Abstract
Synthesis of 1,2,3,6-tetrahydrophosphinine 1-oxides by regioselective reduction of 1,2- dihydrophosphinine 1-oxides was reported in this communication.
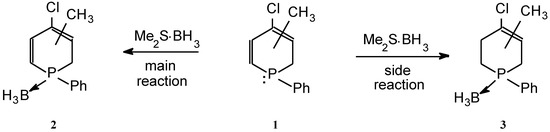
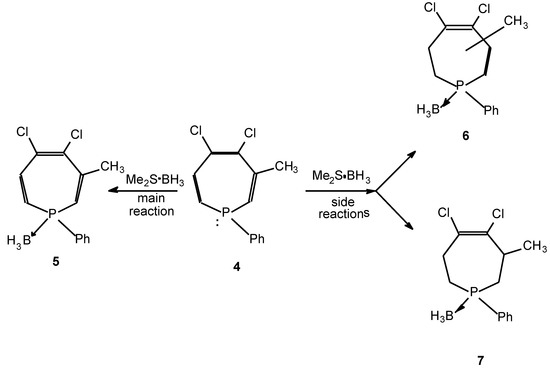
The formation of unsaturated phosphine-borane complexes from the corresponding phosphines and the dimethylsulfide borane (BMS) reagent may be accom- panied by reductive side-reactions giving rise to saturated derivatives. Thus, the preparation of dihydrophosphinine borane 2 from dihydrophosphinine 1 and the BMS reagent was complicated by the formation of tetrahydro derivative 3 (Scheme 1) [1]. The similar synthesis of phosphepine borane 5 was not only accompanied by the formation of the dihydrophosphepine (6), but also by that of the tetrahydro derivative (7) (Scheme 2) [1]. The product compositions were summarized in the Table.
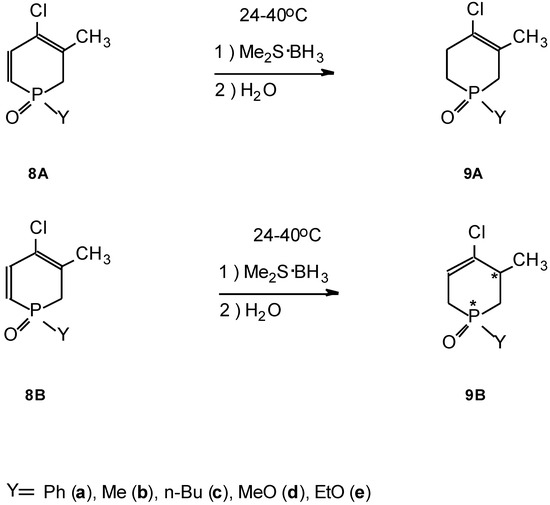
The reducing ability of the BMS reagent is of wider applicability, as was also suitable for the conversion of the dihydrophosphinine oxides (8) to the corresponding tetrahydro derivatives (9) (Scheme 3).
The reductions were complete after stirring at 24–40°C for 20–30 hr followed by hydrolysis. Starting from the isomeric mixture (A and B) of the dihydrophosphinine (8), the products (9) also consisted of two double-bond isomers (A and B). Isomer B was the mixture of two diastereoisomers. After purification by column chromato- graphy, the isolated yield of the reduced product (9A and 9B) fell in the range of 22-61%.
Scheme 1.
Scheme 2.
Scheme 3.

Table 1.
Product compositions of the phosphine-BMS reactions.
| Starting material | Products (%) |
| 1 | 2 (90), 3 (10) |
| 4 | 5 (60), 6 (22), 7 (18) |
The reduction probably takes place through hydrobo- ration of the α, β-double bond of 8. The intermediate so formed must be very unstable, as we could not determine it by means of spectroscopy.
The selectivity of the reduction was assumed to be due to steric and electronic factors. Detailed results will be reported elsewhere [2].
Acknowledgement
The authors thank the OTKA support of this work (Grant No. T 014917).
References
- Keglevich, Gy.; Újszászy, K.; Szölôssy, Á.; Ludányi, K.; Tôke, L. J Organomet. Chem. 1996, 516, 139.
- Keglevich, Gy.; Újszászy, K.; Szölôssy, Á.; Ludányi, K.; Tôke, L. submitted for publication. 1996.
- Sample Availability: Supporting sample 9Aa, MDPI 4684, is available from MDPI.
© 1997 MDPI. All rights reserved