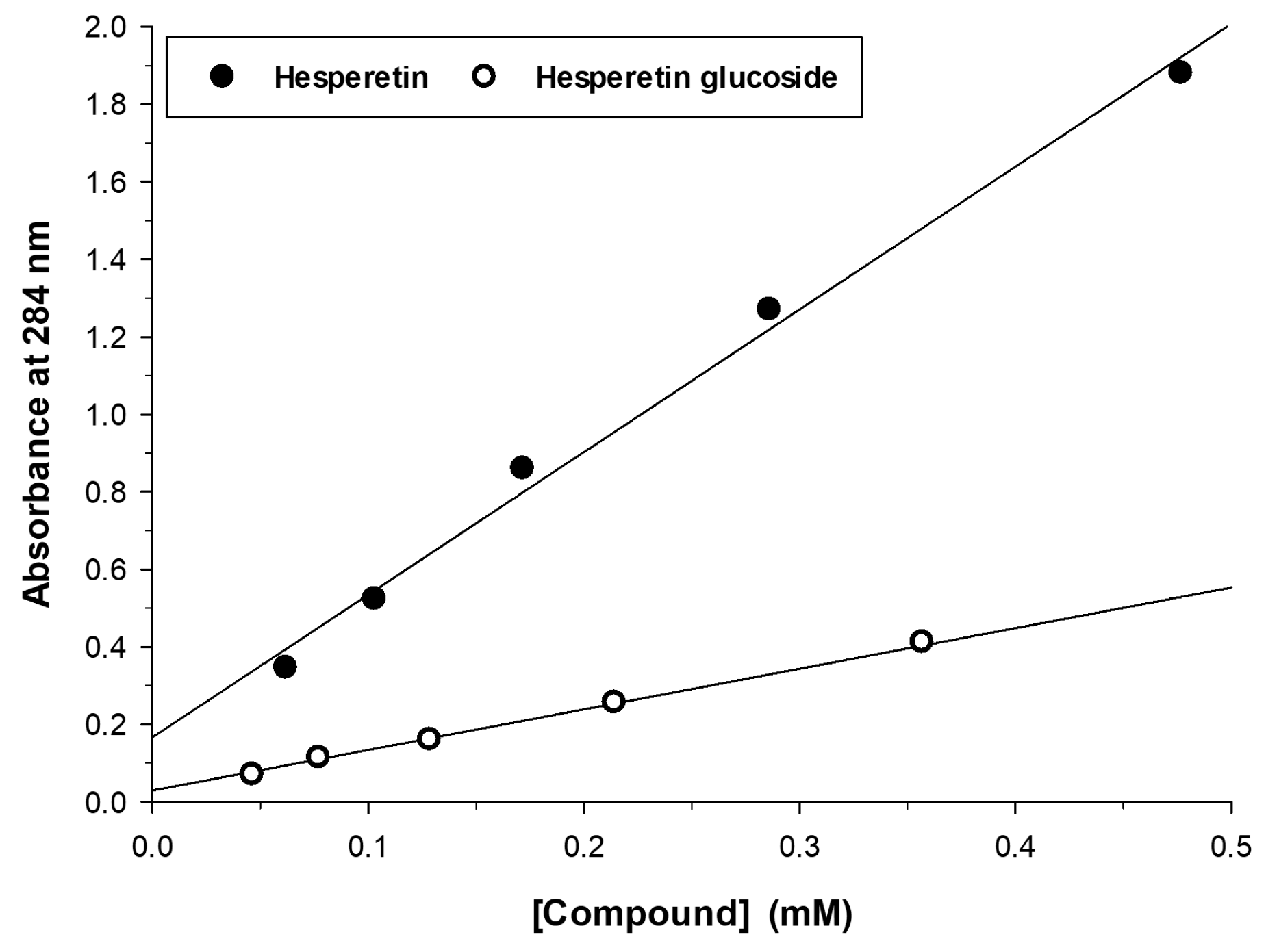
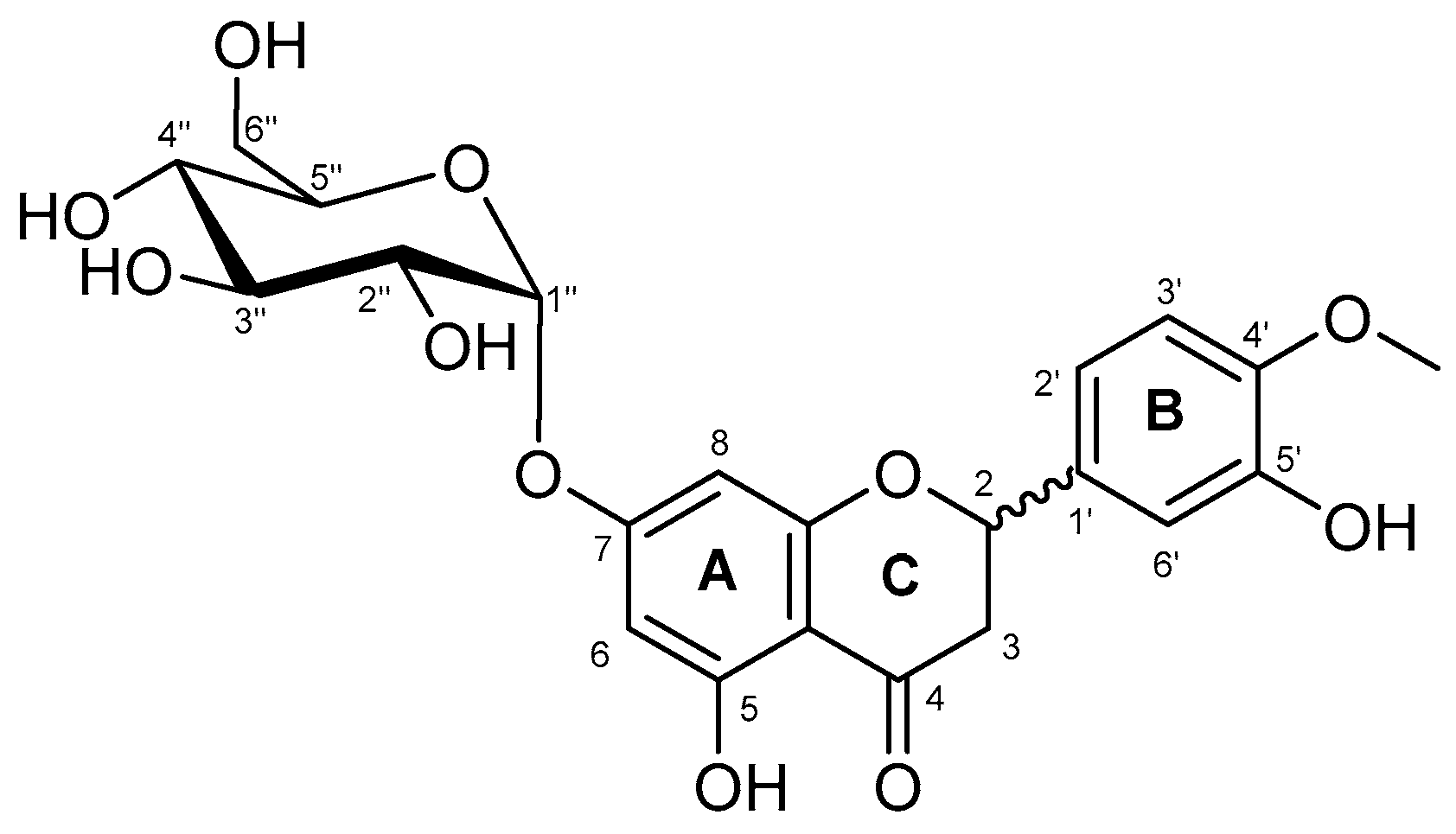
2.2. Characterization of the Monoglucosylated Derivative
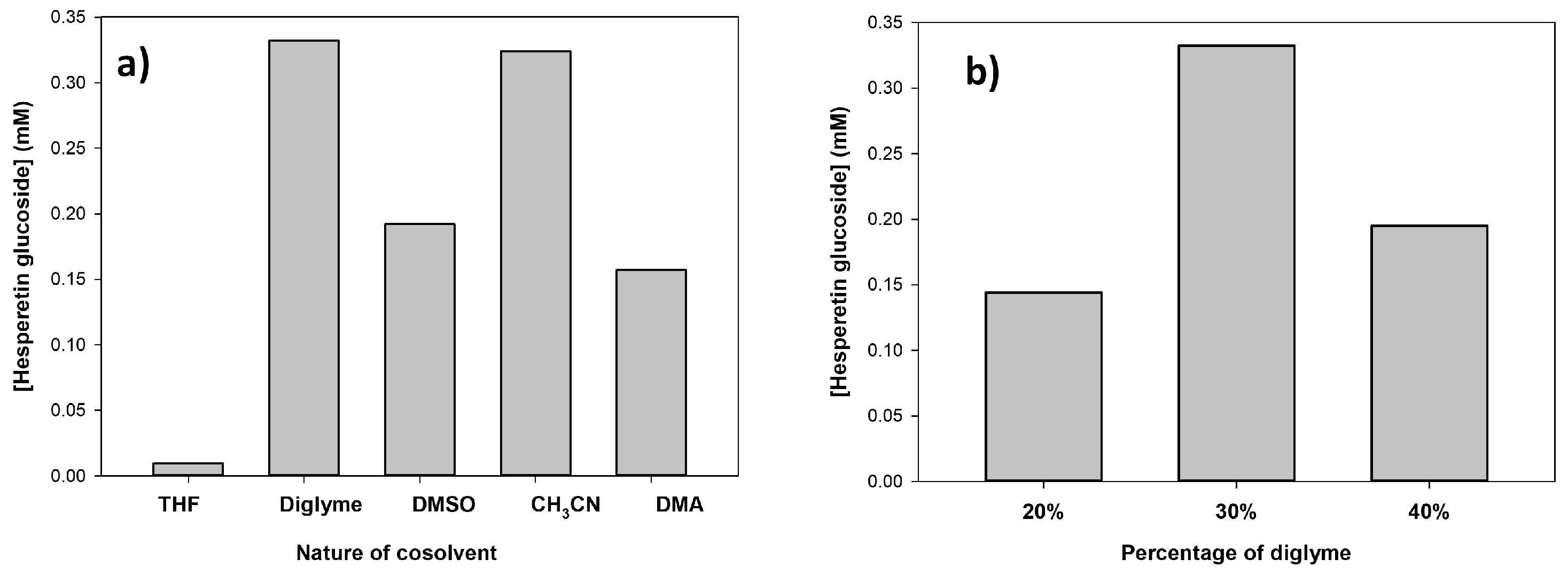
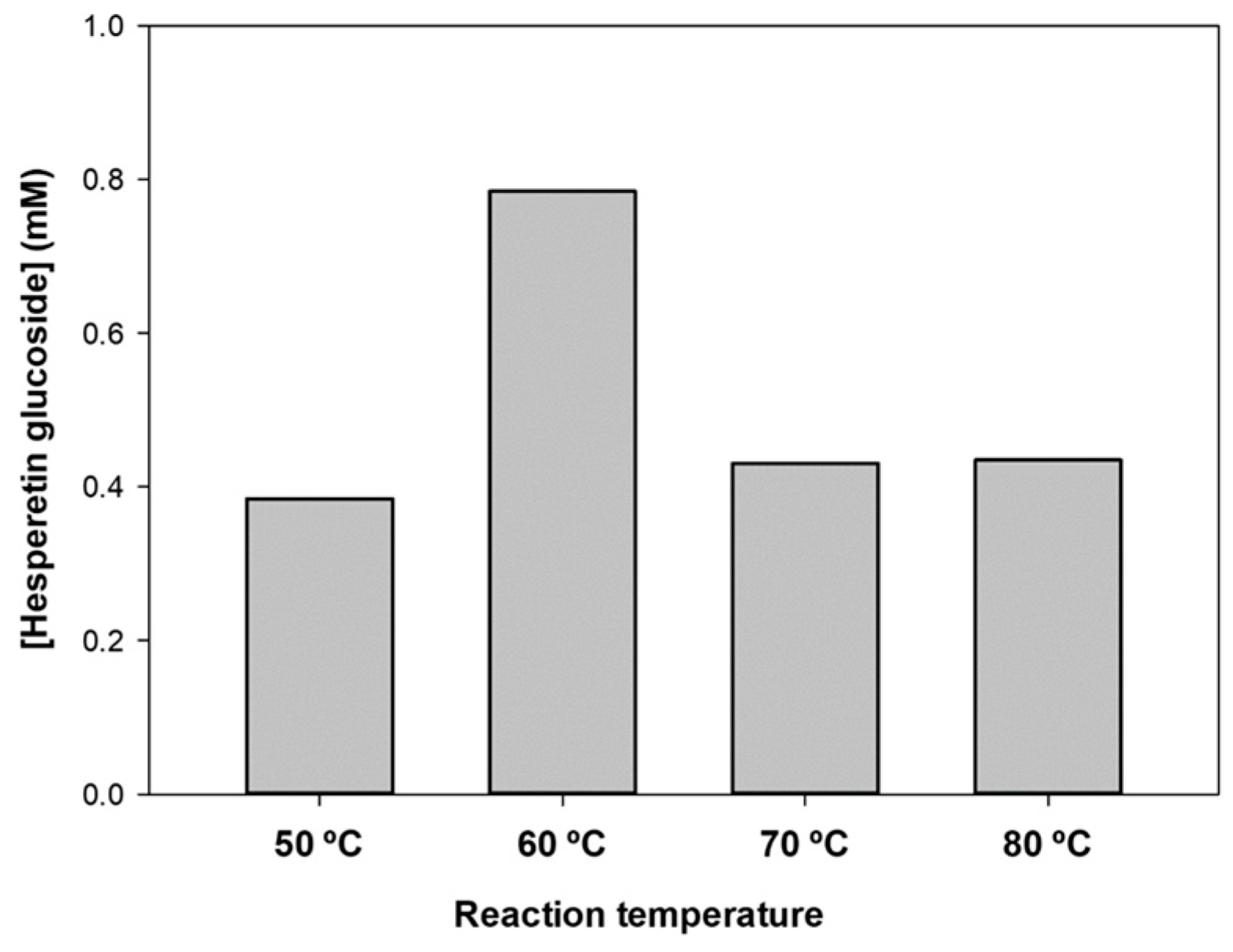
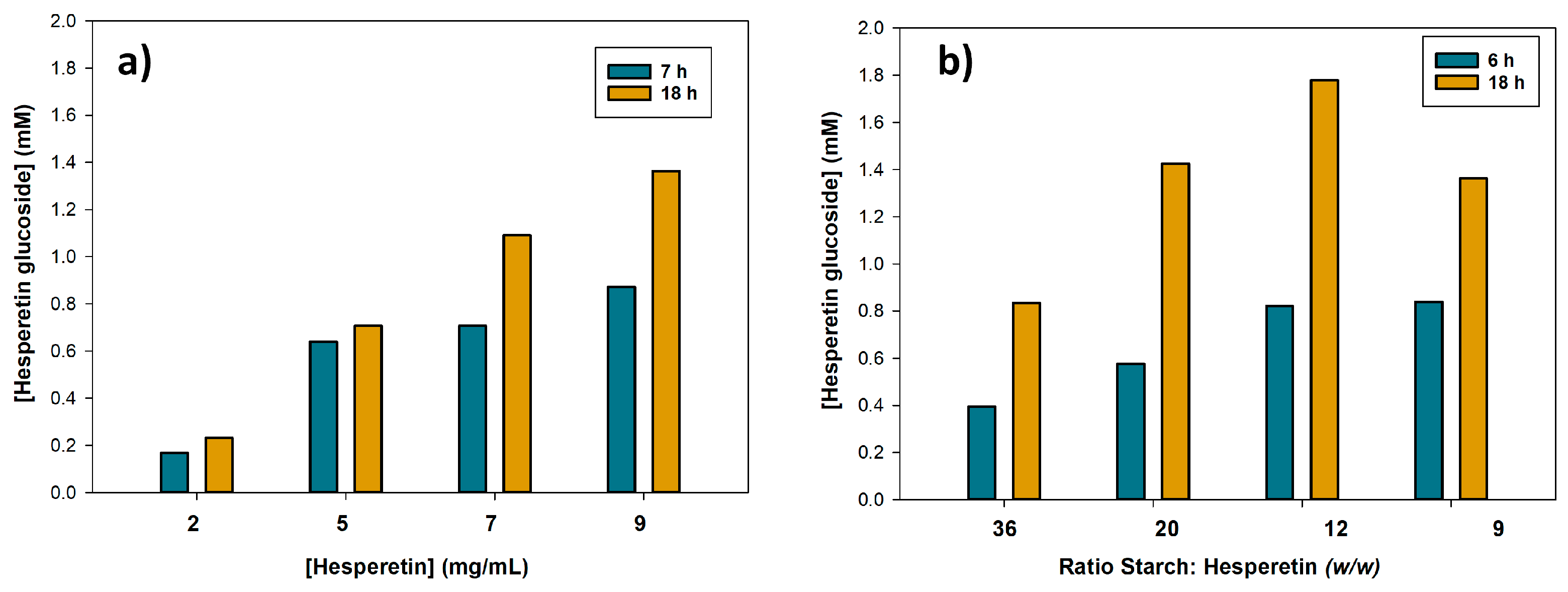
2.3. Optimization of Hesperetin Glucosylation
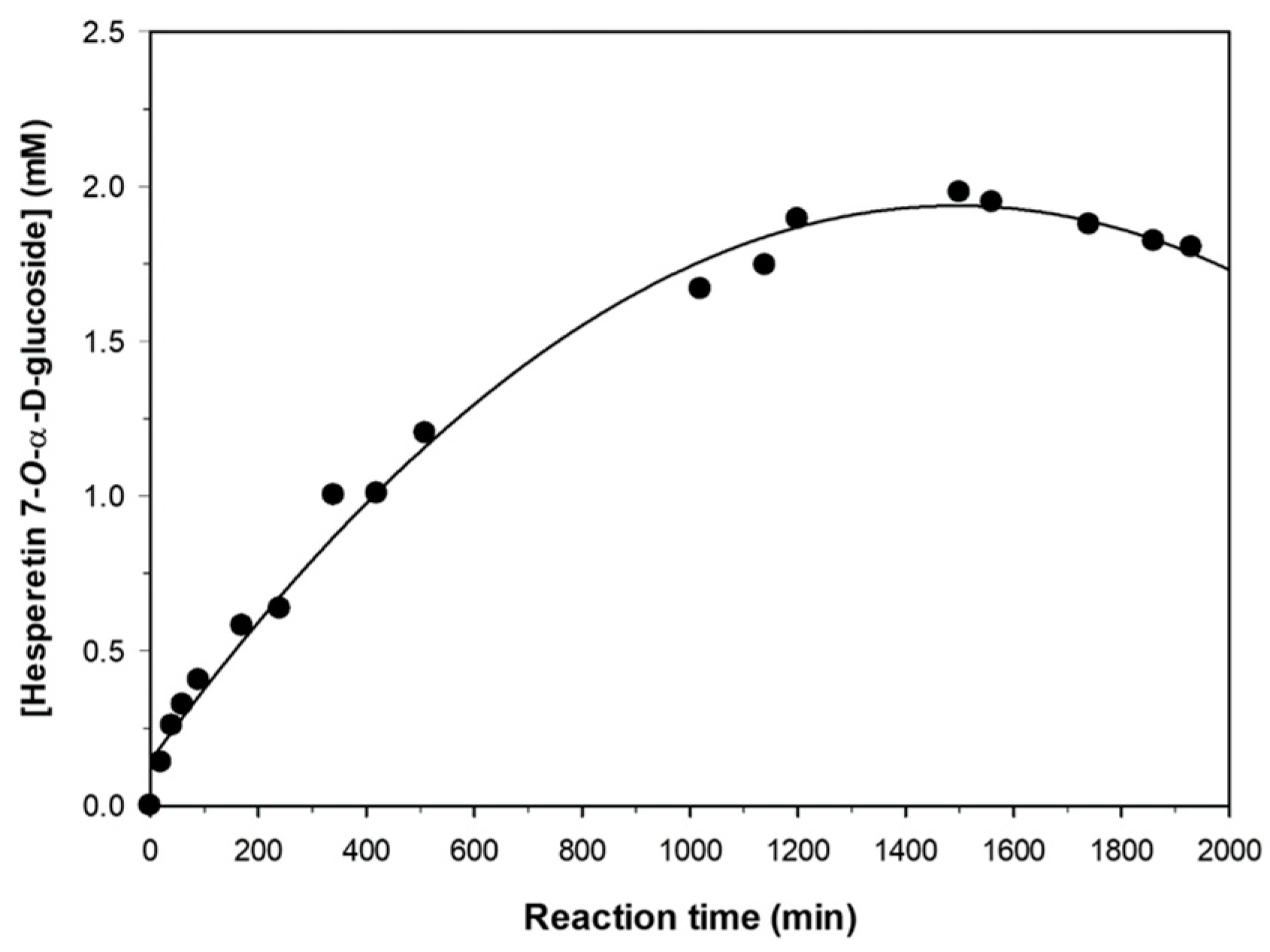
2.4. Progress of Hesperetin Glucosylation under Optimal Conditions.
3. Materials and Methods
3.1. Chemicals
3.2. Enzymes
3.3. Optimal Conditions for Enzymatic Glucosylation of Hesperetin
3.4. High-Performance Liquid Chromatography (HPLC)
3.5. HPLC coupled to Mass Spectrometry (HPLC-MS)
3.6. Purification of the Main Hesperetin Glucoside
3.7. Mass Spectrometry (MS)
3.8. Nuclear Magnetic Resonance (NMR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonzales, G.B.; Van Camp, J.; Vissenaekens, H.; Raes, K.; Smagghe, G.; Grootaert, C. Review on the use of cell cultures to study metabolism, transport, and accumulation of flavonoids: From mono-cultures to co-culture systems. Compr. Rev. Food Sci. Food Saf. 2015, 14, 741–754. [Google Scholar] [CrossRef]
- Souza, P.O.D.; Bianchi, S.E.; Figueiró, F.; Heimfarth, L.; Moresco, K.S.; Gonçalves, R.M.; Hoppe, J.B.; Klein, C.P.; Salbego, C.G.; Gelain, D.P.; et al. Anticancer activity of flavonoids isolated from Achyrocline satureioides in gliomas cell lines. Toxicol. In Vitro 2018, 51, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Núñez-Sánchez, M.A.; Tomás-Barberán, F.A.; Espín, J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Madeira Junior, J.V.; Nakajima, V.M.; Contesini, F.J.; Teixeira, C.B.; Macedo, J.A.; Macedo, G.A. Hesperetin: Simple natural compound with multiple biological activity. In Fruit and Pomace Extracts: Biological Activity, Potential Applications and Beneficial Health Effects; Owen, J.P., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2015; pp. 107–120. ISBN 978-1-63482-510-8. [Google Scholar]
- Liu, X.R.; Zhang, Y.; Lin, Z.Q. Advances in studies on the biological activities of hesperidin and hesperetin. Chin. J. New Drugs 2011, 20, 329–333. [Google Scholar]
- Yu, Y.; Kong, R.; Cao, H.; Yin, Z.; Liu, J.; Nan, X.; Phan, A.T.; Ding, T.; Zhao, H.; Wong, S.T.C. Two birds, one stone: Hesperetin alleviates chemotherapyinduced diarrhea and potentiates tumor inhibition. Oncotarget 2018, 9, 27958–27973. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, A.L.; Zhang, Y.L.; Li, Z.; Ding, H.W.; Huang, C.; Meng, X.M.; Li, J. Design, synthesis and evaluation of hesperetin derivatives as potential multifunctional anti-Alzheimer agents. Molecules 2017, 22, 1067. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Setzer, W.N.; Ahmadi, A.; Mansouri, K.; Nabavi, S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crops Prod. 2015, 76, 582–589. [Google Scholar] [CrossRef]
- Ahmadi, A.; Hassandarvish, P.; Lani, R.; Yadollahi, P.; Jokar, A.; Bakar, S.A.; Zandi, K. Inhibition of chikungunya virus replication by hesperetin and naringenin. RSC Adv. 2016, 6, 69421–69430. [Google Scholar] [CrossRef]
- Vivekanandhan, D.K.; Verma, P.R.P.; Singh, S.K. Emerging technologies for improving bioavailability of polyphenols. Curr. Nutr. Food Sci. 2016, 12, 12–22. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Vrba, J.; Kren, V.; Vacek, J.; Papouskova, B.; Ulrichova, J. Quercetin, quercetin glycosides and taxifolin differ in their ability to induce AhR activation and CYP1A1 expression in HepG2 cells. Phytother. Res. 2012, 26, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Shimizu, R.; Kanemaru, M.; Suzuki, Y.; Moriwaki, M.; Mizukami, H. Enzymatically modified isoquercitrin, α-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol. Pharm. Bull. 2009, 32, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Domínguez, M.; de Eugenio, L.I.; Peñalver, P.; Belmonte-Reche, E.; Morales, J.C.; Poveda, A.; Jiménez-Barbero, J.; Prieto, A.; Plou, F.J.; Martínez, M.J. Enzymatic Synthesis of a novel neuroprotective hydroxytyrosyl glycoside. J. Agric. Food Chem. 2017, 65, 10526–10533. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.; Gutmann, A.; Kulmer, S.T.; Nidetzky, B. Creating a water-soluble resveratrol-based antioxidant by site-selective enzymatic glucosylation. ChemBioChem 2015, 16, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Raab, T.; Barron, D.; Arce Vera, F.; Crespy, V.; Oliveira, M.; Williamson, G. Catechin glucosides: Occurrence, synthesis, and stability. J. Agric. Food Chem. 2010, 58, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, F.; Yoshida, A.; Toyama, T.; Wada-Takahashi, S.; Takahashi, S.S. α-Glucosyl hesperidin suppressed the exacerbation of 5-fluorouracil-induced oral mucositis in the hamster cheek pouch. J. Funct. Foods 2016, 21, 223–231. [Google Scholar] [CrossRef]
- Vijaya Bharathi, B.; Jaya Prakash, G.; Krishna, K.M.; Ravi Krishna, C.H.; Sivanarayana, T.; Madan, K.; Rama Raju, G.A.; Annapurna, A. Protective effect of alpha glucosyl hesperidin (G-hesperidin) on chronic vanadium induced testicular toxicity and sperm nuclear DNA damage in male Sprague Dawley rats. Andrologia 2015, 47, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Tanabe, F.; Arai, N.; Mitsuzumi, H.; Miwa, Y.; Kubota, M.; Chaen, H.; Kibata, M. Bioavailability of glucosyl hesperidin in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Mitsuzumi, H.; Uchida, S.; Maruta, K.; Ariyasu, T.; Fukuda, S.; Shimizu, R.; Takeda, R.; Shikata, C. Dietary glucosyl hesperidin improves skin color and skin conditions in women-placebo-controlled double-blind comparative study. Jpn. Pharmacol. Ther. 2015, 43, 1687–1699. [Google Scholar]
- Desmet, T.; Soetaert, W.; Bojarová, P.; Kren, V.; Dijkhuizen, L.; Eastwick-Field, V.; Schiller, A. Enzymatic glycosylation of small molecules: Challenging substrates require tailored catalysts. Chem. Eur. J. 2012, 18, 10786–10801. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Soto, M.E.; Seibel, J. Biotechnological synthesis and transformation of valuable sugars in the food and pharmaceutical industry. Curr. Org. Chem. 2014, 18, 964–986. [Google Scholar] [CrossRef]
- Plou, F.J.; Gómez de Segura, A.; Ballesteros, A. Application of glycosidases and transglycosidases for the synthesis of oligosaccharides. In Industrial Enzymes: Structure, Function and Application; Polaina, J., MacCabe, A.P., Eds.; Springer: New York, NY, USA, 2007; pp. 141–157. [Google Scholar]
- Gonzalez-Alfonso, J.L.; Leemans, L.; Poveda, A.; Jiménez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Efficient α-glucosylation of epigallocatechin gallate catalyzed by cyclodextrin glucanotransferase from Thermoanaerobacter sp. J. Agric. Food Chem. 2018, 66, 7402–7408. [Google Scholar] [CrossRef] [PubMed]
- Miguez, N.; Ramirez-Escudero, M.; Gimeno-Perez, M.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Fernandez-Lobato, M.; Sanz-Aparicio, J.; Plou, F.J. Fructosylation of hydroxytyrosol by the β-fructofuranosidase from Xanthophyllomyces dendrorhous: Insights into the molecular basis of the enzyme specificity. ChemCatChem 2018. [Google Scholar] [CrossRef]
- Shimoda, K.; Hamada, H. Production of hesperetin glycosides by Xanthomonas campestris and cyclodextrin glucanotransferase and their anti-allergic activities. Nutrients 2010, 2, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Hamada, H.; Hamada, H. Glycosylation of hesperetin by plant cell cultures. Phytochemistry 2008, 69, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, M.; Plou, F.J.; Andersen, C.; Martin, M.T.; Pedersen, S.; Ballesteros, A. Chemical modification of lysine side chains of cyclodextrin glycosyltransferase from Thermoanaerobacter causes a shift from cyclodextrin glycosyltransferase to alpha-amylase specificity. FEBS Lett. 1999, 445, 333–337. [Google Scholar] [CrossRef]
- Torres, P.; Poveda, A.; Jimenez-Barbero, J.; Parra, J.L.; Comelles, F.; Ballesteros, A.O.; Plou, F.J. Enzymatic synthesis of α-glucosides of resveratrol with surfactant activity. Adv. Synth. Catal. 2011, 353, 1077–1086. [Google Scholar] [CrossRef]
- González-Alfonso, J.; Rodrigo-Frutos, D.; Belmonte-Reche, E.; Peñalver, P.; Poveda, A.; Jiménez-Barbero, J.; Ballesteros, A.O.; Hirose, Y.; Polaina, J.; Morales, J.; et al. Enzymatic synthesis of a novel pterostilbene α-glucoside by the combination of cyclodextrin glucanotransferase and amyloglucosidase. Molecules 2018, 23, 1271. [Google Scholar] [CrossRef] [PubMed]
- Uitdehaag, J.C.M.; Van Der Veen, B.A.; Dijkhuizen, L.; Dijkstra, B.W. Catalytic mechanism and product specificity of cyclodextrin glycosyltransferase, a prototypical transglycosylase from the α-amylase family. Enzyme Microb. Technol. 2002, 30, 295–304. [Google Scholar] [CrossRef]
- Marié, T.; Willig, G.; Teixeira, A.R.S.; Gazaneo Barboza, E.; Kotland, A.; Gratia, A.; Courot, E.; Hubert, J.; Renault, J.H.; Allais, F. Enzymatic synthesis of resveratrol α-glycosides from β-cyclodextrin-resveratrol complex in water. ACS Sustain. Chem. Eng. 2018, 6, 5370–5380. [Google Scholar] [CrossRef]
- Funayama, M.; Nishino, T.; Hirota, A.; Murao, S.; Takenishi, S.; Nakano, H. Enzymatic synthesis of (+)catechin-α-glucoside and its effect on tyrosinase activity. Biosci. Biotechnol. Biochem. 1993, 57, 1666–1669. [Google Scholar] [CrossRef]
- Mathew, S.; Adlercreutz, P. Regioselective glycosylation of hydroquinone to α-arbutin by cyclodextrin glucanotransferase from Thermoanaerobacter sp. Biochem. Eng. J. 2013, 79, 187–193. [Google Scholar] [CrossRef]
- Choung, W.J.; Hwang, S.H.; Ko, D.S.; Kim, S.B.; Kim, S.H.; Jeon, S.H.; Choi, H.D.; Lim, S.S.; Shim, J.H. Enzymatic synthesis of a novel kaempferol-3-O-β-d-glucopyranosyl-(1→4)-O-α-d-glucopyranoside using cyclodextrin glucanotransferase and its inhibitory effects on aldose reductase, inflammation, and oxidative stress. J. Agric. Food Chem. 2017, 65, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ge, B.; Jiang, M.; Xu, G.; Dong, J.; Ni, Y. High production of genistein diglucoside derivative using cyclodextrin glycosyltransferase from Paenibacillus macerans. J. Ind. Microbiol. Biotechnol. 2017, 44, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.T.; Cruces, M.A.; Alcalde, M.; Plou, F.J.; Bernabe, M.; Ballesteros, A. Synthesis of maltooligosyl fructofuranosides catalyzed by immobilized cyclodextrin glucosyltransferase using starch as donor. Tetrahedron 2004, 60, 529–534. [Google Scholar] [CrossRef]
- Plou, F.J.; Martin, M.T.; Gomez de Segura, A.; Alcalde, M.; Ballesteros, A. Glucosyltransferases acting on starch or sucrose for the synthesis of oligosaccharides. Can. J. Chem. 2002, 80, 743–752. [Google Scholar] [CrossRef]
- Alcalde, M.; Plou, F.J.; de Segura, A.G.; Remaud-Simeon, M.; Willemot, R.M.; Monsan, P.; Ballesteros, A. Immobilization of native and dextran-free dextransucrases from Leuconostoc mesenteroides NRRL B-512F for the synthesis of glucooligosaccharides. Biotechnol. Tech. 1999, 13, 749–755. [Google Scholar] [CrossRef]
- Li, C.; Gu, H.; Dou, H.; Zhou, L. Identification of flavanones from peel of Citrus changshan-Huyou Y. B. Chang, by HPLC-MS and NMR. Eur. Food Res. Technol. 2007, 225, 777–782. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Berman, E.; Mazur, Y.; Gressel, J. (7) Glucosylation and (1-6) rhamnosylation of exogenous flavanones by undifferentiated Citrus cell cultures. Plant Sci. 1989, 61, 23–28. [Google Scholar] [CrossRef]
- Leemhuis, H.; Kelly, R.M.; Dijkhuizen, L. Engineering of cyclodextrin glucanotransferases and the impact for biotechnological applications. Appl. Microbiol. Biotechnol. 2010, 85, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.T.; Alcalde, M.; Plou, F.J.; Dijkhuizen, L.; Ballesteros, A. Synthesis of malto-oligosaccharides via the acceptor reaction catalyzed by cyclodextrin glycosyltransferases. Biocatal. Biotransform. 2001, 19, 21–35. [Google Scholar] [CrossRef]
- Strompen, S.; Miranda-Molina, A.; López-Munguía, A.; Castillo, E.; Saab-Rincón, G. Acceptor-induced modification of regioselectivity in CGTase-catalyzed glycosylations of p-nitrophenyl-glucopyranosides. Carbohydr. Res. 2015, 404, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Potocká, E.; Mastihubová, M.; Mastihuba, V. Enzymatic synthesis of tyrosol glycosides. J. Mol. Catal. B Enzym. 2015, 113, 23–28. [Google Scholar] [CrossRef]
- Piedrabuena, D.; Míguez, N.; Poveda, A.; Plou, F.J.; Fernández-Lobato, M. Exploring the transferase activity of Ffase from Schwanniomyces occidentalis, a β-fructofuranosidase showing high fructosyl-acceptor promiscuity. Appl. Microbiol. Biotechnol. 2016, 100, 8769–8778. [Google Scholar] [CrossRef] [PubMed]
- Linde, D.; Rodríguez-Colinas, B.; Estévez, M.; Poveda, A.; Plou, F.J.; Fernández Lobato, M. Analysis of neofructooligosaccharides production mediated by the extracellular β-fructofuranosidase from Xanthophyllomyces dendrorhous. Bioresour. Technol. 2012, 109, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Woo, J.B.; Ryu, S.I.; Moon, S.K.; Han, N.S.; Lee, S.B. Glucosylation of flavonol and flavanones by Bacillus cyclodextrin glucosyltransferase to enhance their solubility and stability. Food Chem. 2017, 229, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Letchmanan, K.; Shen, S.C.; Ng, W.K.; Tan, R.B.H. Application of transglycosylated stevia and hesperidin as drug carriers to enhance biopharmaceutical properties of poorly-soluble artemisinin. Colloids Surf. B 2018, 161, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Position | δH | δC |
|---|---|---|
| 2 | 5.48 | 78.2 |
| 3 | 2.8, 3.3 | 42.1 |
| 4 | - | 196.8 |
| 5 a | - | 162.6 |
| 6 b | 6.2 c | 95.6 |
| 7 | - | 164.7 |
| 8 b | 6.18 | 96.5 |
| 9 a | - | 162.2 |
| 10 | - | 103.0 |
| 1′ | - | 130.5 |
| 2′ | 6.9 c | 117.5 |
| 3′ | 6.9 | 111.7 |
| 4′ | - | 147.6 |
| 5′ | - | 146.1 |
| 6′ | 6.9 | 113.8 |
| MeO | 3.8 | 55.4 |
| 1′′ | 5.50, 5.53 (J = 3.4 Hz) c | 96.7 |
| 2′′ | 3.4 | 71 |
| 3′′ | 3.6 | 72.7 |
| 4′′ | 3.2 | 69.4 |
| 5′′ | 3.3 | 73.9 |
| 6′′ | 3.54, 3.46 | 60.2 |
| OH2′′ | 5.1 c | - |
| OH3′′ | 4.97 | - |
| OH4′′ | 5.0 | - |
| OH6′′ | 4.5 | - |
| Time | Methanol (%) | Water (%) |
|---|---|---|
| 0–2 min | 50 | 50 |
| 2–5 min | 52 | 48 |
| 5–10 min | 55 | 45 |
| 10–20 min | 60 | 40 |
| 20–30 min | 70 | 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Alfonso, J.L.; Míguez, N.; Padilla, J.D.; Leemans, L.; Poveda, A.; Jiménez-Barbero, J.; Ballesteros, A.O.; Sandoval, G.; Plou, F.J. Optimization of Regioselective α-Glucosylation of Hesperetin Catalyzed by Cyclodextrin Glucanotransferase. Molecules 2018, 23, 2885. https://doi.org/10.3390/molecules23112885
González-Alfonso JL, Míguez N, Padilla JD, Leemans L, Poveda A, Jiménez-Barbero J, Ballesteros AO, Sandoval G, Plou FJ. Optimization of Regioselective α-Glucosylation of Hesperetin Catalyzed by Cyclodextrin Glucanotransferase. Molecules. 2018; 23(11):2885. https://doi.org/10.3390/molecules23112885
Chicago/Turabian StyleGonzález-Alfonso, José L., Noa Míguez, J. Daniel Padilla, Laura Leemans, Ana Poveda, Jesús Jiménez-Barbero, Antonio O. Ballesteros, Georgina Sandoval, and Francisco J. Plou. 2018. "Optimization of Regioselective α-Glucosylation of Hesperetin Catalyzed by Cyclodextrin Glucanotransferase" Molecules 23, no. 11: 2885. https://doi.org/10.3390/molecules23112885
APA StyleGonzález-Alfonso, J. L., Míguez, N., Padilla, J. D., Leemans, L., Poveda, A., Jiménez-Barbero, J., Ballesteros, A. O., Sandoval, G., & Plou, F. J. (2018). Optimization of Regioselective α-Glucosylation of Hesperetin Catalyzed by Cyclodextrin Glucanotransferase. Molecules, 23(11), 2885. https://doi.org/10.3390/molecules23112885









