Abstract
One lignanoid compound, isoamericanol B (1), along with four triterpenoid compounds—cis-3-O-p-hydroxycinnamoyloleanolic acid (2), trans-3-O-p-hydroxy cinnamoyloleanolic acid (3), cis-3-O-p-hydroxycinnamoylursolic acid (4), trans-3-O-p-hydroxycinnamoylursolic acid (5) have been isolated for the first time from the leaves of Elaeagnus oldhamii Maxim. Compounds 1–4 significantly inhibited the expression of NO (nitric oxide) produced in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. The IC50 value for inhibition of nitrite production of compound 1 was about 10.3 ± 0.4 μg/mL. In the cell viability test, however, among compounds 1–4 compound 1 did not significantly change cell viability. Therefore, in this study compound 1 possessed anti-inflammatory effects. The result suggests compound 1 as a potential lead compound for the treatment of inflammatory diseases.
1. Introduction
There are about 90 species of Elaeagnus around the World. The majority are native to the temperate and subtropical regions in Asia, of which nine species can be found in Taiwan [1]. Many species of Elaeagnus are considered as folk medicinal plants, e.g., E. umbellate [2], E. pungens [3], E. angustifolia [4,5] and E. multiflora [6]. Triterpenoids, steroids and flavonoids have been isolated from several species of Elaeagnus, e.g., E. ungens [7], E. umbellate [7], E. bockii Diels [8], E. orientalis [9] and E. pungens [10,11].
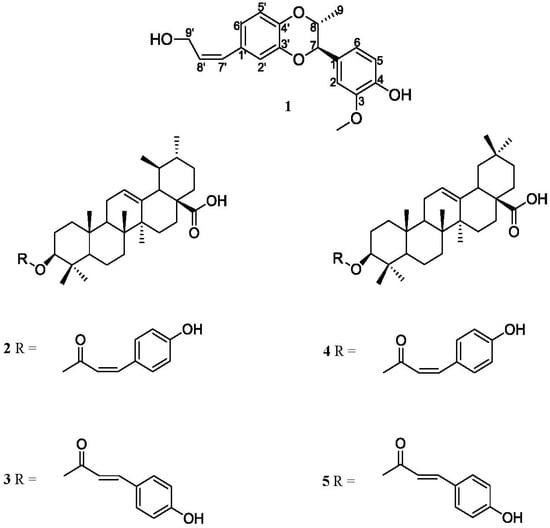
Figure 1.
The chemical structures of compounds 1–5.
Elaeagnus oldhamii Maxim. is a traditional herbal medicine mainly used in Taiwan to treat rheumatoid arthritis. We have investigated the analgesic and anti-inflammatory effects of the methanol extract of E. oldhamii Maxim. in a previous study [12]. In connection with our interest in the chemical components of this plant, in this study the EtOAc-soluble fraction has been isolated. In this study one lignanoid compound isoamericanol B (1) (Figure 1), as well as four triterpenoid compounds—cis-3-O-p-hydroxycinnamoyloleanolic acid (2), trans-3-O-p-hydroxy cinnamoyloleanolic acid (3), cis-3-O-p-hydroxycinnamoylursolic acid (4) and trans-3-O-p-hydroxycinnamoyl-ursolic acid (5) (Figure 1) have been isolated for the first time from the leaves of Elaeagnus oldhamii Maxim. The isolation and detailed structural elucidation of compound 1 and the anti-inflammatory activity of the five isolates are described herein.
2. Results and Discussion
The lignanoid compound isoamericanol B (1) has been isolated from Elaeagnus lanceolata Warb. ex Diels [13]. Even though this compound has been isolated in previous study, we provide here more detailed NMR spectrum information to elucidate the structure of isoamericanol B (1) more clearly and completely in this study.
Isoamericanol B (1), [α]23 D +32.1° (c 0.2, MeOH), was isolated as a yellowish oil. Its molecular formula was assigned as C19H20O5 on the basis of the HR-ESI-MS pseudo molecular peak at m/z 327.1226 [M−H]− (calc. mass for 327.1232). The IR spectrum showed an OH band (3,406 cm−1), and phenyl and olefin groups (3,078, 1,626, 1,610, 1,560, 1,518 and 1,506 cm−1). The UV absorption at λmax 258.7 nm indicated a conjugated double band and an aromatic group.
The 13C-NMR spectrum showed the signals for 19 carbons (Table 1). After deducting one methoxyl carbon from the total of 19 carbons, the remaining 18 carbons consisted of twelve aromatic carbons, two olefinic carbons, and four sp3 carbons to form the structure of lignanoid compound.

Table 1.
NMR data (CD3COCD3) of 1. δ in ppm, J in Hz.
| Position | δH | δC |
|---|---|---|
| 1 | - | 129.39 |
| 2 | 7.06 (d, J = 1.6, 1H) | 111.30 |
| 3 | - | 148.50 |
| 4 | - | 147.57 |
| 5 | 6.87 (d, J = 8.2, 1H) | 117.92 |
| 6 | 6.92 (dd, J = 8.2, 1.6, 1H) | 120.30 |
| 7 | 5.18 (d, J = 2.6, 1H) | 78.13 |
| 8 | 4.60 (qd, J = 6.6, 2.6, 1H) | 73.81 |
| 9 | 1.08 (d, J = 6.6, 3H) | 13.71 |
| Phenyl-OMe | 3.83 (s, 3H) | 56.48 |
| Phenyl-OH | 7.69 (s, H) | - |
| 1′ | - | 132.77 |
| 2′ | 6.87 (d, J = 1.8, 1H) | 118.25 |
| 3′ | - | 143.94 |
| 4′ | - | 142.57 |
| 5′ | 6.86 (d, J = 8.2, 1H) | 115.92 |
| 6′ | 6.79 (dd, J = 8.2, 1.8, 1H) | 123.45 |
| 7′ | 6.38 (d, J = 11.8,1H) | 129.64 |
| 8′ | 5.76 (m, 1H) | 131.54 |
| 9′ | 4.38 (d, J = 5.0, 2H) | 59.83 |
| OH | 3.89 (br s, H) | - |
1H-NMR spectral signals at δ 6.38 (1H, d, J = 11.8 Hz), δ 5.76, (1H. m), δ 4.38 (2H, d, J = 5.0 Hz), δ 3.89 (OH, exchangeable to D2O) and three 13C-NMR signals at δ 129.64, δ 131.54 and δ 59.83 consisted were observed. The COSY spectrum showed that signal of δ 5.76 has correlations with δ 6.38 and δ 4.38, and the HMBKC spectrum (Figure 2) showed the signal at δ 4.38 has a correlation with δ 131.54 (C-8′) and δ 129.64 (C-7′). The evidence confirmed the presence of a 1-hydroxy-2-propenyl moiety which is one of the C3 units attached at one of the aromatic rings.
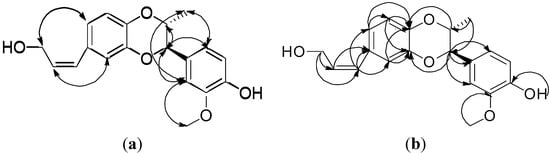
Figure 2.
Key NOESY (↔) (a) correlations and HMBC connectivities (→) (b) of compound 1.
One set of ABX system 1H-NMR signals at δ 6.87 (1H, d, J = 1.8 Hz, H-2′), δ 6.86 (1H, d, J = 8.2 Hz, H-5′), δ 6.79 (1H, dd, J = 8.2, 1.8 Hz, H-6′) were were revealed from COSY correlation. 13C-NMR signals at δ 118.25, δ 115.92 and δ 123.45 were assigned as C-2′, C-5′ and C-6′ due to the corresponding HMQC correlation, and C-1′ was confirmed at δ 132.77 from the HMBC correlation with H-7′ (δ 6.38) and H-8′ (δ 5.76). The NOESY correlation (Figure 2) (H-7′/H-2′, H-6′; H-9′/H-2′, H-6′) was further proof of the location of 1-hydroxy-2-propenyl moiety at the C-1′ position. HMBC correlations between H-2′ to C-3′ (δ 143.94), C-4′ (δ 142.57), between H-5′ to C-3′ (δ 143.94), C-4′ (δ 142.57) and between H-6′ to C-5′ (δ 115.92), C-4′ (δ 142.57) were also observed. Based on the above evidence, one of monolignan units was proved to be cis-cinnammyl alcohol with C-3′ (δ 143.94), and C-4′ (δ 142.57) showing an ortho-dioxygenation pattern.
The aromatic ring on another monolignol contained a set of ABX coupling systems and an ortho-dioxygenated function which was revealed from following NMR spectral signals: two aromatic carbons at δ148.50 and δ147.57 (lower field than δ 150) were judged as ortho-dioxygenated aromatic carbons. A methoxy group (δH 3.83, 3H, s) and a phenolic hydroxy group (δH 7.69, 1H, s, exchangeable with D2O) attached at δ 148.50 and δ 147.57, respectively were proven by HMBC correlations. Three phenyl protons present at δH 7.06 (1H, d, J = 1.6 Hz), 6.87 (1H, d, J = 8.2 Hz) and 6.92 (1H, dd, J = 8.2, 1.6 Hz) resonated at δC 111.30, 117.92, and 120.30, respectively, from the HMQC experiments, and were also seen by COSY correlations.
The remaining C-4 aromatic carbon at δ 129.39 showed it was substituted with an alkyl group. The δH 7.06 signal was assigned at C-2 which was located between the methoxyl and alkyl groups due to the NOSEY correlation with the methoxy group and a meta-coupling constant (J = 1.6 Hz) with H-5. δH 6.92 with doublet of doublet coupling constant (J = 8.2, 1.6 Hz), and δH 6.87 with a doublet coupling constant (J = 8.2 Hz) can be assigned to the two protons detected at C-6 and C-5, respectively. The δH 6.87 signal at higher field than the other two phenyl protons indicated this phenyl proton was ortho to a phenolic hydroxyl group. Three remaining sp3 13C-NMR signals of a propyl group presented at δC 78.13 (C-7), 73.81 (C-8), and 13.71 (C-9), and C-7 and C-8 were proposed to connect with an oxygen atom due to their lower field shift. There corresponding protons at δH at 5.18 (1H. d, J = 2.6 Hz, H-7), δH 4.60 (1H, qd, J = 6.6, 2.6 Hz, H-8) and 1.08 (3H, d, J = 6.6 Hz, H-9) were judged by the HMQC correlation. The COSY spectrum clarified the contiguous sequence. The signal at δH 5.18 (H-7) exhibited a HMBC correlation to C-1 (δC 129.39), C-2 (δC 111.30), and C-6 (δC 120.30). From the abovementioned evidence, the propyl residue was proposed to be linked at the para- position with respect to the hydroxyl group. In addition, the correlation between H-8 to C-4′ and H-7 to C-3′ allowed us to conclude that compound 1 is a 1,4-dioxane-type (8.O.4′7.O.3′) lignan.
As for the relative stereochemistry of isoamericanol B (1), it was judged as a cis-configuration based on the following evidence: The small coupling constant (J = 2.6 Hz) between H-7 and H-8 may be assigned as diequatorial or one axial and one equatorial. If H-7 and H-8 were positioned with a diequatorial orientation, it would be a trans-orientation and the methyl group and aromatic group will be in a diaxial position. In this situation. H3-9 would not give any NOESY correlation with the phenyl protons, but H-6 and H-2 exhibit NOESY correlations to H3-9, therefore, the cis-configuration of 1 was unambiguously confirmed. Therefore, the structure of isoamericanol B (1) was elucidated as shown in Figure 1.
The 1H- and 13C-NMR spectra of the four triterpenoid compounds 2–5, including cis-3-O-p-hydroxycinnamoyloleanolic acid [14], trans-3-O-p-hydroxycinnamoyl-oleanolic acid [15], cis-3-O-p-hydroxycinnamoylursolic acid [16] and trans-3-O-p-hydroxycinnamoylursolic acid [17] were compared with the spectral data reported in the literature, thus confirming their structures.

Table 2.
Cell viability and effect of compounds 1–5 on LPS-induced NO production in macrophages a.
| Compound | Dose (μg/mL) | Cell Viability (% of Control) | No Level | NO Inhibition (% of Control) | IC50 (μg/mL) |
|---|---|---|---|---|---|
| Control | (−) | 98.2 ± 4.4 | −0.1 ± 0.3 | ± | |
| LPS | (+) | 100.5 ± 4.0 | 30.3 ± 2.2 ### | ± | |
| 1 | 2.5 | 100.6 ± 5.9 | 17.6 ± 0.5 *** | 42.0 ± 1.6 | |
| 5 | 97.6 ± 4.3 | 17.5 ± 08 *** | 42.3 ± 2.6 | ||
| 10 | 90.7 ± 3.3 | 15.2 ± 0.4 *** | 49.7 ± 1.4 | 10.3 ± 0.4 | |
| 20 | 88.8 ± 2.6 | 12.0 ± 0.4 *** | 60.4 ± 1.3 | ||
| 2 | 2.5 | 87.2 ± 2.0 | 19.7 ± 1.4 ** | 35.1 ± 4.6 | |
| 5 | 77.9 ± 5.0 | (−) | (−) | ||
| 10 | 43.4 ± 1.6 | (−) | (−) | ||
| 20 | 28.0 ± 2.9 | (−) | (−) | ||
| 3 | 2.5 | 87.6 ± 5.6 | 23.5 ± 1.4 ** | 22.5 ± 4.6 | |
| 5 | 86.6 ± 4.2 | 21.0 ± 0.7 *** | 30.7 ± 2.2 | ||
| 10 | 74.1 ± 3.9 | (−) | (−) | ||
| 20 | 49.7 ± 8.2 | (−) | (−) | ||
| 4 | 2.5 | 90.8 ± 5.4 | 23.0 ± 2.8 *** | 24.2 ± 9.1 | |
| 5 | 87.4 ± 3.1 | 20.8 ± 1.8 *** | 31.3 ± 5.8 | ||
| 10 | 70.3 ± 3.5 | (−) | (−) | ||
| 20 | 47.6 ± 9.7 | (−) | (−) | ||
| 5 | 2.5 | 93.0 ± 8.6 | 20.5 ± 2.6 *** | 32.3 ± 8.6 | |
| 5 | 88.8 ± 5.7 | 19.5 ± 1.8 *** | 35.6 ± 6.1 | ||
| 10 | 88.5 ± 7.4 | 18.4 ± 0.8 *** | 39.3 ± 2.6 | >20 | |
| 20 | 86.5 ± 6.1 | 17.2 ± 0.9 *** | 43.0 ± 3.1 |
a The data were presented as mean ± S.D. for three different experiments performed in triplicate; ### compared with sample of control group; ** p < 0.01, and *** p < 0.001 were compared with LPS-alone group.
Macrophages play an important role in the host defense system. Bacterial lipopolysaccharide (LPS) could stimulate macrophages to secret inducible nitric oxide synthases (iNOS), NO (nitric oxide) production and the release of pro-inflammatory mediators further [18]. On the other hands, NO has the critical role of regulating pro-inflammatory release during inflammatory processes [19]. In this study, the anti-inflammatory activity the compounds 1 to 5 isolated from E. oldhamii Maxim. was tested. Of these compounds 1 was estimated to decrease nitrate of LPS-stimulated production in RAW264.7 cells with an IC50 value of 10.3 ± 0.4 μg/mL. Cell viability was also evaluated (Table 2).
3. Experimental
3.1. General
UV spectra were obtained with a Shimadzu Pharmaspec-1700 (Taichung, Taiwan) UV-Visible spectrophotometer. Optical rotations were obtained with a Jasco P-1020 (Taichung, Taiwan) polarimeter. Infrared spectra were obtained with a Shimadzu IR prestige-21 Fourier transform infrared spectrophotometer. 1D- and 2D-NMR spectra were recorded with a Bruker DRX-500 FT-NMR (Taichung, Taiwan) spectrometer. Mass spectrometric (HREIMS) data were generated at the Mass Spectrometry Laboratory of the Chung Hsing University (Taichung, Taiwan). Column chromatography was performed using LiChroCART Si gel (5 μM; Taichung, Taiwan), and TLC analysis was carried out using aluminum pre-coated Si plates and the spots were visualized using a UV lamp at λ = 254 nm.
3.2. Collection, Extraction and Isolation
E. oldhamii Maxim. was collected from Jin-Shun Chen herbal garden (Nantou, Taiwan) as described in Flora of Taiwan [1]. A plant specimen has been deposited in the School of Chinese Pharmaceutical Sciences and Chinese Medicine Resources. The materials were totally dried in air under dark. Dried leaves of E. oldhamii Maxim. (10.0 kg) were cut into small pieces and soaked in methanol (70 L, 7 days × 3). After filtration, the combined extract was concentrated under reduced pressure to give a dried extract (801.0 g). The dried extract was suspended in H2O (2 L) and extracted with ethyl acetate (2 L, 5 times). The resulting ethyl acetate extract was concentrated to yield 302.5 g of an brown-green thick oil that was purified by 2.2 kg silica gel with particle size 0.063–0.200 mm and internal diameter of column 10 cm packed height 50 cm chromatography with using a gradient of increasing polarity with total n-hexane to total ethyl acetate as mobile phase and separated into 20 fractions on the basis of TLC analysis for random isolation of compounds. Fraction 14 (11.40 g) was re-separated by Sephadex LH 20 column chromatography (chloroform–methanol = 3:7), silica gel column chromatography (n-hexane:acetone = 1:1) and semi-preparative normal phase HPLC (n-hexane:ethyl acetate = 1:1) to afford pure compound 1 (11.8 mg). Fraction 8 (3.85 g) was re-separated by silica gel column chromatography (n-hexane-ethyl acetate = 8:2) and semi-preparative HPLC (n-hexane:acetone = 8:2) to afford pure compounds 2 (22.1 mg), 3 (12.5 mg), 4 (52.0 mg) and 5 (5.3 mg).
3.3. Isoamericanol B (1)
Yellowish oil; {[α]23D +32.1° (c 0.2, MeOH)}; HR-ESI-MS m/z: 327.1226 [M−H]− (calcd. for C19H20O5, 327.1232), UV (MeOH) λmax (log ε ):258.7 (4.13) nm. IR (KBr) νmax: hydroxyl band (3,406 cm−1), phenyl and olefinic groups (3,078, 1,626, 1,610, 1,560, 1,518 and 1,506 cm−1). 1H-NMR and 13C-NMR (500/125 MHZ, in CD3COCD3) were shown on Table 1.
3.4. Chemicals
The solvent used to open column isolation (Sephadex LH 20 and silica gel column) in the study, such as n-hexane, chloroform, ethyl acetate, acetone and methanol were all ACS grade. The HPLC grade n-hexane, ethyl acetate and acetone for HPLC isolation and the deuteriated solvent, acetone-d6, for NMR measurement were purchased from the Merck branch in Taipei, Taiwan. LPS (endotoxin from Escherichia coli, serotype 0127:B8), Carr (type IV), indomethacin, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
3.5. Cell Culture
A murine macrophage cell line RAW264.7 (BCRC No. 60001) was purchased from the Bioresources Collection and Research Center (BCRC, Hsinchu, Taiwan) of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were cultured in plastic dishes containing Dulbecco’s Modified Eagle Medium (DMEM, Sigma) supplemented with 10% fetal bovine serum (FBS, Sigma) in a CO2 incubator (5% CO2 in air) at 37 °C and subcultured every 3 days at a dilution of 1:5 using 0.05% trypsin-0.02% EDTA in Ca2+-, Mg2+-free phosphate-buffered saline (DPBS).
3.6. Cell Viability
Cells (2 × 105) were cultured in 96-well plate containing DMEM supplemented with 10% FBS for 1 day to become nearly confluent. Then cells were cultured with compounds 1–5 in the presence of 100 ng/mL LPS (lipopolysaccharide) for 24 h. After that, the cells were washed twice with DPBS and incubated with 100 μL of 0.5 mg/mL MTT for 2 h at 37 °C testing for cell viability. The medium was then discarded and 100 μL dimethyl sulfoxide (DMSO) was added. After 30-min incubation, absorbance at 570 nm was read using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
3.7. Measurement of Nitric Oxide/Nitrite
NO production was indirectly assessed by measuring the nitrite levels in the cultured media and serum determined by a colorimetric method based on the Griess reaction. The cells were incubated with different concentration of samples in the presence of LPS (100 ng/mL) at 37 °C for 24 h. Then, cells were dispensed into 96-well plates, and 100 μL of each supernatant was mixed with the same volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride and 5% phosphoric acid) and incubated at room temperature for 10 min, the absorbance was measured at 540 nm with a Micro-Reader (Molecular Devices). By using sodium nitrite to generate a standard curve, the concentration of nitrite was measured form absorbance at 540 nm.
3.8. Statistical Analysis
All data of IC50 values in each concentration (5, 10, 15 and 20 μg/mL) were expressed as mean ± SD (n = 4). IC50 values were estimated using a non-linear regression algorithm (Sigma Plot 12.0; SPSS Inc. Chicago, IL, USA). Statistical evaluation was carried out by one-way ANOVA followed by Scheffe’s multiple range tests.
4. Conclusions
The compound isoamericanol B (1), a lignanoid compound, which has been isolated for the first time from the leaves of Elaeagnus oldhamii Maxim. showed an excellent anti-inflammatory activity by decreasing nitrate of LPS-stimulated production in RAW264.7 cell with IC50 values of 10.3 ± 0.4 μg/mL. Compounds 2–4 also displayed an anti-inflammatory activity by inhibiting NO production. As to anti-inflammatory activity, compound 2 is stronger than compounds 3 and 4 is stronger than compound 5. The differences in anti-inflammatory activity may result from the cis- or trans- forms of the 3-O-p-hydroxycinnamoyl side chains of the oleanolic acid (compounds 2 and 3) and ursolic acid (compounds 4 and 5) structures. However, in the cell viability test, compound 1 is the only one that did not change significantly cell viability at the tested concentrations (2.5 to 20 μg/mL) among the compounds 1 to 4. Therefore, for the anti-inflammatory effect and safety reasons, compound 1 may be a useful lead for the development of novel non-steroidal anti-inflammatory drugs.
Acknowledgments
This work was kindly supported by a grant from the Taiwan Department of Health Clinical Trials and Research Center of Excellence (DOH-102-TD-B-111-004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, T.S. Elaeagnaceae. In Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1998; Volume 3, pp. 753–759. [Google Scholar]
- Ahmad, S.D.; Sabir, M.S.; Juma, M.; Asad, H.S. Morphological and biochemical variations in Elaeagnus umbellata Thunb. from mountains of Pakistan. Acta Bot. Croat. 2005, 64, 121–128. [Google Scholar]
- Yuebin, G.; Liu, J.; Su, D. In vivo evaluation of the anti-asthmatic, antitussive and expectorant activities of extract and fractions from Elaeagnus pungens leaf. J. Ethnopharmacol. 2009, 126, 538–542. [Google Scholar]
- Ahmadiani, A.; Hosseiny, J.; Semnanian, S.; Javan, M.; Saeedi, F.; Kamalinejad, M.; Saremi, S. Antinociceptive and anti-inflammatory effects of Elaeagnus angustifolia fruit extract. J. Ethnopharmacol. 2000, 72, 287–292. [Google Scholar] [CrossRef]
- Mehrabani Natanzi, M.; Pasalar, P.; Kamalinejad, M.; Dehpour, A.R.; Tavangar, S.M.; Sharifi, R.; Ghanadian, N.; Rahimi-Balaei, M.; Gerayesh-Nejad, S. Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Med. Iran 2012, 50, 589–596. [Google Scholar]
- Lee, Y.S.; Chang, Z.Q.; Oh, B.C.; Park, S.C.; Shin, S.R.; Kim, N.W. Antioxidant activity, anti-inflammatory activity, and whitening effects of extracts of Elaeagnus multiflora Thunb. J. Med. Food 2007, 10, 126–133. [Google Scholar]
- Fu, Y.C.; Wang, X.J. Advances on chemical constituents and pharmacological activities from plants of Elaeagnus L. Qilu Pharmaceutical Affairs 2007, 26, 232–233. [Google Scholar]
- Lou, F.M.; Yang, J.; Bai, Z.C.; Wu, B.F. Studies on chemical constituents in rhizome of Elaeagnus bockii. Zhongguo Zhong Yao Za Zhi 2006, 31, 988–989. [Google Scholar]
- Ayaz, M.; Riaz, M.; Malik, A.; Ahmad, E.; Fatima, I. Elaeagnoside, chymotrypsin inhibiting steroidal glucoside from Elaeagnus orientalis. Nat. Prod. Res. 2009, 23, 409–414. [Google Scholar] [CrossRef]
- Ge, Y.B.; Li, M.S.; Mei, Z.N.; Yang, G.Z. Two new flavonol glycosides from the leaves of Elaeagnus pungens. J. Asian Nat. Prod. Res. 2013. [Google Scholar] [CrossRef]
- Wu, Y.B.; Gu, Y.; Ouyang, M.A. Water-soluble constituents from the bark of Elaeagnus pungens and their cytotoxic activities. J. Asian Nat. Prod. Res. 2010, 12, 278–285. [Google Scholar] [CrossRef]
- Liao, C.R.; Chang, Y.S.; Peng, W.H.; Lai, S.C.; Ho, Y.L. Analgesic and anti-inflammatory activities of the methanol extract of Elaeagnus oldhamii Maxim. in mice. Am. J. Chin. Med. 2012, 40, 581–597. [Google Scholar] [CrossRef]
- Song, W.W.; Li, B.; Liu, J.K. A new lignan from Elaeagnus lanceolata (Elaeagnaceae). Yunnan Zhiwu Yanjiu 2010, 32, 455–462. [Google Scholar]
- Ishikawa, T.; Seki, M.; Nishigaya, K.; Miura, Y.; Seki, H.; Chen, I.S.; Ishii, H. Studies on the chemical constituents of Xanthoxylum nitidum (Roxb.) D. C. (Fagara nitida Roxb.). III. The chemical constituents of the wood. Chem. Pharm. Bull. 1995, 43, 2014–2018. [Google Scholar] [CrossRef]
- Takahashi, H.; Luchi, M.; Fujita, Y.; Minami, H.; Fukuyama, Y. Coumaroyl triterpenes from Casuarina equisetifolia. Phytochemistry 1999, 51, 543–550. [Google Scholar]
- He, X.; Liu, R.H. Cranberryphytochemicals: Isolation, structureelucidation, and their antiproliferative and antioxidant activities. J. Agric. Food Chem. 2006, 54, 7069–7074. [Google Scholar] [CrossRef]
- Katai, M.; Terai, T.; Meguri, H. Triterpenoids of the barks of Pieris japonica D. Don (Japanese name: Asebi). II. 13C nuclear magnetic resonance of the gamma-lactones of ursane- and oleanane-type triterpenes. Chem. Pharm. Bull. 1983, 31, 1567–1571. [Google Scholar] [CrossRef]
- Pan, C.; Kim, E.S.; Jung, S.H.; Nho, C.W.; Lee, J.K. TectorigenininhibitsIFN-gamma/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells. Arch. Pharm. Res. 2008, 31, 1447–1456. [Google Scholar]
- Lee, Y.J.; Eun, J.R. Cilostazoldecreasesethanol-mediatedTNF alphaexpression in RAW264.7 murine macrophage and in liver from binge drinking mice. Korean J. Physiol. Pharmacol. 2012, 16, 131–138. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).