Abstract
Two new polyphenols, penthorumin C (1) and 2,6-dihydroxyacetophenone-4-O-[4ꞌ,6ꞌ-(S)-hexahydroxydiphenoyl]-β-d-glucose (2), along with four known polyphenolic acids, pinocembrin-7-O-[4ꞌꞌ,6ꞌꞌ-hexahydroxydiphenoyl]-β-d-glucose(3), pinocembrin-7-O-[3ꞌꞌ-O-galloyl- 4ꞌꞌ,6ꞌꞌ-hexahydroxydiphenoyl]-β-d-glucose (4), thonningianin A (5), and thonningianin B (6) were isolated from Penthourm chinense. All compounds were evaluated for their anti-proliferative activity in HSC-T6 cells, and 2 and 5 showed significant activity, with IC50 values of 12.7 and 19.2 μM, respectively.
1. Introduction
Oxidative stress due to the imbalance in oxidant and antioxidant status has been implicated in the pathogenesis of several diseases, including cancer. Several papers have reported the role of free radical mediated oxidative damage in multi-stage events of carcinogenesis. Polyphenolic acids are a complex group of compounds that have attracted attention in the last few years because of their widespread occurrence on plants and their significant biological activities [1,2]. Polyphenols may have therapeutic health effects for a variety of chronic pathological conditions including cancer, neurodegenerative diseases, diabetes, and cardiovascular diseases due to the antioxidant activity. Previous reports have showed that polyphenolic acids are the inhibition of hepatic stellate cells proliferation, considering to be associated to liver fibrosis [3].
Penthorum chinense Pursh, (Saxifragaceae), is widely distributed in eastern Asia, (China, Japan, Korea, and eastern Russia) [4]. In China, where it is both consumed as food and used in traditional Chinese medicine, whole plant products prepared from P. chinense is used to alleviate “heat”-associated disorders, detoxification, to promote circulation, and to treat liver problems, and to protect the spleen [5]. P. chinense metabolites have shown anti-oxidant and antitumor activities [6,7,8,9]. Flavonoids, triterpenoids, polyphenols, and lignans have been isolated from P. chinense [10]. Two new polyphenols (compounds 1 and 2), along with four polyphenols (compounds 3–6) were isolated from the aerial parts of P. chinense (Figure 1). Herein, we describe the separation and structural characterization of these compounds, as well as their anti-proliferative activity in HSC-T6 cells induced by PDGF.
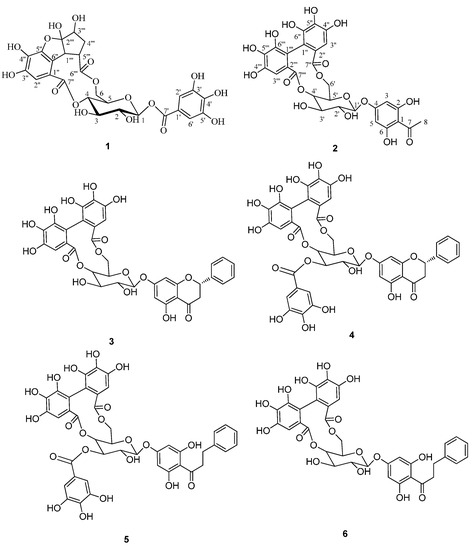
Figure 1.
Compounds 1–6 isolated from Penthourm chinense.
2. Results and Discussion
2.1. Structure Elucidation
An 80% ethanolic extract of dried P. chinense whole plant was suspended in distilled water and extract with petroleum ether, EtOAc and n-BuOH. The EtOAc soluble fraction was concentrated under reduced pressure to produce a residue that was subjected multiple chromatography, two new compounds 1 and 2 and four known compounds were isolated and identified.
Compound 1 was obtained as white powder. The molecular formula C26H24O17 was deduced from the HERSIMS ion peak at m/z 607.0948 [M−H]− (calcd m/z 607.0901), exhibiting fifteen degrees of hydrogen deficiency. Its IR spectrum contained characteristic absorptions for hydroxyl (3407.6 cm−1), carbonyl (1724.0 cm−1) and aromatic ring (1619.9, 1454.0 cm−1) moieties. Interpretation of the NMR spectra (Table 1) suggested the presence of a glucose (C-1 to C-6), and two galloyl (C-1ꞌ to C-7ꞌ; C-1ꞌꞌ to C-7ꞌꞌ) groups (Ikuko et al., 2000). The glucopyranose moiety was determined to have a β-configuration at C-1 with the large coupling constant of H-1 (J = 7.8 Hz). The remaining six carbons were assigned as one carbonyl (δC 173.6), one methylene (δC 35.0), two methines (δC 55.7, 75.4) including one (δC 75.4) connected to an oxygen atom, and one doubly oxygen-substituted quaternary carbon (δC 116.8). The HMBC spectrum (Figure 2) exhibited long-range correlations from H2-4ꞌꞌꞌ to C-1ꞌꞌꞌ, C-2ꞌꞌꞌ, C-3ꞌꞌꞌ, and C-5ꞌꞌꞌ, consistent with a cyclopentyl ring. The presence of a 1,4,6-tri-O-substituted-β-glucopyranose was deduced from HMBC correlations from H-4 to C-7ꞌꞌ, from H-1 to C-7ꞌ, and from H2-6 to C-6ꞌꞌ. The HMBC correlations from H-1ꞌꞌꞌ to C-1ꞌꞌ, C-5ꞌꞌ, C-6ꞌꞌ and from H-1ꞌꞌꞌ, H-4ꞌꞌꞌ, H-5ꞌꞌꞌ to C-6ꞌꞌꞌ indicated that the cyclopentyl ring was connected to C-6ꞌꞌ and C-6ꞌꞌꞌ via C-1ꞌꞌꞌ/C-6ꞌꞌ and C-5ꞌꞌ/C-6ꞌꞌꞌ. The glucopyranose, substituted galloyl moieties, and an additional ring account for 15 degrees of hydrogen deficiency. On the basis of the molecular formula and the chemical shift of C-2ꞌꞌꞌ (δC 116.8), the cyclopentyl ring was also connected to C-5ꞌꞌ via oxygen linkage to form the remaining ring [11]. The absolute configuration of glucose was proposed to be D by comparison of its specific rotation, [α]D −21° (c 0.16, MeOH), with those reported for related compounds [12,13].

Table 1.
1H (600MHz) and13C (150 MHz) NMR spectroscopic data of compound 1 in DMSO-d6.
| Position | δ H | δ C |
|---|---|---|
| 1 | 5.66(1H, d, 7.8) | 94.4 |
| 2 | 3.40(1H, t, 9.0) | 73.0 |
| 3 | 3.58(1H, t, 9.0) | 73.2 |
| 4 | 5.07(1H, t, 9.6) | 74.5 |
| 5 | 4.07(1H, t, 4.2) | 66.8 |
| 6 | 4.49(1H, dd, 3.6, 10.8)3.92(1H, d, 4.2) | 64.6 |
| 1ꞌ | - | 118.4 |
| 2ꞌ, 6ꞌ | 7.03(2H, s) | 109.2 |
| 3ꞌ, 5ꞌ | - | 145.7 |
| 4ꞌ | - | 139.4 |
| 7ꞌ | - | 164.7 |
| 1ꞌꞌ | - | 117.7 |
| 2ꞌꞌ | 6.82(1H, s) | 110.6 |
| 3ꞌꞌ | - | 146.1 |
| 4ꞌꞌ | - | 133.6 |
| 5ꞌꞌ | - | 146.8 |
| 6ꞌꞌ | - | 119.7 |
| 7ꞌꞌ | - | 167.1 |
| 1ꞌꞌꞌ | 3.68(1H, d, 9.0) | 55.7 |
| 2ꞌꞌꞌ | - | 116.8 |
| 3ꞌꞌꞌ | 3.96(1H, dd, 6.6, 12.0) | 75.4 |
| 4ꞌꞌꞌ | 2.04(1H, d, 6.0)1.93(1H, d, 12.0) | 35.0 |
| 5ꞌꞌꞌ | 2.42(1H, d, 6.0) | 45.3 |
| 6ꞌꞌꞌ | - | 173.6 |
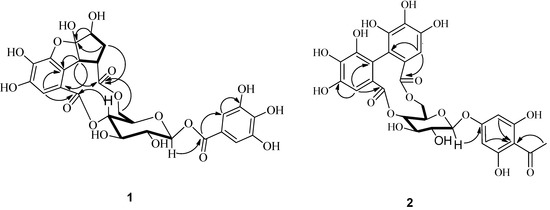
Figure 2.
1H-1H COSY (▬) and key HMBC (→) correlations of compounds 1 and 2.
The relative configuration of 1 was confirmed by observance of NOESY correlations (Figure 3), that were observed between H-3ꞌꞌꞌ to H-4ꞌꞌꞌ α, and H-5ꞌꞌꞌ, H-1ꞌꞌꞌ to H-4ꞌꞌꞌ β. These correlations suggested that H-3ꞌꞌꞌ was α-oriented and both H-5ꞌꞌꞌ and H-1ꞌꞌꞌ were both β-orientated. To minimize the energy of 1 the OH-2ꞌꞌꞌ was assigned as β-orientation. The structure of compound 1 (penthorumin C) was thus assigned as shown in Figure 1.
Compound 2 was obtained as white powder with a molecular formula of C28H24O17 according to HRESIMS (m/z [M+H]+ 633.1088, calcd m/z 633.1019) constant with 17 degrees of hydrogen deficiency. The IR spectrum indicated hydroxyl (3407.6 cm−1), carbonyl (1731.7 cm−1) and aromatic ring (1627.6, 1596.7 cm−1) moieties were present. The 1H-NMR showed resonances for one methyl group (δH 2.61, 3H, s). The 13C-NMR data (Table 2) exhibited resonances for four methines at δC 71.7 (C-4ꞌ), 73.8 (C-2ꞌ), 73.8 (C-3ꞌ) and 71.1 (C-5ꞌ), one methylene at δC 62.9 (C-6ꞌ) and one anomeric methine at δC 99.8 (C-1ꞌ), indicating the presence of a glucopyranose unit. The glucopyranose moiety was determined to have a C-1ꞌ β-orientation by observance of H-1ꞌ (J = 7.8 Hz) coupling constant. The 1H and 13C-NMR spectra of 2 (Table 2) are similar to those of thonningianin B [14], except for the absence of the resonances corresponding to one benzyl group. The HMBC correlations from H3-8 to C-7 and C-1, suggested that the acetyl group (C7-C8) was connected to C-1. The CD spectrum of 2 shows a negative Cotton effect at 262.5 nm (Δ −5.6) and a positive effect at 241.5 nm (Δ +23.0), indicating an S-configuration of the hexahydroxydiphenoyl (HHDP) group [14]. The absolute configuration of glucose was proposed to be D by comparison of its specific rotation, [α]D −31° (c 0.21, MeOH), with those reported for related compounds [12]. The structure of compound 2 (2,6-dihydroxyacetophenone-4-O-[4',6'-(S)-hexahydroxydiphenoyl]-β-d-glucose) was thus assigned as shown in Figure 1.
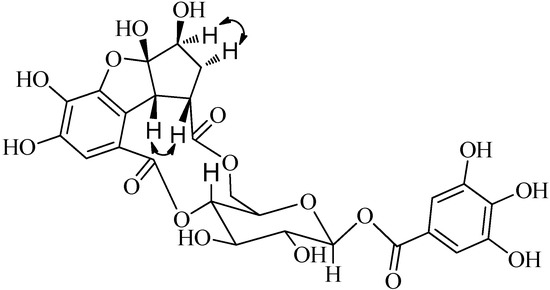
Figure 3.
Selected NOESY correlations (  ) of compound 1.
) of compound 1.
 ) of compound 1.
) of compound 1.

Table 2.
1H (600MHz) and13C (150 MHz) NMR spectroscopic data of compound 2 in DMSO-d6.
| Position | δ H | δ C |
|---|---|---|
| 1 | - | 105.8 |
| 2, 6 | - | 164.0 |
| 3, 5 | 6.08(2H, s) | 95.0 |
| 4 | - | 163.3 |
| 7 | - | 203.6 |
| 8 | 2.61(3H, s) | 32.8 |
| 1ꞌ | 5.04(1H, d, 7.8) | 99.8 |
| 2ꞌ | 3.57(1H, t, 9.6) | 73.8 |
| 3ꞌ | 3.36(1H, t, 9.0) | 73.8 |
| 4ꞌ | 4.61(1H, t, 9.6) | 71.7 |
| 5ꞌ | 4.13(1H, m) | 71.1 |
| 6ꞌ | 4.98(1H, dd, 6.0, 13.2) 3.74(1H, d, 13.2) | 62.9 |
| 1ꞌꞌ | - | 115.3 |
| 2ꞌꞌ | - | 124.5 |
| 3ꞌꞌ | 6.35(1H, s) | 105.3 |
| 4ꞌꞌ | - | 144.3 |
| 5'' | - | 135.1 |
| 6'' | - | 144.6 |
| 7ꞌꞌ | - | 167.9 |
| 1ꞌꞌꞌ | - | 115.6 |
| 2''' | - | 124.8 |
| 3''' | 6.54(1H, s) | 106.3 |
| 4''' | - | 144.4 |
| 5''' | - | 135.4 |
| 6''' | - | 144.6 |
| 7''' | - | 167.1 |
Additionally, the known 3–6 were identified as pinocembrin-7-O-[4ꞌꞌ,6ꞌꞌ-hexahydroxydiphenoyl]-β-d-glucose (3) [15], pinocembrin-7-O-[3ꞌꞌ-O-galloyl-4ꞌꞌ,6ꞌꞌ-hexahydroxydiphenoyl]-β-d-glucose (4) [16], thonningianin A (5) [14], and thonningianin B (6) [14] by comparison of their physicochemical data with the reported values.
2.2. Cytotoxicity of Penthorum Chinense Extract
Penthorum. chinense extracts were evaluated on the HSC-T6 cells for culture periods of 24 h. P. chinense extract and four fractions (Pe, Et, n-BuOH, and W), showed little toxic effect under 80 μg/mL (Figure 4). The cytotoxicity of n-BuOH fraction was stronger than other fractions (P. chinense extract, Pe, Et, and W), and half of the cells were dead when the added concentration was up to 160 μg/mL.
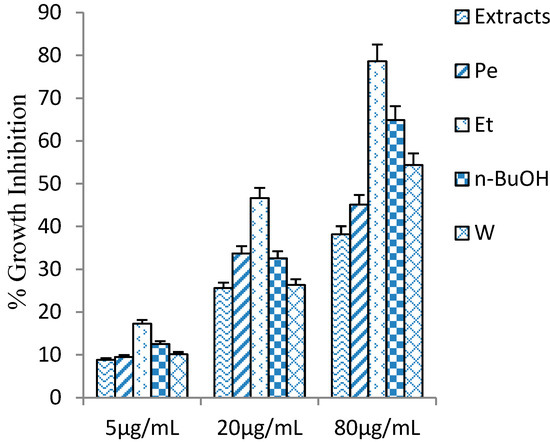
Figure 4.
The inhibitory activity of Penthorum chinense extracts and four fractional extractions on HSC-T6 cells. Values are mean ± SD, n = 6.
2.3. Effect on PDGF Induced Proliferation
Based on the cytotoxic effect on HSC-T6 cells, we evaluated the anti-proliferative activity at 80 μg/mL. In order to figure out the active constituents of P. chinense, P. chinense extract was suspended in water and partitioned successively with petroleum ether (Pe), ethyl acetate (Et), n-BuOH(n-BuOH), and water (W), and these four fractions (Pe, Et, n-BuOH, and W) were tested their inhibitory activity, together with P. chinense extract (Figure 4). In comparison with other parts of P. chinense, Et fraction showed remarkable inhibitory activity, 78.6% at 80 μg/mL. The result suggested that the active ingredients of anti-proliferation may exhibit in Et part, and some isolation and purification methods had been further applied in Et part, which led to isolation of six polyphenols 1–6, including two new isolates 1 and 2. The anti-proliferative activities of 1–6 were evaluated in parallel experiments by measuring their inhibitory activities in PDGF induced HSC-T6. Compounds 2 and 5 displayed moderate inhibitory activity, with IC50 values of 12.7, 19.2 μM, respectively vs. 6.36 μM for Colchicine, whereas the other compounds exhibited weak activity, with IC50 values greater than 200 μM.
3. Experimental
3.1. General
Optical rotations were measured on a Perkin-Elmer 341 digital polarimeter at 589 nm. CD spectra were recorded by a JASCO J-810 spectropolarimeter. IR spectra were recorded as KBr disks on an Intelligent Fourier Nicolet FTIR 6,700 Infrared Spectrometer. The NMR spectra, including 1H, 13C, DEPT and 2D-NMR, were recorded on a Bruker AM-400 and AC-600 spectrometer with chemical shifts reported as δ values, using TMS as internal standard. HRESIMS data were obtained on an Agilent Technologies 6,538 UHD Accurate-Mass Q-TOF LC/MS spectrometer (Agilent Technologies, Waltham, MA, USA). Thin-layer chromatography was performed on TLC plates (Silica gel HSGF254. Jiangyou Company of Yantai, Yantai, China; RP-18 F254. Merck, Darmstadt, Germany) and spots were visualized by heating after dipping into 10% H2SO4. Silica gel (100–200 and 200–300 mesh, Jiangyou Company of Yantai, Yantai, China), Sephadex LH-20 (Pharmacia, Fairfield, NJ, USA), RP-C18 (43–60 μm, Merck), and MCI gel (Mitsubishi Chemical Corporation, Hongkong, China) were used for column chromatography.
3.2. Plant Material
The dry aerial parts of P. chinense were provided by Gulin Gansu Pharmaceutical Co., Ltd (Sichuan, China) in September 2009. The plant was identified by Prof. Wansheng Chen. A voucher specimen (No. IT100629) was deposited in the Department of Pharmacognosy of the Second Military Medical University, Shanghai, China.
3.3. Extraction and Isolation
The dry aerial parts of P. chinense (10 kg) were extracted three times with 80% EtOH (3 × 80 L, 2 h each) at 80 °C, and concentrated under vacuum to give a residue (1,167 g). The residue was suspended in water (1.5 L) and then successively partitioned with petroleum ether (3 × 1.0 L), EtOAc (3 × 2.0 L), and n-BuOH (2 × 1.5 L) to give petroleum ether-soluble (99 g), EtOAc-soluble (245 g), and n-BuOH-soluble (239 g) extracts. The EtOAc extract (100 g) was separated by chromatography using a silica gel column and eluted with a gradient of CH2Cl2–CH3OH (50:1, 30:1, 10:1, 5:1, 2:1, and 1:1, v/v, each 2 L) to give six fractions (F1–F6). Fraction Fr4 (25.4 g) was divided into five subfractions (Fr-4.1–Fr-4.5) through a silica gel column employing a petroleum ether–acetone gradient (20:1, 10:1, 5:1, 2:1, and 1:1, each 150 mL). Compound 1 (25 mg) and 2 (21 mg) were purified from fraction F4.5 (3.2 g) by Sephadex HL-20 with a gradient of MeOH–H2O (50:50, 70:30, each 350 mL). Fraction F4.2 (2.3 g) was applied to ODS column with of MeOH–H2O (50:50, 400 mL) to give 3 (16 mg). Compounds 4 (18 mg) and 6 (15 mg) were obtained from fraction F4.3 (7.0 g) which was subjected to ODS column and eluted with MeOH–H2O (50:50, 70:30, each 550 mL). Compound 5 (11 mg) was isolated from F4.4 (1.2 g) by reverse-phase HPLC with H2O/CH3CN 80/20 to 50/50 over 30 min (2 mL/min). The degrees of all compounds were higher than 95% based on TLC and HPLC-DAD-ELSD.
3.4. Characterization of Compound 1 and Compound 2
Penthorumin C (1). White powder; 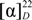 +34.7 (c 0.19, CH3OH); IR (KBr) vmax 3407.6, 1724.0, 1619.9, 1454.0, 1214.9, 1033.6, 763.7, 547.7 cm−1; 1H-NMR and 13C-NMR data, see Table 1; HRESIMS m/z 631.0901 [M+Na]+ (calcd for C22H24O17Na, 631.0906).
+34.7 (c 0.19, CH3OH); IR (KBr) vmax 3407.6, 1724.0, 1619.9, 1454.0, 1214.9, 1033.6, 763.7, 547.7 cm−1; 1H-NMR and 13C-NMR data, see Table 1; HRESIMS m/z 631.0901 [M+Na]+ (calcd for C22H24O17Na, 631.0906).
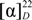 +34.7 (c 0.19, CH3OH); IR (KBr) vmax 3407.6, 1724.0, 1619.9, 1454.0, 1214.9, 1033.6, 763.7, 547.7 cm−1; 1H-NMR and 13C-NMR data, see Table 1; HRESIMS m/z 631.0901 [M+Na]+ (calcd for C22H24O17Na, 631.0906).
+34.7 (c 0.19, CH3OH); IR (KBr) vmax 3407.6, 1724.0, 1619.9, 1454.0, 1214.9, 1033.6, 763.7, 547.7 cm−1; 1H-NMR and 13C-NMR data, see Table 1; HRESIMS m/z 631.0901 [M+Na]+ (calcd for C22H24O17Na, 631.0906).2,6-Dihydroxyacetophenone-4-O-[4ꞌ,6ꞌ-(S)-hexahydroxydiphenoyl]-β-d-glucose (2). White powder; 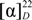 −21.7 (c 0.23, CH3OH); IR (KBr) vmax 3407.6, 1731.7, 1627.6, 1596.8, 1517.7, 1442.5, 1363.4, 1286.3, 1232.3, 1174.4, 1018.2, 962.3, 831.2, 742.5, 566.9 cm−1; 1H-NMR and 13C-NMR data, see Table 2; HRESIMS m/z 633.1083 [M+H]+ (calcd for C28H25O17H, 633.1019).
−21.7 (c 0.23, CH3OH); IR (KBr) vmax 3407.6, 1731.7, 1627.6, 1596.8, 1517.7, 1442.5, 1363.4, 1286.3, 1232.3, 1174.4, 1018.2, 962.3, 831.2, 742.5, 566.9 cm−1; 1H-NMR and 13C-NMR data, see Table 2; HRESIMS m/z 633.1083 [M+H]+ (calcd for C28H25O17H, 633.1019).
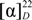 −21.7 (c 0.23, CH3OH); IR (KBr) vmax 3407.6, 1731.7, 1627.6, 1596.8, 1517.7, 1442.5, 1363.4, 1286.3, 1232.3, 1174.4, 1018.2, 962.3, 831.2, 742.5, 566.9 cm−1; 1H-NMR and 13C-NMR data, see Table 2; HRESIMS m/z 633.1083 [M+H]+ (calcd for C28H25O17H, 633.1019).
−21.7 (c 0.23, CH3OH); IR (KBr) vmax 3407.6, 1731.7, 1627.6, 1596.8, 1517.7, 1442.5, 1363.4, 1286.3, 1232.3, 1174.4, 1018.2, 962.3, 831.2, 742.5, 566.9 cm−1; 1H-NMR and 13C-NMR data, see Table 2; HRESIMS m/z 633.1083 [M+H]+ (calcd for C28H25O17H, 633.1019).3.5. Cell Culture
An immortalized rat hepatic stellate cell line, HSC-T6 (obtained from the cell bank of the Chinese Academy of Science, Shanghai, China) was were batch cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS), 100 IU mL−1 and streptomycin at 37 °C in a 5% CO2 incubator.
3.6. Cell Viability
Compounds and P. chinense extract were dissolved in dimethylsulfoxiode (DMSO). Our preliminary study showed that DMSO at a final concentration of 0.1% in media did not affect the cell viability. HSC-T6 cells seeded for 24 h in 96-well plates were exposed to different concentrations of compounds and P. chinense extract. Different concentrations of compounds were carried out in the plate for 5 consecutive wells (final volume 100 μL) and incubated for 24 h. Cell viability was calculated as 100 ° (absorbance of treated compound − absorbance of background light)/(absorbance of control − absorbance of background light).
3.7. Activation of HSC-T6 Cells Induced by PDGF
HSC-T6 cells were plated in a 96-well plate. Initially, cells were cultured with DMEM containing 10% FBS for 6 h. The medium was then replaced with DMEM without FBS to starve the cells for 12 h. The cells were then cultured with DMEM that contained 5.0 ng/mL PDGF (without FBS) for 24 h.
3.8. Anti-Proliferative Activity Assay
Activated HSC, which was induced by some mediators (TGF-β and PDGF, etc.), has been long considered to be associated with liver fibrosis, and inhibition for HSC growth has been proposed as a method for treating liver fibrosis [17,18]. The anti-proliferative activity in PDGF induced HSC-T6 cell was assessed by the MTT assay [19]. Inhibitory activity on cell proliferation was calculated as 100 ° (absorbance of treated compound − absorbance of background light)/(absorbance of model − absorbance of background light). Data were expressed as the mean of the three independent experiments. Colchicine was used as a positive control.
3.9. Statistical Analysis
Results were expressed as mean ± SD for at least three analyses for each sample. Statistical analyses were performed using SPSS 12.0 software. The significance of difference was calculated by one-way ANOVA test, and values with p < 0.05 were considered to be statistically significant. Graphs were drawn with Microsoft Excel 2003 software.
4. Conclusions
In summary, the anti-proliferative activity of P. chinense was tested on HSC-T6 cells. In addition, four fractions were also tested their anti-proliferative activity to figure out the active constituents, and six polyphenols were isolated from the EtOAc fraction. The result showed that EtOAc part possesses stronger inhibitory activity than other parts, and the polyphenols may be the active ingredients of P. chinense based on the current research. Moreover, two new isolates 1 and 2 have been added to the chemical components of this species.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/8/11045/s1.
Acknowledgments
This research project was supported by the National Science Fund for Distinguished Young Scholars (No.81325024), the Science Foundation of Shanghai (No. 13401900106), and the Second Military Medical University Stem Cell and Medical Research Center’s Innovation Research Program (SCMRC1201). The NMR spectra were conducted by the Second Military University School of Pharmacy. The authors also thank Nagle (The University of Mississippi) for his linguistic assistance during the preparation of this manuscript.
Author Contributions
Professor Lianna Sun and Professor Wansheng Chen designed this research; Doudou Huang and Yun Jiang performed the research and analyzed the data; Doudou Huang, Yun Jiang and Fengyan Yao wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Escarpa, A.; González, M.C. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Aata 1974, 427, 119–127. [Google Scholar] [CrossRef]
- Sanli, N.; Fonrodona, G.; Barròn, D.; Özkan, G.; Barbosa, J. Prediction of chromatographic retention, pKa values and optimization of the separation of polyphenolic acids in strawberries. J. Chromatogr. A 2002, 975, 299–309. [Google Scholar] [CrossRef]
- Beltrán, J.L.; Sanli, N.; Fonrodona, G.; Barròn, D.; Özkan, G.; Barbosa, J. Spectrophotometric, potentiometric and chromatographic pKa values of polyphenolic acids in water and acetonitrile–water media. Anal. Chim. Aata 2003, 484, 253–264. [Google Scholar] [CrossRef]
- Ikeda, H.; Itoh, K. Germination and water dispersal of seeds from a threatened plant species Penthorum chinense. Ecol. Res. 2001, 16, 99–106. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xu, X.L.; Jiang, M.H.; Jiang, J.G. Hepatoprotective function of Penthorum chinense pursh. Food Funct. 2013, 4, 1581–1585. [Google Scholar] [CrossRef]
- Mahesh, T.; Menon, V.P. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother. Res. 2004, 18, 123–127. [Google Scholar] [CrossRef]
- Sigurdsson, S.; Ogmundsdottir, H.M.; Hallgrimsson, J.; Gudbjarnason, S. Antitumour activity of Angelica archangelica leaf extract. In Vivo 2005, 19, 191–194. [Google Scholar]
- Moon, Y.J.; Wang, X.D.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef]
- Wang, L.Q.; Yang, J.; Deng, E.; Wang, G.B.; Peng, Z.S. Optimizing the shoot proliferation protocol of Penthorum chinense by axillary buds. Biotechnol. Lett. 2008, 30, 2199–2203. [Google Scholar] [CrossRef]
- Lu, Q.; Jiang, M.H.; Jiang, J.G.; Zhang, R.; Zhang, M.W. Isolation and identification of compounds from Penthorum chinense Pursh with antioxidant and antihepatocarcinoma properties. J. Agric. Food Chem. 2012, 60, 11097–11103. [Google Scholar] [CrossRef]
- Hideyuki, I.; Tsutomu, H.; Osamu, N.; Tadashi, S.; Takuo, O.; Takashi, Y. Modified dehydroellagitannins, geraniinic acids B and C, and phyllanthusiin F. Chem. Pharm. Bull. 1999, 47, 1148–1151. [Google Scholar]
- Huang, Y.L.; Chen, C.C.; Hsu, F.L.; Chen, C.F. Two tannins from Phyllanthus tenellus. J. Nat. Prod. 1998, 61, 523–524. [Google Scholar] [CrossRef]
- Kazunori, H.; Takao, K.; Kazuaki, N.; Yukinobu, I.; Minoru, O.; Hiroshi, M. Two glucosides from roots of Asiasarum Sieboldi. Phytochemistry 1992, 31, 2477–2480. [Google Scholar] [CrossRef]
- Ikuko, I.O.; Naomi, G.; Junichi, T.; Tatsuo, H.; Maxwell, A.G.; Yoko, A. Thonningianins A and B, new antioxidants from the African medicinal herb Thonningia sanguinea. J. Nat. Prod. 2000, 63, 676–679. [Google Scholar] [CrossRef]
- Hegde, V.R.; Pu, H.Y.; Patel, M.; Das, P.R.; Butkiewicz, N.; Arreaze, G.; Gullo, V.P.; Chan, T.M. Two antiviral compounds from the plant Stylogne cauliflora as inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2003, 13, 2925–2928. [Google Scholar]
- Wang, H.W.; Liu, Y.Q.; Feng, C.G. Isolation and identification of a novel flavonoid from Penthorum chinense P. J. Asian Nat. Prod. Res. 2010, 8, 757–761. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- Bartalis, J.; Halaweish, F.T. In vitro and QSAR studies of cucurbitacins on HepG2 and HSC-T6 liver cell lines. Bioorg. Med. Chem. Lett. 2011, 19, 2757–2766. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wang, Z.; Kwong, S.Q.; Lui, E.L.H.; Friedman, S.L.; Li, F.R.; Lam, R.W.C.; Zhang, G.C.; Zhang, H.; Ye, T. Inhibition of PDGF, TGF-β , and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinb. J. Hepatol. 2011, 55, 612–625. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–8 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).