Abstract
The air-dried powdered stem bark of Calophyllum nodusum (Guttiferea) collected from Sandakan (Sabah, Malaysia), was extracted sequentially with hexane, chloroform and methanol. The solvents were removed by rotary evaporator to give dark viscous extracts. Detailed and repeated chromatographic separation of the extracts lead to isolation of two new xanthones, identified as nodusuxanthone (1a) and trapezifolixanthone A (2). Other common terpenoids such as betulinic acid, lupeol, stigmasterol and friedelin were also isolated from the extracts and identified. The structures of the compounds were established by detailed spectral analysis and comparison with previously reported data.
1. Introduction
Calophyllum, locally known as ‘bintangor’, is one of the four genus in the Guttiferae family. It consists of about 150 species found mostly in tropical Asia and the Pacific Islands. Members of the genus are usually rather slender, small to medium trees and a few are reported to have medicinal properties [1,2]. According to previous reports, the genus is a rich source of terpenoids, xanthones, coumarins, benzopyrans, and other phenolic compounds [3,4,5,6]. Some of the well-known compounds having excellent biological activity were isolated from Calophyllum lanigerum collected from Sarawak, Malaysia [7,8]. Dipiranotetracylic coumarins, such as (+)-calanolide A, isolated from this species, have been reported to inhibit human immunodeficiency virus type 1 (HIV-1) replication and cytophaticity, and some of them are currently being developed as chemotherapeutic agents against this virus [9,10]. In this communication we would like report the structural determination of two new xanthones, identified as nodusuxanthone (1a) and trapezifolixanthone A (2), together with four other terpenoids obtained from the crude extracts of Calophyllum nodusum. This is in continuation of our work on bioactive constituents of Malaysian tropical plants [11,12,13,14].
2. Results and Discussion
The air dried, powdered stem bark of Calophyllum nodusum (1.8 kg) was extracted at room temperature sequentially with hexane, chloroform and finally with methanol. The extracts were filtered and solvents removed by rotary evaporator to give 18.6, 31.6 and 38.1 g of dark viscous semisolid extracts, respectively. The chloroform extract was fractionated by column chromatography eluting with different mixtures of hexane, chloroform and methanol to give 168 fractions. Repeated column chromatographic separation of fractions 74-78 yielded nodusuxanthone (1a). Betulinic acid was also obtained from fractions 29-38 after repeated column chromatography separation. Similar fractionation of the methanol extract with vacuum column chromatography and eluting with the same solvent systems gave 118 fractions. Further separation of fractions 87-97 gave trapezifolixanthone A (2). Three common sterols, lupeol, stigmasterol and friedelin, were obtained from the hexane extracts by similar chromatographic separation techniques. The structures of some of the compounds are shown in Figure 1.
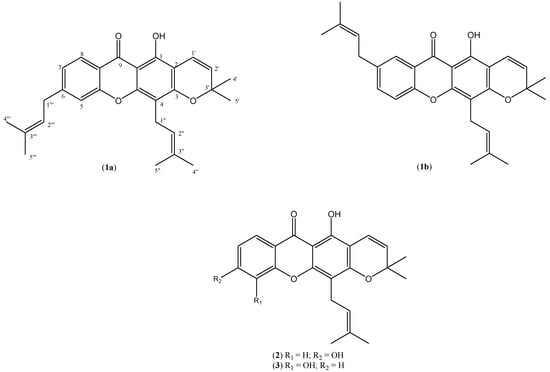
Figure 1.
Structures of compounds 1a, 1b, 2, 3.
Compound 1a was obtained as yellow needle-shaped crystals with m.p. 182–183 °C after recrystallisation from chloroform. The UV spectrum gave λmax absorptions at 247, 284 and 305 nm, which indicated the presence of a xanthone skeleton [15] and the IR spectrum gave a strong absorption at 1,647 cm−1 for the chelated carbonyl group. The EIMS spectrum showed a molecular ion peak at m/z 430 which corresponds to the molecular formula of C28H30O4 with the base peak at m/z 323. HREIMS C28H30O4 gave m/z 430.2146 (calculated value: 430.1656). The integration of the 1H-NMR clearly indicated the presence of 30 protons comprising seven methines, two methylenes, six methyls and a hydroxyl (Table 1). The chelated hydroxyl group occurred at a low field region (δ 13.02). The presence of a chromene ring could be easily rationalised by the occurrence of a set of doublets at δ 5.57 and 6.09 with a common coupling constant of 10.0 Hz and a six proton singlet at δ 1.40. The aromatic A-ring is 1,2,4-trisubstitued, with the observation of two doublets at δ 7.15 (d, J = 2.3 Hz, H-5) and 7.19 (d, J = 8.0 Hz, H-8) and a doublet of doublet at δ 7.53 (dd, J = 2.3 Hz and 8.0 Hz, H-7). The COSY correlations of these methine signals further supported these assignments. The rest of the resonances were due to the presence of two sets of prenylated side chains. The signals for the two methylene groups of the side-chain integrated for four protons occurred as two doublet of doublets at δ 2.94 (dd, J = 2.7, 7.3 Hz, H-1a”, H-1a”’) and 2.96 (dd, 2H, J = 7.3, 10.8 Hz, H-1b”, H-1b”’). Similarly, the protons resonances for the two sp2 carbons also overlapped each other as doublet of doublet at δ 3.61 (dd, 2H, J = 2.7, 10.8 Hz, H-2”, H-2”’). While the sp3 methyl protons were observed as two sharp singlets each integrated for six protons at δ 1.22 and 1.16. The presence of three prenyl substituents in xanthones of Calophyllum species has been reported previously and in one of the compounds the prenyl substituent similarly cyclized to form a chromene ring [4,16].
The DEPT spectrum revealed the existence of a chelated carbonyl signal at low field (δ 182.5), seven methine, two methylene, six methyl carbons and remainder were made up of quarternary carbon atoms. Another low field signal was observed at δ 159.7 due to the hydroxyl group at C-1. The two methyl groups on the chromene ring overlapped each other and occurred at δ 28.7. The attachment of the two prenyl side-chains could be rationalised by examining the HMBC spectrum of the compound (Figure 2).
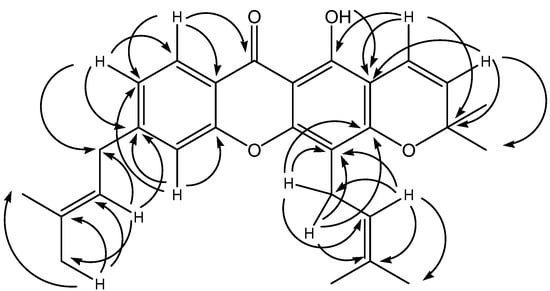
Figure 2.
Selected HMBC correlations of 1a.
One of the side-chains is attached to C-4 of the xanthone skeleton as evidenced by the two-bond and three-bond connectivities of the methylene protons H-1” with C-3 and C-4. The attachment of the other prenyl side-chain can be either at position C-6 (1a) or C-7 (1b), since this aromatic ring is 1,2,4-trisubstituted.

Table 1.
1H-NMR and 13C- NMR spectral data of 1a, 2 and 3.
| H/C | 1a in CD3OD | 2 in CDCl3 | 3 in Me2CO-d6 [17] | |||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | HMBC | δH | δC | HMBC | δH | δC in | |
| 1 | 13.02 (s , OH) | 159.7 | C-2 | 13.03 (s, OH) | 155.9 | C-1, C-9a | 13.33 (s, OH) | 156.0 |
| 2 | 105.3 | 103.2 | 104.7 | |||||
| 3 | 159.7 | 158.6 | 158.2 | |||||
| 4 | 107.3 | 107.0 | 107.4 | |||||
| 4a | 155.9 | 153.6 | 153.7 | |||||
| 5 | 7.15 (d, 2.3 Hz, 1H) | 124.9 | C-7, C-6, C-10a | 7.26 (d, 1.8 Hz, 1H) | 119.7 | C-7, C-10a | 9.22 (s, OH) | 144.4 |
| 6 | 107.3 | 5.76 (s, OH) | 144.4 | C-5 | 7.38 (dd,1.5, 8.0 Hz) | 119.7 | ||
| 7 | 7.53 (dd, 2.3, 8.0 Hz, 1H) | 121.8 | C-8, C-6, C-1”’ | 7.69 (dd, 1.8, 10.0 Hz, 1H) | 116.7 | C-5, C-9, C-10a | 7.28 (t, 8.0 Hz) | 123.9 |
| 8 | 7.19 (d, 8.0 Hz, 1H) | 116.4 | C-8a, C-9, C-7 | 7.19 (d, 10.0 Hz, 1H) | 123.9 | C-8a, C-10a | 7.69 (dd, 1.5, 8.0 Hz) | 116.8 |
| 8a | 122.1 | 120.8 | 120.9 | |||||
| 9 | 182.5 | 180.9 | 181.1 | |||||
| 9a | 104.1 | 104.7 | 103.3 | |||||
| 10a | 147.1 | 144.2 | 144.2 | |||||
| 1’ | 6.09 (d, 10.0 Hz, 1H) | 116.4 | C-1, C-2, C-3’ | 6.70 (d, 10.0 Hz, 1H) | 115.6 | C-3’, C-9a | 6.71 (d, 10.0 Hz) | 115.7 |
| 2’ | 5.57 (d, 10.0 Hz, 1H) | 128.6 | C-2, C-3’, C-4’, C-5’ | 5.58 (d, 10.0 Hz, 1H) | 127.4 | C-3’, C-3 | 5.77 (d, 10.0 Hz) | 127.4 |
| 3’ | 78.4 | 78.2 | 78.3 | |||||
| 4’ | 1.40 (s, 3H) | 28.7 | C-2’, C-3’, C-5’ | 1.45 (s, 3H) | 28.3 | C-1’, C-3’, C-5’ | na | 28.3 |
| 5’ | 1.40 (s, 3H) | 28.7 | C-2’, C-3’, C-4’ | 1.45 (s, 3H | 28.3 | C-1’, C-3’ C4’ | 1.51 (s) | 28.3 |
| 1” a | 2.94 (dd, 2.7, 7.3 Hz, 1H) | 26.0 | C-3, C-4, C-2” | 3.45 (d, 7.32 Hz, 2H) | 21.7 | C-4, C-2”, C-3”, C-4a | 3.57 (d, 6.0 Hz) | 21.7 |
| 1” b | 2.96 (dd, 7.3, 10.8 Hz, 1H) | C-3, C-4, C-2”, C-3” | ||||||
| 2” | 3.61(dd, 2.7, 10.8 Hz, 1H) | 78.0 | C-4, C-1”, C-3”, C-4” | 5.18 (t, 7.36 Hz, 1H) | 122.7 | C-4”, C-5” | 5.31 (t, 6.0 Hz) | 122.6 |
| 3” | 146.0 | 131.6 | 131.6 | |||||
| 4” | 1.22 (s, 3H) | 25.5 | C-2”, C-3”, C-“5 | 1.68 (s, 3H) | 25.6 | C-2”, 3”, C-5” | 1.66 (s) | 25.5 |
| 5” | 1.16 (s, 3H) | 30.5 | C-2”, C-4” | 1.83 (s, 3H) | 17.9 | C-2”, 3”, C-4” | 1.87 (s) | 17.9 |
| 1”’ a | 2.94 (dd, 2.7, 7.3 Hz, 1H) | 26.0 | C-6, C-7, C-2”’ | |||||
| 1”’ b | 2.96 (dd, 7.3, 10.8 Hz, 1H) | C-6, C-2”’ | ||||||
| 2”’ | 3.61 (dd, 2.7, 10.8 Hz, 1H) | 78.0 | C-1”’, C-6, C-4”’ | |||||
| 3”’ | 146.0 | |||||||
| 4’” | 1.22 (s, 3H) | 25.5 | C-2”’, C-3”’, C-5”’ | |||||
| 5”’ | 1.16 (s, 3H) | 30.5 | C-2”’, C-4”’ | |||||
na: not available.
However, structure 1a is more appropriate since the HMBC spectrum revealed three-bond connectivity between proton H-8 to the carbonyl C-9 and this proton also has large ortho coupling constant (J = 8.0 Hz) to H-7. Based on these spectral data, the structure of the new compound 1a is determined as 1-hydroxy-3’,3’-dimethyl-4,6-di(3”-methyl-2”-butenyl)-2H,6H-pyrano[3,2-a]-xanthen-9-one and given the trivial name nodusuxanthone.
Compound 2 was also obtained as yellowish needle-shaped crystals with m.p. 158–160 °C. The IR spectrum exhibited a strong and broad absorption at 3,463 cm−1 which indicated the presence of a hydroxyl group and another strong band at 1,685 cm−1 assigned to a chelated carbonyl. The EIMS gave a molecular ion peak at m/z 378, in agreement with the molecular formula C23H22O5 and with a [M]+-CH3 base peak at m/z 363. HREIMS of C23H22O5 gave m/z 378.1467 (calculated value: 378.1452). The presence of a chromene ring in the compound was similarly rationalised in the 1H-NMR spectrum with the observation of a set of doublets at δ 6.70 and 5.58, each with 10.0 Hz coupling constant and an overlapped six proton singlet at δ 1.45. The protons on the aromatic A-ring also exhibited in an ABX system with the typical occurrence of two doublets at δ 7.26 (d, J = 1.8 Hz) and 7.19 (d, J = 10.0 Hz) and a doublet of doublets at δ 7.69 (dd, J = 1.8 Hz, 10.0 Hz). The xanthone skeleton is also attached to a prenyl side-chain through C-4 with the characteristic presence of signals for two methyls (δ 1.68 and 1.83), methylene (δ 3.45) and methine (δ 5.18). The 13C-NMR revealed the existence of 23 carbon atoms with a low field resonance at δ 180.9 assigned to the chelated carbonyl. The assignments of the proton and carbon resonances to their correct positions were further substantiated by the COSY and HMBC correlation experiments. In comparison, these data are very similar to those of trapezifolixanthone (3), previously reported to occur in roots of Tovomita brevistaminea [17] but with some very obvious differences. Thus, in compound 2 the aromatic protons in ring-A are arranged in an ABX system as can be seen in their couplings (Table 1), but in compound 3 the aromatic ring is in a 1,2,3-trisubstituted arrangement. Hence, the structure of this new compound is determined as 1,6-dihydroxy-3’,3’-dimethyl-4-(3”-methyl-2”-butenyl)-2H,6H-pyrano[3,2-a]-xanthen-9-one and it was given the name trapezifolixanthone A. Besides these two new compounds, betulinic acid, lupeol, stigmasterol and friedelin were also obtained from the hexane and chloroform extracts. Betulinic acid also occurs in other Calophyllum species and was reported to have antiproliferative activity against a number of cell lines which suggests its possible use as a chemotherapeutic agent [18,19]. Xanthones, especially those from Garcinia mangostana, are well known to exhibit strong antioxidant activity [20,21]. However, all three crude extracts and the two new xanthones failed to exhibit any free radical scavenging activity when tested with a DPPH assay (data not shown).
3. Experimental
3.1. General
Melting points were determined on a Leica Galen III apparatus. UV spectra were determined in EtOH using a Shimadzu UV-160A spectrophotometer. NMR spectra were obtained with a JEOL JNM CRX 400 MHz FT-NMR spectrometer in CDCl3 or CD3OD as solvent and tetramethylsilane as internal standard. IR spectra were obtained using a Perkin Elmer FTIR model 1725X spectrometer. EIMS were recorded on a Shimadzu GCMS-QP5050A spectrometer. Silica gel 60H 1.07736 Merck and 60 (0.063–0.200 mm) 1.07734 Merck were used for column chromatography. Precoated sheets of silica gel 60F254 Merck were used for TLC analysis and the spots were visualized either with a UV lamp (254 nm and 356 nm) or by iodine vapor.
3.2. Plant Material, Extraction and Isolation
The stem bark of Calophyllum nodusum was collected in October 2002 from Sabah, East Malaysia and identified by Mr. Julius Kulip of the Forest Department Sandakan, Sabah, and a voucher specimen (FRCSE 554) was deposited here. The air-dried, powdered stem bark of the plant (1.8 kg) was extracted at room temperature sequentially with hexane, chloroform and finally with methanol (5 litres each). The extracts were filtered and solvents were removed by rotary evaporator to give 18.6, 31.6 and 38.1 g of dark viscous semisolid extracts, respectively. The chloroform extract was fractionated with silica gel gravity column chromatography eluting with different mixtures of hexane, chloroform and methanol to give 168 fractions of 100 mL each. Repeated column chromatography of fractions 78-92 yielded a yellowish solid that was recrystallised from chloroform to give yellow needle-shaped crystals of nodusuxanthone (1a, 20 mg). Further column chromatography separation on fractions 29–38 gave betulinic acid (34 mg) as white needles, m.p. 300 °C. Similar fractionation of the methanol extract with vacuum column chromatography and elution with the same solvent systems gave 118 fractions. Further separation of fractions 87-97 yielded a yellow solid that was recrystallised from chloroform to give trapezifolixanthone A (2, 41 mg) as needle-shaped crystals. Three common sterols, lupeol (66 mg), stigmasterol 62 mg) and friedelin (84 mg) were also obtained from the hexane extracts by similar chromatographic separation techniques.
3.3. Spectral Data
Nodusuxanthone (1a). Yellow needle-shaped crystal with m.p. 182–183 °C; UV (EtOH) λmax (log ε) nm: 247 (2.44), 284 (3.65), 305 (1.23); IR (MeOH) νmax cm−1: 3,431, 2,937, 2,278, 1,720, 1,647, 1,586, 1,455, 1,374, 1,055. EIMS m/z (rel. int.): 430 [M]+ (5), 412 (32), 397 (52), 379 (8), 371 (18), 353(29), 323 (100), 309 (34), 305 (38), 302 (15), 281 (14), 269 (25), 189 (18), 59 (62); HREIMS C28H30O4 gave m/z 430.2146 (calculated value: 430.1656); 1H-NMR (CD3OD; 400 MHz) and 13C-NMR (CD3OD; 100 MHz), see Table 1.
Trapezifolixanthone A (2). Needle-shaped cryatals with m.p. 158–160 °C; UV (EtOH) λmax (log ε) nm: 272 (1.61), 293 (2.55); IR (MeOH) νmax cm−1: 3,463, 2,929, 2,018, 1,685, 1,645, 1,451, 1,376, 1,129. EIMS m/z (rel. int.): 378 [M]+ (30), 364 (20), 363 (100), 335 (15), 154 (17) and 55 (12); HREIMS C23H22O5 gave m/z 378.1467 (calculated value: 378.1452); 1H-NMR (CDCl3; 400 MHz) and 13C-NMR (CDCl3; 100 MHz), see Table 1.
Betulinic acid. White powder with m.p. 300 °C; IR vmax cm−1 (CHCl3): 3,698, 2,933, 1,686, 1,453, 1,373, 1,030. EIMS m/z (rel. int.): 456 [M+] (20), 248 (50), 207 (50), 189 (100). The spectral data of the compound were identical to literature values [19].
Lupeol. White powder with m.p. 208 °C; UV (CHCl3) λmax (log ε) nm: 350 (0.74); IR vmax cm−1 (CHCl3): 3,335, 2,938, 1,452, 1,376, 1,033, 879. EIMS m/z (rel. int.): 426 [M+] (30), 218 (55), 95 (100). The spectral data of the compound are identical to literature values [20].
Stigmasterol. White needles with m.p. 255 °C; UV (CHCl3) λmax (log ε) nm: 257 (1.64); IR (CHCl3) νmax cm−1: 3,430, 2,934, 1,456, 1,372, 1,053, 963; EIMS m/z (rel. int.): 412 [M+] (55), 255 (43) and 55 (100). The spectral data of the compound are identical to literature values [20].
Friedelin. White crystals with m.p. 150 °C; UV (CHCl3) λmax (log ε) nm: 287 (0.09); IR (CHCl3) νmax cm−1: 2,927, 1,868, 1,712, 1,453, 1,384, 1,068; EIMS m/z (rel. int.): 426 [M+] (30), 273 (40), 95 (100). The spectral data of the compound are identical to literature values [21].
4. Conclusions
Two new xanthones have been isolated from the stem bark crude extracts of Calophyllum nodusum, together with the common terpenoids, betulinic acid lupeol, stigmasterol and friedelin. The new compounds were spectroscopically identified and given the trivial names nodusuxanthone and trapezifolixanthone A. Both the pure compounds and the extracts failed to exhibit any antioxidant activity when tested in the DDPH assay.
Acknowledgements
The authors wish to express thanks to the Malaysian Government for providing a research grant and Universiti Putra Malaysia for providing research facilities and technical support.
References
- Whitmore, T.C. A manual for foresters. In Tree Flora of Malaya; Longman: London, UK, 1989; pp. 162–195. [Google Scholar]
- Corner, E.J.H. Wayside Trees of Malaya; The Malayan Nature Society: Kuala Lumpur, Malaysia, 1988; pp. 349–359. [Google Scholar]
- Ishikawa, T. Anti HIV-1 active Calophyllum coumarins: Distribution, chemistry and activity. Heterocycles 2000, 53, 453–474. [Google Scholar] [CrossRef]
- Hay, A.-E.; Helesbeux, J.-J.; Duval, O.; Labaied, M.; Grellier, P.; Richomme, P. Aantimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii. Life Sci. 2004, 75, 3077–3085. [Google Scholar] [CrossRef]
- Iinuma, M.; Ito, T.; Tosa, H.; Tanaka, T.; Miyake, R.; Chelladurai, V. Prenylated xanthonoids from Calophyllum apetalum. Phytochemistry 1997, 46, 1423–1429. [Google Scholar]
- Kijjoa, A.; Gonzales, M.J.; Pinto, M.M.M.; Silva, A.M.S.; Anantachoke, C.; Herz, W. Xanthones from Calophyllum teysmanii var inophylloide. Phytochemistry 2000, 55, 833–836. [Google Scholar]
- Kashman, Y.; Gustafson, K.R.; Fuller, R.W.; Cardellina, J.H., II.; McMahon, J.B.; Currens, M.J.; Buckheit, R.W., Jr.; Hughes, S.H.; Cragg, G.M.; Boyd, M.R. The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J. Med. Chem. 1992, 35, 2735–2742. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Egglestone, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R.; Johnson, R.K.; Hertzberg, R.P.; Westley, J.W. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar]
- Kostova, I. Coumarins as inhibitors of HIV reverse transcriptase. Curr. Hiv Res. 2006, 4, 347–363. [Google Scholar] [CrossRef]
- Laure, F.; Raharivelomanana, P.; Butaud, J.-F.; Bianchini, J.-P.; Gaydo, E.M. Screening of anti-HIV-1 inophyllums by HPLC-DAD of Calophyllum inophyllum leaf extracts from Fresh Polynesia Islands. Anal. Chim. Acta 2008, 624, 147–153. [Google Scholar] [CrossRef]
- Komala, I.; Rahmani, M.; Sukari, M.A.; Ismail, H.B.M.; Ee, G.C.L.; Rahmat, A. Furaquinoline alkaloids from Melicope bonwickii (F. Muell.) T. Hartley. Nat. Prod. Res. 2006, 20, 355–360. [Google Scholar] [CrossRef]
- Rahmani, M.; Susidarti, R.A.; Ismail, H.B.M.; Sukari, M.A.; Tuafiq-Yap, Y.H.; Ee, G.C.L.; Ali, A.M.; Kulip, J.; Waterman, P.G. Coumarins from Malaysian Micromelum minutum. Phytochemistry 2003, 64, 873–877. [Google Scholar] [CrossRef]
- Susidarti, R.A.; Rahmani, M.; Ismail, H.B.M.; Sukari; Taufiq-Yap, Y.H.; Ee, G.C.L.; Ali, A.M. Cytotoxic activity of coumarins from Micrmelum minutum. Pharm. Biol. 2009, 47, 182–185. [Google Scholar] [CrossRef]
- Shamaun, S.S.; Rahmani, M.; Hashim, N.M.; Ismail, H.B.M.; Sukari, M.A.; Ee, G.C.L.; Go, R. Prenylated flavones from Artocarpus altilis. J. Nat. Med. 2010, 64, 478–481. [Google Scholar] [CrossRef]
- Iinuma, M.; Tosa, H.; Toriyama, N.; Tanaka, T.; Ito, T.; Chelladurai, V. Six xanthones from Calophyllum austroindicum. Phytochemistry 1996, 43, 681–685. [Google Scholar] [CrossRef]
- Ranjith, H.; Dharmaratne, W.; Nishanthi, W.M.; Wijesinghe, M. A trioxygenated diprenylated chromenxanthone from Calophyllum moonii. Phytochemistry 1997, 46, 1293–1295. [Google Scholar]
- Seo, E.-K.; Wall, M.E.; Wani, M.C.; Navarro, H.; Mukherjee, R.; Farnsworth, N.R.; Kinghorn, A.D. Cytotoxic constituents from the roots of Tovomita brevistaminea. Phytochemistry 1999, 52, 669–674. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Kessler, J.H.; Mullauer, F.B.; de Roo, G.M.; Medema, J.P. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett. 2007, 251, 132–145. [Google Scholar] [CrossRef]
- Jung, H.A.; Su, B.N.; Keller, W.J.; Mehta, R.G.; Kinghorn, A.D. Antioxidant xanthones from pericarp of Garcinia mangostana (Mangosteen). J. Agric. Food Chem. 2006, 54, 2077–2082. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cardenas-Rodriguez, N.; Orozco-Ibarra, M.; Perez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Bastos, D.Z.L.; Pimentel, I.C.; Jesus, D.A.; Oliveira, B.H. Biotrasformation of Betulinic and Betulonic acids by fungi. Phytochemistry 2007, 68, 834–839. [Google Scholar] [CrossRef]
- Jain, P.S.; Bari, S.B. Isolation of lupeol, stigmasterol and champesterol from petroleum ether extract of woody stem of Wrightia tinctoria. Asian J. Plant Sci. 2010, 9, 163–167. [Google Scholar] [CrossRef]
- Klass, J.; Tinto, W.F. Friedelane triterpenoids from Peritassa compta: Complete 1H and 13C assignments by 2D NMR Spectroscopy. J. Nat. Prod. 1992, 55, 1626–1630. [Google Scholar] [CrossRef]
- Samples Availability: Samples of the compounds 1a and 2 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).