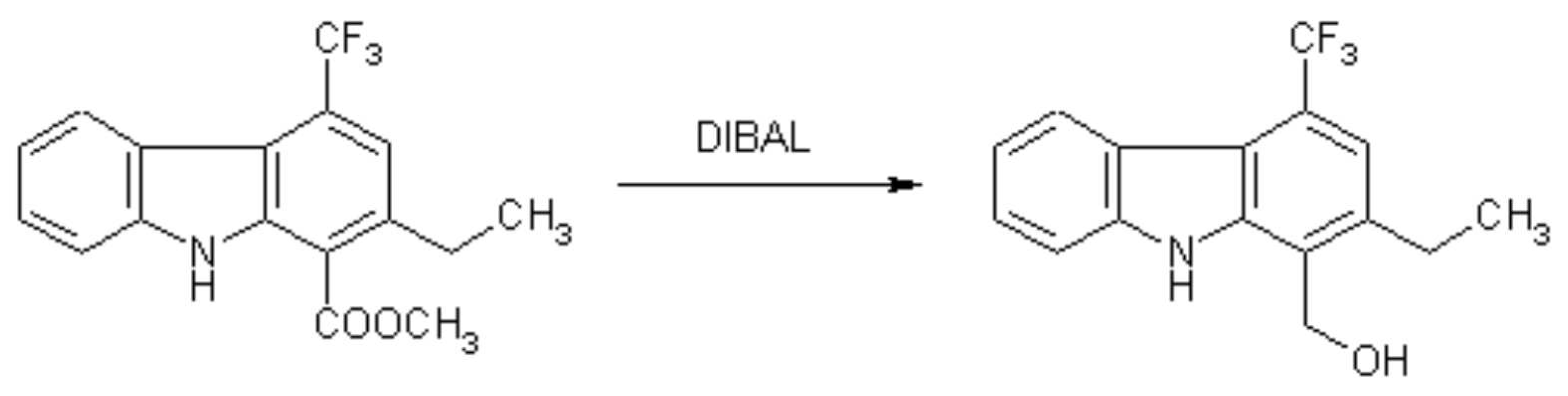
The general part of the experimental section [1] has been presented elsewhere. This reduction was carried out in order to confirm the position of the ethyl substituent in the starting material [1] using NOE difference spectroscopy with the alcohol obtained.
To a solution of methyl 2-ethyl-4-trifluoromethylcarbazole-1-carboxylate [1] (75 mg, 0.23 mmol) in dry THF (11 ml) was added a 1M solution of diisobutylaluminiumhydride in n-hexane (2 ml, 2 mmol), and the mixture was stirred for 5 hr at 65 deg.C under an argon atmosphere. After cooling, HCl (2N, 10 ml) and THF (5 ml) were added, then the mixture was saturated with NaCl. The phases were separated and the organic layer was washed with brine, dried, and evaporated. Recrystallization from ethyl acetate - light petroleum gave the title compound as yellow needles (65 mg, 96%).
M.p. 150-153 deg.C.
1H NMR (CDCl3): 9.29 (bs, 1H, NH; shows NOE on irradiation at 5.20 ppm), 8.29 (d, J=8.1Hz, 1H, H-5), 7.49-7.42 (m, 2H, H-7, H-8), 7.37 (s, 1H, H-3), 7.32-7.22 (m, 1H, H-6), 5.20 (d, J=5.3Hz, 2H, CH2OH), 2.83 (q, J=7.5Hz, 2H, CH2CH3; shows NOE on irradiation at 5.20 ppm), 1.87 (t, J=5.3Hz, 1H, CH2OH), 1.29 (t, J=7.5Hz, 3H, CH2CH3; shows NOE on irradiation at 5.20 ppm).
IR (cm-1, KBr): 3482, 3288, 2964, 1353, 1149, 1105, 990, 892, 738.
MS (m/z, EI): 293 (54%), 291 (26), 275 (100), 262 (16), 248 (17), 206 (19), 204 (17), 191 (10).
Anal. calc. for C16H14F3NO (293.29): C 65.52, H 4.81, N 4.78; found: C 65.22, H 4.57, N 4.57.
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes
- Haider, N.; Wanko, R. Heterocycles 1994, 38, 1805.
- Sample Availability: The product is available from MDPI, 0.015g, MDPI 11845.
© 1997 MDPI. All rights reserved