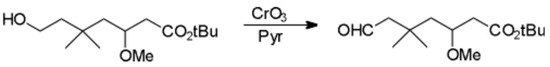
Scheme.
Hydroboration of tert-butyl-3-methoxy-5,5-dimethyl-6-heptenoate [1] with either BH3…THF or BH3…Me2S provided the terminal alcohol tert-butyl-3-methoxy-5,5-dimethyl-7-hydroxy-heptanoate, in unoptimized, nonreproducable and disappointingly low 30% yield [2]. The poor yield is presumably due to isolation problems. Typically, this alcohol was not isolated but was directly oxidized as follows without purification.
To a room temperature solution of pyridine (6.2 ml, 77 mmol) and CrO3 (3.9g, 39 mmol) in CH2Cl2 (60 ml), the title compound (1.65g, 6.4 mmol) in CH2Cl2 (15 ml) was added. After stirring for 30 minutes, the reaction was poured into ether (400 ml), then filtered through Florisil and concentrated to give, after flash chromatography (4 : 1 pet ether : ether) the title aldehyde as a colorless oil, 1.12g, in 75 percent yield. This aldehyde was unstable and typically used immediately and without purification.
1H NMR (CDCl3): δ: 9.87 (t, J = 2.8Hz, 1H), 3.75 (m, 1H), 3.23 (s, 3H), 2.68 (dd, J = 14.7, 4.1Hz, 1H), 2.52 (dd, J = 12.7, 2.8Hz, 1H), 2.25 - 2.21 (m, 2H), 1.78 (dd, J = 12.3, 7.4Hz, 1H), 1.42 (s, 9H), 1.5 - 1.3 (m, 1H), 1.04 (s, 6H).
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes
- Smith, D. tert-Butyl-3-methoxy-5,5-dimethyl-6-heptenoate. Molecules 1997, 2, M30. [Google Scholar] [CrossRef]
- Smith, D. tert-Butyl-3-methoxy-5,5-dimethyl-7-hydroxy-heptanoate. Molecules 1997, 2, M31. [Google Scholar] [CrossRef]
- Sample Availability: No sample available.
© 1997 MDPI. All rights reserved