Abstract
A series of linear furanocoumarins with different substituents have been designed and synthesized. Their structures were confirmed by 1H-NMR spectroscopy, high resolution mass spectra (EI-MS), IR, and X-ray single-crystal diffraction. All of the target compounds were evaluated in vitro for their antifungal activity against Rhizoctorzia solani, Botrytis cinerea, Alternaria solani, Gibberella zeae, Cucumber anthrax, and Alternaria leaf spot at 100 μg/mL, and some of the designed compounds exhibited potential antifungal activities. Compound 3a (67.9%) exhibited higher activity than the control Osthole (66.1%) against Botrytis cinerea. Furthermore, compound 4b (62.4%) represented equivalent antifungal activity as Osthole (69.5%) against Rhizoctonia solani. The structure-activity relationship (SAR) study demonstrates that linear furanocoumarin moiety has an important effect on the antifungal activity, promoting the idea of the coumarin ring as a framework that might be exploited in the future.
1. Introduction
Plant diseases cause severe crop yield reduction and result in significant economic losses every year. How to control them in modern agriculture is still a big challenge [1,2]. Although many chemical agents were developed and applied to control these diseases, most of them cannot fully protect the crops or completely cure the crops’ tissues from fungal infection under field conditions. The botanical fungicide is one of the plant protection alternatives, generally considered safe for the environment and health. The development botanical fungicide is important [3].
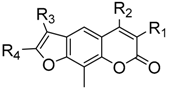
Coumarins widely exist in nature and can be found in all parts of plants, especially in grasses, orchids, citrus fruits, and legumes [4]. Furanocoumarins are one of the main groups in coumarins, based on their chemical structure, they can be generally classified as linear (e.g., Psoralen, Figure 1) and angular (e.g., Angelicin, Figure 1) type. Angular furanocoumarins are always present together with linear furanocoumarins, but in lower contents [5]. As a structural core, furanocoumarins is used regularly as a scaffold in medicinal and agricultural chemistry, this is highlighted by Angelica dahurica and Psoralen (Figure 1), the traditional Chinese herbs, usually possess a broad scope of pharmacological and biochemical activities, including anti-Alzheimer’s disease, anticancer [6], anti-HIV (human immunodeficiency virus), antitumor [7], antitumour, antidiabetic [8], anti-inflammatory, antidepressant [9], antiprotozoal, insecticidal [10], antibacterial [11], and antifungal [12,13] activities, and they are active photosensitizers for the treatment of several skin diseases [14,15,16].
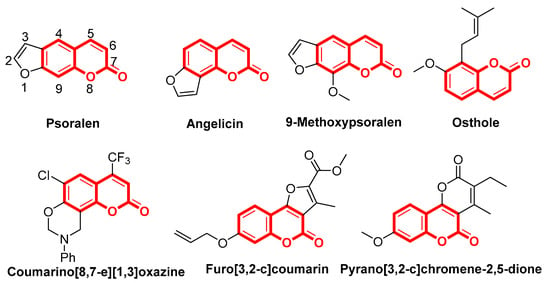
Figure 1.
Structures of coumarin-containing compounds.
To date, in order to decrease toxicity and explore the potential biological activity of linear furanocoumarins there has been accomplished in three different ways: first, using angular furanocoumarins, which on account of their geometry cannot crosslink with DNA; second, blocking of the photo reactive α-pyrone double bond by appropriate substituents or by annelation of an additional aromatic ring; third, incorporating an additional benzene ring between active double bonds of the a-pyrone and furan moiety [16]. Based on our previous work (Figure 1) [12,13,17,18,19,20], a series of different substituted linear furanocoumarins were designed and synthesized by construction of a furan ring on the benzene moiety of coumarin (Scheme 1). To the best of our knowledge, there was less research systematically investigated the antifungal activity of linear furanocoumarins against plant pathogenic fungi. Aiming to discover promising botanical candidates, we screened the antifungal activity of the synthesized linear furanocoumarins against six botanical fungi, including Rhizoctorzia solani, Botrytis cinerea, Alternaria solani, Gibberella zeae, Cucumber anthrax and Alternaria leaf spot, which are often encountered in plants.
Scheme 1.
The structures of the designed and synthesized compounds.
2. Materials and Methods
2.1. Chemicals and Methods
All materials were obtained from commercial sources and used as received. The evidence for the formation of all the synthesized compounds can be achieved by the melting point, 1H-NMR, 13C-NMR, high resolution mass spectra (HR-MS) and IR spectra. Melting points were obtained on a melting-point apparatus (BUCHI, Flawil, Switzerland) and are uncorrected. NMR spectra were performed on a Bruker DRX-400 instrument (Bruker, Karlsruhe, Germany) in CDCl3 or DMSO-d6 with TMS as the internal reference (400 and 100 MHz for 1H-NMR and 13C-NMR respectively). Infrared spectra were recorded on a Bruker Tensor 27 spectrometer, and samples were prepared as KBr plates. HR-MS were acquired in positive mode on a JMS-AX505HA (JEOL, Akishima, Japan), and the detailed physical and analytical data are listed in the Supplementary Information. The course of reactions and the purity of products were monitored by thin-layer chromatography (TLC) using silica gel GF/UV 254 (YUHUA, Gongyi, China). Reaction yields were not optimized. The single-crystal structures of compound 1a and 6f were determined by X-ray crystallography as illustrated (Figure 2), respectively.
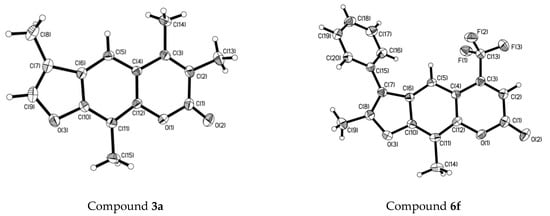
Figure 2.
X-ray single crystal structures of compounds 3a and 6f.
In this study, a series of psoralen derivatives (compounds 3a–3f, 4a–4f, 5a–5f, and 6a–6f) were designed and synthesized by forming a furan ring on the benzene moiety of coumarin. Aiming to improve the levels of antifungal activity, we substituted the hydrogen on C-2 and C-3 positions for methyl or phenyl to block the double bond on furan moiety. In addition, to enrich the compound group, the hydrogen on C-5 and C-6 positions were substituted by methyl, ethyl, trifluoromethyl, fluorine, chlorine, or formed with an additional hexatomic ring on the both position.
2.1.1. General Procedure for the Preparation of Compounds 2a–2f
The initial coumarins 2a–2f (Scheme 2) were synthesized from commercially available 2-methylresorcinol and β-ketoester through Pechmann reaction. The mixture of 2-methylresorcinol (100 mmol, 12.41 g) and β-ketoester (ethyl 3-oxobutanoate) (120 mmol, 13.26 g) were dropwise into the concentrated sulfuric acid at iced water with stirring for 12 h, and the crude product was recrystallized to generate compound 2a. Yields for compounds 2a–2f vary from 30% to 60%.
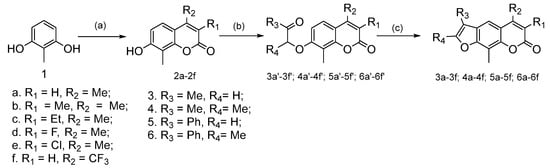
Scheme 2.
Synthetic routes for the target compounds. Reagents and conditions: (a) R4COCHR3COOCH2CH3, H2SO4, 0 °C; (b) α-chloroacetone, hydrous K2CO3, KI, tetra-n-butylammonium bromide (TBAB), hydrous Acetone, 80 °C, 6 h; (c) NaOH, H2O, N2, reflux, 4 h.
2.1.2. Preparation of 2-Bromo-1-phenylpropan-1-one
All the α-chloroacetone used in the Scheme 2 (b) except 2-bromo-1-phenylpropan-1-one was obtained from commercial sources without further purification. 2-bromo-1-phenylpropan-1-one was synthesized through the reported method [21,22] (Scheme 3).
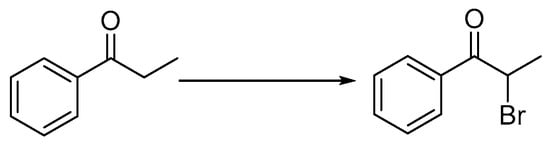
Scheme 3.
The synthetic method for the preparation of 2-bromo-1-phenylpropan-1-one. Reagents and conditions: 30% H2O2, 40% HBr, r.t., 24 h.
2.1.3. General Procedure for the Preparation of Compounds 3a′–3f′, 4a′–4f′, 5a′–5f′ and 6a′–6f′
Ether derivatives (compounds 3a′–3f′, 4a′–4f′, 5a′–5f′, and 6a′–6f′, shown in Scheme 2) were synthesized by etherification from compounds 2a–2f through Williamson conditions [23], compound 2a (10 mmol, 1.90 g) was dissolved in acetone at reflux. K2CO3 (30 mmol, 4.15 g), tetra-n-butylammonium bromide (TBAB, 0.2 equiv., 0.64 g) and KI (10 mmol, 1.66 g) were added gradually with stirring for 15 min, and then 1-chloropropan-2-one (10 mmol, 0.92 g) was added to the system. The mixture was continuing stirred at reflux for 6 h. After the reaction solution cooling, the filtrate was concentrated under reduced pressure and purified by recrystallization to generate compound 3a′. Yields for compounds 3a′–3f′, 4a′–4f′, 5a′–5f′, and 6a′–6f′ vary 50% to 90%.
2.1.4. General Procedure for the Preparation of Compounds 3a–3f, 4a–4f, 5a–5f, and 6a–6f
Thereafter, cyclization of oxo ether derivative compound 3a′ (5 mmol, 1.20 g) was accomplished by heating with strong alkaline solution in the dark for 3 h under N2 protection. The solution was diluted with iced water, and acidified with 10% HCl solution. The precipitate obtained was collected and crystallized from MeOH to generate compound 3a. Yields for the furanocoumarin derivatives compounds 3a–3f, 4a–4f, 5a–5f and 6a–6f vary 60% to 90%.
3,5,9-Trimethyl-7H-furo[3,2-g]chromen-7-one (3a): White solid; m.p.: 183.2~183.7 °C; Yield: 83.1%; 1H-NMR (400 MHz, CDCl3) δ 7.54 (s, 1H), 7.51 (d, J = 23.4 Hz, 2H), 6.26 (s, 1H), 2.59 (s, 3H), 2.52 (s, 3H), 2.29 (d, J = 1.0 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 161.43, 155.80, 153.19, 149.41, 142.81, 125.25, 115.99, 115.89, 112.80, 111.72, 109.56, 19.32, 8.48, 7.93; IR (KBr) ν/cm−1: 3100, 2917, 1707, 1594, 1383, 1102, 859, 817, 758; HR-MS (ESI): m/z calcd for C14H12O3 ([M + H]+) 229.0865, found 229.0857.
3,5,6,9-Tetramethyl-7H-furo[3,2-g]chromen-7-one (3b): White solid; m.p.: 219.4~219.6 °C; Yield: 94.3%; 1H-NMR (400 MHz, DMSO) δ 7.86 (s, 1H), 7.79 (s, 1H), 2.46 (s, 6H), 2.26 (s, 3H), 2.12 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 162.48, 154.94, 147.97, 146.69, 142.49, 125.02, 119.49, 116.57, 115.86, 111.24, 108.96, 15.58, 13.40, 8.46, 7.94; IR (KBr) ν/cm−1: 3103, 1699, 1593, 1378, 1112, 1074, 855, 758; HR-MS (ESI): m/z calcd for C15H14O3 ([M + H]+) 243.1021, found 243.1016.
6-Ethyl-3,5,9-trimethyl-7H-furo[3,2-g]chromen-7-one (3c): White solid; m.p.: 165.6~165.7 °C; Yield: 93.5%; 1H-NMR (400 MHz, CDCl3) δ 7.52 (s, 1H), 7.46 (d, J = 1.1 Hz, 1H), 2.73 (q, J = 7.5 Hz, 2H), 2.58 (s, 3H), 2.50 (s, 3H), 2.28 (d, J = 1.1 Hz, 3H), 1.17 (t, J = 7.5 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 162.06, 155.02, 148.11, 146.28, 142.51, 125.51, 125.05, 116.79, 115.85, 111.42, 109.04, 21.02, 15.08, 13.25, 8.48, 7.97; IR (KBr) ν/cm−1: 2963, 1686, 1591, 1394, 1122, 898, 812, 778; HR-MS (ESI): m/z calcd for C16H16O3 ([M + H]+) 257.1178, found 257.1172.
6-Fluoro-3,5,9-trimethyl-7H-furo[3,2-g]chromen-7-one (3d): White solid; m.p.: 220.1~220.5 °C; Yield: 82.4%; 1H-NMR (400 MHz, CDCl3) δ 7.50 (d, J = 1.3 Hz, 1H), 7.49 (s, 1H), 2.58 (s, 3H), 2.49 (d, J = 2.9 Hz, 3H), 2.29 (d, J = 1.2 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ155.07 (d, J = 2.3 Hz), 146.22, 143.90, 143.09, 141.44, 131.56 (d, J = 12.4 Hz), 126.02, 115.77, 115.48 (d, J = 2.4 Hz), 111.65 (d, J = 6.8 Hz), 109.88, 10.59 (d, J = 4.0 Hz), 8.57, 7.93; HR-MS (ESI): m/z calcd for C14H11FO3 ([M + H]+) 247.0770, found 247.0765.
6-Chloro-3,5,9-trimethyl-7H-furo[3,2-g]chromen-7-one (3e): Yellow solid; m.p.: 271.9~272.1 °C; Yield: 93.4%; 1H-NMR (400 MHz, DMSO) δ 7.90 (s, 1H), 7.87 (s, 1H), 2.63 (s, 3H), 2.45 (s, 3H), 2.26 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 157.46, 155.59, 148.43, 147.38, 143.17, 125.85, 118.65, 115.94, 115.83, 112.07, 109.74, 16.77, 8.54, 7.93; IR (KBr) ν/cm−1: 3096, 2926, 1706, 1613, 1574, 1391, 1115, 882, 801, 757; HR-MS (ESI): m/z calcd for C14H11ClO3 ([M + H]+) 263.0475, found 263.0470.
3,9-Dimethyl-5-(trifluoromethyl)-7H-furo[3,2-g]chromen-7-one (3f): Yellow solid.; m.p.: 188.4~189.0 °C; Yield: 94.5%; 1H-NMR (400 MHz, DMSO) δ 7.97 (s, 1H), 7.65 (s, 1H), 7.01 (s, 1H), 2.50 (s, 7H), 2.26 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.55, 156.22, 150.10, 143.51, 142.33 (d, J = 32.2 Hz), 126.08, 123.24, 120.50, 113.44 (q, J = 5.8 Hz), 112.87 (q, J = 2.3 Hz), 110.59, 109.36, 8.57, 7.78; IR (KBr) ν/cm−1: 3130, 2931, 1730, 1595, 1389, 1272, 1137, 1074, 870, 795; HR-MS (ESI): m/z calcd for C14H9F3O3 ([M + H]+) 283.0582, found 283.0577.
2,3,5,9-Tetramethyl-7H-furo[3,2-g]chromen-7-one (4a): White solid; mp.: 199.9~200.3 °C; Yield: 92.0%; 1H-NMR (400 MHz, CDCl3) δ 7.37 (s, 1H), 6.22 (s, 1H), 2.55 (s, 3H), 2.49 (d, J = 0.6 Hz, 3H), 2.41 (s, 3H), 2.18 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 161.52, 154.32, 153.27, 152.24, 148.78, 126.63, 115.41, 112.25, 110.39, 109.76, 108.48, 19.25, 11.93, 8.36, 7.88; IR (KBr) ν/cm−1: 3055, 2985, 2931, 1704, 1595, 1368, 1274, 1103, 839, 758; HR-MS (ESI): m/z calcd for C15H14O3 ([M + H]+) 243.1021, found 243.1010.
2,3,5,6,9-Pentamethyl-7H-furo[3,2-g]chromen-7-one (4b): White solid; m.p.: 201.5~202.2 °C; Yield: 82.3%; 1H-NMR (400 MHz, CDCl3) δ 7.37 (s, 1H), 2.55 (s, 3H), 2.47 (s, 3H), 2.41 (s, 3H), 2.23 (s, 3H), 2.18 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 162.49, 153.46, 151.81, 147.34, 146.78, 126.39, 118.88, 116.01, 109.97, 109.74, 107.93, 15.46, 13.29, 11.91, 8.37, 7.88; IR (KBr) ν/cm−1: 2920, 1698, 1593, 1435, 1122, 758; HR-MS (ESI): m/z calcd for C16H16O3 ([M + H]+) 257.1178, found 257.1166.
6-Ethyl-2,3,5,9-tetramethyl-7H-furo[3,2-g]chromen-7-one (4c): White solid; m.p.: 201.7~201.8 °C; Yield: 55.2%; 1H-NMR (400 MHz, CDCl3) δ 7.38 (s, 1H), 2.72 (q, J = 7.5 Hz, 2H), 2.55 (s, 3H), 2.49 (s, 3H), 2.41 (s, 3H), 2.18 (s, 3H), 1.17 (t, J = 7.5 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 162.24, 153.64, 151.91, 147.58, 146.47, 126.53, 125.08, 116.34, 110.26, 109.77, 108.16, 77.40, 77.08, 76.76, 20.97, 15.06, 13.26, 11.97, 8.44, 7.97; IR (KBr) ν/cm−1: 2924, 1701, 1686, 1574, 1402, 1117, 895, 777; HR-MS (ESI): m/z calcd for C17H18O3 ([M + H]+) 271.1334, found 271.1324.
6-Fluoro-2,3,5,9-tetramethyl-7H-furo[3,2-g]chromen-7-one (4d): White solid; m.p.: 238.2~238.4 °C; Yield: 64.1%; 1H-NMR (400 MHz, CDCl3) δ 7.32 (s, 1H), 2.55 (s, 3H), 2.46 (d, J = 2.9 Hz, 3H), 2.42 (s, 3H), 2.19 (d, J = 0.6 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 155.73, 153.61, 152.72, 145.62, 141.23, 131.65 (d, J = 12.6 Hz), 127.44, 114.91, 110.32 (d, J = 6.7 Hz), 109.72, 108.84, 11.96, 10.50 (d, J = 4.0 Hz), 8.48, 7.90; IR (KBr) ν/cm−1: 2920, 1706, 1573, 1402, 1115, 818, 756; HR-MS (ESI): m/z calcd for C15H13FO3 ([M + H]+) 261.0927, found 261.0916.
6-Chloro-2,3,5,9-tetramethyl-7H-furo[3,2-g]chromen-7-one (4e): White solid; m.p.: 253.8~253.9 °C; Yield: 47.3%; 1H-NMR (400 MHz, CDCl3) δ 7.40 (s, 1H), 2.66 (s, 3H), 2.56 (s, 3H), 2.43 (s, 3H), 2.19 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 157.44, 154.09, 152.84, 148.43, 146.70, 127.21, 118.04, 115.16, 110.64, 109.85, 108.56, 77.40, 77.08, 76.76, 16.62, 12.00, 8.41, 7.88; IR (KBr) ν/cm−1: 3091, 2920, 1704, 1573, 1115, 886, 750; HR-MS (ESI): m/z calcd for C15H13ClO3 ([M + H]+) 277.0631, found 277.0622.
2,3,9-Trimethyl-5-(trifluoromethyl)-7H-furo[3,2-g]chromen-7-one (4f): Yellow solid; m.p.: 212.1~212.2 °C; Yield: 80.1%; 1H-NMR (400 MHz, CDCl3) δ 7.53 (s, 1H), 6.74 (s, 1H), 2.58 (s, 3H), 2.43 (s, 3H), 2.19 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.81, 154.96, 153.26, 149.75, 142.45 (d, J = 32.2 Hz), 127.60, 121.93 (d, J = 275.7 Hz), 112.99 (q, J = 5.9 Hz), 111.91–110.95 (m), 110.04, 109.71, 109.03, 12.02, 8.55, 7.82; IR (KBr) ν/cm−1: 3073, 2920, 1730, 1703, 1410, 1288, 1116, 718; HR-MS (ESI): m/z calcd for C15H11F3O3 ([M + H]+) 297.0739, found 297.0729.
5,9-Dimethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (5a): White solid; m.p.: 197.5~197.9 °C; Yield: 41.7%; 1H-NMR (400 MHz, CDCl3) δ 8.01 (d, J = 8.0 Hz, 2H), 7.65 (t, J = 7.4 Hz, 1H), 7.52 (t, J = 7.7 Hz, 2H), 7.37 (d, J = 8.8 Hz, 1H), 6.71 (d, J = 8.8 Hz, 1H), 6.15 (s, 1H), 5.41 (s, 2H), 2.39 (s, 3H), 2.38 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 161.25, 156.26, 153.10, 149.55, 142.60, 131.29, 129.22, 127.95, 127.52, 122.78, 122.50, 116.66, 113.22, 112.77, 110.12, 77.40, 77.08, 76.76, 19.38, 8.58; IR (KBr) ν/cm−1: 3073, 1707, 1590, 1386, 1113, 1085, 911, 754, 698; HR-MS (ESI): m/z calcd for C19H14O3 ([M + H]+) 291.1021, found 291.1013.
5,6,9-Trimethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (5b): White solid; m.p.: 191.0~191.6 °C; Yield: 70.8%; 1H-NMR (400 MHz, CDCl3) δ 7.83 (s, 2H), 7.65 (d, J = 7.2 Hz, 2H), 7.53 (t, J = 7.6 Hz, 2H), 7.43 (t, J = 7.4 Hz, 1H), 2.64 (s, 3H), 2.48 (s, 3H), 2.26 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 162.38, 155.46, 148.16, 146.66, 142.37, 131.52, 129.20, 127.86, 127.55, 122.59, 122.52, 120.01, 117.31, 112.32, 109.57, 15.68, 13.51, 8.57; IR (KBr) ν/cm−1: 2921, 1695, 1592, 1118, 757, 693; HR-MS (ESI): m/z calcd for C20H16O3 ([M + H]+) 305.1178, found 305.1169.
6-Ethyl-5,9-dimethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (5c): White solid; m.p.: 180.0~180.1 °C; Yield: 63.2%; 1H-NMR (400 MHz, CDCl3) δ 7.81 (s, 2H), 7.64 (d, J = 7.2 Hz, 2H), 7.52 (t, J = 7.6 Hz, 2H), 7.42 (t, J = 7.4 Hz, 1H), 2.73 (q, J = 7.5 Hz, 2H), 2.62 (s, 3H), 2.48 (s, 3H), 1.16 (d, J = 7.5 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 161.80, 155.40, 148.14, 146.15, 142.30, 131.45, 129.17, 127.82, 127.43, 125.86, 122.43, 122.40, 117.33, 112.39, 109.40, 21.07, 15.07, 13.23, 8.50; IR (KBr) ν/cm−1: 3050, 1695, 1591, 1356, 1126, 1101, 766, 705; HR-MS (ESI): m/z calcd for C21H18O3 ([M + H]+) 319.1334, found 319.1329.
6-Fluoro-5,9-dimethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (5d): Yellow solid; m.p.: 200.3~200.9 °C; Yield: 37.2%; 1H-NMR (400 MHz, CDCl3) δ 7.86 (s, 1H), 7.78 (s, 1H), 7.66–7.61 (m, 2H), 7.53 (t, J = 7.6 Hz, 2H), 7.47–7.40 (m, 1H), 2.65 (s, 3H), 2.48 (d, J = 2.8 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 155.51, 146.36, 142.84, 141.60, 131.58, 131.14, 129.26, 128.03, 127.53, 123.56, 122.39, 116.19, 112.65 (d, J = 7.1 Hz), 110.39, 10.64 (d, J = 3.9 Hz), 8.66; IR (KBr) ν/cm−1: 2926, 2849, 1729, 1599, 1134, 754; HR-MS (ESI): m/z calcd for C19H13FO3 ([M + H]+) 309.0927, found 309.0922.
6-Chloro-5,9-dimethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (5e): White solid; m.p.: 187.2~188.3 °C; Yield: 70.8%; 1H-NMR (400 MHz, CDCl3) δ 7.86 (d, J = 2.3 Hz, 2H), 7.67–7.61 (m, 2H), 7.54 (t, J = 7.6 Hz, 2H), 7.45 (t, J = 7.4 Hz, 1H), 2.67 (s, 3H), 2.65 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 157.42, 156.03, 148.47, 147.51, 142.95, 131.08, 129.30, 128.09, 127.58, 123.45, 122.58, 119.05, 116.49, 113.14, 110.30, 16.87, 8.66; IR (KBr) ν/cm−1: 3051, 1720, 1608, 1120, 998, 153, 692; HR-MS (ESI): m/z calcd for C19H13ClO3 ([M + H]+) 325.0631, found 325.0629.
9-Methyl-3-phenyl-5-(trifluoromethyl)-7H-furo[3,2-g]chromen-7-one (5f): Red solid; m.p.: 201.9~202.0 °C; Yield: 78.6%; 1H-NMR (400 MHz, CDCl3) δ 7.99 (s, 1H), 7.89 (s, 1H), 7.61 (d, J = 7.2 Hz, 2H), 7.54 (t, J = 7.6 Hz, 2H), 7.44 (t, J = 7.3 Hz, 1H), 6.79 (s, 1H), 2.66 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.42 (d, J = 0.5 Hz), 156.64, 150.16, 143.24, 130.67, 129.34, 128.19, 127.43, 123.57, 123.16, 122.64, 120.42, 114.02 (q, J = 2.7 Hz), 113.93–113.77 (m), 111.09, 109.99, 8.70; IR (KBr) ν/cm−1: 3078, 2911, 1713, 1613, 1394, 1243, 1116, 953, 854, 752; HR-MS (ESI): m/z calcd for C19H11F3O3 ([M + H]+) 345.0739, found 345.0735.
2,5,9-Trimethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (6a): White solid; m.p.: 261.9~261.9 °C; Yield: 89.2%; 1H-NMR (400 MHz, CDCl3) δ 7.58–7.48 (m, 5H), 7.42 (t, J = 6.9 Hz, 1H), 6.24 (s, 1H), 2.63 (s, 3H), 2.57 (s, 3H), 2.46 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 161.57, 154.64, 153.37, 153.06, 149.16, 132.10, 129.04, 128.88, 127.43, 125.12, 116.97, 116.22, 112.77, 111.47, 109.22, 19.37, 12.96, 8.56; IR (KBr) ν/cm−1: 3062, 2917, 1707, 1592, 1396, 1104, 938, 868, 754; HR-MS (ESI): m/z calcd for C20H16O3 ([M + H]+) 305.1178, found 305.1172.
2,5,6,9-Tetramethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (6b): White solid; m.p.: 216.7~218.2 °C; Yield: 78.8%; 1H-NMR (400 MHz, CDCl3) δ 7.57 (s, 1H), 7.52 (t, J = 7.1 Hz, 4H), 7.42 (dd, J = 10.9, 4.3 Hz, 1H), 2.62 (s, 3H), 2.56 (s, 3H), 2.42 (s, 3H), 2.24 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 162.38, 155.46, 148.16, 146.66, 142.37, 131.52, 129.20, 127.86, 127.55, 122.59, 122.52, 120.01, 117.31, 112.32, 109.57, 15.68, 14.6, 13.51, 8.57; IR (KBr) ν/cm−1: 2956, 2920, 2850, 1697, 1592, 1396, 1324, 1120, 861, 759; HR-MS (ESI): m/z calcd for C21H18O3 ([M + H]+) 319.1334, found 319.1329.
6-Ethyl-2,5,9-trimethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (6c): Yellow solid; m.p.: 167.1~167.8 °C; Yield: 75.8%; 1H-NMR (400 MHz, CDCl3) δ 7.57 (s, 1H), 7.52 (t, J = 7.2 Hz, 4H), 7.42 (dd, J = 10.9, 4.3 Hz, 1H), 2.72 (q, J = 7.5 Hz, 2H), 2.62 (s, 3H), 2.56 (s, 3H), 2.44 (s, 3H), 1.16 (t, J = 7.5 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 162.13, 153.84, 152.72, 147.84, 146.43, 132.34, 129.00, 128.92, 127.32, 125.50, 124.88, 117.01, 111.21, 108.67, 21.03, 15.12, 13.25, 12.96, 8.55; IR (KBr) ν/cm−1: 2949, 1697, 1591, 1369, 1331, 1120, 940, 753, 701; HR-MS (ESI): m/z calcd for C22H20O3 ([M + H]+) 333.1491, found 333.1482.
6-Fluoro-2,5,9-trimethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (6d): White solid; m.p.: 221.1~221.4 °C; Yield: 60.3%; 1H-NMR (400 MHz, CDCl3) δ 7.53 (dd, J = 15.5, 4.2 Hz, 6H), 2.63 (s, 3H), 2.58 (s, 3H), 2.42 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 153.85 (d, J = 2.0 Hz), 153.39, 145.92, 141.44, 131.94, 131.72 (d, J = 13.1 Hz), 129.08, 128.87, 127.51, 125.85, 116.88, 115.66, 111.34 (d, J = 6.8 Hz), 109.43, 99.99, 12.97, 10.60 (d, J = 3.9 Hz), 8.61; IR (KBr) ν/cm−1: 2932, 1723, 1398, 1181, 1136, 868, 754; HR-MS (ESI): m/z calcd for C20H15FO3 ([M + H]+) 323.1083, found 323.1075.
6-Chloro-2,5,9-trimethyl-3-phenyl-7H-furo[3,2-g]chromen-7-one (6e): Yellow solid.; m.p.: 237.6~238.3 °C; Yield: 71.4%; 1H-NMR (400 MHz, CDCl3) δ 7.58 (s, 1H), 7.53 (dt, J = 14.5, 7.3 Hz, 4H), 7.43 (t, J = 7.1 Hz, 1H), 2.63 (s, 3H), 2.61 (s, 3H), 2.58 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 157.60, 154.42, 153.50, 148.65, 147.12, 131.89, 129.11, 128.90, 127.55, 125.72, 118.61, 117.03, 116.04, 111.80, 109.35, 16.85, 13.01, 8.63; IR (KBr) ν/cm−1: 2922, 1715, 1607, 1570, 1320, 1113, 943, 856, 756; HR-MS (ESI): m/z calcd for C20H15ClO3 ([M + H]+) 339.0788, found 339.0783.
2,9-Dimethyl-3-phenyl-5-(trifluoromethyl)-7H-furo[3,2-g]chromen-7-one (6f): Yellow solid; m.p.: 208.7~209.3 °C; Yield: 40.4%; 1H-NMR (400 MHz, CDCl3) δ 7.72 (s, 1H), 7.54 (t, J = 7.6 Hz, 2H), 7.48 (d, J = 7.6 Hz, 2H), 7.43 (t, J = 7.2 Hz, 1H), 6.75 (s, 1H), 2.64 (s, 3H), 2.59 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.60, 155.04, 153.92, 149.83, 142.37 (q, J = 32.5 Hz), 131.43, 128.92 (d, J = 37.6 Hz), 127.63, 125.86, 123.18, 120.43, 117.05, 113.32 (q, J = 5.8 Hz), 112.56 (d, J = 2.6 Hz), 110.09, 109.55, 12.99, 8.60; IR (KBr) ν/cm−1: 3089, 1726, 1600, 1276, 1126, 879, 758; HR-MS (ESI): m/z calcd for C20H13F3O3 ([M + H]+) 359.0895, found 359.0890.
2.2. The Crystal Structure of Compounds 3a and 6f
The crystallographic data have been deposited with the Cambridge Crystallographic Data Centre (CCDC) as supplementary publication; number CCDC 1448022 (compound 3a), CCDC 1448050 (compound 6f). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44 1223 336033 or E-mail: deposit@ccdc.cam.ac.uk).
2.3. Biological Assays
All the synthesized compounds were evaluated in vitro against six plants pathogenic fungi (Rhizoctorzia solani, Botrytis cinerea, Alternaria solani, Gibberella zeae, Cucumber anthrax, and Alternaria leaf spot), which were widely found in plants, using the mycelium growth inhibitory rate methods on PDA (a kind of culture medium within potato, agar and water), with Osthole used as the positive control. The tested compounds were dissolved in dimethylformamide (DMF) to prepare the stock solution before mixing with molten agar below 55 °C. The medium containing compounds at a concentration of 100 μg/mL for the preliminary screening was poured into sterilized petri dishes. Mycelial disks (5 mm in diameter) were then inoculated in the center of the Petri dishes and incubated at 25 °C for 3 to 5 days. Each experiment was carried out in triplicates. DMF served as the negative control. The colony diameter of each strain was measured by cross bracketing method, and the inhibitory rates of the compounds were summarized in Table 1.

Table 1.
Preliminary antifungal activities of all target compounds.
3. Results and Discussion
3.1. Synthetic Chemistry
In the synthesis of linear furancoumarins, furan rings were usually formed through 7-hydroxycoumarins and alkyne or α-halogenated ketone, with heavy metals often used as a catalyst. The approach leaded to environmental pollution and a higher cost. This study adopted the cheaper materials and the more simple method to generate the product. Ether derivatives were synthesized by etherification from different substituted 7-hydroxycoumarins through Williamson conditions Thereafter, cyclization of oxo ether derivatives were accomplished by heating with NaOH aqueous solution in the dark under N2 protection. The carbonyl substituents of α-halogenated ketone were different, so the time required for the subsequent etherified product was also different. When R3 position was methyl, the cyclization took a short time, usually in 2 h. When R3 position was phenyl, it took a longer time. This could be due to the large steric hindrance.
3.2. Antifungal Activity and the Structure-Activity Relationships
Data of Table 1 summarized the antifungal activities of all synthesized furancoumarin derivatives against six phytopathogenic fungi at the concentration of 100 μg/mL, respectively. Although the antifungal activity of most of the fused furancoumarin derivatives was not satisfactory, some structure-activity relationships still can be discovered. First, the synthesized compounds noticeably were more efficient to Rhizoctonia solani and Botrytis cinerea than other tested fungi, which indicated that the introduction of a fused-furan moiety to the coumarin core is important for designing coumarin based fungicides for Rhizoctonia solani and Botrytis cinerea. Compound 3a showed a broad antifungal spectrum against all tested six phytopathogenic fungi and exhibited higher inhibitory activity (67.9%) than the control Osthole (66.1%) against Botrytis cinerea. Besides, compound 4b (62.4%) showed equivalent antifungal activity with Osthole (69.5%) against Rhizoctonia solani. Compound 3a bears the smallest substituents among our synthesized compounds, with only two methyl groups at the periphery of the furancoumarin core, we envisioned that the size of our other designed molecules is an important issue in developing fungicides with high activity. Second, regardless of differences at the R3 and R4 position, the high inhibitory rate of compounds 4e, 5e and 6e suggest that the chlorine atom on R1 position was essential for the antifungal activity. Third, by comparing compounds 3 and 5, the phenyl on R3 position weakened the antifungal activity of the synthesized compounds. We found that compound 3f with a trifluoromethyl group substituted at the R2 position also showed very high activities against Rhizoctonia solani and Botrytis cinerea, suggesting that the introduction of a trifluoromethyl is meaningful to improve the activity of these kinds of fungicides.
4. Conclusions
In order to find potential activity from furanocoumarin derivatives for further structural optimization, we designed and synthesized a series of psoralen derivatives in a simple and efficient way. Most of the synthesized compounds displayed potential antifungal activity against certain phytopathogenic fungi in vitro. Some of the fused funancoumarin analogues exhibited good antifungal activity against Botrytis cinerea and Rhizoctonia solani, such as compounds 3a (67.9%), 3b (52.4%), 3f (61.5%), 4e (58.2%), 5c (58.2%), 5e (50.0%) and 5g (52.9%). Furthermore, compound 4b (62.4%) represented equivalent antifungal activity with Osthole (66.1%) against Rhizoctonia solani. Compound 3a was identified as the most active and therefore the most promising candidate for further study. In addition, it is reported that 9-methoxypsoralen can be used as photo-antimicrobial against Colletotrichum acutatum conidia under UV light exposure without any damage on the leaves [5]. Therefore, the antifungal activities of our synthesized psoralen derivatives may be further enhanced under UV light, the detailed investigation is now under way in our lab. However, in spite of the absence of UV radiation, the inhibitory rates of some synthesized compounds are appreciable. Further structural optimization of fused furancoumarin analogues is well under way, aiming to prepare analogues with improved antifungal activity.
Supplementary Materials
The following are available online: 1H-NMR and 13C-NMR Spectra of Products, HRMS Spectra of Target Compounds.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (No. 21272116) and the Funds for the Central Univers Fundamental Research Funds for the Central Universities (KYZ201604). We also thank John Clough from Syngenta Jealott’s Hill International Research Centre (UK) for suggestion.
Author Contributions
W.H.Z. and J.L. performed the molecular design; J.L., X.Y., Y.W. and C.G.L. performed the experimental work; X.Y., J.L. and Y.D. wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Z.; Chen, S.; Zhu, S.; Luo, J.; Zhang, Y.; Weng, Q. Synthesis and Fungicidal Activity of β-Carboline Alkaloids and Their Derivatives. Molecules 2015, 20, 13941–13957. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Talbot, N.J. Fungal physiology—A future perspective. Microbiology 2009, 155, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Vrchotová, N. Insecticidal effect of furanocoumarins from fruits of Angelica archangelica L. against larvae Spodoptera littoralis. Boisd. Ind. Crops Prod. 2013, 43, 33–39. [Google Scholar] [CrossRef]
- Marumoto, S.; Miyazawa, M. Structure-activity relationships for naturally occurring coumarins as β-secretase inhibitor. Bioorg. Med. Chem. 2012, 20, 784–788. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, H.D.; Pereira, A.C.; Brancini, G.T.P.; de Leão, H.C.; Júnior, N.S.M.; Bachmann, L. Furocoumarins and coumarins photoinactivate Colletotrichum acutatum and Aspergillus nidulans fungi under solar radiation. J. Photochem. Photobiol. B 2014, 131, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Chauthe, S.K.; Mahajan, S.; Rachamalla, M.; Tikoo, K.; Singh, I.P. Synthesis and evaluation of linear furanocoumarins as potential anti-breast and anti-prostate cancer agents. Med. Chem. Res. 2015, 24, 2476–2484. [Google Scholar] [CrossRef]
- Hafez, O.M.A.; Amin, K.M.; Abdel-Latif, N.A.; Mohamed, T.K.; Ahmed, E.Y.; Maher, T. Synthesis and antitumor activity of some new xanthotoxin derivatives. Eur. J. Med. Chem. 2009, 44, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, N.M.M.; Abd-Alla, H.I.; Aly, H.F.; Albalawy, M.A.; Shaker, K.H.; Bouajila, J. Preliminary In Vitro and In Vivo Evaluation of Antidiabetic Activity of Ducrosia anethifolia Boiss. and Its Linear Furanocoumarins. Biomed. Res. Int. 2014, 2014, 480545. [Google Scholar] [CrossRef] [PubMed]
- Karamat, F.I.; Olry, A.; Doerper, S.; Vialart, G.; Ullmann, P.; Werck-Reichhart, D. CYP98A22, a phenolic ester 30-hydroxylase specialized in the synthesis of chlorogenic acid, as a new tool for enhancing the furanocoumarin concentration in Rutagraveolens. BMC Plant Biol. 2012, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Sardari, S.; Mori, Y.; Horita, K.; Micetich, R.G.; Nishibe, S.; Daneshtalab, M. Synthesis and Antifungal Activity of Coumarins and Angular Furanocoumarins. Bioorg. Med. Chem. 1999, 7, 1933–1940. [Google Scholar] [CrossRef]
- Verotta, L.; Lovagli, E.; Vidari, G.; Finzi, P.V.; Neri, M.G.; Raimondi, A.; Parapini, S.; Taramelli, D.; Riva, A.; Bombardelli, E. Raimondi, 4-Alkyl- and 4-phenylcoumarins from Mesua ferrea as promising multidrug resistant antibacterials. Phytochemistry 2004, 65, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Zhang, R.R.; Wang, J.Q. Microwave-assisted synthesis and antifungal activity of novel fused Osthole derivatives. Eur. J. Med. Chem. 2016, 97, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Xu, Z.C. Microwave-Assisted Synthesis and Antifungal Activities of Polysubstituted Furo[3,2-c]chromen-4-ones and 7,8,9,10-Tetrahydro-6H-benzofuro[3,2-c]chromen-6-ones. Synth. Commun. 2015, 46, 3257–3263. [Google Scholar] [CrossRef]
- Shen, Q.; Peng, Q.; Shao, J.; Liu, X.; Huang, Z.; Pu, X. Synthesis and biological evaluation of functionalized coumarins as acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2005, 40, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Finn, G.J.; Creaven, B.; Egan, D.A. Study of the in vitro cytotoxic potential of natural and synthetic coumarin derivatives using human normal and neoplastic skin cell lines. Melanoma Res. 2001, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- El-Gogary, S.; Hashem, N.; Khodeir, M.N. Synthesis and photooxygenation of angular furocoumarins: Isopsedopsoralen and allopsoralen. Res. Chem. Intermed. 2015, 41, 1591–1600. [Google Scholar] [CrossRef]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Zhang, R.R.; Yin, W.Z.; Yu, X.; Zhang, Y.L.; Liu, P.; Gu, Y.C.; Zhang, W.H. Microwave-assisted Synthesis and antifungal activity of coumarin[8,7-e][1,3]oxazine derivatives. Mol. Divers. 2016, 20, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Zhang, Y.; Wang, J.Q.; Zhang, W.H. Design, Synthesis and Antifungal Activity of Coumarin Ring-Opening Derivatives. Molecules 2016, 21, 1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Liu, J.; Zhang, Y.; Hou, M.Q.; Zhang, M.Z.; Zhou, F.; Zhang, W.H. Microwave-assisted synthesis and antifungal activity of novel coumarin derivatives: Pyrano[3,2-c]chromene-2,5-diones. Eur. J. Med. Chem. 2016, 116, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Podgoršek, A.; Stavber, S.; Zupan, M.; Iskra, J. Bromination of ketones with H2O2-HBr “on water”. Green Chem. 2007, 9, 1212–1218. [Google Scholar] [CrossRef]
- Jiang, Q.; Sheng, W.; Guo, C. Synthesis of phenacyl bromides via K2S2O8-mediated tandem hydroxybromination and oxidation of styrenes in water. Green Chem. 2013, 8, 2175–2179. [Google Scholar] [CrossRef]
- Zhang, W.H.; Jiang, M.G. Synthesis and antifungal activity of umbelliferone analogues. Chin. J. Org. Chem. 2010, 30, 254–259. [Google Scholar]
Sample Availability: Samples of the compounds 3a-3f are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).