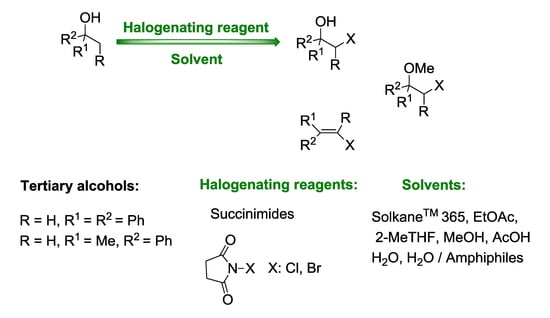
Transformation of Tertiary Benzyl Alcohols into the Vicinal Halo-Substituted Derivatives Using N-Halosuccinimides
Abstract
:1. Introduction
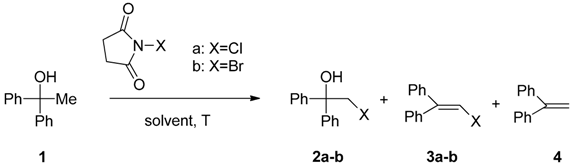
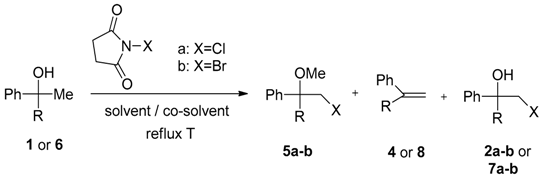
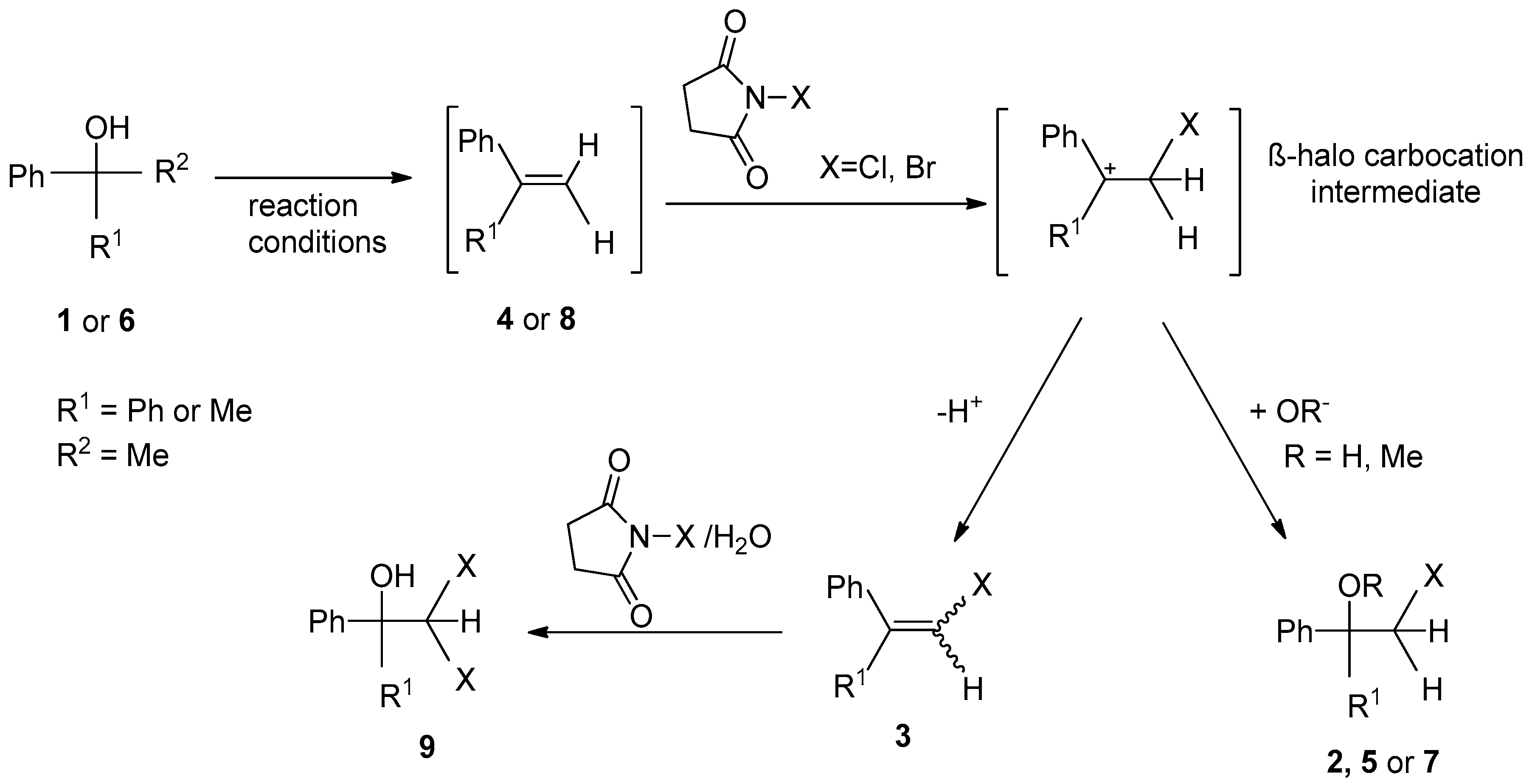
2. Results and Discussion
3. Materials and Methods
General Procedure for Vicinal Halogenation of Tertiary Benzyl Alcohols Using NXS on mmol Scale
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anastas, P.T. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Federsel, H.-J. En route to full implementation: Driving the green chemistry agenda in the pharmaceutical industry. Green Chem. 2013, 15, 3105–3115. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbuhler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Pollet, P.; Davey, E.A.; Urena-Benavides, E.E.; Eckert, C.A.; Liotta, C.L. Solvents for sustainable chemical processes. Green Chem. 2014, 16, 1034–1055. [Google Scholar] [CrossRef]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green Solvents in Carbohydrate Chemistry: From Raw Materials to Fine Chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Li, C.-J.; Chen, L. Organic chemistry in water. Chem. Soc. Rev. 2006, 35, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Grieco, P.A. Organic Synthesis in Water; Blackie Academic & Professional: London, UK, 1998. [Google Scholar]
- Lipshutz, B.H.; Ghorai, S. Transitioning organic synthesis from organic solvents to water. What’s your E Factor? Green Chem. 2014, 16, 3660–3679. [Google Scholar] [CrossRef] [PubMed]
- La Sorella, G.; Strukul, G.; Scarso, A. Recent advances in catalysis in micellar media. Green Chem. 2015, 17, 644–683. [Google Scholar] [CrossRef]
- Stavber, G. The Road to Greener Applied Organic Synthesis: Performing Organic Reactions in Micelle-Based and Host-Guest Aqueous Nanoreactors. Aust. J. Chem. 2010, 63, 849–849. [Google Scholar] [CrossRef]
- Agatsuma, T.; Ogawa, H.; Akasaka, K.; Asai, A.; Yamashita, Y.; Mizukami, T.; Akinaga, S.; Saitoh, Y. Halohydrin and Oxime Derivatives of Radicicol: Synthesis and Antitumor Activities. Bioorgan. Med. Chem. 2002, 10, 3445–3454. [Google Scholar] [CrossRef]
- Ros, A.; Magriz, A.; Dietrich, H.; Fernández, R.; Alvarez, E.; Lassaletta, J.M. Enantioselective Synthesis of Vicinal Halohydrins via Dynamic Kinetic Resolution. Org. Lett. 2006, 8, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Thorat, P.B.; Goswami, S.V.; Sondankar, V.P.; Bhusare, S.R. Stereoselective synthesis of vic-halohydrins and an unusual Knoevenagel product from an organocatalyzed aldol reaction: A non-enamine mode. Chin. J. Catal. 2015, 36, 1093–1100. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemisty: Reactions, Mechanisms, and Structure, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Bonini, C.; Righi, G. Regio- and Chemoselective Synthesis of Halohydrins by Cleavage of Oxiranes with Metal-Halides. Synthesis 1994, 1994, 225–238. [Google Scholar] [CrossRef]
- Haufe, G. Regio- and stereoselective synthesis of vicinal fluorohydrins. J. Fluorine Chem. 2004, 125, 875–894. [Google Scholar] [CrossRef]
- Narender, M.; Reddy, M.S.; Nageswar, Y.D.; Rao, K.R. Aqueous phase synthesis of vic-halohydrins from olefins and N-halosuccinimides in the presence of beta-cyclodextrin. J. Mol. Catal. A Chem. 2006, 258, 10–14. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Baishya, G.; Harshavardhan, S.J.; Janardhana Chary, C.; Gupta, M.K. Green approach for the conversion of olefins into vic-halohydrins using N-halosuccinimides in ionic liquids. Tetrahedron Lett. 2005, 46, 3569–3572. [Google Scholar] [CrossRef]
- Bentley, P.A.; Mei, Y.; Du, J. Thiourea catalysis of NCS in the synthesis of chlorohydrins. Tetrahedron Lett. 2008, 49, 1425–1427. [Google Scholar] [CrossRef]
- Das, B.; Venkateswarlu, K.; Damodar, K.; Suneel, K. Ammonium acetate catalyzed improved method for the regioselective conversion of olefins into halohydrins and haloethers at room temperature. J. Mol. Catal. A Chem. 2007, 269, 17–21. [Google Scholar] [CrossRef]
- Podgorsek, A.; Stavber, S.; Zupan, M.; Iskra, J. Environmentally benign electrophilic and radical bromination ‘on water’: H2O2-HBr system versus N-bromosuccinimide. Tetrahedron 2009, 65, 4429–4439. [Google Scholar] [CrossRef]
- Jereb, M.; Zupan, M.; Stavber, S. Effective and selective iodofunctionalisation of organic molecules in water using the iodine-hydrogen peroxide tandem. Chem. Commun. 2004, 22, 2614–2615. [Google Scholar] [CrossRef] [PubMed]
- Stavber, G.; Zupan, M.; Jereb, M.; Stavber, S. Selective and effective fluorination of organic compounds in water using Selectfluor F-TEDA-BF4. Org. Lett. 2004, 6, 4973–4976. [Google Scholar] [CrossRef] [PubMed]
- Pavlinac, J.; Zupan, M.; Stavber, S. Effect of water on the functionalization of substituted anisoles with iodine in the presence of F-TEDA-BF4 or hydrogen peroxide. J. Org. Chem. 2006, 71, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Pravst, I.; Zupan, M.; Stavber, S. Directed regioselectivity of bromination of ketones with NBS: Solvent-free conditions versus water. Tetrahedron Lett. 2006, 47, 4707–4710. [Google Scholar] [CrossRef]
- Pavlinac, J.; Zupan, M.; Stavber, S. ‘Green’ iodination of dimethoxy- and trimethoxy-substituted aromatic compounds using an iodine-hydrogen peroxide combination in water. Synthesis 2006, 15, 2603–2607. [Google Scholar] [CrossRef]
- Podgorsek, A.; Stavber, S.; Zupan, M.; Iskra, J. Free radical bromination by the H2O2-HBr system on water. Tetrahedron Lett. 2006, 47, 7245–7247. [Google Scholar] [CrossRef]
- Stavber, G.; Zupan, M.; Stavber, S. Micellar-System-Mediated Direct Fluorination of Ketones in Water. Synlett 2009, 4, 589–594. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic oxidative iodination of ketones catalysed by sodium nitrite “on water” or in a micelle-based aqueous system. Green Chem. 2009, 11, 1262–1267. [Google Scholar] [CrossRef]
- Stavber, G.; Stavber, S. Towards Greener Fluorine Organic Chemistry: Direct Electrophilic Fluorination of Carbonyl Compounds in Water and Under Solvent-Free Reaction Conditions. Adv. Synth. Catal. 2010, 352, 2838–2846. [Google Scholar] [CrossRef]
- Stavber, S.; Zupan, M. A New, Selective Method for Conversion of Alcohols to Vicinal Fluorohydrins. J. Chem. Soc. Chem. Comm. 1994, 2, 149–150. [Google Scholar] [CrossRef]
- Vražić, D.; Stavber, G.; Jereb, M.; Stavber, S. Micellar system-mediated direct conversion of tert. alcohols to vicinal fluorohydrins in water using SELECTFLUORTM F-TEDA-BF4. In Proceedings of the 16th European Symposium on Fluorine Chemisty-ESFC, Ljubljana, Slovenia, 18–23 July 2010; p. 345.
- Lindstrom, U.M. Organic Reactions in Water: Principles, Strategies and Applications; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Rideout, D.C.; Breslow, R. Hydrophobic acceleration of Diels-Alder reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Koebrich, G.; Trapp, H.; Flory, K.; Drischel, W. Zur Synthese von Halogenolefinen durch Wittig-Reaktion und mittels Dichlormethyllithium. Chem. Ber. 1966, 99, 689. (In German) [Google Scholar] [CrossRef]
- Grant, D.W.; Shilton, R. Reaction of bromine with phenyl-substituted tertiary alcohols. J. Chem. Soc. Perkin Trans. 1 1974, 135–137. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2a–b, 3a–b, 5a–b and 7a–b are available from the authors.
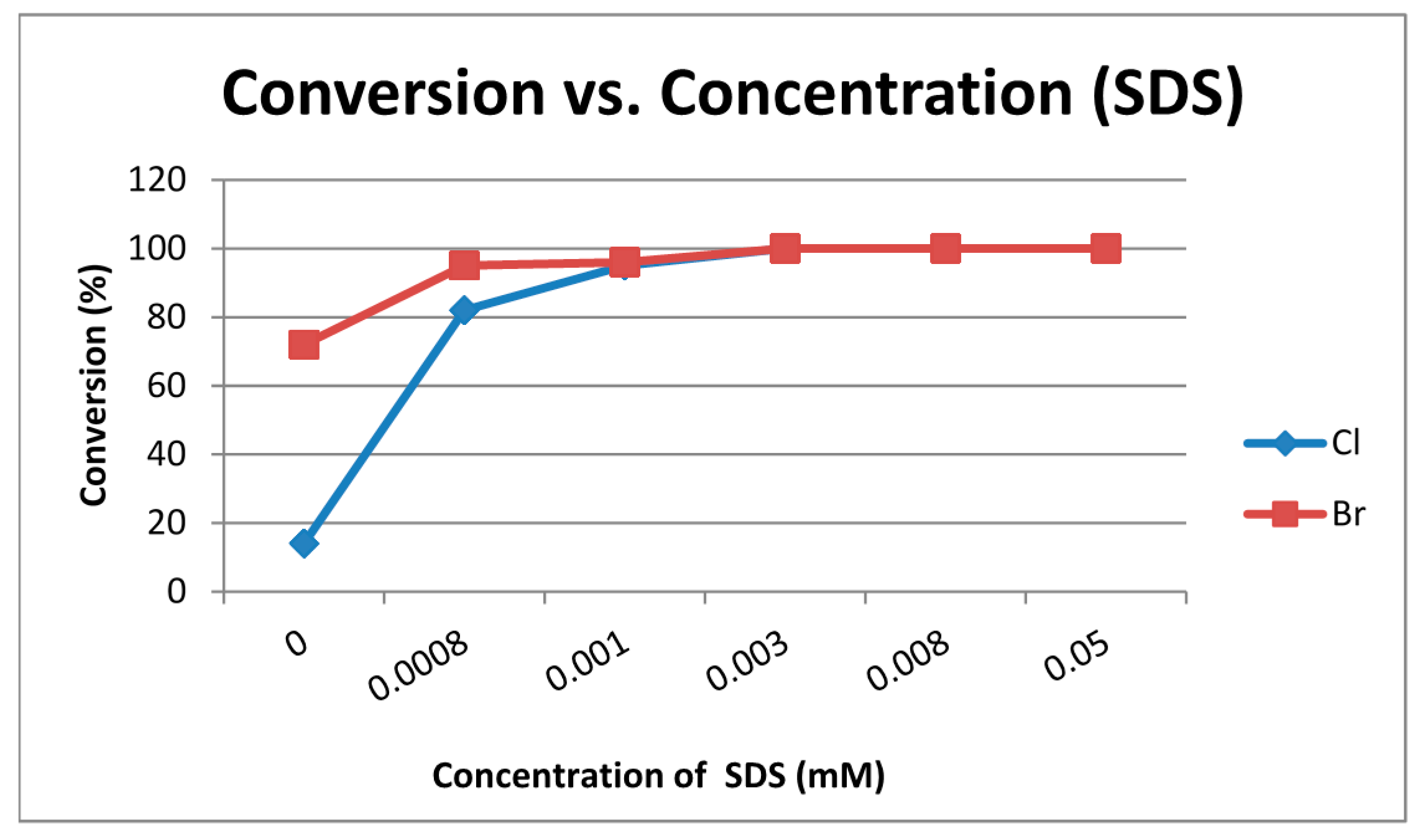
| Entry | X | Solvent | Reaction Conditions | Conversion b (%) of 1 | Relative Distribution of Products b (%) | ||
|---|---|---|---|---|---|---|---|
| 2a–b | 3a–b | 4 | |||||
| 1 c | Cl | SolkaneTM 365 | reflux, 18 h | / | / | / | / |
| 2 c | Br | SolkaneTM 365 | reflux, 18 h | / | / | / | / |
| 3 | Cl | EtOAc | reflux, 18 h | / | / | / | / |
| 4 | Br | EtOAc | reflux, 18 h | 32 | 3 | 5 | 24 |
| 5 d | Cl | EtOAc/H2O | 75–80 °C, 18 h | 100 | / | / | 100 |
| 6 d | Br | EtOAc/H2O | 75–80 °C, 18 h | 100 | / | / | 100 |
| 7 | Cl | 2-MeTHF | reflux, 18 h | 81 | 2 | 1 | 78 |
| 8 | Br | 2-MeTHF | reflux, 18 h | 89 | 7 | / | 82 |
| 9 | Cl | AcOH | reflux, 24 h | 100 | / | 98 | 2 |
| 10 | Br | AcOH | reflux, 24 h | 100 | / | 100 | / |
| 11 | Cl | AcOH | reflux, 4 h | 100 | / | 96 | 4 |
| 12 | Br | AcOH | reflux, 4 h | 100 | / | 100 | / |
| 13 | Cl | AcOH | 70–75 °C, 4 h | 100 | / | 100 | / |
| 14 | Br | AcOH | 70–75 °C, 4 h | 100 | / | 60 | 40 |
| 15 | Cl | AcOH | 40 °C, 4 h | / | / | / | / |
| 16 | Br | AcOH | 40 °C, 4 h | / | / | / | / |
| Entry | R | X | Solvent/Co-Solvent | Conversion b (%) of 1 | Relative Distribution b of Products (%) | ||
|---|---|---|---|---|---|---|---|
| 5a–b | 4 or 8 | 2a–b or 7a–b | |||||
| 1 | Ph | Cl | MeOH (5 mL) | 100 | 73 | 23 | / c |
| 2 | Ph | Br | MeOH (5 mL) | 100 | 40 | 60 | / |
| 3 | Ph | Cl | MeOH (5 mL)/H2O (20 mmol) | 98 | 60 | 30 | 5 c |
| 4 | Ph | Br | MeOH (5 mL)/H2O (20 mmol) | 100 | 13 | 84 | 3 |
| 5 | Ph | Cl | MeOH (2.5 mL)/H2O (2.5 mL) | 100 | 43 | 25 | 29 |
| 6 | Ph | Br | MeOH (2.5 mL)/H2O (2.5 mL) | 100 | 9 | 74 | 7 c |
| 7 | Me | Cl | AcOH (2.5 mL)/H2O (2.5 mL) | 100 | / | / | 93 d |
| 8 | Me | Br | AcOH (2.5 mL)/H2O (2.5 mL) | 100 | / | / | 95 e |
| 9 | Ph | Cl | H2O (5 mL) | 14 | / | / | 14 |
| 10 | Ph | Br | H2O (5 mL) | 72 | / | / | 54 f |
| 11 | Me | Cl | H2O (5 mL) | 100 | / | / | 100 |
| 12 | Me | Br | H2O (5 mL) | 96 | / | 44 | 52 |
| Entry | X | H2O/SDS | Conversion b (%) of 1 | Relative Distribution of Products b (%) | |||
|---|---|---|---|---|---|---|---|
| 2a–b | 3a–b | 4 | 9a–b | ||||
| 1 | Cl | SDS (c = 0.0008 M) | 82 | 47 | 11 | 24 | / |
| 2 | Br | SDS (c = 0.0008 M) | 95 | 53 | 12 | 24 | 6 |
| 3 | Cl | SDS (c = 0.001 M) | 95 | 47 | 10 | 32 | 6 |
| 4 | Br | SDS (c = 0.001 M) | 96 | 49 | 10 | 27 | 10 |
| 5 | Cl | SDS (c = 0.003 M) | 100 | 72 | 8 | 20 | / |
| 6 | Br | SDS (c = 0.003 M) | 100 | 56 | 5 | 28 | 12 |
| 7 | Cl | SDS (c = 0.008 M) | 100 | 86 | 7 | 7 | / |
| 8 | Br | SDS (c = 0.008 M) | 100 | 67 | 5 | 17 | 11 |
| 9 | Cl | SDS (c = 0.05 M) | 100 | 89 | 4 | 7 | / |
| 10 | Br | SDS (c = 0.05 M) | 100 | 70 | / | 23 | 7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajvazi, N.; Stavber, S. Transformation of Tertiary Benzyl Alcohols into the Vicinal Halo-Substituted Derivatives Using N-Halosuccinimides. Molecules 2016, 21, 1325. https://doi.org/10.3390/molecules21101325
Ajvazi N, Stavber S. Transformation of Tertiary Benzyl Alcohols into the Vicinal Halo-Substituted Derivatives Using N-Halosuccinimides. Molecules. 2016; 21(10):1325. https://doi.org/10.3390/molecules21101325
Chicago/Turabian StyleAjvazi, Njomza, and Stojan Stavber. 2016. "Transformation of Tertiary Benzyl Alcohols into the Vicinal Halo-Substituted Derivatives Using N-Halosuccinimides" Molecules 21, no. 10: 1325. https://doi.org/10.3390/molecules21101325
APA StyleAjvazi, N., & Stavber, S. (2016). Transformation of Tertiary Benzyl Alcohols into the Vicinal Halo-Substituted Derivatives Using N-Halosuccinimides. Molecules, 21(10), 1325. https://doi.org/10.3390/molecules21101325