Abstract
Sorbicillinoids are important hexaketide metabolites derived from fungi. They have a variety of biological activities including cytotoxic, antioxidant, antiviral and antimicrobial activity. The unique structural features of the sorbicillinoids make them attractive candidates for developing new pharmaceutical and agrochemical agents. About 90 sorbicillinoids have been reported in the past few decades. This mini-review aims to briefly summarize their occurrence, structures, and biological activities.
1. Introduction
Sorbicillinoids (also called vertinoids) belong to hexaketide metabolites in which the cyclization has taken place on the carboxylate terminus [1]. They have highly diverse bioactivities and have been isolated from either marine [2,3,4] or terrestrial fungi [5,6,7]. Many of them possess elaborate bicyclic or tricyclic systems that appear to arise from the oxidative dearomatizaton and subsequent dimerization/trimerization of sorbicillin (5). The presence of the C1’–C6’ sorbyl sidechain is another structural feature of these compounds. The term “sorbicillinoid” has come to encompass the family as a whole and generally refers to any compound that contains the carbon skeleton of sorbicillin.
Since first reported in 1948 by Cram et al., sorbicillinoids have been extensively studied [8,9]. In 2011, Harned and Volp reviewed the structures of 62 sorbicillinoids [1]. Since then, many new members of this family were isolated and great progress has been made [4,10,11,12,13]. According to the structural features, sorbicillinoids can be divided into four groups: monomeric sorbicillinoids, bisorbicillinoids, trisorbicillinoids, and hybrid sorbicillinoids. Biosynthesis and chemical synthesis have been extensively studied and reviewed [1,11,14,15,16,17]. In this mini-review, we focus on the occurrence and biological activities of sorbicillinoids, and 28 additional sorbicillinoids were added on the basis of the previous review [1].
2. Occurrence
Sorbicillinoids have a diverse distribution in fungi (Table 1, Table 2, Table 3 and Table 4). Accordingly, their structures are shown in Figure 1, Figure 2, Figure 3 and Figure 4. In total, about 90 sorbicillinoids have been isolated, and they were found mainly in terrestrial fungi, which contained nine genera, namely Acremonium, Aspergillus, Clonostachys, Emericella, Penicillium, Phaeoacremonium, Scytalidium, Trichoderma, and Verticillium, and partly in marine fungi that included five genera (i.e., Paecilomyces, Penicillium, Phialocephala, Trichoderma and Trichothecium). All these fungi belong to ascomycetes.

Table 1.
Occurrence of the monomeric sorbicillinoids (1–30) in fungi.

Table 2.
Occurrence of the bisorbicillinoids (31–60) in fungi.

Table 3.
Occurrence of the trimeric sorbicillinoids (61–65) in fungi.

Table 4.
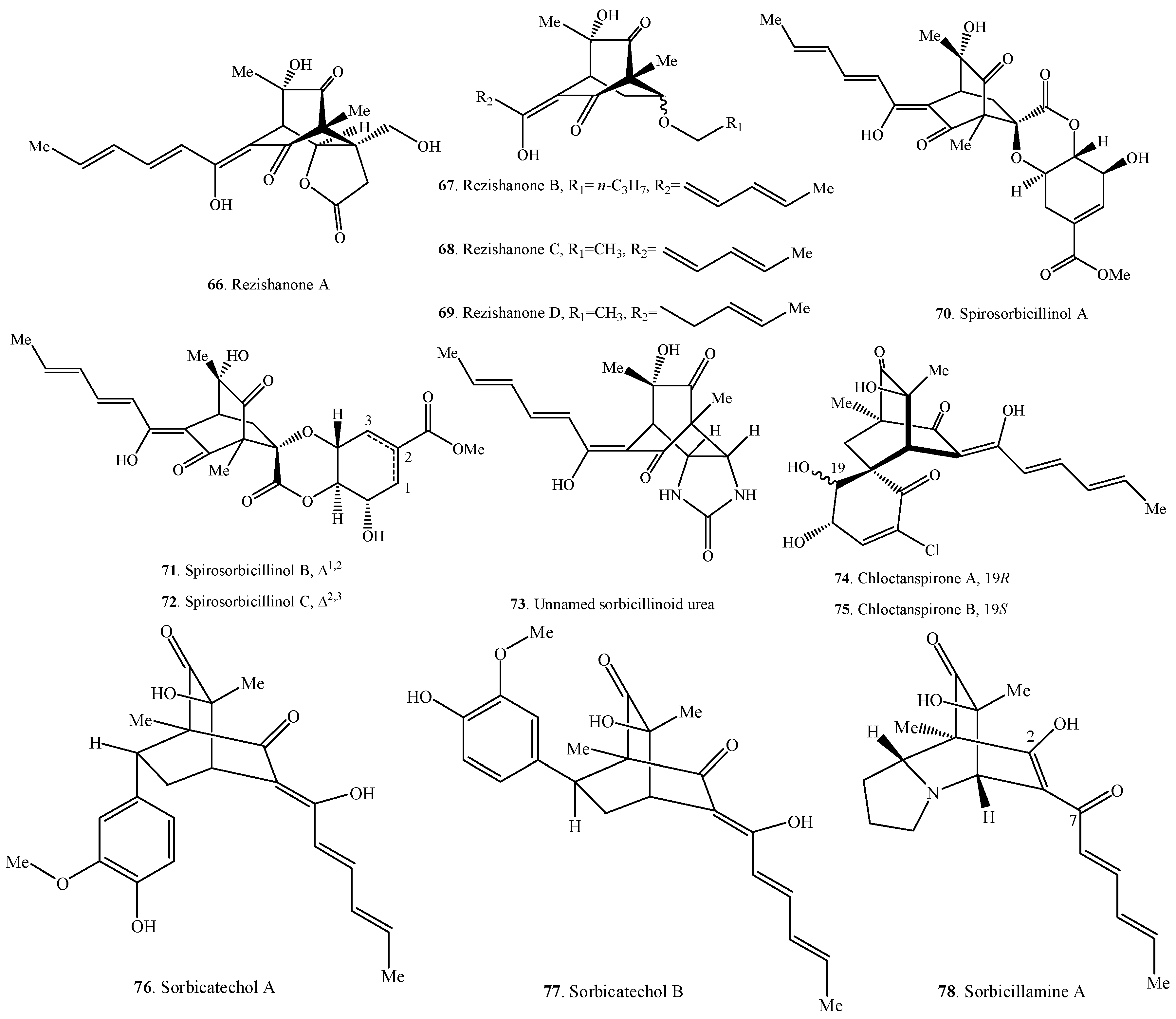
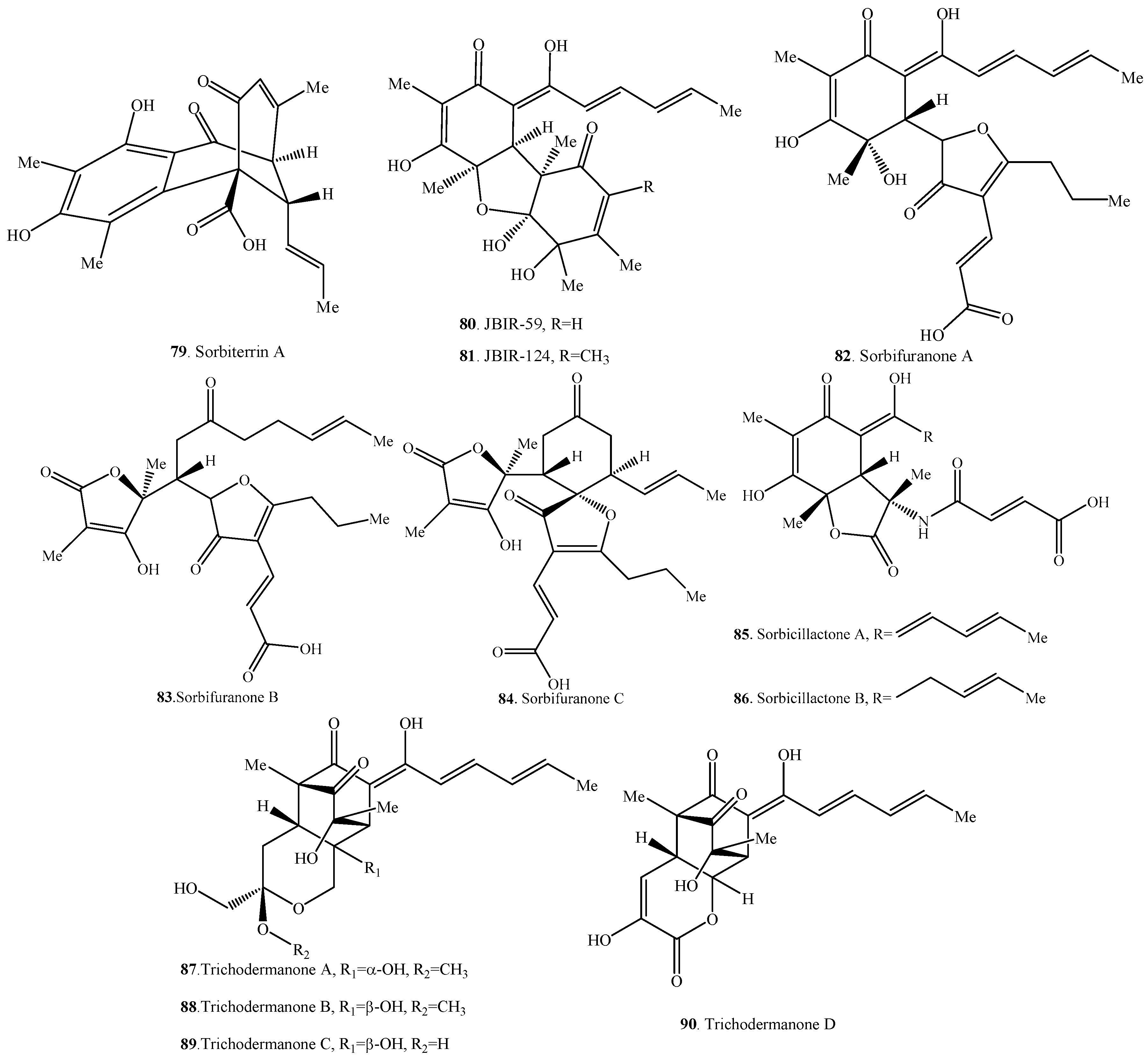
Occurrence of the hybrid sorbicillinoids (66–90) in fungi.
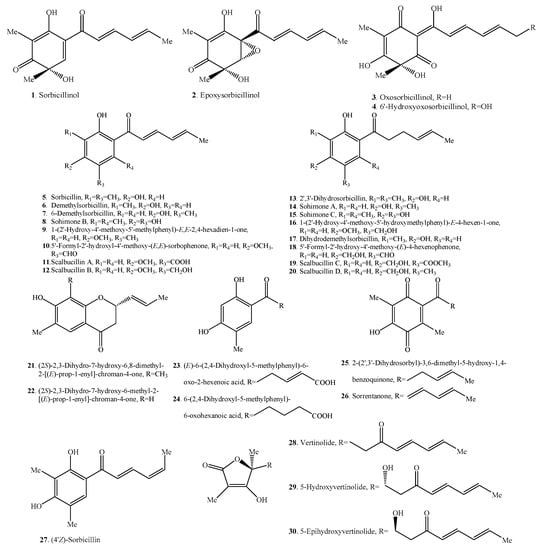
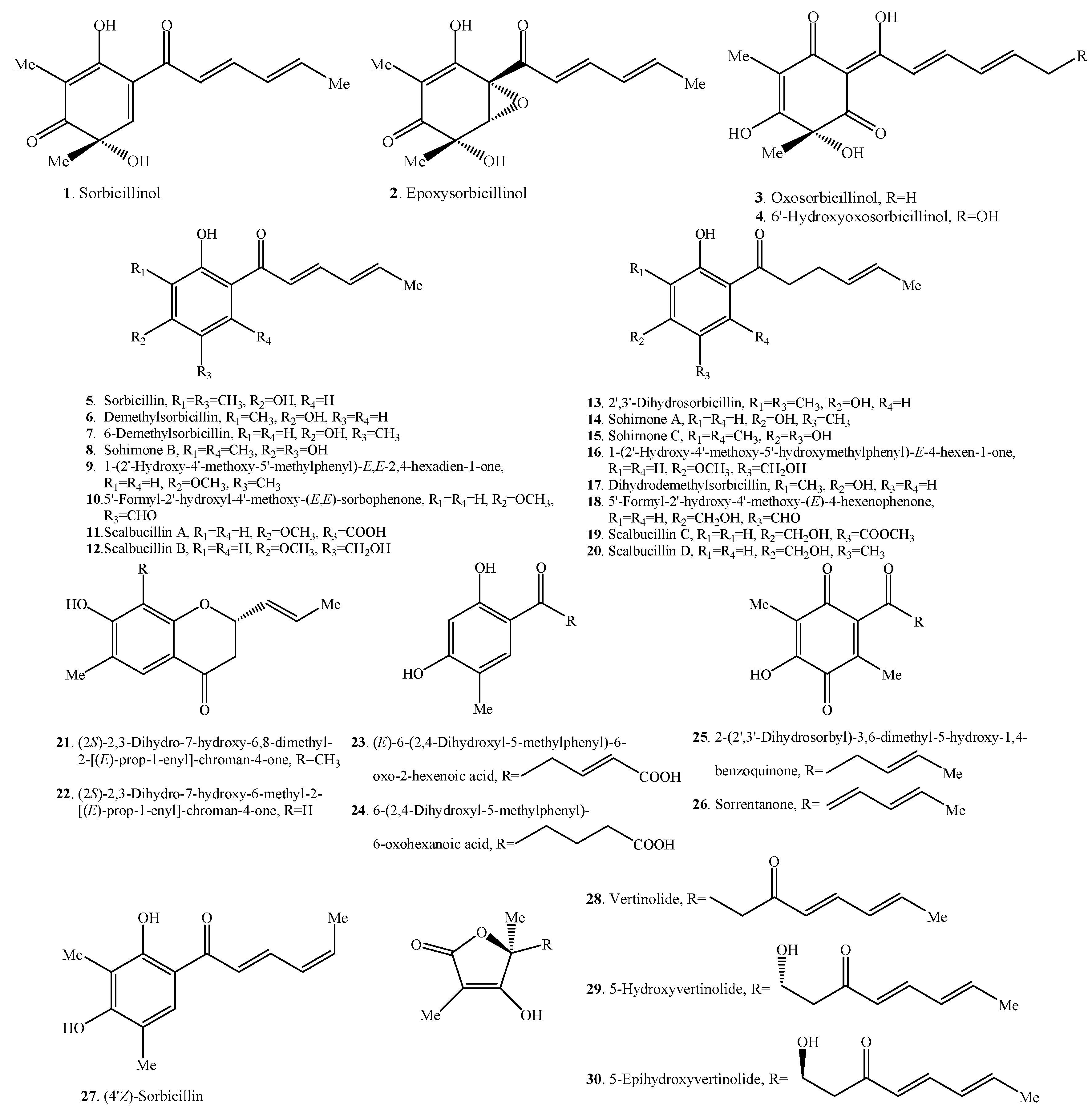
Figure 1.
Structures of the monomeric sorbicillinoids (1–30) isolated from fungi.
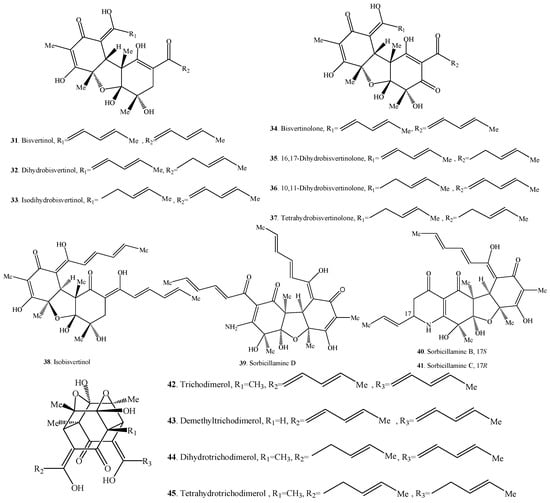
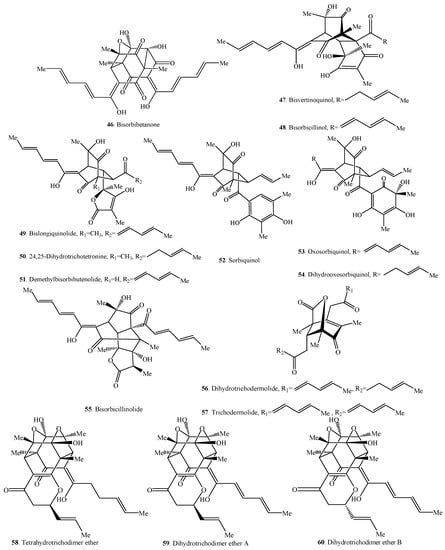
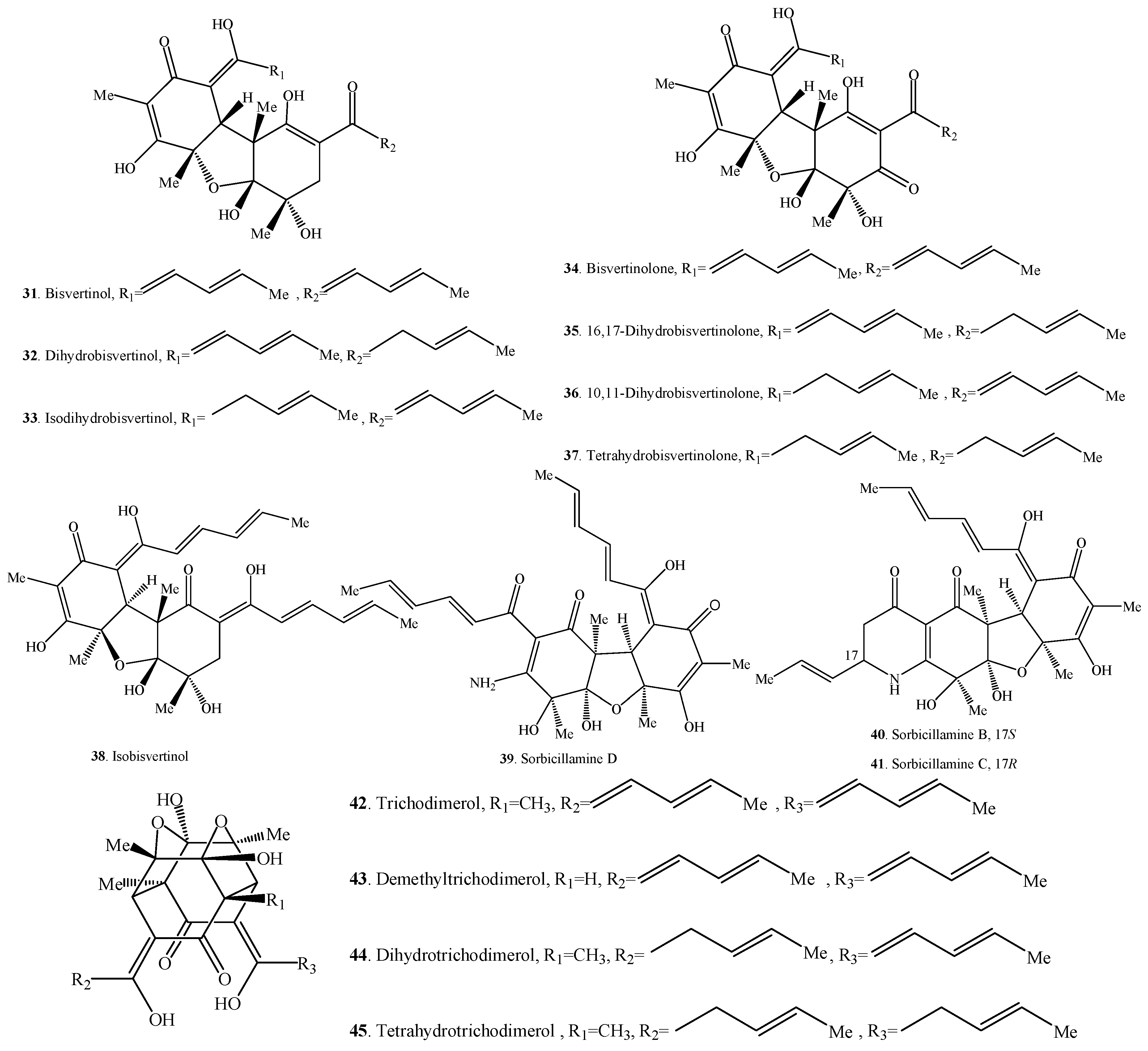
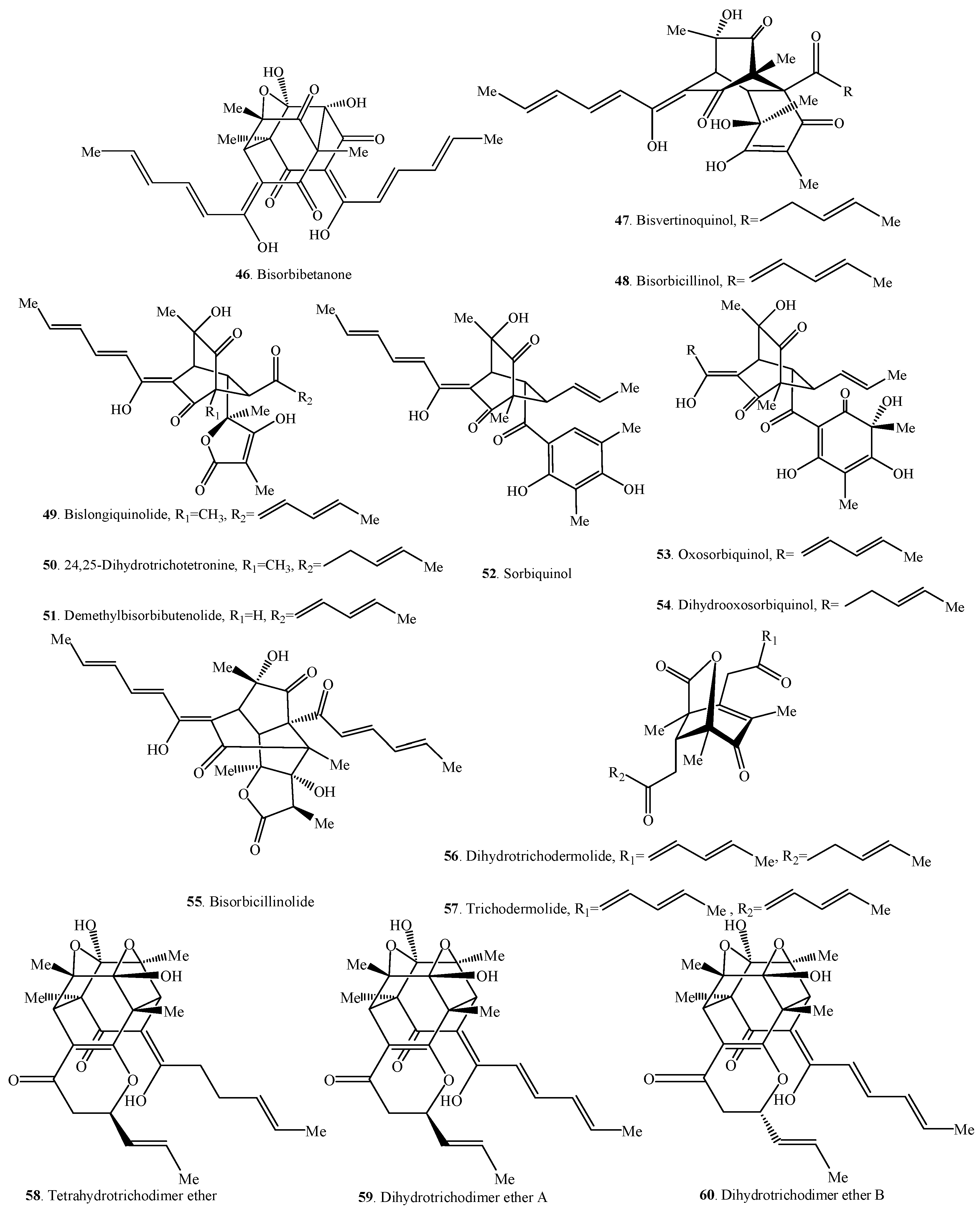
Figure 2.
Structures of the bisorbicillinoids (31–60) isolated from fungi.
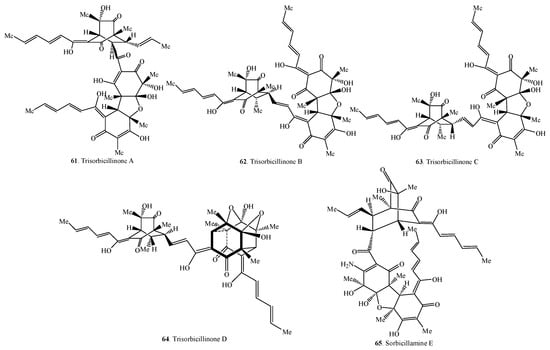
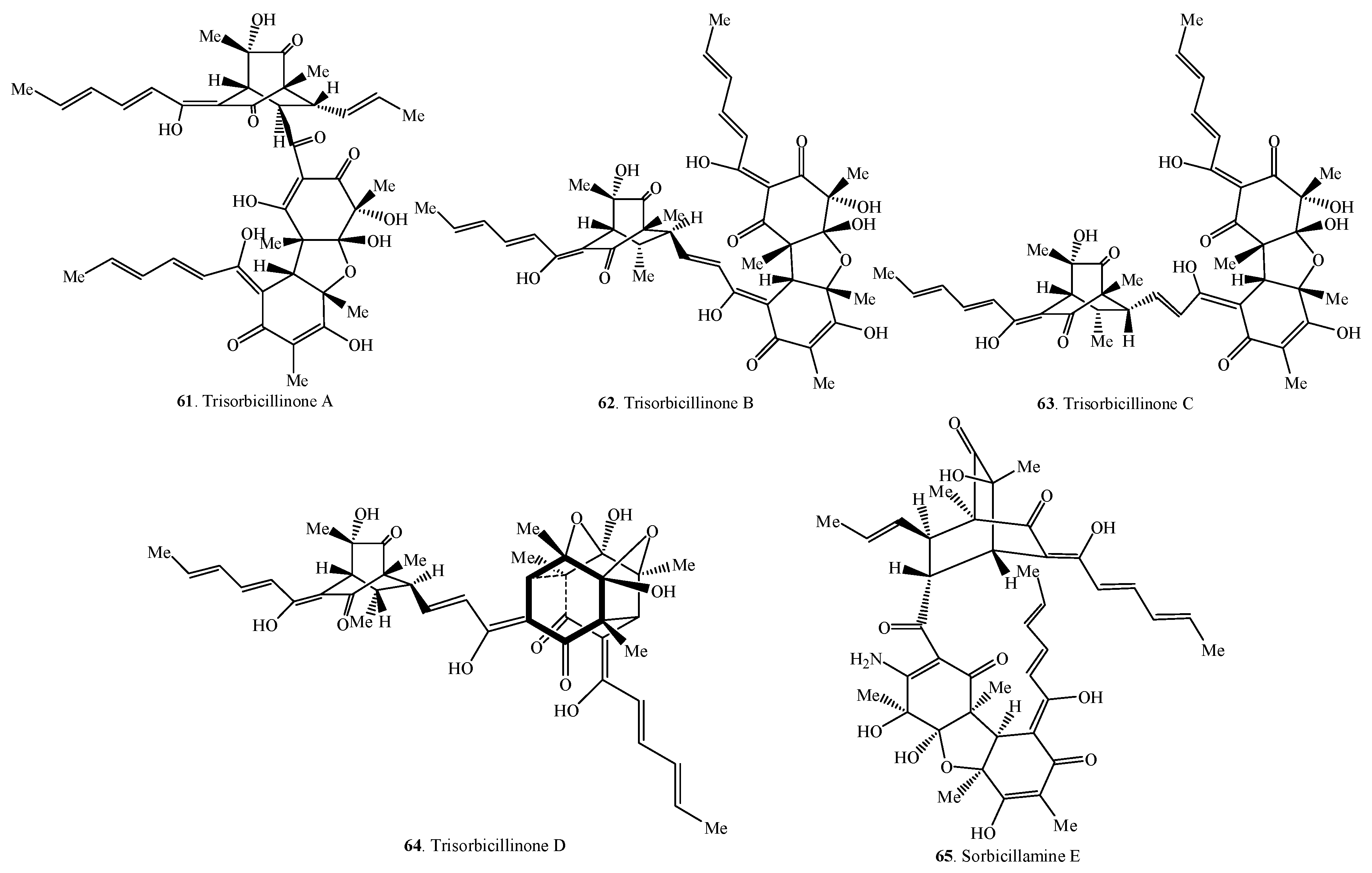
Figure 3.
Structures of the trimeric sorbicillinoids (61–65) isolated from fungi.
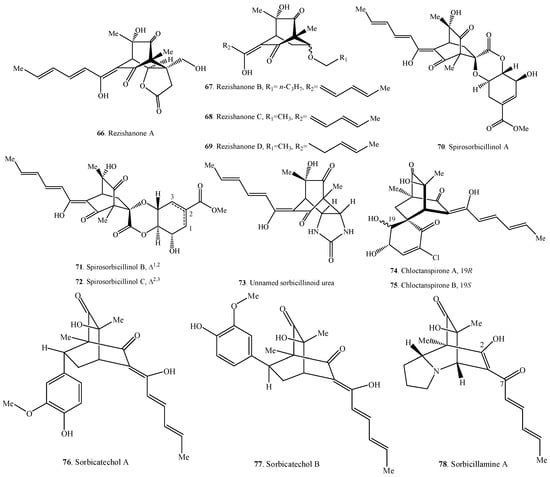
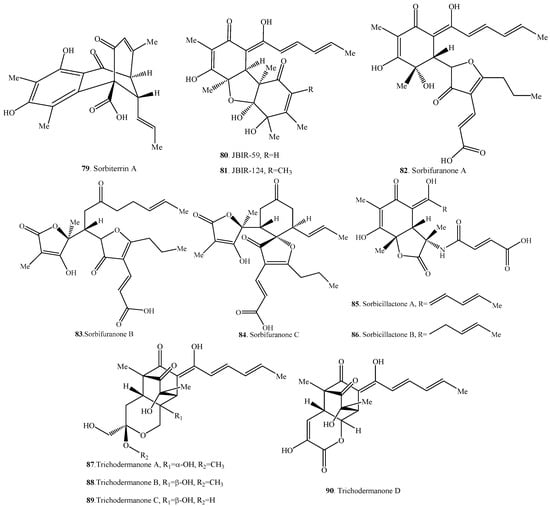
Figure 4.
Structures of the hybrid sorbicillinoids (66–90) isolated from fungi.
2.1. Monomeric Sorbicillinoids
To date, 30 monomeric sorbicillinoids (Table 1 and Figure 1) have been isolated from Clonostachys, Emericella, Penicillium, Phaeoacremonium, Phialocephala, Scytalidium, Trichoderma, Trichothecium and Verticillium species.
Sorbicillinol (1) was found to be highly reactive and it was the biosynthetic precursor of the other sorbicillinoid family members [11].
Sorrentanone (=3-hydroxy-2,5-dimethyl-6-(1′-oxo-2′,4′-dienylhexyl)-1,4-benzoquinone, 26) was the benzoquinone structure of sohirnone B (8), meaning that it was imagined arising from the oxidation of sohirnone B (8) [5,18]. Similarly, 2-(2′,3′-dihydrosorbyl)-3,6-dimethyl-5-hydroxy-1,4-benzoquinone (25) was the benzoquinone of sohirnone C (15) [5,19].
2.2. Bisorbicillinoids
Bisorbicillinoids are also called dimeric sorbicillinoids, which consist of two sorbicillinoid monomers (Table 2), whose structures are shown in Figure 2. Up to now, 30 bisorbicillinoids have been isolated from fungi. These compounds are mainly distributed in the genera Acremonium, Aspergillus, Clonostachys, Penicillium, Phialocephala, Trichoderma, Trichothecium and Verticillium.
2.3. Trisorbicillinoids
Trisorbicillinoids are also called trimeric sorbicillinoids. Up to date, only five trimeric sorbicillinoids have been isolated from marine fungi (i.e., Penicillium sp. F23-2 and Phialocephala sp. FL30r) (Table 3 and Figure 3). Among them, sorbicillamine E (65) was a compound containing N element [10].
2.4. Hybrid Sorbicillinoids
Hybrid sorbicillinoids are proposed to be derived from either a Diels-Alder or a Michael reaction of a monomeric sorbicillinoid diene and a second non-sorbicillinoid dienophile. About 25 hybrid sorbicillinoids have been isolated from fungi so far.
The structure of sorbicillamine A (78) was a tentative assignment for the C-2/C-7 unit, which might exist as either enol or keto tautomers, and they were interconverting on the NMR timescale in solution [10].
Compound 73 from an intertidal marine fungus Paecilomyces marquandii was an unnamed sorbicillinoid urea [57]. Chloctanspirones A (74) and B (75) containing chlorine were isolated from Penicillium terrestre derived from a marine sediment. The differences between them were their absolute configuration at C-19 [58]. Similarly, both sorbicatechols A (76) and B (77) were isolated from the marine sediment-derived fungus Penicillium chrysogenum PJX-17, and their differences were the absolute configuration at C-7 [59].
Unnamed urea (73), sorbicillamine A (78), sorbicillactone A (85), and sorbicillactone B (86) were a class of N-containing compounds [10,21,57]. Interestingly, the N-containing sorbicillinoids including dimeric sorbicillamines D (39), B (40), C (41), and trimeric sorbicillamine E (65) were all isolated from marine fungi (Table 2, Table 3 and Table 4). Except urea 73 from the genus Paecilomyces, others were isolated from the genus Penicillium.
3. Biological Activities
3.1. Cytotoxic Activity
Many sorbicillinoids were screened to have cytotoxic activities, which are summarized in Table 5. (2S)-2,3-Dihydro-7-hydroxy-6,8-dimethyl-2-[(E)-prop-1-enyl]-chroman-4-one (21) and (2S)-2,3-dihydro-7-hydroxy-6-methyl-2-[(E)-prop-1-enyl]-chroman-4-one (22) displayed significant activities against the human breast cancer cell line MCF-7 with IC50 values of 9.51 and 7.82 μg/mL, respectively, and 2′,3′-dihydrosorbicillin (13) showed moderate cytotoxicity against various human cancer cell lines (colon cancer cell line Lovo, hepatic cancer cell line Bel-7402, lung cancer line A549, nasopharyngeal carcinoma cell lines CNE1, CNE2, KB and SUNE1) with IC50 values ranging from 9.19 to 21.93 μg/mL [4].

Table 5.
Cytotoxic activity of the screened sorbicillinoids from fungi.
5′-Formyl-2′-hydroxyl-4′-methoxy-(E,E)-sorbophenone (10) showed cytotoxic activity on OSU-CLL (lymphocytic leukemia) cell lines with IC50 value of 3.1 µM at 48 h, on MDA-MB-435 (melanoma) and SW-620 (colon) cell lines with IC50 values of 1.5 and 0.5 µM at 72 h, respectively. Similarly, 1-(2′-hydroxy-4′-methoxy-5′-methylphenyl)-E,E-2,4-hexadien-1-one (9) on MDA-MB-435 and SW-620 cell lines with IC50 values of 65.2 and 15.1 µM, scalbucillin B (12) on MDA-MB-435 and SW-620 cell lines with IC50 values of 67.9 and 16.0 µM, and 5′-formyl-2′-hydroxy-4′-methoxy-(E)-4-hexenophenone (18) on MDA-MB-435 and SW-620 cell lines with IC50 values of 2.3 and 2.5 µM at 72 h, respectively [12].
(E)-6-(2,4-Dihydroxyl-5-methylphenyl)-6-oxo-2-hexenoic acid (23) and 6-(2,4-dihydroxyl-5-methylphenyl)-6-oxohexanoic acid (24) from a saline lands-derived fungus Trichoderma sp. showed cytotoxic effects on P388 cell line with IC50 values of 72.8 and 44.5 μM, and on HL-60 cell line with IC50 values of 52.5 and 81.2 μM, respectively [6].
Dihydrotrichodermolide (56) and dihydrodemethylsorbicillin (17) displayed cytotoxic effects against P388 cell line (IC50 values of 11.5 and 0.1 μM, respectively) and K562 cell line (IC50 values of of 22.9 and 4.8 μM, respectively) [36].
Chloctansprirone A (74) was active against HL-60 and A549 cell lines with IC50 values of 9.2 and 39.7 μM, respectively. Chloctansprirone B (75) showed relatively weak activity against HL-60 cells with IC50 value of 37.8 μM [58].
By comparing the structure-activity relationships of the compounds, the sorbyl sidechain was very important. Sorbicillinoids with their C2′-C3′ double bond being reduced were less active. For example, sorbicllin (5) showed significant inhibitory activity on HeLa and HepG2 cells with IC50 values of 1.6 and 27.2 μM, respectively. On the contrary, 2′,3′-dihydrosorbicillin (13) with the C2′-C3′ double bond being reduced showed less activity on HeLa and HepG2 cells with IC50 values of 7.4 and 44.4 μM, respectively. The same phenomena were observed for the compounds 6-demethylsorbicillin (7) vs. sohirnone A (14) [27], bisvertinolone (34) vs. 10,11-dihydrobisvertinolone (36) [27], and 5′-formyl-2′-hydroxyl-4′-methoxy-(E,E)-sorbophenone (10) vs. 5′-formyl-2′-hydroxy-4′-methoxy-(E)-4-hexenophenone (18) [12].
3.2. Antimicrobial Activity
Some sorbicillinoids exhibited antimicrobial activities that are shown in Table 6. 5′-Formyl-2′-hydroxyl-4′-methoxy-(E,E)-sorbophenone (10) and 5′-formyl-2′-hydroxy-4′-methoxy-(E)-4-hexenophenone (18) displayed strong antifungal activity on A. niger with MIC values of 0.05 and 0.04 μg/mL (0.20 and 0.16 μM), respectively, much more potent than the positive control (amphotericin B, MIC value of 31 μg/mL). Scalbucillin B (12) showed an MIC value of 0.60 μg/mL (2.42 μM) against Aspergillus niger. Considering the potent antimicrobial activity, a hemolytic assay toward sheep red blood cells in vitro was carried out to assess the toxicity of these compounds (10, 12, 18). They showed a similarly low toxicity on sheep red blood cells, which indicated the promising safety for their potential application as the anti-Aspergillus agents [12].

Table 6.
Antimicrobial activity of the screened sorbicillinoids from fungi.
Dihydrotrichodimerol (44) and tetrahydrotrichodimerol (45) exhibited strong antibacterial activity on Bacillus megaterium with MIC values of 25 and 12.5 μg/mL, respectively. Dihydrotrichodimer ether A (59) and dihydrotrichodimer ether B (60) had strong antibacterial activity on Escherichia coli with MIC values of 25 and 50 μg/mL, respectively. Furthermore, dihydrotrichodimer ether B (60) showed preferable antibacterial activity against Ballus subtilis with MIC value of 50 μg/mL [13].
3.3. Antiviral Activity
Sorbicatechols A (76) and B (77) from the marine-derived fungus Penicillium chrysogenum PJX-17 showed potent antiviral activity against influenza A virus (H1N1) with IC50 values of 85 and 113 μM, respectively (ribavirin as the positive control with IC50 value of 84 μM) [59].
Sorbicillactone A (85) from a sponge-derived fungus Penicillium chrysogenum displayed anti-HIV activity. It protected human T lymphocytes (H9 cells) against the cytopathic effect of HIV-1 in the concentration range of 0.3 and 3.0 μg/mL [21]. This hybrid sorbicillinoid was considered to be a potential inhibitor to VP40 matrix protein of the Ebola virus [63].
3.4. Antioxidant Activity
Active oxygen species cause many diseases such as atherosclerosis, inflammation, ischemia-reperfusion injury, rheumatioid arthritis and central nervous diseases. Furthermore, senility, cancer initiation and progression are also believed to involve active oxygen species [64,65]. Thus, it is expected that the effective antioxidant agents may prevent the onset and development of these diseases. Some sorbicillinoids exhibited obviously antioxidant activity. The DPPH radical scavenging activity of the sorbicillinoids isolated before 2011 was well summarized [1]. After 2011, only one sorbicillinoid JBIR-124 (81) from Penicillium citrinum Sp1080624G1f01 was screened to have DPPH radical scavenging activity with IC50 value of 30 µM [62].
3.5. Other Biological Activities
Other biological activities of the sorbicillinoids are shown in Table 7. Dihydrotrichodimerol (44) and bislongiquinolide (=bisorbibutenolide=trichotetronine, 49) from Trichoderma citrinoviridev influenced aphid feeding preferences [48]. Isobisvertinol (38) from Aspergillus sp. FKI-1746 inhibited lipid droplet accumulation in macrophages [40].

Table 7.
Other biological activities of the screened sorbicillinoids from fungi.
In addition, dihydrotrichodimerol (44) from an unidentified fungus activated peroxisome proliferator-activated receptor γ (PPAR γ) with an ED50 value of 80 ng/mL [50]. Bisvertinolone (34) from Verticillium intertextum inhibited the biosynthesis of β-l,6-glucan [42].
Trichodimerol (=MS-182123, 42) from Penicillium chrysogenum strain V39673 inhibited the production of tumor necrosis factor-α (TNF-α) by macrophages (IC50 value of 200 ng/mL) and monocytes (IC50 value of 200 ng/mL) [46]. Subsequently, trichodimerol was screened to show an inhibitory effect on lipopolysaccharide-induced eicosanoid secretion in THP-1 human monocytic cells [66].
6′-Hydroxyoxosorbicillinol (4) showed inhibition on soybean lipoxygenase activity with an IC50 value of 16 µM, about 10 folds higher than oxosorbicillinol (3). 6′-Hydroxyoxosorbicillinol (4) also exhibited prostaglandin D2 and leukotriene B4 release suppression activity with IC50 values of 10 and 100 µM, respectively [22].
Sorbiterrin A (79) showed moderate acetylcholinesterase (AChE) inhibitory effect with IC50 value of 25 µg/mL [61].
4. Conclusions
About 90 sorbicillinoids have been isolated from terrestrial and marine ascomycetous fungi in the past few decades. Some of them exhibited promising bioactivities, especially cytotoxic, antioxidant, antimicrobial, and antiviral activities. In recent years, more and more new members of sorbicillinoids have been isolated. All these sorbicillinoids could be the rich resources of biologically active substances with significant medicinal and agricultural potential.
The biosynthesis studies of sorbicillinoids have been carried out [11,14,15,16,17] and well summarized [1]. Sorbicillinol (1) has been hypothesized as a precursor of most sorbicillinoids that were biosynthesized by polyketide synthases (PKs) [14]. In addition, the PKS gene cluster containing SorbA, SorbB and SorbC has been characterized for sorbicillin (5) biosynthesis, and sorbicillinol (1) was proved as a key intermediate [11]. The extensive 13C enrichment studies carried out by Abe and co-workers have unequivocally demonstrated that many of biosynthetic hypotheses of sorbicillinoids are correct [14,15,16,17]. There are still some uncertainties. Furthermore, the specific polyketide synthases in the biosynthetic pathway of sorbicillinoids in fungi have not been characterized. Chemical syntheses of sorbicillinoids have attracted pharmaceutical chemists as they have potential applications in the agriculture, pharmaceutical and food industries. Some sorbicillinoids such as sorbicillin (5), vertinolide (28), epoxysorbicillinol (2), and trichodimerol (=MS-182123, 42) have been synthesized successfully, and well summarized [1].
In most cases, biological activities, structure-activity relations, and mode of action of sorbicillinoids have been investigated based on in vitro studies or animal models. Few studies have been performed at the level of clinical trials in patients. Future studies should be emphasized on the improvement in methodological quality and warrant further clinical research on the effects of these compounds. The applications of sorbicillinoids as antitumor agents, antimicrobials, antivirus agents and antioxidants, as well as their underlying bioactivities, have led to considerable interest within the pharmaceutical community and health-care industry. With a good understanding of the biosynthetic pathways of some sorbicillinoids, we can not only increase outputs of the bioactive sorbicillinoids but also block biosynthesis of some harmful sorbicillinoids by specific interferences.
Acknowledgments
This work was co-financed by the grants from the National Natural Science Foundation of China (31271996 and 31471729), and the Hi-Tech R&D Program of China (2011AA10A202).
Author Contributions
Jiajia Meng performed bibliographic research, drafted and corrected the manuscript. Xiaoxiang Fu, Xiaohan Wang, Dan Xu and Xuping Zhang retrieved literature, participated in the discussions and supported manuscript corrections. Daowan Lai reviewed the manuscript and helped to revise it. Ligang Zhou and Guozhen Zhang conceived the idea, designed the review structure, supervised manuscript drafting, and revised the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harned, A.M.; Volp, K.A. The sorbicillinoid family of natural products: Isolation, biosynthesis and synthetic studies. Nat. Prod. Rep. 2011, 28, 1790–1810. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, F.; Cai, S.; Zeng, X.; Xiao, X.; Gu, Q.; Zhu, W. Two new bisorbicillinoids isolated from a deep-sea fungus, Phialocephala sp. FL30r. J. Antibiot. 2007, 60, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateff, A.; Fisch, K.; Wright, A.D. Trichopyrone and other constituents from the marine sponge-derived fungus Trichoderma sp. Z. Naturforsch. 2009, 64c, 186–192. [Google Scholar] [CrossRef]
- Lan, W.-J.; Zhao, Y.; Xie, Z.-L.; Liang, L.-Z.; Shao, W.-Y.; Zhu, L.-P.; Yang, D.-P.; Zhu, X.-F.; Li, H.-J. Novel sorbicillin analogues from the marine fungus Trichoderma sp. associated with the seastar Acanthaster planci. Nat. Prod. Commun. 2012, 7, 1337–1340. [Google Scholar] [PubMed]
- Maskey, R.P.; Grün-Wollny, I.; Grün-Wollny, H. Sorbicillin analogues and related dimeric compounds from Penicillium notatum. J. Nat. Prod. 2005, 68, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, W.; Huang, Y.; Rong, X. Two acid sorbicillin analogues from saline lands-derived fungus Trichoderma sp. J. Antibiot. 2011, 64, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.-M.; Zhan, Z.-J.; Ding, Z.-S.; Shan, W.-G. Bioactive metabolites from Penicillium sp. P-1, a fungal endophyte in Huperzia serrata. Chem. Nat. Compd. 2011, 47, 541–544. [Google Scholar] [CrossRef]
- Cram, D.J.; Tishler, M. Mold metabolites. I. Isolation of several compounds from clinical penicillin. J. Am. Chem. Soc. 1948, 70, 4238–4239. [Google Scholar] [CrossRef] [PubMed]
- Cram, D.J. Mold metabolites. II. The structure of sorbicillin, a pigment produced by the mold Penicillium notatum. J. Am. Chem. Soc. 1948, 70, 4240–4243. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Peng, J.; Zhu, T.; Gu, Q.; Keyzers, R.A.; Li, D. Sorbicillamines A−E, nitrogen-containing sorbicillinoids from the deep-sea-derived fungus Penicillium sp. F23-2. J. Nat. Prod. 2013, 76, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Fahad, A.; Abood, A.; Fisch, K.M.; Osipow, A.; Davison, J.; Avramovic, M.; Butts, C.P.; Piel, J.; Simpson, T.J.; Cox, R.J. Oxidative dearomatisation: The key step of sorbicillinoid biosynthesis. Chem. Sci. 2014, 5, 523–527. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; Raja, H.A.; Figueroa, M.; Swanson, S.M.; Falkinham, J.O., III; Lucas, D.M.; Grever, M.R.; Wani, M.C.; Pearce, C.J.; Oberlies, N.H. Sorbicillinoid analogs with cytotoxic and selective anti-Aspergillus activities from Scytalidium album. J. Antibiot. 2015, 68, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.-M.; Qi, F.-M.; Li, J.; Jiang, C.-X.; Hou, Y.; Shi, Y.-P.; Di, D.-L.; Zhang, J.-W.; Wu, Q.-X. Isolation of secondary metabolites from the soil-derived fungus Clonostachys rosea YRS-06, a biological control agent, and evaluation of antibacterial activity. J. Agric. Food Chem. 2016, 64, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Sugimoto, O.; Tanji, K.; Hirota, A. Identification of the quinol metabolite “Sorbicillinol”, a key intermediate postulated in bisorbicillinoid biosynthesis. J. Am. Chem. Soc. 2000, 122, 12606–12607. [Google Scholar] [CrossRef]
- Abe, N.; Yamamoto, K.; Arakawa, T.; Hirota, A. The biosynthesis of bisorbicillinoids: Evidence for a biosynthetic route from bisorbibutenolide and bisorbicillinolide. Chem. Commun. 2001, 2001, 23–24. [Google Scholar] [CrossRef]
- Abe, N.; Arakawa, T.; Yamamoto, K.; Hirota, A. Biosynthesis of bisorbicillinoid in Trichoderma sp. USF-2690; evidence for the biosynthetic pathway, via sorbicillinol, of sorbicillin, bisorbicillinol, bisorbibutenolide, and bisorbicillinolide. Bisosci. Biotechnol. Biochem. 2002, 66, 2090–2099. [Google Scholar] [CrossRef]
- Sugaya, K.; Koshino, H.; Hongo, Y.; Yasunaga, K.; Onose, J.; Yoshikawa, K.; Abe, N. The biosynthesis of sorbicillinoids in Trichoderma sp. USF-2690: Prospect for the existence of a common precursor to sorbicillinol and 5-epihydroxyvertinolide, a new sorbicillinoid member. Tetrahedron Lett. 2008, 49, 654–657. [Google Scholar] [CrossRef]
- Miller, R.F.; Huang, S. Isolation and structure of sorrentanone: A new tetrasubstituted quinone from Penicillium chrysogenum. J. Antibiot. 1995, 48, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, Q.; Zhu, W.; Cui, C.; Fan, G. Two new benzoquinone derivatives and two new bisorbicillinoids were isolated from a marine-derived fungus Penicillium terrestre. J. Antibiot. 2005, 58, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sperry, S.; Samuels, G.J.; Crews, P. Vertinoid polyketides from the saltwater culture of the fungus Trichoderma longibrachiatum separated from a Haliclona marine sponge. J. Org. Chem. 1998, 63, 10011–10014. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Gulder, T.A.M.; Tsuruta, H.; Mühlbacher, J.; Maksimenka, K.; Steffens, S.; Schaumann, K.; Stohr, R.; Wiese, J.; et al. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron 2005, 61, 7252–7265. [Google Scholar] [CrossRef]
- Komoda, T.; Nishikawa, M. 6′-Hydroxyoxosorbicillinol, a new lipoxygenase inhibitor and PGD2/LTB4 release suppressor from Penicillium sp. Biosci. Biotechnol. Biochem. 2012, 76, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Yamamoto, K.; Hirota, A. Novel fungal metabolites, demethylsorbicillin and oxosorbicillinol, isolated from Trichoderma sp. USF-2690. Biosci. Biotechnol. Biochem. 2000, 64, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Itabashi, T.; Wakana, D.; Takeda, H.; Yaguchi, T.; Kawai, K.; Hosoe, T. Isolation and structure elucidation of new phthalide and phthalane derivatives, isolated as antimicrobial agents form Emericella sp. IFM57991. J. Antibiot. 2016, 69, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Nakamura, H.; Komagata, K. Studies on variation of penicillin producing mold. Part II. Biochemical genetical studies on the yellow pigments losing mutation of chrysogenum Q 176 to pigmentless sultant Pen. chrysogenum Q 176. J. Agric. Chem. Soc. Jpn. 1953, 27, 345–348. [Google Scholar]
- Andrade, R.; Ayer, W.A.; Mebe, P.P. The metabolites of Trichoderma longibrachiatum. Part 1. Isolation of the metabolites and the structure of trichodimerol. Can. J. Chem. 1992, 70, 2526–2535. [Google Scholar] [CrossRef]
- Du, L.; Zhu, T.; Li, L.Y.; Cai, S.; Zhao, B.; Gu, Q. Cytotoxic sorbicillinoids and bisorbicillinoids from a marine-derived fungus Trichoderma sp. Chem. Pharm. Bull. 2009, 57, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Zhao, L.X.; Chen, Y.W.; Huang, R.; Miao, C.P.; Wang, J. Sesquiterpenoids from the endophytic fungus Trichoderma sp. PR-35 of Paeonia delavayi. Chem. Biodivers. 2011, 8, 1717–1723. [Google Scholar]
- Abe, N.; Murata, T.; Hirota, A. Novel oxidized sorbicillin dimers with 1,1-diphenyl-2-picrylhydrazyl-radial scavenging activity from a fungus. Biosci. Biotechnol. Biochem. 1998, 62, 2120–2126. [Google Scholar] [CrossRef]
- Yao, Y.; Li, J.; Jiang, C.-S.; Zhao, X.-X.; Miao, Z.-H.; Liu, H.-T.; Zheng, P.; Yao, W.-X.; Li, W.-Q. Trichodimerol and sorbicillin induced apoptosis of HL-60 cells is mediated by reactive oxygen species. Pharmazie 2015, 70, 394–398. [Google Scholar] [PubMed]
- Trifonov, L.S.; Dreiding, A.S.; Hoesch, L.; Rast, D.M. Isolation of four hexaketides from Verticillium intertextum. Helv. Chim. Acta 1981, 64, 1843–1846. [Google Scholar] [CrossRef]
- Trifonov, L.S.; Bieri, J.H.; Prewo, R.; Dreiding, A.S. Isolation and structure elucidation of three metabolites from Verticillium intertextum: Sorbicillin, dihydrosorbicillin and bisvertinoquinol. Tetrahedron 1983, 39, 4243–4256. [Google Scholar] [CrossRef]
- Reátegui, R.F.; Wicklow, D.T.; Gloer, J.B. Phaeofurans and sorbicillin analogues from a fungicolous Phaeoacremonium species (NRRL 32148). J. Nat. Prod. 2006, 69, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Geigert, J.; Stermitz, F.R.; Schroeder, H.A. Two new natural substituted hexenophenones from the fungus Scytalidium. Tetrahedron 1973, 29, 2343–2345. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Bruhn, T.; Schäffler, K.; Steffens, S.; Schmaljohann, R.; Wiese, J.; Imhoff, J.F. Sorbifuranones A-C, sorbicillinoid metabolites from Penicillium strains isolated from Mediterranean sponges. Tetrahedron 2010, 66, 9894–9901. [Google Scholar] [CrossRef]
- Li, D.; Cai, S.; Zhu, T.; Wang, F.; Xiao, X.; Gu, Q. New cytotoxic metabolites from a deep-sea-derived fungus, Phialocephala sp., strain FL30r. Chem. Biodivers. 2011, 8, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.; Abdel-Lateff, A.; Wright, A.D.; Kehraus, S.; Krick, A.; König, G.M. Novel sorbicillin derivatives with an unprecedented carbon skeleton from the sponge-derived fungus Trichoderma species. Eur. J. Org. Chem. 2007, 2007, 2268–2275. [Google Scholar] [CrossRef]
- Trifonov, L.S.; Bieri, J.H.; Prewo, R.; Dreiding, A.S.; Rast, D.M.; Hoesch, L. The constitution of vertinolide, a new derivative of tetronic acid, produced by Verticillium intertextum. Tetrahedron 1982, 38, 397–403. [Google Scholar] [CrossRef]
- Andrade, R.; Ayer, W.A.; Trifonov, L.S. The metabolites of Trichoderma longibrachiatum III. Two new tetronic acids: 5-hydroxyvertinolide and bislongiquinolide. Aust. J. Chem. 1997, 50, 255–257. [Google Scholar] [CrossRef]
- Koyama, N.; Ohshiro, T.; Tomoda, H.; Ōmura, S. Fungal isobisvertinol, a new inhibitor of lipid droplet accumulation in mouse macrophages. Org. Lett. 2007, 9, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, L.S.; Hilpert, H.; Floersheim, P.; Dreiding, A.S.; Rast, D.M.; Skrivanova, R.; Hoesch, L. Bisvertinols: A new group of dimeric vertinoids from Verticillium intertextum. Tetrahedron 1986, 42, 3157–3179. [Google Scholar] [CrossRef]
- Kontani, M.; Sakagami, Y.; Marumo, S. Frist β-1,6-giucan biosynthesis inhibitor, bisvertinolone isolated from fungus, Acremonium stricturn and its absolute stereochemistry. Tetrahedron Lett. 1994, 35, 2577–2580. [Google Scholar] [CrossRef]
- Ueda, J.; Hashimoto, J.; Inaba, S.; Takagi, M.; Shin-ya, K. JBIR-59, a new sorbicillinoid, from a marine-derived fungus Penicillium citrinum SpI080624G1f01. J. Antibiot. 2010, 63, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Murata, T.; Hirota, A. Novel DPPH radical scavengers, bisorbicillinol and demethyltrichodimerol, from a fungus. Biosci. Biotechnol. Biochem. 1998, 62, 661–666. [Google Scholar] [CrossRef]
- Gao, Q.; Leet, J.E.; Thomas, S.T.; Matson, J.A. Crystal structure of trichodimerol. J. Nat. Prod. 1995, 58, 1817–1821. [Google Scholar] [CrossRef]
- Warr, G.A.; Veitch, J.A.; Walsh, A.W.; Hesler, G.A.; Pirnik, D.M.; Leet, J.E.; Lin, P.-F.M.; Medina, I.A.; McBrien, K.D.; Forenza, S.; et al. BMS-182123, a fungal metabolite that inhibits the production of TNF-α by macrophage and monocytes. J. Antibiot. 1996, 49, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, Q.; Zhu, W.; Cui, C.; Fan, G. Dihydrotrichodimerol and tetrahydrotrichodimerol, two new bisorbicillinoids, from a marine-derived Penicillium terrestre. J. Antibiot. 2005, 58, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Cimmino, A.; Ganassi, S.; Altomare, C.; Favilla, M.; Cristofaro, A.D.; Vitagliano, S.; Sabatini, M.A. Bisorbicillinoids produced by the fungus Trichoderma citrinoviride affect feeding preference of the aphid Schizaphis graminum. J. Chem. Ecol. 2009, 35, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Shirota, O.; Pathak, V.; Hossain, C.F.; Sekita, S.; Takatori, K.; Satake, M. Structural elucidation of trichotetronines: Polyketides possessing a bicycle [2.2.2] octane skeleton with a tetronic acid moiety isolated from Trichoderma sp. J. Chem. Soc. Perk. Trans. 1 1997, 1997, 2961–2964. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.H.; Cai, X.F.; Shin, J.C.; Lee, K.; Hong, Y.-S.; Lee, J.J. Fungal metabolites, sorbicillinoid polyketides and their effects on the activation of peroxisome proliferator-activated receptor γ. J. Antibiot. 2005, 58, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Balde, E.S.; Andolfi, A.; Bruyère, C.; Cimmino, A.; Lamoral-Theys, D.; Vurro, M.; Damme, M.V.; Altomare, C.; Mathieu, V.; Kiss, R.; et al. Investigations of fungal secondary metabolites with potential anticancer activity. J. Nat. Prod. 2010, 73, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Murata, T.; Yamamoto, K.; Hirota, A. Bisorbibetanone, a novel oxidized sorbicillin dimer, with 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity from a fungus. Tetrahedron Lett. 1999, 40, 5203–5206. [Google Scholar] [CrossRef]
- Washida, K.; Abe, N.; Sugiyama, Y.; Hirota, A. Novel DPPH radical scavengers, demethylbisorbibutenolide and trichopyrone, from a fungus. Biosci. Biotech. Biochem. 2007, 71, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.; Ayer, W.A.; Trifonov, L.S. The metabolites of Trichoderma longibrachiatum Part II. The structures of trichodermolide and sorbiquinol. Can. J. Chem. 1996, 74, 371–379. [Google Scholar] [CrossRef]
- Li, D.; Wang, F.; Xiao, X.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. Trisorbicillinone A, a novel sorbicillin trimer, from a deep sea fungus, Phialocephala sp. FL30r. Tetrahedron Lett. 2007, 48, 5235–5238. [Google Scholar] [CrossRef]
- Li, D.; Cai, S.; Zhu, T.; Wang, F.; Xiao, X.; Gu, Q. Three new sorbicillin trimers, trisorbicillinones B, C, and D, from a deep ocean sediment derived fungus, Phialocephala sp. FL30r. Tetrahedron 2010, 66, 5101–5106. [Google Scholar] [CrossRef]
- Cabrera, G.M.; Butler, M.; Rodriguez, A.; Godeas, A.; Haddad, R.; Eberlin, M.N. A sorbicillinoid urea from an intertidal Paecilomyces marquandii. J. Nat. Prod. 2006, 69, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, L.; Zhu, T.; Kurtán, T.; Mándi, A.; Zhao, Z.; Li, J.; Gu, Q. Chloctanspirones A and B, novel chlorinated polyketides with an unprecedented skeleton, from marine sediment derived fungus Penicillium terrestre. Tetrahedron 2011, 67, 7913–7918. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, X.; Du, L.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Sorbicatechols A and B, antiviral sorbicillinoids from the marine- derived fungus Penicillium chrysogenum PJX-17. J. Nat. Prod. 2014, 77, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Washida, K.; Abe, N.; Sugiyama, Y.; Hirta, A. Novel secondary metabolites, spirosorbicillinols A, B, and C, from a fungus. Biosci. Biotech. Biochem. 2009, 73, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, T.; Ding, Y.; Khan, I.A.; Gu, Q.; Li, D. Sorbiterrin A, a novel sorbicillin derivative with cholinesterase inhibition activity from the marine-derived fungus Penicillium terrestre. Tetrahedron Lett. 2012, 53, 325–328. [Google Scholar] [CrossRef]
- Kawahara, T.; Takagi, M.; Shin-ya, K. JBIR-124: A novel antioxidative agent from a marine sponge-derived fungus Penicillium citrinum SpI080624G1f01. J. Antibiot. 2012, 65, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; Acharya, A.B.; Subramaniyan, S.; Babu, S.; Kulkarni, S.; Narayanappa, R. Secondary metabolites extracted from marine sponge associated Comamonas testosterone and Citrobacter freundii as potential antimicrobials against MDR pathogens and hypothetical leads for VP40 matrix protein of Ebola virus: An in vitro and in silico investigation. J. Biomol. Struct. Dyn. 2016, 34. [Google Scholar]
- Finkel, T. Radical medicine: Treating ageing to cure disease. Nat. Rev. Mol. Cell Biol. 2005, 6, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Hirota, A. Chemical studies of the radical scavenging mechanism of bisorbicillinol using the 1,1-diphenyl-2-picrylhydrzyl radical. Chem. Commun. 2002, 2002, 662–663. [Google Scholar] [CrossRef]
- Mazzucco, C.E.; Warr, G. Trichodimerol (BMS-182123) inhibits lipopolysaccharide-induced eicosanoid secretion in THP-1 human monocytic cells. J. Leukocyte Biol. 1996, 60, 271–277. [Google Scholar] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).