Abstract
Iridoids are a class of monoterpenoid compounds constructed from 10-carbon skeleton of isoprene building units. These compounds in their aglycones and glycosylated forms exist in nature to contribute to mechanisms related to plant defenses and diverse plant-animal interactions. Recent studies have also shown that iridoids and other structurally related monoterpenes display a vast array of pharmacological effects that make them potential modulators of the Alzheimer’s disease (AD). This review critically evaluates the therapeutic potential of these natural products by assessing key in vitro and in vivo data published in the scientific literature. Mechanistic approach of scrutiny addressing their effects in the Alzheimer’s brain including the τ-protein phosphorylation signaling, amyloid beta (Aβ) formation, aggregation, toxicity and clearance along with various effects from antioxidant to antiinflammatory mechanisms are discussed. The drug likeness of these compounds and future prospects to consider in their development as potential leads are addressed.
1. Introduction
Alzheimer's disease (AD) is one of the most prevalent age-related diseases mostly affecting the elderly population. Of the estimated 5.5 million Americans with AD in 2017, 5.3 million comprising about 96% of the patients’ population, were 65 years of age or older [1]. The disease also accounts for up to 70% of all cases of dementia and has a global prevalence of about 47 million people in 2015 [2]. Moreover, the projected figure for dementia by the year 2050 is 131.5 million highlighting the rapid rate of increase in its importance. As life expectancy continues to increase all over the world in parallel with economic development, the risk of AD along with its cost and social burden will be felt even more in the future. The disease is characterized by progressive cognitive deficit and irreversible neuronal deterioration. To date, there is no cure for AD and the average lifespan between the manifestation of clinical symptoms and death is about 8.5 years [3]. The memory deficits in AD are also associated with behavioral changes making patient care management very challenging.
The therapeutic options for AD are very limited with drug therapy mainly directed at the cholinergic system by using cholinesterase inhibitors such as donepezil, galantamine, and rivastigmine. A limited benefit by using non-competitive N-methyl-d-aspartate (NMDA) receptor antagonists including memantine have also been employed [4,5]. In the recent review article, the role of natural products in ameliorating the various neurodegenerative diseases has been outlined [6]. Significant advances in understanding the therapeutic potential of polyphenolic compounds such as flavonoids [7,8,9,10,11,12], caffeic acid derivatives [13] and aromatic diterpenoids [14] that benefit AD through multiple mechanisms of actions have also been presented. One class of compounds that has not received much attention as potential therapy for AD is the monoterpene class. In the present communication, a systematic review of these compounds with special emphasis on iridoids is presented.
2. Overview of Iridoids Chemistry
Iridoids are the monoterpenoid class of natural products that are constructed with 10 carbon skeleton. One structural marker of this compounds is the cis-fused cyclopenta[c]pyran system that exist in nature as glycosides, aglycones, in the form of secoiridoids or bisiridoids forms (Figure 1). In the case of secoiridoids, the C7–C8 bond of the iridoid skeleton is cleaved following a series of oxidation steps to give rise to compounds like secologanin (Figure 1), which also serves as a precursor to the synthesis of alkaloids.
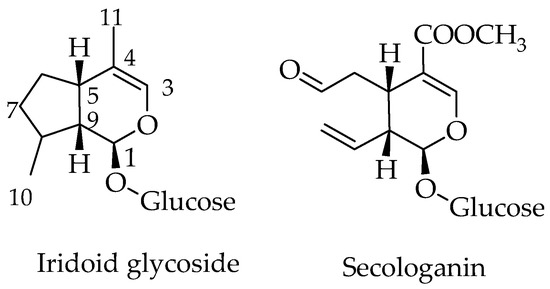
Figure 1.
General structure of iridoids and secoiridoids.
The biosynthesis pathway of terpenoids has been reviewed in the various literatures [15,16] and involves some key biosynthetic intermediates like the mevalonic acid. Even though the starting primary metabolite goes as far back as a two-carbon metabolite, acetyl-CoA, the basic skeleton of all terpenoids is defined by the 5-carbon isoprene units in the form of isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The precursor of all terpenoids in the further steps of reaction is the geranyl pyrophosphate (GPP) that is made from two isoprene units (Figure 2). The sesquiterpenes (15 carbon), diterpenes (20 carbon) and triterpenes (30 carbons) are classical examples of terpenoids that arise from condensation of these isoprene units through a serious of enzyme-catalyzed reactions. The GPP gives rise to a range of cyclic and acyclic monoterpenes of biological significance [17], while the 8-hydroxygeraniol unique pathways leads to iridoids and their derivatives (Figure 2). The compounds scrutinized for their potential effect in ameliorating the biochemical and behavioral symptoms of AD are shown in Figure 3.
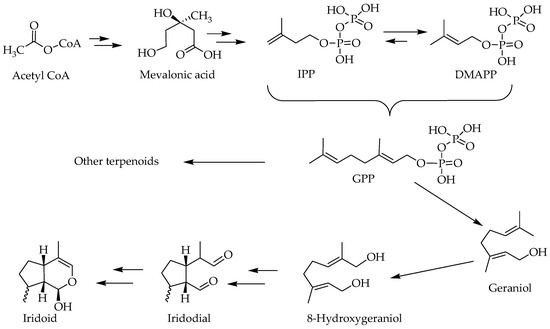
Figure 2.
An overview of biosynthesis pathway of iridoids.
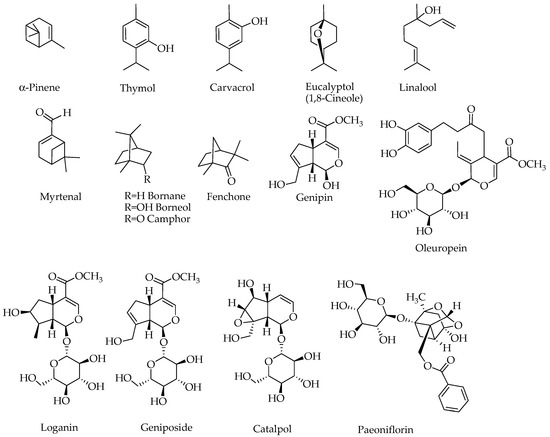
Figure 3.
Structures of compounds with potential effects on the Alzheimer’s brain. Note that compounds containing a sugar moiety (oleuropein, loganin, geniposide, catalpol and paeoniflorin) are highly polar and hence are not components of essential oils.
3. General Function of Iridoids and other Monoterpenes in Nature
Why plants and animals produce secondary metabolites has been a century-old question that has not yet been fully answered. A number of general arguments presented in the last few decades have been based on the role of such compounds in cell-cell communication within the organism or plant-animal interactions including defense against pathogens [15,18,19,20]. With respect to chemical defense against herbivores and pathogens, the role of iridoids is well defined as these compounds have been demonstrated to be bitter and show good sets of biological activities [21,22]. Interestingly, animals such as butterfly are known to accumulate these chemicals as defense against pathogens [23,24,25]. The biological activities of iridoids in mammalian system have also been the subject of intense scrutiny in recent years and effects including antidiabetic properties have recently been reviewed along with other monoterpenes [17]. In this communication, the promise of monoterpenes, but primarily iridoids, (Figure 3) for treating AD is scrutinized by assessing published literature on their in vitro and in vivo effects.
4. Therapeutic Potential for Alzheimer’s Disease
4.1. In Vitro Protective Effects
The vast arrays of neuroprotective effects of iridoids and some monoterpenes are shown in Table 1 [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. The Aβ formation, aggregation and function have been the major target areas of AD for in vitro experiments. In a study by Marumoto et al. [26], the β-secretase (recombinant human BACE1) inhibitory activities of some monoterpenes have been evaluated. Even though the inhibitory activity of these compounds were confirmed, their activity was moderate (above 50 μM) with geranyl acetone being the most active (IC50 value of 51.9 ± 3.9 μM) followed by (+)-camphor (95.9 ± 11.0 μM), (−)-fenchone (106.3 ± 14.9 μM), (+)-fenchone (117.0 ± 18.6 μM), and (−)-camphor (134.1 ± 16.4 μM). A number of in vitro experiments have also been devoted to studying the inhibitory effects of monoterpenes against Aβ-induced cytotoxicity in neuronal cells in vitro. Treatment of cells with borneaol suppressed the Aβ-induced cytotoxicity and oxidative stress in the SH-SY5Y (human neuroblastoma) cells [31] while 1,8-cineole (eucalyptol) showed similar effect in PC12 (rat pheochromocytoma) cells [32]; and genipin in cultured hippocampal neurons [34]. The antioxidant activity of these monoterpenes is also evident from their ameliorating effect on the H2O2-induced oxidative stress as shown by catalpol in astrocytes [29], and α-pinene and 1,8-cineole in PC12 cells [33]. Cultured primary cortical neurons exposed to Aβ could also be rescued by geniposide from toxicity and oxidative stress [37]. In a further experiment to show the mechanism of action of geniposide in primary cultured cortical neurons’ protection, the geniposide-induced τ protein phosphorylation and phosphorylation of Akt at Ser-473 site and GSK-3β at Ser-9 site were shown to be inhibited by leptin antagonists [38]. The role of leptin as potential mechanism of iridoids action on the Alzheimer’s brain is discussed in detail in the following section.

Table 1.
In vitro effects of iridoids and other monoterpenes related to AD pathology.
With respect to the Aβ-induced toxicity in the central neuronal cells, the role of insulin-degrading enzyme (IDE) has been highlighted in recent years. In addition to degradation and clearance of Aβ, the IDE play pivotal role in the regulation of Aβ activity. By using primary cortical neurons in a culture media, Zhang et al. [39] have demonstrated that geniposide enhance the phosphorylation of peroxisome proliferator-activated receptor γ (PPARγ). The effect of geniposide in the activation of the IDE promoter was also shown to be mediated via the glucagon-like peptide-1 (GLP-1) receptor while other pathways confirmed to be involved by inhibitor studies (see Table 1) where phosphatidyl inositol 3-kinase, PI3K, proto-oncogene tyrosine-protein kinase Src (c-Src), PPARγ, protein kinase A (PKA) and epidermal growth factor receptor (EGFR) [39,40]. Furthermore, in the SH-SY5Y cells, geniposide has been shown to ameliorate the cytotoxicity of Aβ along with its oligomer assembly and cytotoxicity [41]. The protective effect of geniposide in the SH-SY5Y cells treated with other toxicants such as formaldehyde has also been reported [42]. The effect of paeoniflorin in PC12 cells protection from Aβ was similar with geniposide in that its activity was correlated with upregulation of the protein kinase B (Akt) phosphorylation level, B-cell lymphoma 2 (Bcl-2) protein expression, reducing Bax protein expression and is inhibited by LY294002 [52]. The protective effect of paeoniflorin from the 6-hydroxydopamine-induced apoptosis in PC12 cells was also correlated with enhanced antioxidant capacity (GSH (glutathione - reduced form) level) and suppression of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) translocation [53]. A number of other studies (Table 1) also showed the protective effect of paeoniflorin against Aβ cytotoxicity in PC12 cells [52,53,54] and SH-SY5Y cells [55]; as well as glutamate-induced cytotoxicity in PC12 cells [56].
Hydrogen peroxide (H2O2)-induced cytotoxicity in PC12 cells could be inhibited by geniposide through the PI3K-dependent pathway as evidenced from the study using a selective inhibitor, LY294002 [43]. In the same cell system, Liu et al. [44] also showed that the effect of geniposide in reversing the oxidative stress induced by H2O2 involves an increased level of Bcl-2 by activation of the mitogen-activated protein kinase (MAPK), mitogen-activated protein kinase kinase (MEK) and rapidly accelerated fibrosarcoma proto-oncogene serine/threonine-protein (c-Raf) phosphorylation along with the phosphorylation of the p90 variant of the ribosomal s6 kinase (p90RSK) [43]. The requirement of the PI3K and GLP-1 receptor activation has also been confirmed in the PC12 cells protection from the H2O2-induced cytotoxicity [44].
The antiinflammatory effect of these compounds in the CNS came from evidences in vitro showing the inhibition of nitric oxide (NO) release from the lipopolysaccharide (LPS)-stimulated microglia by genipin along with suppression of microglial cells activation [34]. Beyond suppression of NO production, genipin also ameliorated the LPS-induced tumour necrosis factor-α (TNF), interleukin-1β (IL-1), prostaglandin E2 (PGE-2), intracellular reactive oxygen species (iROS), and NF-kB activation in microglial cells in vitro [35].
In an organotypic cultured hippocampal tissues, the scopolamine-induced functional changes was shown to be inhibited by loganin along with inhibition of acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and β-secretase (BACE1) [45]. An effect on β-secretase (BACE1) inhibitory activity of loganin has also been reported by Youn et al. [49]. A direct effect on one of the most prevalent AD target, AChE, for loganin with IC50 value in sub-micromolar range was particularly impressive [46]. A further molecular docking studies have shown that loganin’s non-competitive type of interaction generate a negative binding energies for cholinesterase as well as BACE1 suggesting a high affinity and tighter binding capacity for the active site of the enzymes [46]. As BChE (though to a lesser extent, see Table 1) is also inhibited, loganin appear to target AChE, BChE, and BACE1 that are all important in AD pathology. The Aβ-induced inflammatory changed in PC12 cells could also be inhibited by loganin as evidenced from a reduction in the level of TNF-α and protein expression of iNOS and cyclooxygenase-2 (COX-2) [47,48]. These effects were also correlated with inhibition of NF-κB along with the closely related regulatory pathways including the phosphorylation of MAPKs (ERK1/2 (Extracellular signal–regulated kinase ½), p38 and JNK (c-Jun N-terminal kinase) [47].
A number of other studies (Table 1) have shown that monoterpenes possess direct inhibitory effect against AChE activity. This includes a report by Kaufmann et al. [50] on 8-cineole, carvacrol, myrtenal and verbenone, although the best activity in this study was observed at relatively high concentration (IC50 = 170 μM for myrtenal). On the other hand, oleuropein, thymol and carvacrol have been shown to have a much better activity but the best activity (IC50 < 5 μM) was obtained when a carbamate moiety was added to carvacrol through a synthesis approach [51]. In the latter case, there has also been a drive to improve the biological activity of existing anti-Alzheimer’s drugs by incorporating the monoterpene skeleton through synthesis. For example, with the help of a docking-based design, galantamine-camphane hybrids have been shown to display over a 100-fold better activity in AChE inhibition than galantamine [27].
All the in vitro data is shown in Table 1, which clearly indicates the therapeutic potential of iridoids as well as other monoterpenes in AD. The gross inhibition of cytotoxicity in neuronal cells induced by Aβ and other toxic agents have been demonstrated to be ameliorated. The reactive oxygen species (ROS), proinflammatory cytokines and many mediators could also be suppressed while mitochondrial deterioration was inhibited. At the molecular level, a range of antioxidant proteins and enzymes could be enhanced by these natural products along with anti-apoptotic genes and proteins, while proapoptotic genes and proteins appear to be suppressed (Table 1).
4.2. Evidence of Efficacy Demonstrated through In Vivo Studies
In parallel with the overwhelming in vitro data, animal studies on iridoids and some other monoterpenes (Table 2) have shown potential therapeutic effects for treating AD [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. The neuroprotective effect of carvacrol in vivo was studied by Zhong et al. [55] using the intracerebral hemorrhage mouse model, where a significant reduction of the aquaporin-4 (AQP4)-dependent oedema was observed. It is worth noting that AQP4 is a water channel in the brain that plays major role in the development of cerebral oedema. The structural, physiological and pathological significance of ACQ4 has been extensively reviewed [78,79,80,81,82]. Considering the pathophysiological role of AQP4 in a range of CNS disorders including ischemic stroke [83], neuroinflammation [84] and autoimmune neurodegenerative diseases [85], the reversal of cerebral oedema induced through AQP4 activity by monoterpenes is an interesting observation.

Table 2.
In vivo effects of iridoids and some other monoterpenes as potential modulators of AD.
A number of studies have targeted the oxidative stress and associated disorders by inducing the pathology with d-(+)-galactose injection into experimental animals. In this model, catalpol has been shown to reduce the level of Aβ in the cerebral cortex along with improvement of learning and memory; while the level of antioxidant defenses (SOD and GPx) were boosted [58]. In senescent mice treated with D-galactose, Zhang et al. [60] also reported neuroprotection by catalpol as evidenced by the increased level and activity of choline acetyltransferase (CHAT). Moreover, catalpol in this model has been shown to reverse the suppressed level of muscarinic acetylcholine (ACh) receptor M1 while concomitantly suppressing the level of inflammatory and oxidant markers (TNF-α, IL-1 and advanced glycation end products (AGEs)) [60]. Improvement of memory deficit along with antioxidant markers (glutathione S-transferase (GSH-ST), glutamine synthetase (GS) and creatine kinase (CK) have also been shown for catalpol [61,63].
In other experiments, Aβ was directly injected into the brain to study the biochemical and behavioral changes in animals. Catalpol was among the iridoids showing activity in this model where prevention of the ACh neuronal damage was noted from the increased level of choline CHAT positive cells density in cerebral cortex as well as increased level of ChAT activity [28]. Geniposide also ameliorated the Aβ-induced neuronal abnormalities including cellular densities and synaptic proteins level in the transgenic mice model [63]. On the other hand, linalool has been shown to reverse cognitive deficits and altered the level of the antioxidant and protein (SOD, GPx, AChE) levels/activity in mice injected with Aβ [69]. The effect of paeoniflorin in memory improvement and protection of animals from Aβ through mechanisms including enhancing antioxidant defenses (e.g., GSH) and calcium homeostasis have also been reported [76,77].
Zhang et al. [62] employed the APP/PS1 Transgenic mouse model of AD to study the potential benefit of geniposide. The insulin deficiency induced by streptozotocin (STZ) in these wild-type transgenic animals appeared to enhance the GSK-3β level/activity which was suppressed by geniposide administration in a dose dependent manor. It is worth noting that the doses employed here were very small (5, 10, and 20 mg/kg). The data were also in line with the broader effect of geniposide in signal transduction pathways related to insulin resistance reviewed recently [17]. The GSK-3β plays direct role in τ protein hyperphosphorylation [86,87]. The role of the Akt in the regulation of GSK-3β is also well understood and its phosphorylation initiates its inactivation that appeared to be modulated by geniposide. In agreement with this data, geniposide can also regulate the phosphorylation of τ protein both in the insulin-dependent and independent manor in primary cultured cortical neurons [63]. It does also enhance the phosphorylation of Akt at Ser473 and Thr308 sites [63]. The dual effect of geniposide both in diabetes and AD is thus evident from its effect on the phosphorylation of τ protein via the PI3K-GSK-3β kinase pathway. To date, hyperphosphorylated τ protein is one of the pathological hallmark of AD as it is the principal component of neurofibrillary tangles (NFTs) [87]. The structural integrity of τ protein is regulated by a cascade of phosphorylation-related pathways, and hence both kinases and phosphatases play important roles in stable NFT formation. The GSK-3β being the key player in the kinase—mediated hyperphosphorylation of τ protein, its regulation by geniposide seems to shed some light into the possible mechanism of iridoids’ action. The crosstalk between diabetes and AD was also highlighted by Gao et al. [67] who confirmed the potential role of geniposide through GSK-3β regulation. Similarly, in the study by Liu et al. [68], geniposide has been shown to decrease the Aβ1-42 level while improving the expression of IDE in Aβ-treated STZ-induced diabetic rats. In the further experiment on transgenic mice model, geniposide was shown to improve learning and memory along with antiinflammatory effect (through suppression of RAGE-dependent signaling in activation of ERK and IκB/NF-κB and the production of TNF-α, IL-1β) and lowering the Aβ level in the cerebrum [65]. Other compounds which have been shown to improve learning and memory in transgenic model of AD include linalool that could suppress pro-inflammatory proteins such as p38 MAPK, NOS-2, cyclooxygenase-2 (COX2) and IL-1β [70]. The effect of paeoniflorin in the transgenic mouse model of AD was also studied by Gu et al. [75]. In addition to improvement of the memory deficit, a reduction in the level of inflammation (NF-κB, TNF-α, IL-1β, IL-6) and apoptotic (caspase-3) markers were observed. As demonstrated for geniposide (above), paeoniflorin also modulate the GSK-3β signaling in transgenic animal model of AD [75].
Other behavioral models of AD included the scopolamine-induced AD model where loganin showed beneficial effect through the route of administration [71]. The neuroprotective effect of monoterpenes in other in vivo models has also been documented. For example, oleuropein could ameliorate the pentylenetetrazole (PTZ)-induced seizures in mice or colchicine-induced learning and memory deficits [73].
5. Insights into the Mechanism of Action of Iridoids and Other Monoterpenes in AD
The previous sections on the in vitro and in vivo effects of monoterpenes provided a plethora of evidences linking these compounds with key pathological pathways of AD. The general mechanism of action of monoterpenes in the AD brain is depicted in Figure 4. Some of the key features of monoterpenes, particularly iridoids, as an emerging class of compounds as anti-AD agents are shown below.
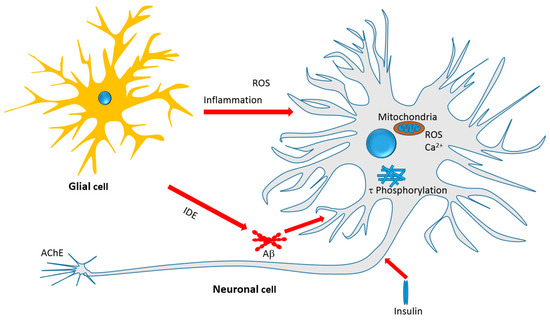
Figure 4.
Therapeutic targets of iridoids and other monoterpenes discussed in this review. Antiinflammatory effect, amelioration of oxidative stress, mechanisms related to Aβ formation, aggregation and clearance, τ-protein phosphorylation and aggregation, and neurotoxicity associated with mitochondrial dependent and independent mechanisms are among the therapeutic targets.
The role of Aβ in the pathology and as therapeutic target for AD has been reviewed in the various literatures (e.g., [88,89,90]). Recent review articles from our laboratories have also shown that many polyphenolic compounds such the flavonoids, diterpenoids and cinamate derivatives display therapeutic potential for AD through multiple mechanisms involving Aβ [6,7,8,9,10,11,12,13,14]. Hence, the formation, aggregation and toxicity of Aβ can all serve as targets for therapeutic agents. The direct role of monoterpenes in the formation and aggregation of Aβ is however less clear and the observed activity at moderate concentration may not be of a high degree of therapeutic relevance. Never the less, direct effect on APP processing enzymes has been shown. The predominant forms of the pathological Aβ in the brain are Aβ1–40 and to a lesser extent Aβ1–42 which are formed through the amyloidgenic β-secretase-dependent pathway. The selective inhibition of this enzyme by monoterpenes (Table 1) without much effect on the non-amyloidogenic marker enzyme (α-secretase) is an interesting finding. A large body of evidence also suggests that monoterpenes (Table 1 and Table 2) ameliorate the Aβ-induced cytotoxicity both in cultured neuronal cells and various animal models of AD [6,7,8,9,10,11,12,13,14]. Upon aggregation, the Aβ oligomers induce neurotoxicity leading to cell death, impairment of synaptic function and behavioral deficits that are commonly observed in AD animal models. Hence, one major target of the iridoids as well as the selected other monoterpenes appear to be mediated through mechanisms related to Aβ formation and/or toxicity.
The role of ROS in Aβ-induced neurotoxicity has been well established from evidences mostly linking redox metals like copper, zinc, and iron coordinating the generation of toxic free radicals and/or ROS [91,92,93,94,95,96,97]. As inhibitors of ROS generation through direct metal chelation and ROS scavenging, the role of polyphenols as potential therapeutic agents for AD has been extensively studied. In this direction, our own studies on catechol functional group and the flavonoid skeleton as optimized structural moieties for biological effects have been exhaustively researched [98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115]. The monoterpenes presented in this communication however lack such structural moiety unless additional skeleton as that shown in oleuropein is added (Figure 3). Their effect on the amelioration of the Aβ-toxicity as well as neurotoxicity induced by H2O2 suggest a mechanism of action beyond direct ROS scavenging. This can include boosting antioxidant defenses, and in this connection, numerous studies have shown an increased antioxidant status in the AD brain following treatment by monoterpenes (Table 2).
Another well-defined mechanism of action of monoterpenes in the AD brain appears to be linked to anti-inflammatory effect. In view of neuroinflammation as the major pathological hallmark of AD, the role of inflammatory cells activation in the brain, primarily astrocyte and microglial cells, have been investigated in the last few decades. Readers are thus directed to excellent reviews in the field [116,117,118,119,120,121,122]. Interestingly, all of the best-characterised inflammatory markers such as TNF, IL-1, COX and NOS have been shown to be suppressed by the studied compounds in this review. Since inhibition of these proinflammatory cytokines such TNF is known to provide favorable outcome in AD [123,124], the suppressive effect of numerous monoterpenes on proinflammatory level in the Alzheimer’s brain is in line with potential benefit in AD. Among the regulators of cytokines in their proinflammatory effect is the NF-κB which has been demonstrated to play key role in AD [125]. As modulators of the NF-κB, monoterpenes appear to also link their potential therapeutic mechanism through such an effect.
Leptin is one of the hormones produced by adipocytes with primary function in body weight and fat regulation through diverse mechanisms including modulation of food intake and metabolism [126]. Diverse other functions of leptin were however emerging in recent years; these include modulation of the immune response and broad range of neuronal regulation from neuroprotection to cognition [127,128]. The role of leptin receptor-mediated regulation in the cerebral cortex and hippocampus and dysregulation in AD has also been well recognized [129,130,131,132]. In addition to neurons, immune cells in the brain such as astrocytes and glial cells do also express leptin receptors and are regulated by this adipocytes’ hormone [133,134]. Considering evidences showing the potential neuroprotective effect of leptin under pathological condition as well as many other in vitro and in vivo experiments (e.g., [135,136,137]), the modulatory effects of monoterpenes in this system is an exciting development. As leptin antagonist abolished the effect of geniposide on τ phosphorylation and phosphorylation of Akt at Ser-473 site and GSK-3β at Ser-9 in the Alzheimer’s brain (Table 1), part of the iridoids action is likely to be mediated through leptin regulation.
As with their formation, the degradation of Aβ peptides and plaques must be tightly regulated to avoid pathological disorders such the AD. Among the various mechanisms involved in Aβ degradation and clearance include the Aβ proteases, low-density lipoprotein receptor-related protein 1, and the apolipoprotein E systems [138]. Of the protease enzymes, neprilysin (also known as membrane metallo-endopeptidase) is a zinc-dependent metalloprotease that cleaves Aβ and have shown a good correlation with Aβ accumulation [139]. The endothelin-converting enzyme, and angiotensin-converting enzyme do also function as Aβ degrading enzymes. The role of IDE in Aβ degradation has recently been clarified and its dysregulation is now known to contribute to the pathology of the AD [138,140,141]. In fact, IDE is considered to be the main extracellular protease enzyme for the degradation of Aβ [142,143] and its expression, as with neprilysin, in the hippocampus has been shown to decrease with increasing age [144]. Hence, upregulation of the Aβ degrading enzymes is among the therapeutic approaches for AD [145,146,147]. In the brain, glial cells such as the microglia and astrocytes are the main source of IDE secretion [113] and their dysregulation could thus contribute to AD pathology; while promotion of IDE secretion from these cells could be implicated in AD therapy through enhancing Aβ clearance. The astrocytes and microglial cells are also primary phagocytes in the brain that recognize Aβ through membrane receptors to remove through phagocytosis [148]. The therapeutic approach of AD by upregulating IDE is however a tricky one, as IDE also selectively degrades insulin and its inhibitors are needed to improve glucose homeostasis (e.g., in diabetes). The role of iridoids in this regards is very interesting as geniposide has been shown to upregulate IDE [39] while displaying potent antidiabetic effect [17]. As IDE is degrading the monomeric form of Aβ, it is preventing the formation of oligomers or aggregates that is prerequisite to Aβ cytotoxicity in neuronal cells. Hence, a clear line of evidence is now available for geniposide and/or other iridoids that showed a promise in the Alzheimer’s brain.
The dual effect of iridoids in diabetes and AD is also manifested from the possible mechanism of action related to the τ protein phosphorylation pathway. The formation of intracellular NFTs is a result of aggregation of the hyperphosphorylated τ-protein. As a major component of the neuronal cytoskeleton, τ-protein is closely associated with microtubules and aids a number of neuronal functions from axonal transport to neurite outgrowth [87,149]. The function of τ protein in stabilizing the microtubule to facilitate the normal neuronal function is governed by its phosphorylation which is regulated by a number of cellular kinases and phosphatases [150]. Consequently, τ protein dysregulation is among the pathological hallmark of AD as in NFTs and hence serves as a target for drug therapy. Hyperphosphorylation of τ-protein quickly initiates the formation of helical filaments and aggregates as seen in the NFTs of AD. This intern leads to microtubule disassembly and destabilization [151]. The signaling cascade in τ-protein hyperphosphorylation has been shown to involve the GSK-3β that directly act on the protein (to phosphorylate it) and make it to disassociate with the microtubules [152,153]. Hence, downregulating GSK-3β by drugs is essential in AD not only to regulate τ-protein hyperphosphorylation but also to manage other deleterious effect of GSK-3β such as in ROS generation from the mitochondria. For example, GSK-3β has been shown to down-regulate the transcription factor Nrf2 after oxidative damage [154]. The GSK-3β itself is regulated by other kinases such as the Akt that phosphorylate GSK-3β at different sites to negatively regulate its activity. Furthermore, activation of PI3K triggers the activation of Akt that phosphorylates GSK-3β leading to inhibition of τ-protein phosphorylation. Hence, the dysfunction of PI3K/Akt signaling is linked to τ-protein phosphorylation or NFT formation in AD. The p38 MAPK is also emerged as anther kinase involved in τ-protein phosphorylation and hence can be targeted by drugs [155,156,157]. A review article of such signal transduction pathways and possible pharmacological regulations is eloquently presented by Medina et al. [158]. The observation of iridoids to regulate τ-protein phosphorylation by inhibiting GSK-3β and regulation of the associated system primarily the PI3K/Akt signaling (Table 1 and Table 2) is a remarkable documentation of record for this group of compounds. The pioneering compound in this regard is geniposide (e.g., [38,39,67]). Other natural products such phenolics including resveratrol [159], curcumin [160], hyperforin [161] and capsaicin [162] have been shown to display inhibitory effect against τ protein hyperphosphorylation as well as affect in vivo models of AD. Hence, iridoids with structural feature distinctively different from polyphenols appear to share one common feature of mechanism in their potential AD modulations.
Overall, it appears that the iridoids and some other monoterpenoids target the various cellular and biochemical features of AD pathology depicted in Figure 4. They target oxidative stress by boosting antioxidant defenses; inhibit the Aβ cascades particularly neurotoxicity; inhibit τ-protein phosphorylation and hence NFTs formation; promote the clearance of toxic proteins (Aβ) through IDE; modulate the insulin signaling pathway and insulin resistance as antidiabetic agents; and display a range of anti-inflammatory effects by suppressing the expression of numerous key proinflammatory proteins. Another interesting development is the direct effect of monoterpenes on AChE enzyme and further possible opportunity of potency optimization through chemical synthesis.
6. Drug-Likeness and Structural Perspectives
A range of qualitative and quantitative measures of drug-likeness parameters have been employed in recent years to identify leads in drug discovery researches as well as improving the efficiency of known bioactive compounds. In the in silico drug-likeness predictions, the undesirable properties of small molecular weight compounds assessed by poor ADMET (absorption, distribution, metabolism, excretion, and toxicity) characteristics are used as a screening tool [163]. In this regard, monoterpenes (unless glycosylated, see Figure 3) act as a component of essential oils with list solubility profile in water falls within the poor drug-likeness profile. Accordingly, their absorption, distribution, metabolism, and excretion profiles were not in line with what one expects as ideal drug molecules. Hence, all in vitro and in vivo data so far suggest that they are absorbed and distributed to tissues but with far slower rate than that ideally expected [164,165,166,167,168]. Human trial also confirmed these observations but the iridoid glycosides, with sugar attachment thereby increasing their polarity, appear to be a good compromise in vivo [169,170,171,172,173]. Like many other sugar-linked natural products, the iridoid glycosides such as geniposide have been shown to be metabolized by intestinal bacteria to release their aglycone (e.g., genipin) [174,175] which also give rise to conjugated products (e.g., with glucuronic acid) [176]. Increasing water solubility by glycosylation to release a bioactive aglycone in the intestine has been reported to be one way of enhancing bioavailability for natural products [177]. Even for glycosides such as geniposide, however, the absolute oral bioavailability after oral administration remains to be poor ~9.67% [178]. Nevertheless, both in vitro and in vivo experiments have shown good effects in ameliorating the biochemical and behavioral markers of AD. Hence, despite their predicted poor drug-likeness profile, iridoids and other monoterpenes have shown potent activity to be seriously considered as potential lead compounds in future studies.
7. Future Prospects
One common advantage of employing compounds of natural origin (e.g., monoterpenes) is that they are associated with common foods and beverages that are already in use for human consumption. As neuromodulators, particularly in AD, the beneficial effects of some essential oils as crude mixtures of small molecular weight fragrant compounds including monoterpenes have been reported in the various literature [see review article, 179]. As indicated in the preceding section, however, the drug likeness of these molecules has not been in favor of their development as drugs given their poor water solubility and bioavailability. The iridoids glycosides appear to offer a better bioavailability profile and pharmacology as evidenced from their activity profile in vitro and in vivo. The fact that both the glycosylated and the aglycones are active in vitro suggests that the glycosides being a better bioavailable compounds could be more preferable as drug candidates. One should bear in mind that research on this class of compounds is still at its infant stage and more work is needed on optimization of their pharmacology through medicinal chemistry. The effect of some monoterpenes, for example, could be enhanced by over 100-fold when other functional groups such as a carbamate moiety were added or they being incorporated into the existing anti-AD drugs such as galantamine [27,51]. Naturally, human clinical trials would offer not only valuable data on efficacy but also pharmacokinetic profile that are desperately needed for these compounds. Such study of course would be preferred once a lead compound is identified and optimized through future research. In the meantime, all the available data now suggest that small molecules of the iridoids class and related monoterpenes could be considered as potential leads for AD therapy.
Conflicts of Interest
The author declare no conflict of interest. No funding from internal or external sources were used for this contribution.
References
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Available online: https://www.alz.org/facts/ (accessed on 22 December 2017).
- Alzheimer’s Disease International. World Alzheimer Report 2016, Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future. Available online: https://www.alz.co.uk/research/world-report-2016 (accessed on 22 December 2017).
- Jost, B.C.; Grossberg, G.T. The natural history of Alzheimer’s disease: A brain bank study. J. Am. Geriatr. Soc. 1995, 43, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Haas, C. Strategies, development, and pitfalls of therapeutic options for Alzheimer’s disease. J. Alzheimers Dis. 2012, 28, 241–281. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Zhu, X.; Nunomura, A.; Smith, M.A.; Perry, G. Therapeutic options in Alzheimer’s disease. Expert Rev. Neurother. 2006, 6, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Elufioye, T.O.; Berida, T.I.; Habtemariam, S. Plants-derived neuroprotective agents: Cutting the cycle of cell death through multiple mechanisms. eCAM 2017, 2017, 3574012. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E.; Nabavi, S.F. Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in Alzheimer’s and Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2016, 16, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Rutin as a natural therapy for Alzheimer’s disease: Insights into its mechanisms of action. Curr. Med. Chem. 2016, 23, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Lentini, G. The therapeutic potential of rutin for diabetes: An update. Mini Rev. Med. Chem. 2015, 15, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Castilla, S.A.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Sureda, A.; Manayi, A.; Nabavi, S.M. Neuroprotective effects of fisetin in Alzheimer’s and Parkinson’s Diseases: From chemistry to medicine. Curr. Top. Med. Chem. 2016, 16, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Protective effects of caffeic acid and the Alzheimer’s brain: An update. Mini Rev. Med. Chem. 2017, 17, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. eCAM 2016, 2016, 2680409. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The biosynthesis of C5–C25 terpenoid compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Antidiabetic potential of monoterpenes: A case of small molecules punching above their weight. Int. J. Mol. Sci. 2018, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2016. [CrossRef] [PubMed]
- Llusià, J.; Estiarte, M.; Peñuelas, J. Terpenoids and plant communication. Butll. Inst. Catalana Hist. Nat. 1996, 64, 125–133. [Google Scholar]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Biere, A.; Marak, H.B.; van Damme, J.M. Plant chemical defense against herbivores and pathogens: Generalized defense or trade-offs? Oecologia 2004, 140, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Reudler, J.H.; Lindstedt, C.; Pakkanen, H.; Lehtinen, I.; Mappes, J. Costs and benefits of plant allelochemicals in herbivore diet in a multi enemy world. Oecologia 2015, 179, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.A.; Deane-Bowers, M. Localization of defensive chemicals in two congeneric butterflies (Euphydryas, Nymphalidae). J. Chem. Ecol. 2017, 43, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Laurentz, M.; Reudler, J.H.; Mappes, J.; Friman, V.; Ikonen, S.; Lindstedt, C. Diet quality can play a critical role in defense efficacy against parasitoids and pathogens in the Glanville fritillary (Melitaea cinxia). J. Chem. Ecol. 2012, 2012, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, N. The phylogenetics and biochemistry of host-plant specialization in Melitaeine butterflies (Lepidoptera: Nymphalidae). Evolution 2001, 55, 522–537. [Google Scholar] [CrossRef]
- Marumoto, S.; Okuno, Y.; Miyazawa, M. Inhibition of β-Secretase activity by monoterpenes, sesquiterpenes, and C13 norisoprenoids. J. Oleo Sci. 2017, 66, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Stavrakov, G.; Philipova, I.; Zheleva-Dimitrova, D.; Valkova, I.; Salamanova, E.; Konstantinov, S.; Doytchinova, I. Docking-based design and synthesis of galantamine-camphane hybrids as inhibitors of acetylcholinesterase. Chem. Biol. Drug Des. 2017, 90, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Xie, H.; Zhao, T.K.; Kang, B. Catalpol regulates cholinergic nerve system function through effect on choline acetyl-transferase not M receptor affinity. Biomed. Pharmacother. 2015, 69, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Jiang, B.; Liu, J.H.; Lei, C.; Zhang, X.L.; An, L.J. Protective effects of catalpol against H2O2-induced oxidative stress in astrocytes primary cultures. Neurosci. Lett. 2008, 442, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Sun, Q.X.; Xia, Z.Q.; Hu, Y.E. Regulatory effect of catalpol from Radix Rehmanniae on M2 receptor density in M2 receptor transfected CHO cells. Chin. Pharmacol. Bull. 2006, 22, 1462–1466. [Google Scholar]
- Hur, J.; Pak, S.C.; Koo, B.S.; Jeon, S. Borneol alleviates oxidative stress via upregulation of Nrf2 and Bcl-2 in SH-SY5Y cells. Pharm. Biol. 2013, 51, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Vaibhav, K.; Javed, H.; Tabassum, R.; Ahmed, M.E.; Khan, M.M.; Khan, M.B.; Shrivastava, P.; Islam, F.; Siddiqui, M.S.; et al. 1,8-cineole (eucalyptol) mitigates inflammation in amyloid beta toxicated PC12 cells: Relevance to Alzheimer’s disease. Neurochem. Res. 2014, 39, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Naturforsch. C. 2016, 71, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Sakura, N.; Chiba, K.; Mohri, T. Prevention of the neurotoxicity of the amyloid beta protein by genipin. Biol. Pharm. Bull. 2001, 24, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.N.; Choi, Y.S.; Jung, H.J.; Park, G.H.; Park, J.M.; Moon, S.K.; Cho, K.H.; Kang, C.; Kang, I.; Oh, M.S.; et al. Genipin inhibits the inflammatory response of rat brain microglial cells. Int. Immunopharmacol. 2010, 10, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Chiba, K.; Yoshikawa, C. Genipin suppresses A23187-induced cytotoxicity in neuro2a cells. Biol. Pharm. Bull. 2009, 32, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lv, C.; Li, H.; Du, S.; Liu, X.; Li, Z.; Xin, W.; Zhang, W. Geniposide protects primary cortical neurons against oligomeric Aβ1-42-induced neurotoxicity through a mitochondrial pathway. PLoS ONE 2016, 11, e0152551. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Zhang, Y.; Yin, F. Leptin signaling plays a critical role in the geniposide-induced decrease of tau phosphorylation. Acta Biochim. Biophys. Sin. 2015, 47, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Z.; Liu, J.; Yin, F. Cell signaling mechanisms by which geniposide regulates insulin-degrading enzyme expression in primary cortical neurons. CNS Neurol. Disord. Drug Targets 2015, 14, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Liu, J.; Yin, F. Geniposide attenuates the level of Aβ1-42 via enhancing leptin signaling in cellular and APP/PS1 transgenic mice. Arch. Pharm. Res. 2017, 40, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Ding, H.; Liang, M.; Li, X.; Mo, W.; Wang, X.; Liu, Y.; He, R.; Hua, Q. Neuroprotective effects of geniposide in SH-SY5Y cells and primary hippocampal neurons exposed to Aβ42. Biomed. Res. Int. 2014, 2014, 284314. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Chen, J.Y.; Li, J.; Sun, M.R.; Mo, W.C.; Liu, K.L.; Meng, Y.Y.; Liu, Y.; Wang, F.; He, R.Q.; et al. The protective effect of geniposide on human neuroblastoma cells in the presence of formaldehyde. BMC Complement. Altern. Med. 2013, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Yin, F.; Guo, L.X.; Deng, X.H.; Hu, Y.H. Neuroprotection of geniposide against hydrogen peroxide induced PC12 cells injury: Involvement of PI3 kinase signal pathway. Acta Pharmacol. Sin. 2009, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, F.; Zheng, X.; Jing, J.; Hu, Y. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem. Int. 2007, 51, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Kim, H.-B.; Lee, S.; Kim, M.-J.; Lee, S.-O.; Han, S.-M.; Maeng, S.; Park, J.-H. Loganin enhances long-term potentiation and recovers scopolamine-induced learning and memory impairments. Physiol. Behav. 2017, 171, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, H.K.; Park, C.H.; Yokozawa, T.; Min, B.S.; Jung, H.A.; Choi, J.S. Kinetics and molecular docking studies of loganin, morroniside and 7-O-galloyl-d-sedoheptulose derived from Corni fructus as cholinesterase and β-secretase 1 inhibitors. Arch. Pharm. Res. 2016, 39, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Youn, K.; Ahn, M.R.; Kim, O.Y.; Jeong, W.S.; Ho, C.T.; Jun, M. Neuroprotective effect of loganin against Aβ25-35-induced injury via the NF-κB-dependent signaling pathway in PC12 cells. Food Funct. 2015, 6, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Wang, C.F.; Wang, Q.H.; Xiao, Y.; Wang, Z.B.; Kuang, H.X. Study on active constituents against Alzheimer’s disease from Valeriana amurensis. Zhongguo Zhong Yao Za Zhi. 2016, 41, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Jeong, W.S.; Jun, M. β-Secretase (BACE1) inhibitory property of loganin isolated from Corni fructus. Nat. Prod. Res. 2013, 27, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.; Dogra, A.K.; Wink, M. Myrtenal inhibits acetylcholinesterase, a known Alzheimer target. J. Pharm. Pharmacol. 2011, 63, 1368–1371. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.Z.; Gazioglu, I.; Dag, A.; Salmas, R.E.; Kayık, G.; Durdagi, S.; Sonmez, F. Synthesis, anticholinesterase activity and molecular modelling study of novel carbamate-substituted thymol/carvacrol derivatives. Bioorg. Med. Chem. 2017, 25, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, S.Y.; Wang, J.G. Role of PI3K/Akt pathway in effect of paeoniflorin against Aβ25-35-induced PC12 cell injury. Zhongguo Zhong Yao Za Zhi 2014, 39, 4045–4049. [Google Scholar] [PubMed]
- Dong, H.; Li, R.; Yu, C.; Xu, T.; Zhang, X.; Dong, M. Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis in PC12 cells via suppressing reactive oxygen species-mediated PKCδ/NF-κB pathway. Neuroscience 2015, 285, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ji, X.; Zhang, J.; Shi, G.; Zhu, X.; Wang, K. Paeoniflorin attenuates Aβ25-35-induced neurotoxicity in PC12 cells by preventing mitochondrial dysfunction. Folia Neuropathol. 2014, 52, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, L.; Zhu, X.; Zhang, K.; Huang, B.; Zhang, J.; Zhang, Y.; Zhu, L.; Zhou, B.; Zhou, F. Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol. Neurobiol. 2014, 34, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, K.; Wu, D.; Li, X.; Ou, Y. Protective effect of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via Bcl-2/Bax signal pathway. Folia Neuropathol. 2012, 50, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, B.; Dai, M.; Sun, Y.; Sun, Q.; Yang, G.; Bian, L. Carvacrol alleviates cerebral edema by modulating AQP4 expression after intracerebral hemorrhage in mice. Neurosci. Lett. 2013, 555, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, J.; Xiang, S.; Sheng, S.; Jiang, Y.; Yang, Z.; Hua, F. Catalpol preserves neural function and attenuates the pathology of Alzheimer’s disease in mice. Mol. Med. Rep. 2016, 13, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Li, W.T.; Yu, S.T.; Xie, H.; Han, H.R. Catalpol regulates function of hypothalamic-pituitary-adrenocortical-axis in an Alzheimer’s disease rat model. Pharmazie 2014, 69, 688–693. [Google Scholar] [PubMed]
- Zhang, X.; Jin, C.; Li, Y.; Guan, S.; Han, F.; Zhang, S. Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by d-galactose. Food Chem. Toxicol. 2013, 58, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; An, L.J.; Bao, Y.M.; Wang, J.Y.; Jiang, B. d-galactose administration induces memory loss and energy metabolism disturbance in mice: Protective effects of catalpol. Food Chem. Toxicol. 2008, 46, 2888–2894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, A.; Jiang, B.; Bao, Y.; Wang, J.; An, L. Further pharmacological evidence of the neuroprotective effect of catalpol from Rehmannia glutinosa. Phytomedicine 2008, 15, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, F.; Liu, J.; Liu, Z.; Guo, L.; Xia, Z.; Zidichouski, J. Geniposide attenuates insulin-deficiency-induced acceleration of β-amyloidosis in an APP/PS1 transgenic model of Alzheimer’s disease. Neurochem. Int. 2015, 89, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, F.; Liu, J.; Liu, Z. Geniposide attenuates the phosphorylation of tau protein in cellular and insulin-deficient APP/PS1 transgenic mouse model of Alzheimer’s disease. Chem. Biol. Drug Des. 2016, 87, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Wang, L.; Liu, X.; Yan, S.; Yan, S.S.; Wang, Y.; Zhang, W. Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology 2015, 89, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Liu, X.; Liu, H.; Chen, T.; Zhang, W. Geniposide attenuates mitochondrial dysfunction and memory deficits in APP/PS1 transgenic mice. Curr. Alzheimer Res. 2014, 11, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, Y.; Jiang, Y.; Ding, J.; Li, L. Geniposide ameliorates learning memory deficits, reduces tau phosphorylation and decreases apoptosis via GSK3β pathway in streptozotocin-induced Alzheimer rat model. Brain Pathol. 2014, 24, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Deng, X.; Yin, F. Geniposide decreases the level of Aβ1-42 in the hippocampus of streptozotocin-induced diabetic rats. Acta Biochim. Biophys. Sin. 2013, 45, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Sci. 2017, 174, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Kim, H.C.; Lee, S.Y.; Jang, C.G. Loganin improves learning and memory impairments induced by scopolamine in mice. Eur. J. Pharmacol. 2009, 619, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N.; Delfan, B.; Motamed-Gorji, N.; Dehpour, A.R. Effects of oleuropein on pentylenetetrazol-induced seizures in mice: Involvement of opioidergic and nitrergic systems. J. Nat. Med. 2017, 71, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Pourkhodadad, S.; Alirezaei, M.; Moghaddasi, M.; Ahmadvand, H.; Karami, M.; Delfan, B.; Khanipour, Z. Neuroprotective effects of oleuropein against cognitive dysfunction induced by colchicine in hippocampal CA1 area in rats. J. Physiol. Sci. 2016, 66, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Cai, Z.; Cai, M.; Liu, K.; Liu, D.; Zhang, Q.; Tan, J.; Ma, Q. Protective effect of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2016, 13, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.R.; Peng, J.H.; Cheng, X.B.; Shi, B.Z.; Zhang, M.Y.; Xu, R.X. Paeoniflorin attenuates amyloidogenesis and the inflammatory responses in a transgenic mouse model of Alzheimer’s disease. Neurochem. Res. 2015, 40, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.Z.; Ma, S.P.; Hong, Z.Y. Peoniflorin activates Nrf2/ARE pathway to alleviate the Aβ(1-42)-induced hippocampal neuron injury in rats. Yao Xue Xue Bao 2013, 48, 1353–1357. [Google Scholar] [PubMed]
- Zhong, S.Z.; Ge, Q.H.; Li, Q.; Qu, R.; Ma, S.P. Paeoniflorin attenuates Aβ(1-42)-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J. Neurol. Sci. 2009, 280, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.D.; Yeh, R.; Sandstrom, A.; Chorny, I.; Harries, W.E.; Robbins, R.A.; Miercke, L.J.; Stroud, R.M. Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance. Proc. Natl. Acad. Sci. USA 2009, 106, 7437–7442. [Google Scholar] [CrossRef] [PubMed]
- Strand, L.; Moe, S.E.; Solbu, T.T.; Vaadal, M.; Holen, T. Roles of aquaporin-4 isoforms and amino acids in square array assembly. Biochemistry 2009, 48, 5785–5793. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.E.; Sorbo, J.G.; Sogaard, R.; Zeuthen, T.; Petter, O.O.; Holen, T. New isoforms of rat Aquaporin-4. Genomics 2008, 91, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Potokar, M.; Stenovec, M.; Jorgacevski, J.; Holen, T.; Kreft, M.; Ottersen, O.P.; Zorec, R. Regulation of AQP4 surface expression via vesicle mobility in astrocytes. Glia 2013, 61, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Smith, A.J.; Phuan, P.W.; Tradtrantip, L.; Anderson, M.O. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert. Opin. Ther. Targets 2017, 21, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Vella, J.; Zammit, C.; Giovanni, G.D.; Muscat, R.; Valentino, M. The central role of aquaporins in the pathophysiology of ischemic stroke. Front. Cell Neurosci. 2015, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.M.; Badaut, J. Aquaporin 4: A player in cerebral edema and neuroinflammation. J. Neuroinflamm. 2012, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Fukuda, A.M.; Jullienne, A.; Petry, K.G. Aquaporin and brain diseases. Biochim. Biophys. Acta 2014, 1840, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, Y.; Limaye, A.R.; Levey, A.I.; Srinivasan, S. Glycogen synthase kinase 3β and Alzheimer’s disease: Pathophysiological and therapeutic significance. Cell Mol. Life Sci. 2006, 63, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Costa, M.; de Almeida, M.S.C.; da Cruz E Silva, O.A.B.; Henriques, A.G. Protein phosphorylation is a key mechanism in Alzheimer’s disease. J. Alzheimers Dis. 2017, 58, 953–978. [Google Scholar] [CrossRef] [PubMed]
- León, R.; Garcia, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.M.; Roberts, B.R.; Streltsov, V.A.; Nuttall, S.D.; Masters, C.L. The role of Aβ in Alzheimer’s disease. In Amyloid Fibrils and Prefibrillar Aggregates: Molecular and Biological Properties; Otzen, D.E., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 263–293. [Google Scholar] [CrossRef]
- Salomone, S.; Caraci, F.; Leggio, G.M.; Fedotova, J.; Drago, F. New pharmacological strategies for treatment of Alzheimer’s disease: Focus on disease modifying drugs. Br. J. Clin. Pharmacol. 2012, 73, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Bousejra-ElGarah, F.; Bijani, C.; Coppel, Y.; Faller, P.; Hureau, C. Iron(II) binding to amyloid-β, the Alzheimer’s peptide. Inorg. Chem. 2011, 50, 9024–9030. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebinia, F.; Emadi, S. Effect of metal chelators on the aggregation of beta-amyloid peptides in the presence of copper and iron. Biometals 2017, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Reybier, K.; Ayala, S.; Alies, B.; Rodrigues, J.V.; Bustos-Rodriguez, S.; La Penna, G.; Collin, F.; Gomes, C.M.; Hureau, C.; Faller, P. Free superoxide is an intermediate in the production of H2O2 by copper(I)-Aβ peptide and O2. Angew. Chem. Int. Ed. Engl. 2016, 55, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Harris, P.L.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef] [PubMed]
- Zawisza, I.; Rózga, M.; Bal, W. Affinity of copper and zinc ions to proteins and peptides related to neurodegenerative conditions (Aβ, APP, α-synuclein, PrP). Coord. Chem. Rev. 2012, 256, 2297–2307. [Google Scholar] [CrossRef]
- Migliorini, C.; Porciatti, E.; Luczkowski, M.; Valensin, D. Structural characterization of Cu2+, Ni2+ and Zn2+ binding sites of model peptides associated with neurodegenerative diseases. Coord. Chem. Rev. 2012, 256, 352–368. [Google Scholar] [CrossRef]
- Noel, S.; Bustos Rodriguez, S.; Sayen, S.; Guillon, E.; Faller, P.; Hureau, C. Use of a new water-soluble Zn sensor to determine Zn affinity for the amyloid-β peptide and relevant mutants. Metallomics 2014, 6, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Varghese, G.K. A novel diterpene skeleton: Identification of a highly aromatic, cytotoxic and antioxidant 5-methyl-10-demethyl-abietane-type diterpene from Premna serratifolia. Phyther. Res. 2015, 29, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Investigation into the antioxidant and antidiabetic potential of Moringa stenopetala: Identification of the active principles. Nat. Prod. Commun. 2015, 10, 475–478. [Google Scholar] [PubMed]
- Habtemariam, S.; Varghese, G.K. Extractability of rutin in herbal tea preparations of Moringa stenopetala leaves. Beverages 2015, 1, 169–182. [Google Scholar] [CrossRef]
- Habtemariam, S.; Varghese, G.K. The antidiabetic therapeutic potential of dietary polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Cowley, R.A. Cowley, Antioxidant and anti-α-glucosidase ccompounds from the rhizome of Peltiphyllum peltatum (Torr.) Engl. Phytother. Res. 2012, 26, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Lentini, G.; Habtemariam, S. Phytochemical, antioxidant and anti-α-glucosidase activity evaluations of Bergenia cordifolia. Phyther. Res. 2012, 26, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Methyl-3-O-methyl gallate and gallic acid from the leaves of Peltiphyllum peltatum: Isolation and comparative antioxidant, prooxidant, and cytotoxic effects in neuronal cells. J. Med. Food 2011, 14, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Juan-Badaturuge, M.; Habtemariam, S.; Thomas, M.J.K. Antioxidant compounds from a South Asian beverage and medicinal plant, Cassia auriculata. Food. Chem. 2011, 125, 221–225. [Google Scholar] [CrossRef]
- Habtemariam, S.; Dagne, E. Comparative antioxidant, prooxidant and cytotoxic activity of sigmoidin A and eriodictyol. Planta Med. 2010, 76, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Juan-Badaturugea, M.; Habtemariam, S.; Jackson, C.; Thomas, M.J.K. Antioxidant principles of Tanacetum vulgare L. aerial part. Nat. Prod. Commun. 2009, 4, 1561–1564. [Google Scholar]
- Habtemariam, S. Activity-guided isolation and identification of free radical-scavenging components from ethanolic extract of boneset (Leaves of Eupatorium perfoliatum). Nat. Prod. Commun. 2008, 3, 1317–1320. [Google Scholar]
- Habtemariam, S.; Jackson, C. Antioxidant and cytoprotective activity of leaves of Peltiphyllum peltatum (Torr.) Engl. Food Chem. 2007, 105, 498–503. [Google Scholar] [CrossRef]
- Habtemariam, S. Modulation of tumour necrosis factor-α-induced cytotoxicity by polyphenols. Phyther. Res. 1997, 11, 277–280. [Google Scholar] [CrossRef]
- Habtemariam, S. Flavonoids as inhibitors or enhancers of the cytotoxicity of tumor necrosis factor-alpha in L-929 tumor cells. J. Nat. Prod. 1997, 60, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Catechols and quercetin reduce MTT through iron ions: A possible artefact in cell viability assay. Phyther. Res. 1995, 9, 603–605. [Google Scholar] [CrossRef]
- Varghese, G.K.; Bose, L.V.; Habtemariam, S. Antidiabetic components of Cassia alata leaves: Identification through α-glucosidase inhibition studies. Pharm. Biol. 2013, 51, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Antihyperlipidemic components of Cassia auriculata aerial parts: Identification through in vitro studies. Phytother. Res. 2013, 27, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. α-Glucosidase inhibitory activity of kaempferol-3-O-rutinoside. Nat. Prod. Commun. 2011, 6, 201–203. [Google Scholar] [PubMed]
- Li, C.; Zhao, R.; Gao, K.; Wei, Z.; Yin, M.Y.; Lau, L.T.; Chui, D.; Yu, A.C. Astrocytes: Implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Garwood, C.J.; Ratcliffe, L.E.; Simpson, J.E.; Heath, P.R.; Ince, P.G.; Wharton, S.B. Astrocytes in Alzheimer’s disease and other age-associated dementias: A supporting player with a central role. Neuropathol. Appl. Neurobiol. 2017, 43, 281–298. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Tago, H.; Mcgeer, E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987, 79, 195–200. [Google Scholar] [CrossRef]
- Schwab, C.; McGeer, P.L. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J. Alzheimers Dis. 2008, 13, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, L.; Heinen, Y.; Van Dam, A.M.; Lucassen, P.J.; Korosi, A. Microglial priming and Alzheimer’s disease: A possible role for (early) immune challenges and epigenetics? Front. Hum. Neurosci. 2016, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of cerebral Aβ in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Shamim, D.; Laskowski, M. Inhibition of inflammation mediated through the tumor Necrosis factor-α biochemical pathway can lead to favorable outcomes in Alzheimer disease. J. Cent. Nerv. Syst. Dis. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Decourt, B.; Lahiri, D.K.; Sabbagh, M.N. Targeting tumor necrosis factor-α for Alzheimer’s disease. Curr. Alzheimer Res. 2017, 14, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; Han, Y.W.; Han, X.H.; Zhang, K.; Chang, Y.N.; Hu, Z.M.; Qi, H.X.; Ting, C.; Zhen, Z.; Hong, W. Upstream regulators and downstream effectors of NF-κB in Alzheimer’s disease. J. Neurol. Sci. 2016, 366, 127–134. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.; MacDonald, N.; Mizielinska, S.; Connolly, C.N.; Irving, A.J.; Harvey, J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol. Cell Neurosci. 2007, 35, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.M.; Mantzoros, C.S. Drug insight: The role of leptin in human physiology and pathophysiology-emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.C.; Guo, M.; Zhang, W.; Lu, X.Y. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J. Biol. Chem. 2008, 283, 18238–18247. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Stone, J.G.; Torres, S.L.; Siedlak, S.L.; Perry, G.; Kryscio, R.; Jicha, G.; Casadesus, G.; Smith, M.A.; Zhu, X.; et al. Dysregulation of leptin signaling in Alzheimer disease: Evidence for neuronal leptin resistance. J. Neurochem. 2014, 128, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Holden, K.F.; Lindquist, K.; Tylavsky, F.A.; Rosano, C.; Harris, T.B.; Yaffe, K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol. Aging 2009, 30, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Khemka, V.K.; Bagchi, D.; Bandyopadhyay, K.; Bir, A.; Chattopadhyay, M.; Biswas, A.; Basu, D.; Chakrabarti, S. Altered serum levels of adipokines and insulin in probable Alzheimer’s disease. J. Alzheimers Dis. 2014, 41, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Lieb, W.; Beiser, A.S.; Vasan, R.S.; Tan, Z.S.; Au, R.; Harris, T.B.; Roubenoff, R.; Auerbach, S.; DeCarli, C.; Wolf, P.A.; et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 2009, 302, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Bjorbaek, C.; Osei, S.; Flier, J.S. Regulation of neuronal and glial proteins by leptin: Implications for brain development. Endocrinology 1999, 140, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Suyama, S.; Koch, M.; Jin, S.; Argente-Arizon, P.; Argente, J.; Liu, Z.W.; Zimmer, M.R.; Jeong, J.K.; Szigeti-Buck, K.; et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 2014, 17, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Dicou, E.; Attoub, S.; Gressens, P. Neuroprotective effects of leptin in vivo and in vitro. Neuroreport 2001, 12, 3947–3951. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Park, C.S.; Lee, S.K.; Shin, D.W.; Kang, J.H. Leptin inhibits 1-methyl-4-phenylpyridinium-induced cell death in SH-SY5Y cells. Neurosci. Lett. 2006, 407, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, J. Leptin protects hippocampal CA1 neurons against ischemic injury. J. Neurochem. 2008, 107, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.G.; Leverenz, J.B.; McMillan, P.J.; Kulstad, J.J.; Ericksen, S.; Roth, R.A.; Schellenberg, G.D.; Jin, L.W.; Kovacina, K.S.; Craft, S. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-epsilon4 allele. Am. J. Pathol. 2003, 162, 313–319. [Google Scholar] [CrossRef]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Shirotani, K.; Lu, B.; Gerard, N.P.; Gerard, C.; Hama, E.; Lee, H.J.; Saido, T.C. Metabolic regulation of brain Aβ by neprilysin. Science 2001, 292, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Dorfman, V.B.; Gamba, A.F.; Frangione, B.; Wisniewski, T.; Castaño, E.M.; Sigurdsson, E.M.; Morelli, L. Plaque-associated overexpression of insulin-degrading enzyme in the cerebral cortex of aged transgenic tg2576 mice with Alzheimer pathology. J. Neuropathol. Exp. Neurol. 2006, 65, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Vekrellis, K.; Ye, Z.; Qiu, W.Q.; Walsh, D.; Hartley, D.; Chesneau, V.; Rosner, M.R.; Selkoe, D.J. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J. Neurosci. 2000, 20, 1657–1665. [Google Scholar] [PubMed]
- Qiu, W.Q.; Walsh, D.M.; Ye, Z.; Vekrellis, K.; Zhang, J.; Podlisny, M.B.; Rosner, M.R.; Safavi, A.; Hersh, L.B.; Selkoe, D.J. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J. Biol. Chem. 1998, 273, 32730–32738. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Cha, M.Y.; Choi, H.; Kang, S.; Choi, H.; Lee, M.S.; Park, S.A.; Mook-Jung, I. Insulin-degrading enzyme secretion from astrocytes is mediated by an autophagy-based unconventional secretory pathway in Alzheimer disease. Autophagy 2016, 12, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Oddo, S.; Sugarman, M.C.; Akbari, Y.; LaFerla, F.M. Age- and region-dependent alterations in Aβ-degrading enzymes: Implications for Aβ-induced disorders. Neurobiol. Aging. 2005, 26, 645–654. [Google Scholar] [CrossRef] [PubMed]
- El-Amouri, S.S.; Zhu, H.; Yu, J.; Marr, R.; Verma, I.M.; Kindy, M.S. Neprilysin: An enzyme candidate to slow the progression of Alzheimer’s disease. Am. J. Pathol. 2008, 172, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Beckett, C.; Belyaev, N.D.; Turner, A.J. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease? J. Neurochem. 2012, 120, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Nalivaeva, N.N. New insights into the roles of metalloproteinases in neurodegeneration and neuroprotection. Int. Rev. Neurobiol. 2007, 82, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.; Sastre, M. Mechanisms of Aβ clearance and degradation by glial cells. Front. Aging Neurosci. 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Martinez, L.; Farías, G.A.; Maccioni, R.B. Tau oligomers as potential targets for Alzheimer’s diagnosis and novel drugs. Front. Neurol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Mi, K.; Johnson, G.V. The role of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2006, 3, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.C.; Zaidi, T.; Grundke-Iqbal, I.; Iqbal, K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 5562–5566. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Johnson, G.V. Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau’s ability to bind and stabilize microtubules. J. Neurochem. 2004, 88, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.; Utton, M.; Gallo, J.M.; Miller, C.C. Cellular phosphorylation of tau by GSK-3β influences tau binding to microtubules and microtubule organization. J. Cell Sci. 1996, 109, 1537–1543. [Google Scholar] [PubMed]
- Rojo, A.I.; Sagarra, M.R.; Cuadrado, A. GSK-3β down-regulates the transcription factor Nrf2 after oxidant damage: Relevance to exposure of neuronal cells to oxidative stress. J. Neurochem. 2008, 105, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Munoz, L.; Ammit, A.J. Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology 2010, 58, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Garrido, J.J.; Wandosell, F.G. Modulation of GSK-3 as a therapeutic strategy on tau pathologies. Front. Mol. Neurosci. 2011, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Jhang, K.A.; Park, J.S.; Kim, H.S.; Chong, Y.H. Resveratrol Ameliorates Tau Hyperphosphorylation at Ser396 Site and Oxidative Damage in Rat Hippocampal Slices Exposed to Vanadate: Implication of ERK1/2 and GSK-3β Signaling Cascades. J. Agric. Food. Chem. 2017, 65, 9626–9634. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Wang, C.; Teng, Z.; Li, Y. Curcumin decreases hyperphosphorylation of tau by down-regulating caveolin-1/GSK-3β in N2a/APP695swe cells and APP/PS1 double transgenic Alzheimer’s disease mice. Am. J. Chin. Med. 2017, 45, 1667–1682. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cheng, P.; Yu, K.; Han, Y.; Song, M.; Li, Y. Hyperforin attenuates aluminum-induced Aβ production and tau phosphorylation via regulating Akt/GSK-3β signaling pathway in PC12 cells. Biomed. Pharmacother. 2017, 96, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, J.; Ma, D.; Yuan, G.; Lu, Y.; Yang, Y. Capsaicin reduces Alzheimer-associated tau changes in the hippocampus of type 2 diabetes rats. PLoS ONE 2017, 12, e0172477. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Jiang, Z.H.; Liu, L.; Hu, M. Mechanisms responsible for poor oral bioavailability of paeoniflorin: Role of intestinal disposition and interactions with sinomenine. Pharm. Res. 2006, 23, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Lin, J.-Z.; Li, L.; Yang, J.-L.; Jia, W.-W.; Huang, W.-H.; Du, F.-F.; Wang, F.-Q.; Li, M.-J.; Li, Y.-F.; et al. Pharmacokinetics and disposition of monoterpene glycosides derived from Paeonia lactiflora roots (Chishao) after intravenous dosing of antiseptic XueBiJing injection in human subjects and rats. Acta Pharmacol. Sin. 2016, 37, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Martey, O.N.K.; Shi, X.; He, X. Advance in pre-clinical pharmacokinetics of paeoniflorin, a major monoterpene glucoside from the root of Paeonia lactiflora. Pharmacol. Pharm. 2013, 4, 4–14. [Google Scholar] [CrossRef]
- Austgulen, L.T.; Solheim, E.; Scheline, R.R. Metabolism in rats of p-cymene derivatives: Carvacrol and thymol. Pharmacol. Toxicol. 1987, 61, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.H.; Fang, Z.Z.; Zhu, L.L.; Liang, S.C.; Ge, G.B.; Liu, Z.Y. Investigation of UDP-glucuronosyltransferases (UGTs) inhibitory properties of carvacrol. Phytother. Res. 2012, 26, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kohlert, C.; Schindler, G.; März, R.W.; Abel, G.; Brinkhaus, B.; Derendorf, H.; Gräfe, E.U.; Veit, M. Systemic availability and pharmacokinetics of thymol in humans. J. Clin. Pharmacol. 2002, 42, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Lang, J.E.; Ley, M.; Nagle, R.; Hsu, C.H.; Thompson, P.A.; Cordova, C.; Waer, A.; Chow, H.H. Human breast tissue disposition and bioactivity of limonene in women with early stage breast cancer. Cancer Prev. Res. 2013, 6, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Hakim, I.A.; Chew, W.; Thompson, P.; Thomson, C.A.; Chow, H.H. Adipose tissue accumulation of d-limonene with the consumption of a lemonade preparation rich in d-limonene content. Nutr. Cancer 2010, 62, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; He, J.C.; Bai, M.; Song, Q.Y.; Feng, E.F.; Rao, G.X.; Xu, G.L. Determination of the plasma pharmacokinetic and tissue distributions of swertiamarin in rats by liquid chromatography with tandem mass spectrometry. Arzneimittelforschung 2012, 62, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lin, L.C.; Lin, C.H.; Tsai, T.H. Comparative oral bioavailability of geniposide following oral administration of geniposide, Gardenia jasminoides Ellis fruits extracts and Gardenia herbal formulation in rats. J. Pharm. Pharmacol. 2014, 66, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kobashi, K.; Aburasa, M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol. Pharm. Bull. 1994, 17, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, F.; Zhang, Y.; Lu, J.; Shi, Y. Simultaneous determination of geniposide and its metabolites genipin and genipinine in culture of Aspergillus niger by HPLC. Biomed. Chromatogr. 2008, 22, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yang, L.; Xu, Y.; Ding, Y.; Annie Bligh, S.W.; Zhang, T.; Wang, Z.T. Identification of metabolites of geniposide in rat urine using ultra-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Belai, A. Natural therapies of the inflammatory bowel disease: The case of rutin and its aglycone, quercetin. Mini Rev. Med. Chem. 2017, 17. in press. [Google Scholar] [CrossRef]
- Yu, B.; Ruan, M.; Cui, X.B.; Guo, J.M.; Xu, L.; Dong, X.P. Effect of borneol on the pharmacokinetics of geniposide in cortex, hippocampus, hypothalamus and striatum of conscious rat by simultaneous microdialysis coupled with UPLC-MS. J. Pharm. Biomed. Anal. 2013, 77, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Rosselli, S.; Bruno, M. Essential oils and pure volatile compounds as potential drugs in Alzheimer’s disease therapy: An updated review of the literature. Curr. Pharm. Des. 2016, 22, 4011–4027. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).