Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment
Abstract
:1. Background
2. Turmeric History
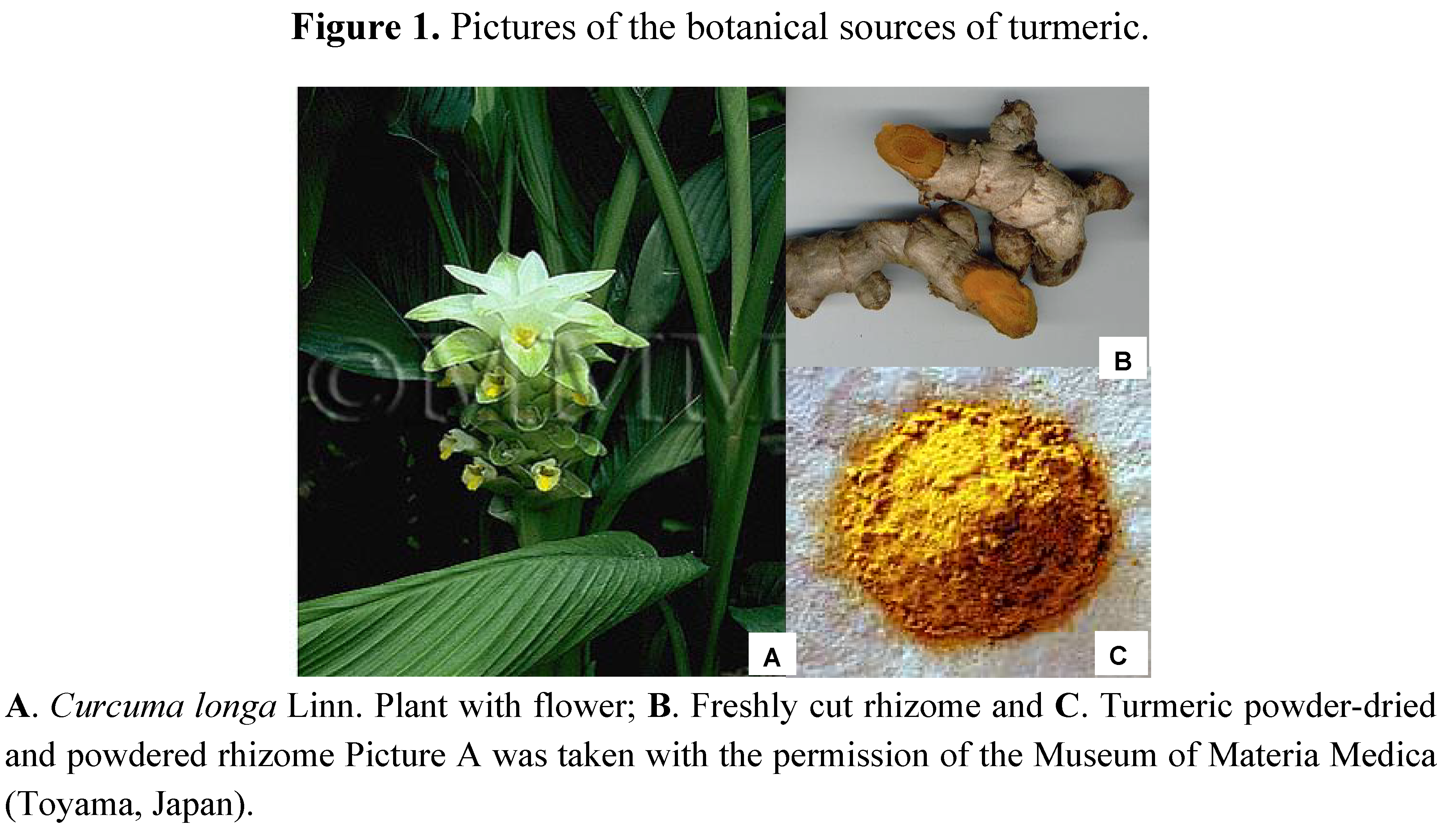
3. Curcumin Chemistry
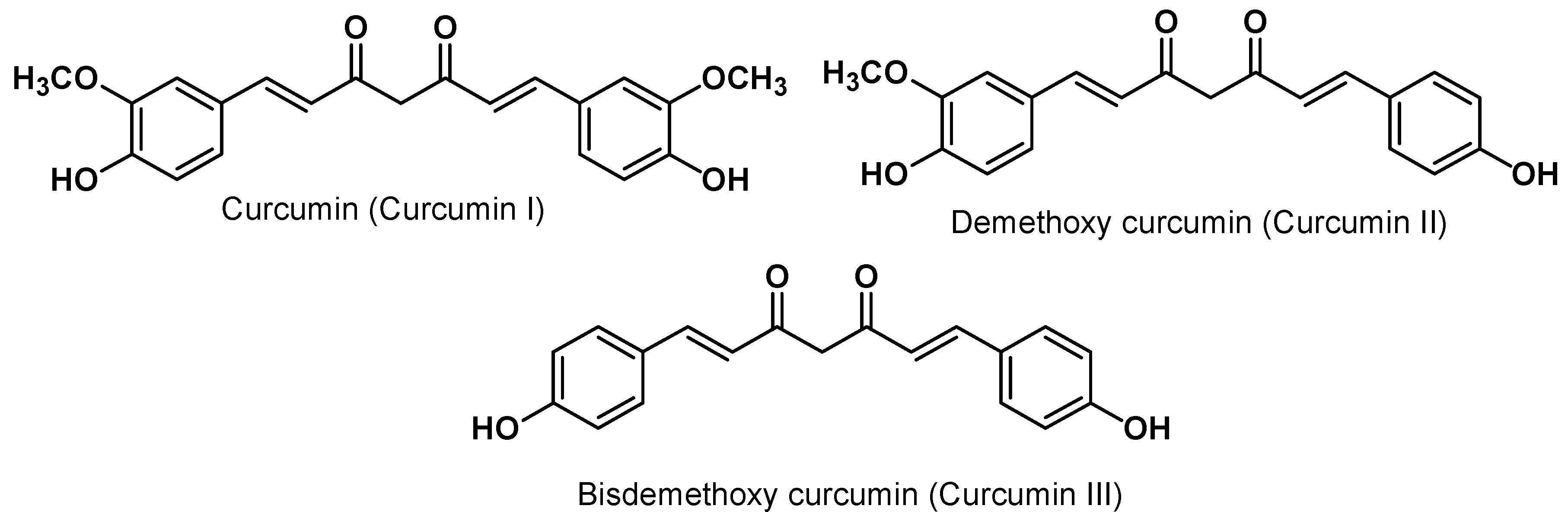
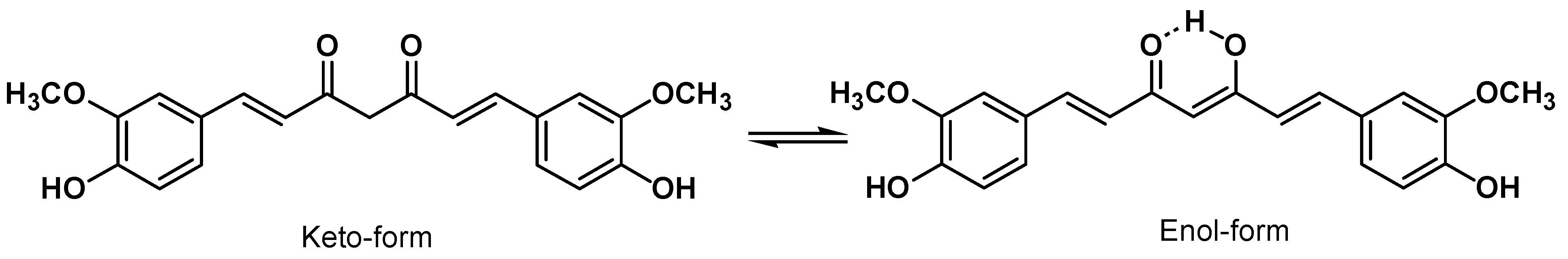
4. Curcumin Safety and Toxicity
5. Curcumin Bioavailability
5.1. Animal Model Pharmacokinetics
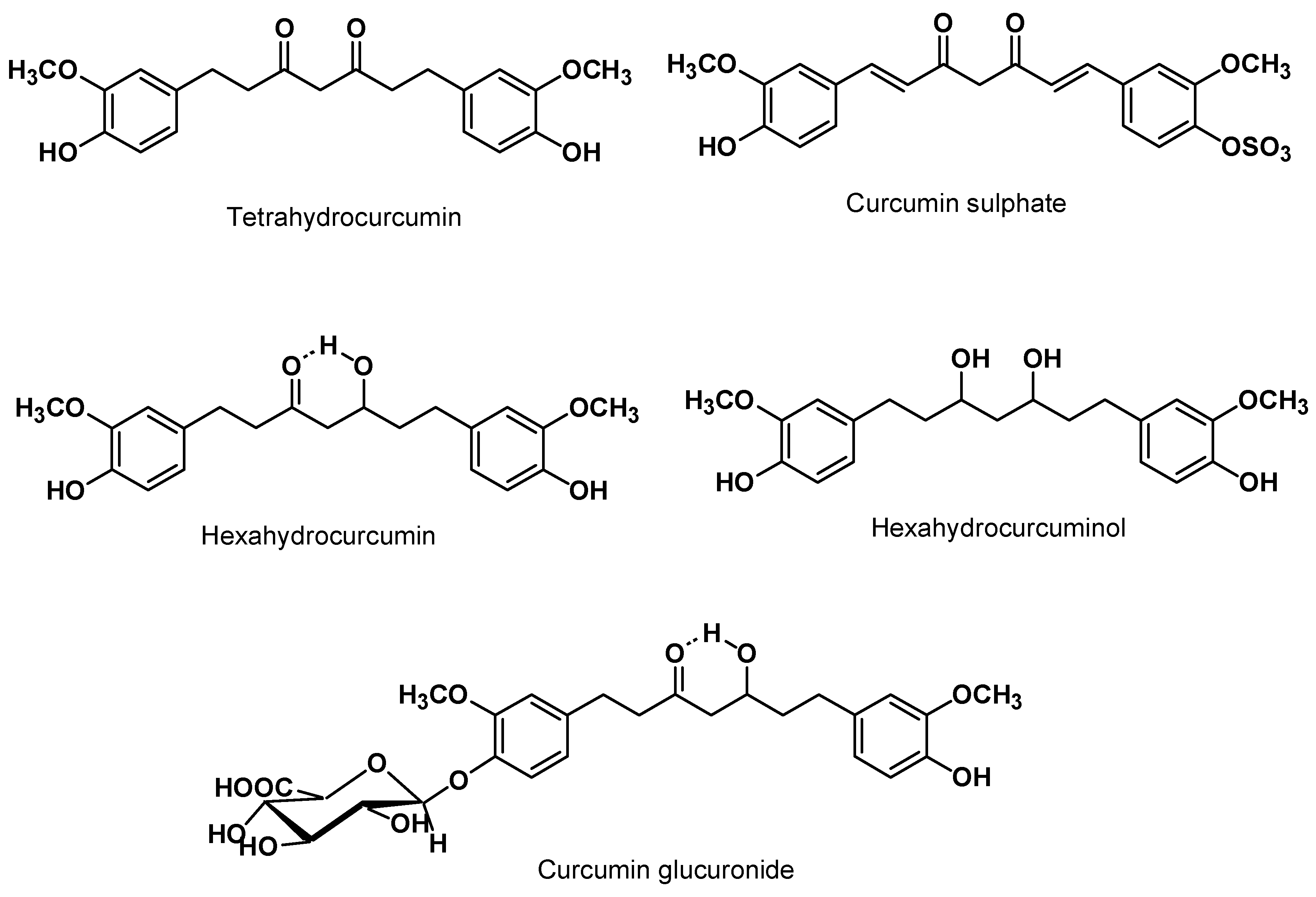
5.2. Clinical Pharmacokinetics
6. Curcumin in Inflammation and Cancer
6.1. Inflammation and Cancer
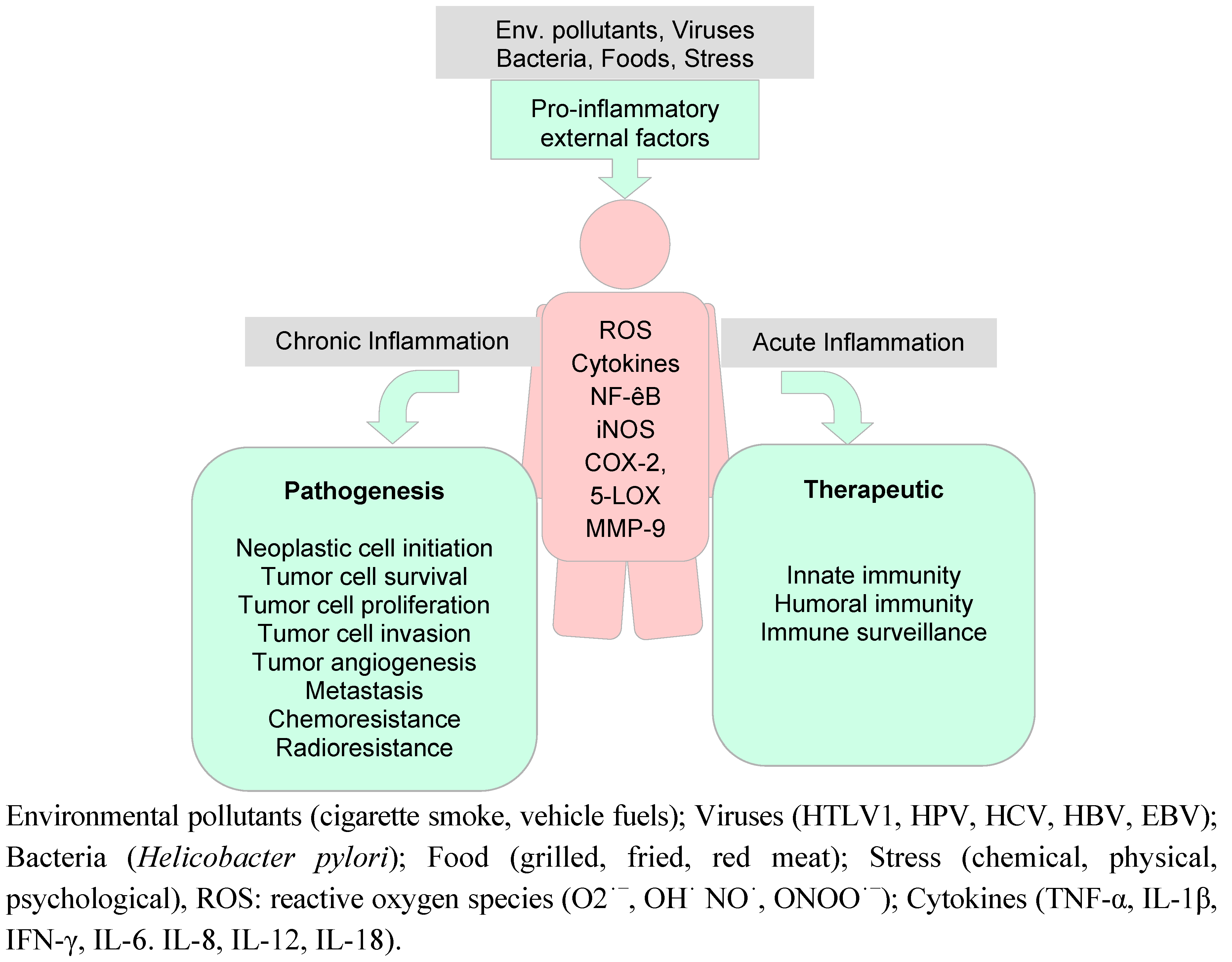
6.2. Preclinical Anti-Oxidant and Anti-Inflammatory Activities on Curcumin
6.2.1. Anti-oxidant activity
6.2.2. Anti-inflammatory effects by inhibition of arachidonic acid pathways
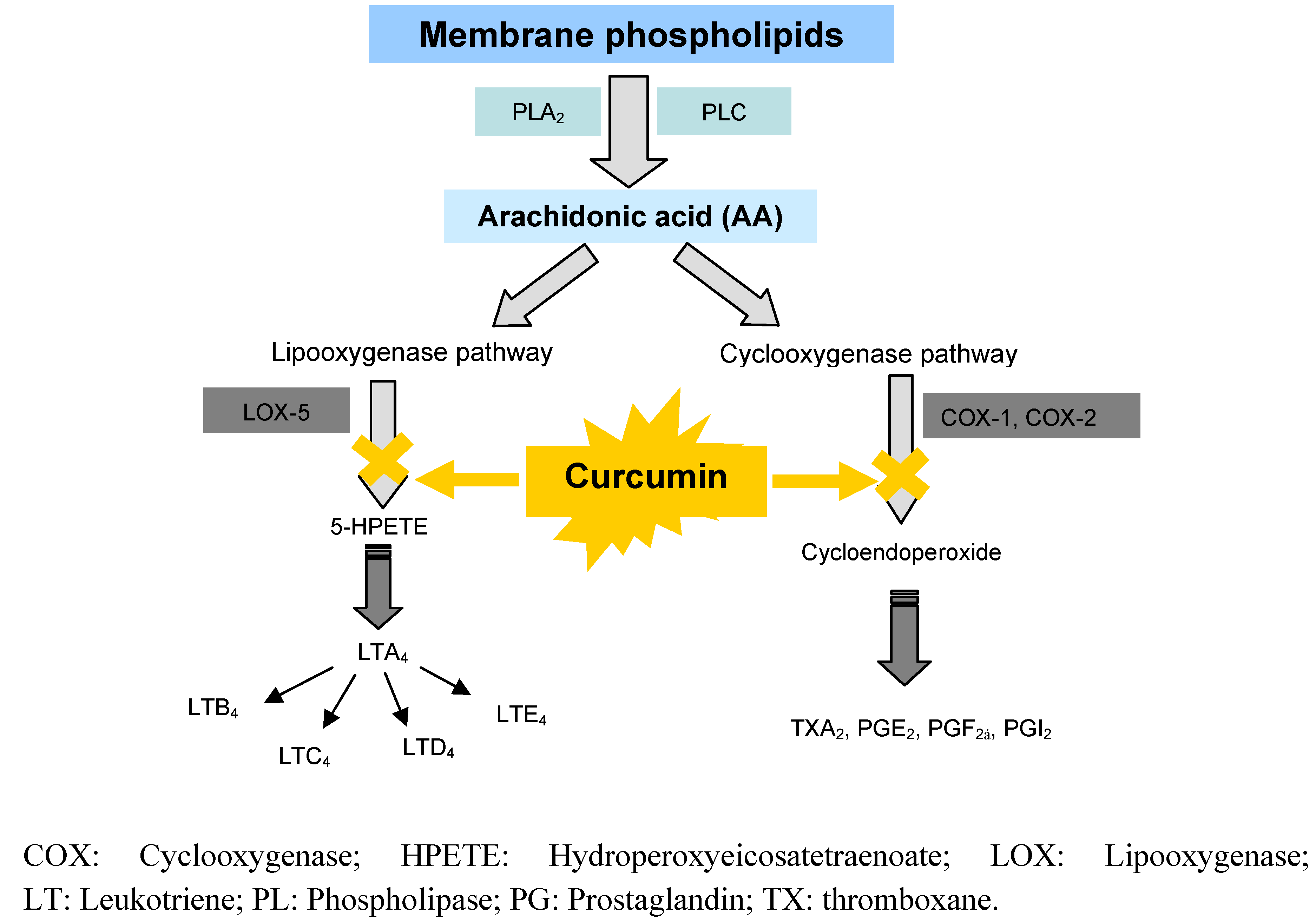
6.3. Clinical Studies on Curcumin
6.3.1. Anti-oxidant activity
6.3.2. Anti-inflammatory activity
6.3.3. Clinical studies: Anti-cancer effects
7. Recent Advancements on Curcumin Formulations and Delivery Systems
- i) Polymeric implantable delivery systems for curcumin: Bansal et al. prepared curcumin in poly-(ε-caprolactone)-based implants aimed at subcutaneous grafting and evaluated the implants in rats. The maximum concentration of curcumin in liver was detected on day 4 post-implantation and the plateau was observed after seven days. The study confirmed the potential of polymeric implants to avoid oral route and provide sustained release of incorporated curcumin [123].
- ii) Micelles: injectable curcumin-loaded poly(ethyleneoxide)-b-poly(ε-caprolactone) micelles for controlled delivery of curcumin prepared by Ma et al. confirmed that micelle-encapsulated curcumin retained its cytotoxicity in both mouse melanoma and human mantle cell lymphoma cell lines [135]. Curcumin-loaded poly(D,L-lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(DL-lactide-co-glycolide; PLGA-PEG-PLGA) micelles, developed by Song et al. showed improved area under the curve (AUC) and t1/2 in vivo. Moreover, micelles decreased curcumin uptake by liver and spleen, and at the same time, enhanced distribution of curcumin in lung and brain [136].
- iii) Nano-delivery systems: Nanotechnology and nanomedicine offer potentials for development of nano sized delivery systems for curcumin. Shaikh et al. developed nanoparticles encapsulating curcumin prepared by the emulsion technique. The in vivo pharmacokinetics revealed that nanoparticles-incorportaed curcumin achieved a 9-fold increase in oral bioavailability as compared to curcumin administered with piperine as absorption enhancer [137]. Tsai et al. prepared an optimized polylactic-co-glycolic acid (PLGA) nano-formulation of curcumin which resulted in a 22-fold higher oral bioavailability in rats as compared to conventional curcumin [138]. Curcumin loaded dextran sulphate-chitosan nanoparticles showed preferential killing of cancer cells compared to normal cells, indicating potential in targeting [139].A very promising delivery system appears to be “nanocurcumin”, polymeric nanoparticle-encapsulated curcumin, readily dispersed in aqueous media and with confirmed anti-cancer potentials in preclinical in vivo models. Nanocurcumin retained the mechanistic specificity of free curcumin, inhibiting the activation of the seminal transcription factor NF-κB and reducing steady state levels of pro-inflammatory cytokines like ILs and TNF-α [140]. Nanocurcumin developed by Bhawana et al. and prepared by wet-milling technique, in size range of 2–40 nm was shown to express stronger antimicrobial potential. It remains to be seen whether the same nanocurcumin will display enhanced anti-cancer activity as well [141]. A similar approach in reducing the size of curcumin crystals was proposed by Gao et al. to produce nanosupsensions for intravenous delivery [142].Wu et al. developed water-dispersible hybrid nanogels for intracellular delivery of curcumin aiming at photodermal therapy [143]. The hybrid combines optical label (Au/Ag bimetallic nanoparticle, polystyrene gel layer, polyethylene gel and provides potent cytotoxicity against B16F10 cells by combined chemo-phototermal treatment. Anti-inflammatory activity of curcumin was also found to be enhanced through delivery via o/w nanoemulsions, as evaluated in mouse ear inflammation model. In comparison, Tween-based formulations failed to achieve the same effect [144].
- iv) Although phospholipid-based delivery systems, based on their size, mostly fall in the category of nanomedicine, due to the specificity of the carrier material, phospholipid vesicles are treated here a separate category. Several research groups have proposed curcumin-phospholipid complexes as means to improve curcumin delivery. Complexation of curcumin with phosphatidylcholine resulted in enhanced bioavailability, improved pharmacokinetics and increased hepatoprotective activity as compared to physical mixtures of curcumin and phosphatidylcholine [145]. Curcumin formulated with Meriva® (phosphatidylcholine) showed increased bioavailability in rats [146]. Curcumin-phospholipid complex administered orally resulted in higher serum concentrations of curcumin as compared to uncomplexed curcumin. Moreover, the complex maintained the effective concentrations of curcumin over longer period of time [147]. However, the content of curcumin in complexes was found to be limited to about 17 and 32% (w/w), respectively, which is much lower than what could be achieved by liposomal encapsulation of curcumin.Phospholipid vesicles and lipid-nanospheres embedding curcumin improved intravenous delivery of curcumin to tissue macrophages, especially bone marrow and spleen macrophages [148]. Solid lipid nanoparticles (SLN) were also proposed as mean to enhance oral bioavailability of curcumin. Pharmacokinetic profile of curcumin on SLN in rats showed significant improvement as compared to solubilized curcumin [149]. In order to further enhance anti-cancer potential of curcumin, transferrin-mediated SLN containing curcumin were developed and their superiority confirmed in breast cancer cells [150]. Solid lipid nanoparticles were proposed for topical application of curcumin as well [151]. Solid lipid nanoparticles developed by Yadav et al., were evaluated in rat model of inflammatory bowel disease and showed enhanced anti-angiogenic and anti-inflammatory activities [152].Probably one of the most studied delivery systems for curcumin delivery is liposomes. Liposomes are well established delivery system able to incorporate poorly soluble drugs and enable their aqueous medium-based administration [153]. Curcumin is expected to accommodate itself inside the hydrophobic interior of liposomes, resulting in higher drug loading capacity [154]. Liposomal curcumin was also reported to have higher stability than free curcumin in phosphate buffer saline, human blood, plasma and RPMI-1640 medium with 10% calf serum [155]. Li et al. developed liposomal delivery system for curcumin aiming at intravenous administration. Liposomal curcumin consistently suppressed NF-κB binding and decreased the expression of NF-κB-regulated gene products, including COX-2 and IL-8, both of which have been implicated in tumour growth/invasiveness. The activity of liposomal curcumin was equal to or better than that of free curcumin at equimolar concentration. Antitumor and anti-angiogenesis effects were suppressed in vivo and based on their results the authors propose that liposomal curcumin for systemic delivery provides the rationale for the treatment of patients suffering from pancreatic carcinoma [156]. Li et al. developed liposomal curcumin which showed dose-dependent growth inhibition and apoptosis in the two human colorectal cancer cell lines (LoVo and Colo205 cells) and synergetic effect with oxaliplatin, a standard chemotherapy for the malignancy. In in vivo study, liposomal curcumin significantly inhibited tumour growth in Colo205 and LoVo xenografts in mice [157]. Thangapazham et al. developed liposomal delivery system for curcumin in which liposomes were coated with prostate membrane specific antigen specific antibodies to achieve targeting. The superiority of such a system was evaluated in two human prostate cancer cell lines. Antibody-coated liposomes showed 10-fold more anti-proliferative activity in human prostate cancer cell lines (LNCaP and C4-2B) compared to non-liposomal curcumin. It was also observed that LNCaP cells were relatively more sensitive to liposomal curcumin than C4-2B cells [158]. Wang et al. reported on liposomal formulation of curcumin able to suppress the growth of head and neck squamous cell carcinoma (HNSCC) in in vitro study in dose-dependent manner and also able to suppress the activation of NF-κB without affecting the expression of pAKT. The expression of cyclin D1, COX-2, MMP-9, Bcl-2, Bcl-xL, Mcl-1L and Mcl-1S were reduced. Nude mice xenograft tumours were suppressed after 3.5 weeks of treatment with i.v. liposomal curcumin, and no demonstrable toxicity of liposomal curcumin was detected. The authors speculated that liposomal curcumin is a viable non-toxic therapeutic agent for HNSCC [159]. Takahashi et al. developed liposomal delivery system for oral administration of curcumin, incorporating up to 68% of curcumin. Faster rate and better absorption after oral administration in rats where achieved as compared to non-liposomal curcumin. These results indicated that liposomal encapsulation enhanced the gastrointestinal absorption of curcumin [160]. A liposome-based intravenous formulation of bis-demethoxy curcumin analogue showed better hepatoprotective activity comparing to its free form [161]. Narayanan et al. proposed interesting approach for the treatment of prostatic adenocarcinoma. Combination of liposomal forms of curcumin and resveratrol significantly decreased prostatic adenocarcinoma in vivo. In vitro studies revealed that curcumin in combination with resveratrol effectively inhibited cell growth and induced apoptosis. These findings suggested that liposomal phytochemicals-in-combination may reduce prostate cancer incidence [162]. Mourtas et al. proposed novel curcumin-decorated nanoliposomes with very high affinity for amyloid-β-42 peptide as vectors for targeted delivery of Alzheimer disease treatment. This approach opens the possibility to further explore the potential of vesicle-surface available curcumin in various cancer treatments [163].
8. Conclusions
| Therapeutic targeting | Effects on |
|---|---|
| Selective anti-inflammatory drugs | Tumour promoting inflammation |
| Telomerase inhibitors | Enabling replicate immortality |
| Inhibitors of HGF/c-Met | Activating invasion and metastasis |
| Inhibitors of VEGF signalling | Inducing anti-angiogenesis |
| Inhibitors of PARP | Genome instability and mutation |
| Proapoptic BH3 mimetics | Resisting cell death |
| Aerobic glycolysis inhibitors | Deregulating cellular energetics |
| EGFR inhibitors | Sustaining proliferative signaling |
| Cyclin-dependent kinase inhibitors | Evading growth suppressor |
| Immune activating anti-CTLA4 mAb | Avoiding immune destruction |
Acknowledgments
References
- Conney, A.H. Enzyme induction and dietary chemicals as approaches to cancer chemoprevention: The seventh DeWitt S. Goodman lecture. Cancer Res. 2003, 63, 7005–7031. [Google Scholar]
- Advances in experimental medicine and biology. In The Molecular Target and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B.; Surh, Y.J.; Shishodia, S. (Eds.) Springer: New York, NY, USA, 2007.
- National Cancer Institute. Clinical development plan: Curcumin. J. Cell. Biochem. Suppl. 1996, 26, 72–85.
- Oppenheimer, A. Turmeric (curcumin) in biliary diseases. Lancet 1937, 229, 619–621. [Google Scholar] [CrossRef]
- US National Institute of Health. Available online: http://clinicaltrials.gov/ct/search?term=curcumin/ accessed on 23 March 2011.
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventative and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Sign. 2008, 10, 511–545. [Google Scholar] [CrossRef]
- World Health Organization, Rhizoma curcumae longae. In WHO Monographs on Selected Medicinal Plants; WHO: Geneva, Switzerland, 1999; Volume 1, pp. 115–124.
- Ploto, A. Turmeric: Post-Production Management for Improved Market Access for Herbs and Spices-Turmeric; Food andAgriculture Organization of the United Nations (FAO): Rome, Italy, 2003. [Google Scholar]
- Spices Board of India. Ministry of Commerce. Available online: http://www.indianspices.com/ accessed on 23 March 2011.
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004, 87, 44–53. [Google Scholar]
- Jayaprakasha, G.K.; Rao, L.J.M.; Sakariah, K.K. Chemistry and biological activities of C. longa. Trends Food Sci. Technol. 2005, 16, 533–548. [Google Scholar] [CrossRef]
- Vogel, H.A.; Pelletier, J. Curcumin-biological and medicinal properties. J. Pharma. 1815, 2, 50. [Google Scholar]
- Milobedzka, J.; Kostanecki, V.; Lampe, V. Structure of curcumin. Ber. Dtsch. Chem. Ges. 1910, 43, 2163–2170. [Google Scholar] [CrossRef]
- Roughley, P.J.; Whiting, D.A. Experiments in the biosynthesis of curcumin. J. Chem. Soc. Perkin Trans. 1 1973, 20, 2379–2388. [Google Scholar] [CrossRef]
- Payton, F.; Sandusky, P.; Alworth, W.L. NMR study of the solution structure of curcumin. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar]
- Basnet, P.; Tho, I.; Skalko-Basnet, N. Curcumin, A Wonder Drug of 21st Century: Liposomal Delivery System Targeting Vaginal Inflammation. In 5th International Congress on Complementary Medicine Research, Tromsø, Norway, May 19–21, 2010. Abstract Number A9M2K9C.
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar]
- Lin, J.K.; Pan, M.H.; Lin-Shiau, S.Y. Recent studies on the biofunctions and biotransformations of curcumin. Biofactors 2000, 13, 153–158. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Karlsen, J.; van Henegouwen, G.B. Studies on curcumin and curcuminoids, VIII: Photochemical stability of curcumin. Z. Lebensm. Unters Forsch. 1986, 183, 116–122. [Google Scholar]
- Eigner, D.; Scholz, D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J. Ethnopharmacol. 1999, 67, 1–6. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of turmeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Sharma, R.A.; Ireson, C.R.; Verschoyle, R.D.; Hill, K.A.; Williams, M.L.; Leuratti, C.; Manson, M.M.; Marnett, L.J.; Steward, W.P.; Gescher, A. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: Relationship with drug levels. Clin. Cancer Res. 2001, 7, 1452–1458. [Google Scholar]
- Perkins, S.; Verschoyle, R.D.; Hill, K.; Parveen, I.; Threadgill, M.D.; Sharma, R.A.; Williams, M.L.; Steward, W.P.; Gescher, A.J. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomarker. Prev. 2002, 11, 535–540. [Google Scholar]
- Ganiger, S.; Malleshappa, H.N.; Krishnappa, H.; Rajashekhar, G.; Rao, R.V.; Sullivan, F. A two generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem. Toxicol. 2007, 45, 64–69. [Google Scholar]
- Deodhar, S.D.; Sethi, R.; Srimal, R.C. Preliminary study on anti-rheumatic activity of curcumin (diferuloyl methane). Indian J. Med. Res. 1980, 71, 632–634. [Google Scholar]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Goh, C.L.; Ng, S.K. Allergic contact dermatitis to Curcuma longa (turmeric). Contact Dermatitis 1987, 17, 186. [Google Scholar] [CrossRef]
- Liddle, M.; Hull, C.; Liu, C.; Powell, D. Contact urticaria from curcumin. Dermatitis 2006, 17, 196–197. [Google Scholar] [CrossRef]
- Thompson, D.A.; Tan, B.B. Tetrahydrocurcumin-related allergic contact dermatitis. Contact Dermatitis 2006, 55, 254–255. [Google Scholar] [CrossRef]
- Joshi, J.; Ghaisas, S.; Vaidya, A.; Vaidya, R.; Kamat, D.V.; Bhagwat, A.N.; Bhide, S. Early human safety study of turmeric oil (Curcuma longa oil) administered orally in healthy volunteers. J. Assoc.Phys. India 2003, 51, 1055–1060. [Google Scholar]
- Kim, D.C.; Kim, S.H.; Choi, B.H.; Baek, N.I.; Kim, D.; Kim, M.J.; Kim, K.T. Curcuma longa extract protects against gastric ulcers by blocking H2 histamine receptors. Biol. Pharm. Bull. 2005, 28, 2220–2224. [Google Scholar] [CrossRef]
- Babu, P.S.; Srinivasan, K. Hypolipidemic action of curcumin: the active principle of turmeric (Curcuma longa) in streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 1997, 166, 169–175. [Google Scholar] [CrossRef]
- Fan, C.; Wo, X.; Qian, Y.; Yin, J.; Gao, L. Effect of curcumin on the expression of LDL receptor in mouse macrophages. J. Ethnopharmacol. 2006, 105, 251–254. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh) 1980, 16, 259–265. [Google Scholar]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. In vitro studies on the intestinal absorption of curcumin in rats. Toxicology 1981, 20, 251–257. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. Metabolism of curcumin: Studies with [3H]curcumin. Toxicology 1981, 22, 337–344. [Google Scholar] [CrossRef]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6- heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar]
- Ireson, C.R.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.K.; Luo, J.L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.L.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar]
- Ireson, C.R.; Jones, D.J.; Orr, S.; Coughtrie, M.W.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomarker. Prev. 2002, 11, 105–111. [Google Scholar]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarker. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomarker. Prev. 2005, 14, 120–125. [Google Scholar]
- Garcea, G.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J.; Berry, D.P. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Mukhtar, H. Cancer chemoprevention through dietary antioxidants: Progress and promise. Antioxid. Redox Sign. 2008, 10, 475–510. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Biesalski, H.K. Polyphenols and inflammation: Basic interactions. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 724–728. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed; Clarendon Press: Oxford, UK, 1998. [Google Scholar]
- Weber, W.M.; Hunsaker, L.A.; Gonzales, A.M.; Heynekamp, J.J.; Orlando, R.A.; Deck, L.M.; Vander Jagt, D.L. TPA-induced up-regulation of activator protein-1 can be inhibited or enhanced by analogs of the natural product curcumin. Biochem. Pharmacol. 2006, 72, 928–940. [Google Scholar] [CrossRef]
- Lala, P.K.; Chakraborty, C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001, 2, 149–156. [Google Scholar] [CrossRef]
- deRojas-Walker, T.; Tamir, S.; Ji, H.; Wishnok, J.S.; Tannenbaum, S.R. Nitric oxide induces oxidative damage in addition to deamination in macrophage DNA. Chem. Res. Toxicol. 1995, 8, 473–477. [Google Scholar] [CrossRef]
- Graziewicz, M.; Wink, D.A.; Laval, F. Nitric oxide inhibits DNA ligase activity: Potential mechanisms for NO-mediated DNA damage. Carcinogenesis 1996, 17, 2501–2505. [Google Scholar] [CrossRef]
- Chan, M.M.; Huang, H.I.; Fenton, M.R.; Fong, D. In vivo inhibition of nitric oxide synthase gene expression by curcumin: A cancer preventive natural product with anti-inflammatory properties. Biochem. Pharmacol. 1998, 55, 1955–1962. [Google Scholar]
- Von Knethen, A.; Brüne, B. Cyclooxygenase-2: An essential regulator of NO-mediated apoptosis. FASEB J. 1997, 11, 887–895. [Google Scholar]
- von Knethen, A.; Callsen, D.; Brune, B. NF-κB and AP-1 activation by nitric oxide attenuated apoptotic cell death in RAW 264.7 macrophages. Mol. Biol. Cell 1999, 10, 361–372. [Google Scholar]
- Chung, S.W.; Hall, S.R.; Perella, M.A. Role of hame oxygenase-1 in microbial host defense. Cell. Microbiol. 2009, 11, 199–207. [Google Scholar] [CrossRef]
- Baron, J.A.; Sandler, R.S. Non-steroidal anti-inflammatory drugs and cancer prevention. Annu. Rev. Med. 2000, 51, 511–523. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, L.A.; Huerta-Alvarez, C. Reduced risk of colorectal cancer among long-term users of aspirin and non-aspirin non-steroidal anti-inflammatory drugs. Epidemiology 2001, 12, 88–93. [Google Scholar] [CrossRef]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer and development. Oncogene 1999, 18, 7908–7916. [Google Scholar]
- Zidi, I.; Mestri, S.; Bartegi, A.; Ben Amor, N. TNF-α and its inhibitors in cancer. Med. Oncol. 2010, 27, 185–198. [Google Scholar]
- Joe, B.; Lokesh, B.R. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim. Biophys. Acta 1994, 1224, 255–263. [Google Scholar]
- Kunchandy, E.; Rao, M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Sreejayan, N.; Rao, M.N. Free radical scavenging activity of curcuminoids. Arzneimittelforschung 1996, 46, 169–171. [Google Scholar]
- Ahsan, H.; Parveen, N.; Khan, N.U.; Hadi, S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives, demethoxycurcumin and bisdemethoxycurcumi. Chem. Biol. Interact. 1999, 121, 161–175. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Greenhill, J.V. Studies on curcumin and curcuminoids: 22. curcumin as a reducing agent and as a radical scavenger. Int. J. Pharm. 1992, 87, 79–87. [Google Scholar]
- Barik, A.; Mishra, B.; Shen, L.; Mohan, H.; Kadam, R.M.; Dutta, S.; Zhang, H.Y.; Priyadarsini, K.I. Evaluation of a new copper(II)-curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radical Biol. Med. 2005, 39, 811–822. [Google Scholar]
- Brouet, I.; Ohshima, H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem. Biophys. Res. Commun. 1995, 206, 533–540. [Google Scholar] [CrossRef]
- Hill-Kapturczak, N.; Thamilselvan, V.; Liu, F.; Nick, H.S.; Agarwal, A. Mechanism of heme oxygenase-1 gene induction by curcumin in human renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 2001, 281, F851–F859. [Google Scholar]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radical Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Kelkel, M.; Jacob, C.; Dicato, M.; Diederich, M. Potential of the dietary antioxidants resveratrol and curcumin in prevention and treatment of hematologic malignancies. Molecules 2010, 15, 7035–7074. [Google Scholar] [CrossRef]
- Bhaumik, S.; Anjum, R.; Rangaraj, N.; Pardhasaradhi, B.V.; Khar, A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999, 456, 311–314. [Google Scholar] [CrossRef]
- Chen, J.; Wanming, D.; Zhang, D.; Liu, Q.; Kang, J. Water-soluble antioxidants improve the antioxidant and anticancer activity of low concentrations of curcumin in human leukemia cells. Pharmazie 2005, 60, 57–61. [Google Scholar]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O’Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar]
- Nair, J.; Strand, S.; Frank, N.; Knauft, J.; Wesch, H.; Galle, P.R.; Bartsch, H. Apoptosis and age-dependant induction of nuclear and mitochondrial etheno-DNA adducts in Long-Evans Cinnamon (LEC) rats: Enhanced DNA damage by dietary curcumin upon copper accumulation. Carcinogenesis 2005, 26, 1307–1315. [Google Scholar]
- Taketo, M.M. Cyclooxygenase-2 inhibitors in tumorigenesis (Part II). J. Natl. Cancer Inst. 1998, 90, 1609–1620. [Google Scholar] [CrossRef]
- Sharma, R.A. Translational medicine: targeting cyclo-oxygenase isozymes to prevent cancer. QJM 2002, 95, 267–273. [Google Scholar] [CrossRef]
- Zhang, F.; Altorki, N.K.; Mestre, J.R.; Subbaramaiah, K.; Dannenberg, A.J. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis 1999, 20, 445–451. [Google Scholar]
- Lev-Ari, S.; Maimon, Y.; Strier, L.; Kazanov, D.; Arber, N. Down-regulation of prostaglandin E2 by curcumin is correlated with inhibition of cell growth and induction of apoptosis in human colon carcinoma cell lines. J. Soc. Integr. Oncol. 2006, 4, 21–26. [Google Scholar]
- Plummer, S.M.; Holloway, K.A.; Manson, M.M.; Munks, R.J.; Kaptein, A.; Farrow, S.; Howells, L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-κB activation via the NIK/IKK signalling complex. Oncogene 1999, 18, 6013–6020. [Google Scholar] [CrossRef]
- Yin, M.J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998, 396, 77–80. [Google Scholar] [CrossRef]
- Handler, N.; Jaeger, W.; Puschacher, H.; Leisser, K.; Erker, T. Synthesis of novel curcumin analogues and their evaluation as selective cyclooxygenase-1 (COX-1) inhibitors. Chem. Pharm. Bull. 2007, 55, 64–71. [Google Scholar] [CrossRef]
- Su, C.C.; Chen, G.W.; Lin, J.G.; Wu, L.T.; Chung, J.G. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B/p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res. 2006, 26, 1281–1288. [Google Scholar]
- Tsujii, M.; Kawano, S.; Tsuji, S.; Sawaoka, H.; Hori, M.; DuBois, R.N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998, 93, 705–716. [Google Scholar]
- Wang, W.; Abbruzzese, J.L.; Evans, D.B.; Larry, L.; Cleary, K.R.; Chiao, P.J. The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin. Cancer Res. 1999, 5, 119–127. [Google Scholar]
- Aggarwal, B.B. Nuclear factor-κB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef]
- Molina, M.A.; Sitja-Arnau, M.; Lemoine, M.G.; Frazier, M.L.; Sinicrope, F.A. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: Growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999, 59, 4356–4362. [Google Scholar]
- Tucker, O.N.; Dannenberg, A.J.; Yang, E.K.; Zhang, F.; Teng, L.; Daly, J.M.; Soslow, R.A.; Masferrer, J.L.; Woerner, B.M.; Koki, A.T.; et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999, 59, 987–990. [Google Scholar]
- Yip-Schneider, M.T.; Barnard, D.S.; Billings, S.D.; Cheng, L.; Heilman, D.K.; Lin, A.; Marshall, S.J.; Crowell, P.L.; Marshall, M.S.; Sweeney, C.J. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis 2000, 21, 139–146. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 36398. [Google Scholar]
- Chen, Y.R.; Tan, T.H. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 1998, 17, 173–178. [Google Scholar]
- Huang, T.S.; Lee, S.C.; Lin, J.K. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc. Natl. Acad. Sci. USA 1991, 88, 5292–5296. [Google Scholar]
- Shoskes, D.; Lapierre, C.; Cruz-Correa, M.; Muruve, N.; Rosario, R.; Fromkin, B.; Braun, M.; Copley, J. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: A randomized placebo controlled trial. Transplantation 2005, 80, 1556–1559. [Google Scholar] [CrossRef]
- Joosten, S.A.; Sijpkens, Y.W.; van Kooten, C.; Paul, L.C. Chronic renal allograft rejection: Pathophysiologic considerations. Kidney Int. 2005, 68, 1–13. [Google Scholar]
- Shaikewitz, S.T.; Chan, L. Chronic renal transplant rejection. Am. J. Kidney Dis. 1994, 23, 884–893. [Google Scholar]
- Avihingsanon, Y.; Ma, N.; Csizmadia, E.; Wang, C.; Pavlakis, M.; Giraldo, M.; Strom, T.B.; Soares, M.P.; Ferran, C. Expression of protective genes in human renal allografts: A regulatory response to injury associated with graft rejection. Transplantation 2002, 73, 1079–1085. [Google Scholar]
- Durgaprasad, S.; Ganesh Pai, C.; Vasanthkumar; Alvres, J.F.; Sanjeeva; Namitha. A pilot study of the anti-oxidant effect of curcumin in tropical pancreatitis. Indian J. Med. Res. 2005, 80, 1556–1559. [Google Scholar]
- Bharti, A.C.; Donato, N.; Singh, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-κB and IκBα kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 2003, 101, 1053–1062. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, E.J.; Joe, E.H.; Jou, I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J. Immunol. 2003, 171, 6072–6079. [Google Scholar]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Satoskar, R.R.; Shah, S.J.; Shenoy, S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654. [Google Scholar]
- Lal, B., Kapoor; Asthana, O.P.; Agrawal, P.K.; Prasad, R.; Kumar, P.; Srimal, R.C. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother. Res. 1999, 13, 318–322. [Google Scholar]
- Lal, B.; Kapoor, A.K.; Agrawal, P.K.; Asthana, O.P.; Srimal, R.C. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother. Res. 2000, 14, 443–447. [Google Scholar] [CrossRef]
- Reddy, S.; Aggarwal, B.B. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett. 1994, 341, 19–22. [Google Scholar]
- Heng, M.C.; Song, M.K.; Harker, J.; Heng, M.K. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br. J. Dermatol. 2000, 143, 937–949. [Google Scholar]
- Schallreuter, K.U.; Rokos, H. Turmeric (curcumin): A widely used curry ingredient, can contribute to oxidative stress in Asian patients with acute vitiligo. Indian J. Dermatol. Venereol. Leprol. 2006, 72, 57–59. [Google Scholar] [CrossRef]
- Prucksunand, C.; Indrasukhsri, B.; Leethochawalit, M.; Hungspreugs, K. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J. Trop. Med. Pub. Health 2001, 32, 208–215. [Google Scholar]
- Kuttan, R.; Sudheeran, P.C.; Josph, C.D. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987, 73, 29–31. [Google Scholar]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolf, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Archiv Pharm. Chem. Life Sci. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Goel, A.; Aggarwal, B.B. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer 2010, 62, 919–930. [Google Scholar] [CrossRef]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamouscell carcinoma. Mol. Cancer 2011, 10, 1–19. [Google Scholar]
- Bansal, S.S.; Vadhanam, M.V.; Gupta, R.C. Development and in vitro-in vivo evaluation of polymeric implants for continuous systemic delivery of curcumin. Pharm. Res. 2011, 28, 1121–1130. [Google Scholar] [CrossRef]
- Kurien, B.T.; Scofield, R.H. Oral administration of heat-solubilized curcumin for potentially increasing curcumin bioavailability in experimental animals. Int. J. Cancer 2009, 125, 1992–1993. [Google Scholar]
- Zebib, B.; Mouloungui, Z.; Noirot, V. Stabilization of curcumin by complexation with divalent cations in glycerol/water system. Bioinorg. Chem. Appl. 2010. [Google Scholar] [CrossRef]
- Kudva, A.K.; Manoj, M.N.; Swamy, B.N.; Ramadoss, C.S. Complexation of amphoterecin B and curcumin with serum albumin: Solubility and effect on erythrocyte membrane damage. J. Expt. Pharmacol. 2011, 3, 1–6. [Google Scholar]
- Qiu, X.; Du, Y.; Lou, B.; Zuo, Y.; Shao, W.; Huo, Y.; Huang, J.; Yu, Y.; Zhou, B.; Du, J.; Fu, H.; Bu, X. Synthesis and identification of new 4-arylidene curcumin analogues as potential anticancer agents targeting nuclear factor-κB signaling pathway. J. Med. Chem. 2010, 53, 8260–8273. [Google Scholar]
- Ohtsu, H.; Xiao, Z.; Ishida, J.; Nagai, M.; Wang, H.; Itokawa, H.; Su, C.; Shih, C.; Chiang, T.; Chang, E. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J. Med. Chem. 2002, 45, 5037–5042. [Google Scholar]
- Lin, L.; Shi, Q.; Nyarko, A.; Bastow, K.; Wu, C.; Su, C.; Shih, C.; Lee, K. Antitumor agents. 250. Design and synthesis of new curcumin analogs as potential anti-prostate cancer agents. J. Med. Chem. 2006, 49, 3963–3972. [Google Scholar] [CrossRef]
- Zambre, A.P.; Kulkarni, V.M.; Padhye, S.; Sandur, S.K.; Aggarwal, B.B. Novel curcumin analogs and proliferation targeting TNF-induced NF-κB activation in human leukemic KBM-5 cells. Bioorg. Med. Chem. 2006, 14, 7196–7204. [Google Scholar] [CrossRef]
- Weber, W.M.; Hunsaker, L.A.; Roybal, C.N.; Bobrovnikova-Marjon, E.V.; Abcouwer, S.F.; Royer, R.E.; Deck, L.M.; Vander Jagt, D.L. Activation of NF-κB is inhibited by curcumin and related enones. Bioorg. Med. Chem. 2006, 14, 2450–2461. [Google Scholar]
- Adams, B.; Ferstl, E.; Davis, M.; Herold, M.; Kurtkaya, S.; Camalier, R.; Hollingshead, M.; Kaur, G.; Sausville, E.; Rickles, F. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg. Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef]
- Padhye, S.; Chavan, D.; Pandey, S.; Deshpande, J.; Swamy, K.V.; Sarkar, F.H. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Med. Chem. 2010, 10, 372–387. [Google Scholar]
- Safavy, A.; Raisch, K.P.; Mantena, S.; Sanford, L.L.; Sham, S.W.; Rama Krishna, N.; Bonner, J.A. Design and development of water-soluble curcumin conjugates as potential anti-cancer agents. J. Med. Chem. 2007, 50, 6284–6288. [Google Scholar] [CrossRef]
- Ma, Z.; Haddadi, A.; Molavi, O.; Lavasanifar, A.; Lai, R.; Samuel, J. Micelles of poly(ethylene oxide)-b-poly(ε-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Materials Res. Part A 2008, 86A, 300–310. [Google Scholar] [CrossRef]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Ravi Kumar, M.N.V. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Tsai, Y.M.; Jan, W.C.; Chien, C.F.; Lee, W.C.; Lin, L.C.; Tsai, T.H. Optimized nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011, 127, 918–925. [Google Scholar] [CrossRef]
- Anitha, A.; Deepagan, V.G.; Divya Rani, V.V.; Menon, D.; Nair, S.V.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate-chitosan nanoparticles. Carbohyd. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 1–18. [Google Scholar] [CrossRef]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar]
- Gao, Y.; Li, Z.; Sun, M.; Guo, C.; Yu, A.; Xi, Y.; Cui, J.; Lou, H.; Zhai, G. Preparation and characterization of intravenously injectable curcumin nanosuspension. Drug Deliv. 2011, 18, 131–142. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials 2011, 32, 598–609. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.W.; Huang, M.T.; Ho, C.T.; Huang, Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar]
- Gupta, N.K.; Dixit, V.K. Bioavailability enhancement of curcumin by complexation with phosphatidylcholine. J. Pharm. Sci. 2011, 100, 1987–1995. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef]
- Sou, K.; Inenaga, S.; Takeoka, S.; Tsuchida, E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int. J. Pharm. 2008, 352, 287–293. [Google Scholar] [CrossRef]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar]
- Mulik, R.S.; Moenkkoenen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. Transferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anti-cancer activity by induction of apoptosis. Int. J. Pharm. 2010, 398, 190–203. [Google Scholar] [CrossRef]
- Tiyaboonchai, W.; Tungpradit, W.; Plianbangchang, P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 2007, 337, 299–306. [Google Scholar] [CrossRef]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Novel formulation of solid lipid microparticles of curcumin for anti-angiogenic and anti-inflammatory activity for optimization of therapy of inflammatory bowel disease. J. Pharm. Pharmacol. 2009, 61, 311–321. [Google Scholar]
- di Cagno, M.; Styskala, J.; Hlaváč, J.; Brandl, M.; Bauer-Brandl, A.; Skalko-Basnet, N. Liposomal solubilization of new 3-hydroxy-quinolinone derivatives with promising anti-cancer activity: A screening method to identify maximum incorporation capacity. J. Liposome Res. 2011. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Pandey, R.; Priyadarsini, I.K. Transport of liposomal and albumin loaded curcumin to living cells: An absorption and fluorescence spectroscopic study. BBA-Gen. Subjects 2006, 1760, 1513–1520. [Google Scholar] [CrossRef]
- Chen, C.; Johnston, T.D.; Jeon, H.; Gedaly, R.; McHugh, P.P.; Burke, T.G.; Ranjan, D. An in vitro study of liposomal curcumin: Stability, toxicity and biological activity in human lymphocytes and Epstein-Barr virus-transformed human B-cells. Int. J. Pharma. 2009, 366, 133–139. [Google Scholar] [CrossRef]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signalling, and angiogenesis. Cancer 2005, 104, 1322–1331. [Google Scholar] [CrossRef]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar]
- Thangapazham, R.L.; Puri, A.; Tele, S.; Blumenthal, R.; Maheshwari, R.K. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008, 32, 1119–1123. [Google Scholar]
- Wang, D.; Veena, M.S.; Stevenson, K.; Tang, C.; Ho, B.; Suh, J.D.; Duarte, V.M.; Faull, K.F.; Mehta, K.; Srivatsan, E.S.; Wang, M.B. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor-κB by an AKT-independent pathway. Clin. Cancer Res. 2008, 14, 6228–6236. [Google Scholar] [CrossRef]
- Takahashi, M.; Inafuku, K.; Miyagi, T.; Oku, H.; Wada, K.; Imura, T.; Kitamoto, D. Efficient preparation of liposomes encapsulating food materials using lecithins by a mechanochemical method. J. Oleo Sci. 2007, 56, 35–42. [Google Scholar] [CrossRef]
- Aukunuru, J.; Joginapally, S.; Gaddam, N.; Burra, M.; Bonepally, C.R.; Prabhakar, K. Preparation, characterization and evaluation of hepatoprotective activity of an intravenous liposomal formulation of bis-demethoxy curcumin analogue (BDMCA). Int. J. Drug Dev. Res. 2009, 1, 37–46. [Google Scholar]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar]
- Mourtas, S.; Canovi, M.; Zona, C.; Aurilia, D.; Niarakis, A; La Ferla, B.; Salmona, M.; Nicotra, F.; Gobbi, M.; Antimisiaris, S.G. Curcumin-decorated nanoliposomes with very high affinity for amyloid-β1-42 peptide. Biomaterials 2011, 32, 1635–1645. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmark of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Azam, F.; Mehta, S.; Harris, A.L. Mechanisms of resistance to anti-angiogenesis therapy. Eur. J. Cancer 2010, 46, 1323–1332. [Google Scholar] [CrossRef]
- Ebos, J.M.; Lee, C.R.; Kerbel, R.S. Tumor and host-mediated pathways of resistance and disease progression in response to anti-angiogenic therapy. Clin. Cancer Res. 2009, 15, 5020–5025. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Villegas, I.; Sanchez-Fidalgo, S.; de la Lastra, C.A. New mechanism and therapeutic potential of curcumin for colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1040–1061. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Ribnicky, D.M.; Lipsky, P.E.; Raskin, I. Revisiting the ancient concept of botanical therpeutics. Nat. Chem. Biol. 2007, 3, 360–366. [Google Scholar]
- Khafif, A.; Schantz, S.P.; Chou, T.C.; Edelstein, D.; Sacks, P.G. Quantitation of chemopreventive synergism between (−)-epigallocatechin-3-gallate and curcumin in normal, premalignant and malignant human oral epithelial cells. Carcinogenesis 1998, 19, 419–424. [Google Scholar] [CrossRef]
- Verma, S.P.; Salamone, E.; Goldin, B. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochem. Biophys. Res. Commun. 1997, 233, 692–696. [Google Scholar] [CrossRef]
- Du, B.; Jiang, L.; Xia, Q.; Zhong, L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy 2006, 52, 23–28. [Google Scholar] [CrossRef]
- Sen, S.; Sharma, H.; Singh, N. Curcumin enhances vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem. Biophys. Res. Commun. 2005, 331, 1245–1252. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules 2011, 16, 4567-4598. https://doi.org/10.3390/molecules16064567
Basnet P, Skalko-Basnet N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules. 2011; 16(6):4567-4598. https://doi.org/10.3390/molecules16064567
Chicago/Turabian StyleBasnet, Purusotam, and Natasa Skalko-Basnet. 2011. "Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment" Molecules 16, no. 6: 4567-4598. https://doi.org/10.3390/molecules16064567
APA StyleBasnet, P., & Skalko-Basnet, N. (2011). Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules, 16(6), 4567-4598. https://doi.org/10.3390/molecules16064567






