Abstract
Our recent study on the stem bark extracts of Mesua beccariana has led to the isolation of two new pyranoxanthones, mesuarianone (1) and mesuasinone (2), two anthraquinones, 4-methoxy-1,3,5-trihydroxyanthraquinone (3) and 2,5-dihydroxy-1,3,4-trimethoxyanthraquinone (4), one coumarin, mammea A/AB (5) and three common triterpenes, stigmasterol (6), friedelin (7) and betulinic acid (8). Structural elucidations of these compounds were achieved using 1D and 2D NMR and MS techniques. This is the first report on the phytochemistry of Mesua beccariana.
1. Introduction
Mesua is a flowering genus consisting of about 48 species indigenous to south tropical Asia [1]. It is commonly known to the locals in Malaysia as ironwood due to its very hard and heavy wood, which is used for railroad ties and structural timber. Although there are some phytochemical reports on the genus Mesua, focused on Mesua ferrea [2,3,4,5], and a few on Mesua racemosa [6], Mesua thwaitesii [7] and Mesua daphnifolia [8,9], no study has been carried out on the chemistry of Mesua beccariana. These reports revealed the presence of xanthones, coumarins, biflavanoids and triterpenoids. This paper reports our recent phytochemical discovery of two new pyranoxanthones – mesuarianone (1) and mesuasinone (2) from the stem bark of Mesua beccariana (Figure 1).
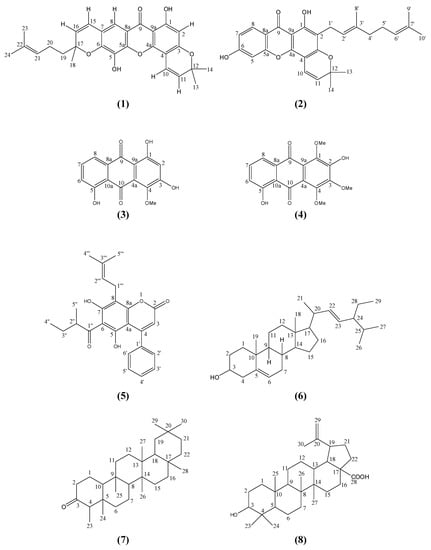
Figure 1.
Structures of compounds.
2. Results and Discussion
Compound 1, mesuarianone (Figure 1), which was isolated from the hexane stem bark extract of Mesua beccariana, is a yellow powder with melting point 166-167 °C. The EIMS spectrum gave a molecular ion (M+) peak at m/z 460, while the HRESIMS spectrum gave 460.1827 (calc’d. 460.1886) consistent with the molecular formula C28H28O6. The IR showed νmax : 3392 (OH), 2971 (CH3), 2923 (CH2), 1639 (C=O), 1574 and 1475 (C=C aromatic) cm-1. The UV maximum absorptions at 208 (6.41), 280 (6.49), 333.8 (6.28) nm were characteristic of a xanthone skeleton.
The 1H- and 13C-NMR spectral information of 1 revealed the existence of 28 hydrogens and 28 carbons, supporting the molecular formula above. These spectroscopic data, along with the DEPT analysis indicated the presence of a xanthone skeleton with all positions substituted, except for C-2 (δ 99.6) and C-8 (δ 113.7). Further examination of the DEPT experiment revealed the existence of four non-oxygenated aromatic carbons which are C-4 (δ 101.6), C-7 (δ 117.8), C-8a (δ 114.8) and C-9a (δ 103.4), along with six oxygenated aromatic carbons which are C-1 (δ 163.3), C-3 (δ 160.7), C-4a (δ 151.8), C-5a (δ 145.3), C-5 (δ 132.5) and C-6 (δ 145.3). The presence of a chelated carbonyl group was indicated by a downfield carbon signal at δ 180.5 (C-9). In the 1H-NMR spectrum, a chelated phenolic hydroxyl group (δ 13.05, 1H, s) and two one-proton aromatic singlets (δ 6.23 and 7.44) were observed.
The HMBC spectrum demonstrated the 2J and 3J multiple bond 1H-13C correlations in the molecule. The sharp singlet in the downfield region at δ 13.05 indicated a chelated hydroxyl group. Its multiple-bond correlations to δ 99.6 (C-2), 103.4 (C-9a) and 163.3 (C-1) in the HMBC spectrum confirmed its position at C-1. Moreover, 3J correlations between δ 7.44 (H-8) and C-9 (δ 180.5) and C-5a (δ 145.3) were clearly seen. Meanwhile, the singlet at δ 6.23 (H-2) gave a 3J correlation to δ 103.4 (C-9a) and a 2J correlation to δ 160.7 (C-3). This confirmed the position of these two aromatic methines to be at C-8 and C-2, respectivel.
The characterization of chemical shifts for protons at δ 2.10 (m, 2H, H-20), 5.06 (t, 1H, H-21), 1.53 (s, 3H, H-23), 1.63 (s, 3H, H-24) and carbons at δ 22.9 (C-20), 123.6 (C-21), 132.6 (C-22), 17.9 (C-23) and 25.9 (C-24) suggested the presence of a prenyl moiety in the molecule (Figure 2). This hypotesis was further confirmed by the establishment of the connectivity of the two vinylic methyl proton singlets at δ 1.53 (H-23) and δ 1.63 (H-24) to their neighbouring carbons at δ 132.3 (C-22) and 123.6 (C-21) in the HMBC spectrum. On the other hand, HMBC experiment indicated the linkage between two aliphatic methyls resonating at δ 1.46 (H-13 & H-14) to carbon signals at δ 78.4 (C-12) and 127.3 (C-11). Long-range 3J and 2J correlations between doublet protons at δ 6.87 (H-10, J = 10.1 Hz) and 5.56 (J = 10.1 Hz, H-11) and the carbon signal at δ 78.4 (C-12) respectively were also seen. These data together with the COSY spectrum analysis suggested the existence of a pyrano ring (ring A; Figure 2). Meanwhile, HMBC correlations of H-19 (δ 1.79) and H-18 (δ 1.48) to C-17 (δ 81.7) and the two ortho coupled vinylic protons H-15 and H-16 (δ 6.46 and 5.65) to C-17 (δ 81.7) were also observed (Figure 3). The latter correlation pattern is similar to that of ring A, thus implying another pyrano ring (ring B) in the molecule. However, the latter pyrano ring carries only one methyl group. Two methylene hydrogens which gave a multiplet signal at δ 2.10 (H-20) demonstrated a 2J correlation to the carbon signal at δ 41.8 (C-19). This information allowed the placement of the prenyl moiety at C-19 which is attached to the pyrano ring B. The HMBC spectrum confirms the attachment of C-19 to C-17. 2J and 3J linkages of H-19 to C-17 and C-16 respectively were observed.
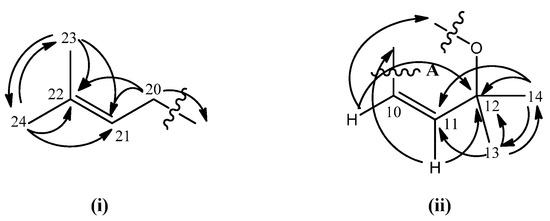
Figure 2.
Prenyl moiety (i) and pyrano ring system (ii) and their HMBC correlations.
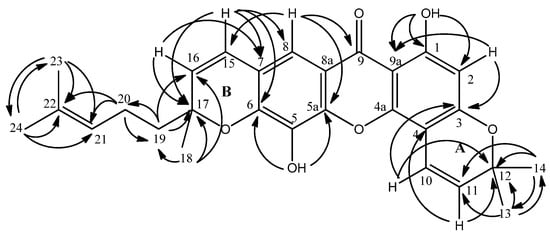
Figure 3.
2J and 3J correlations between 1H and 13C in HMBC analysis.
The connectivity of δ 6.87 (H-10) to C-3 (δ 160.7) and δ 5.56 (H-11) to C-4 (δ 101.6) as observed in the HMBC experiment led to the attachment of pyrano ring A to position C-3 and C-4 in the xanthone skeleton. Similarly, the other prenylated pyrano ring (ring B) is attached to position C-6 and C-7 following the observation of 3J and 2J long-range connectivity between H-15 (δ 6.46) and C-6 (δ 145.3), C-7 (δ 117.8) and C-8 (δ 113.7). The non-chelated hydroxyl singlet at δ 5.52 gave 3J linkages to two quartenary aromatic carbons with overlapped chemical shifts at δ 145.3 (C-5a and C-6). These evidences led to the assignment of the hydroxyl group at C-5 (δ 132.5) (Figure 3).
Hence, the structure of compound 1 was elucidated to be 1,5-dihydroxy-6’,6’-dimethylpyrano[2’,3’: 3,4]-6”-(2-methyl-2-pentenyl)-6”-methylpyrano[2”,3”:6,7]-xanthone and named as mesuarianone. The NMR data of this compound are summarized in Table 1.

Table 1.
1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3) data of mesuarianone (1) and mesuasinone (2).
Compound 2, mesuasinone (Figure 1), which was obtained as a yellow solid with m.p. 118-119 °C, and molecular formula C28H30O5 (EIMS m/z 446), as confirmed by HREISIMS 446.2041 (calc’d. 446.2093) had UV and IR spectral data suggestive of a xanthone derivative. The UV spectrum gave maximum absorptions at 208 (3.82), 254 (3.96), 274.0 (3.93), 334 (3.53) nm, while the IR spectrum showed absorptions at 3229 (OH), 2925 (CH3), 2865 (CH2), 1640 (C=O), 1576 and 1496 (C=C aromatic) cm-1.
The 13C-NMR signals showed the presence of a carbonyl carbon (δ 180.7) along with the presence of twelve quaternary carbons (δ 80.7, 100.3, 103.0, 111.8, 121.2, 131.7, 132.1, 144.1, 144.2, 149.2, 158.9, 160.6), seven methines (δ 115.4, 117.1, 120.1, 121.9, 123.6, 123.9, 126.1), three methylenes (δ 21.2, 22.8, 41.8) and five methyls (δ 17.6, 17.9, 25.6, 25.8, 27.1). The 13C and DEPT NMR spectra of compound 2 showed some similarity to that of 1, suggesting 2 also has a pyranoxanthone structure.
Moreover, the 1H-NMR spectra also supported the existence of a pyrano ring. Two vinylic protons were observed at δ 6.83 (1H, d, J = 10.1 Hz, H-10) and 5.58 (1H, d, J = 10.1 Hz, H-11) along with two methyl signals at δ 1.45 (3H, s, H-14) and 1.66 (3H, s, H-13). The methyl protons H-13 (δ 1.66) demonstrated 3J correlations with δ 27.1 (C-14) and H-14 (δ 1.45) showed 2J correlations with δ 80.7 (C-12) and 3J correlations with δ 126.1 (C-11) in the HMBC experiment. This pyrano ring was connected to the xanthone skeleton at C-3 and C-4 as evidenced by the 3J HMBC correlations between the vinylic proton at δ 6.83 (H-10) and δ 149.2 (C-4a) and δ 158.9 (C-3), while the other vinylic proton at δ 5.58 (H-11) correlates to δ 100.3 (C-4) via another 3J HMBC correlation.
The 1H NMR spectra also gave a sharp singlet at δ 13.02 indicating a chelated hydroxyl group, while the broad singlet at δ 5.73 was due to the free hydroxyl group. In the HMBC spectra, the chelated hydroxyl proton (δ 13.02) showed cross-peaks with three quaternary carbon signals at δ 103.0 (C-9a, 3J), 111.8 (C-2, 3J) and 160.6 (C-1, 2J). The deshielded carbon signal at 160.6 (C-1) was found to be attached to the hydroxyl group.
Three aromatic protons which are arranged in the ortho and meta positions were revealed by the resonances at δ 7.22 (1H, d, J = 8.3 Hz), 7.77 (1H, d, J = 8.3 Hz) and 7.29 (1H, s). The lowest-field aromatic proton (δ 7.77) was assigned to H-8 due to the anisotropic effect of the carbonyl group. This was supported by the 3J and 2J HMBC correlations of H-8 to a carbonyl signal at δ 180.7 (C-9) and two aromatic carbons C-8a (δ 121.2) and C-7 (δ 120.1), while its ortho-coupled proton H-7 correlates to δ 123.9 (C-5) and 121.2 (C-8a) as shown in Figure 4. The position of the proton resonating at δ 7.29 (1H, s) was confirmed to be at C-5 via its HMBC correlations to the carbon signals at δ 120.1 (C-7, 3J) and 144.1 (C-6, 2J).
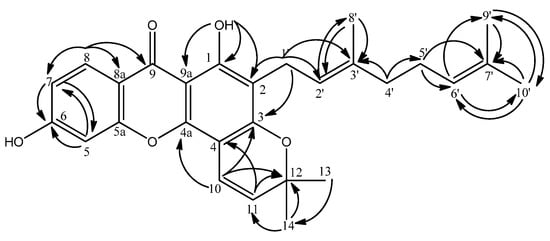
Figure 4.
HMBC Correlations in Compound 2.
The 1H-NMR spectra revealed a geranyl moiety at δ 3.36 (2H, d, J = 7.3 Hz, H-1’), 5.24 (1H, t, J = 7.3 Hz, H-2’), 1.84 (2H, m, H-4’), 2.10 (2H, m, H-5’), 5.09 (1H, t, J = 6.4 Hz, H-6’), 1.80 (3H, s, H-9’), 1.68 (3H, s, H-10’), 1.57 (3H, s, H-8’). This was further confirmed using the long-range correlation system observed in the HMBC spectra as illustrated in Figure 5. The benzylic allylic methylene protons (δ 3.36, H-1’) of the geranyl group showed correlations with δ 111.8 (C-2, 2J) and 158.9 (C-3, 3J) allowing its placement at C-2.
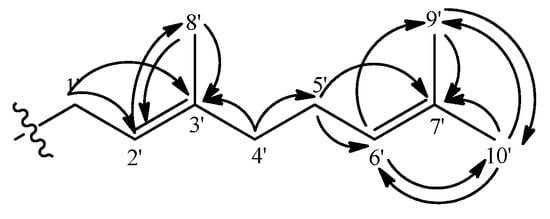
Figure 5.
The geranyl moiety and its long-range Heteroatom Correlations.
Hence, the structure of compound 2 was elucidated to be 1,6-dihydroxy-2-(3’,7’-dimethyl-2’,6’-octenyl)-6”,6”-dimethylpyrano-[2”,3”:3,4]-xanthone and named mesuasinone. The NMR data of this compound are summarized in Table 1.
4-Methoxy-1,3,5-trihydroxyanthraquinone (3) [10], 2,5-dihydroxy-1,3,4-trimethoxyanthraquinone (4) [10], mammea A/AB (5) [11], stigmasterol (6) [13], friedelin (7) [13] and betulinic acid (8) [16,17] were identified by comparing their spectral data with literature.
3. Experimental
3.1. General
Infrared spectra were measured using the universal attenuated total reflection (UATR) technique on a Perkin-Elmer 100 Series FT-IR spectrometer. EIMS were recorded on a Shimadzu GCMS-QP5050A spectrometer. NMR spectra were obtained using a Unity JEOL 400 MHz FTNMR spectrometer using CDCl3 as solvent and tetramethylsilane (TMS) as internal standard. Ultraviolet spectra were recorded in EtOH on a Shimadzu UV-160A, UV-Visible Recording Spectrophotometer.
3.2. Plant Material
The stem bark of Mesua beccariana was collected from the Sri Aman district in Sarawak, Malaysia. The plant material was identified by Associate Professor Dr Rusea Go, Biology Department, Faculty of Science, Universiti Putra Malaysia.
3.3. Extraction and Isolation
The air-dried and powdered sample was extracted successively with n-hexane, dichloromethane, ethyl acetate and methanol. The extracts were dried under reduced pressure using a rotary evaporator to yield hexane (15.6 g), dichloromethane (21.2 g), ethyl acetate (15.8 g) and methanol (80.5 g) extracts. Each of these extracts was chromatographed over a silica gel column using a stepwise gradient system (hexane/chloroform, chloroform/ethyl acetate, and ethyl acetate/methanol). The column chromatography of the hexane extract gave mesuarianone (1, 110 mg), mesuasinone (2, 76 mg) and friedelin (7, 20 mg). Meanwhile, the dichloromethane extract gave stigmasterol (6, 23 mg) and betulinic acid (8, 45 mg) while the ethyl acetate extract gave two anthraquinones and a coumarin identifed as 4-methoxy-1,3,5-trihydroxyanthraquinone (3, 9 mg), 2,5-dihydroxy-1,3,4-trimethoxy-anthraquinone (4, 8 mg) and mammea A/AB (5, 11 mg).
3.4. Spectral Data
Mesuarianone (1). Yellow solid. m.p. 166-167 °C. (CHCl3) +22.5. UV nm (log ε): 208 (6.41), 280 (6.49), 333.8 (6.28). IR νmax (cm-1): 3392, 2971, 2923, 1639, 1574, 1475. For 1H- and 13C- NMR spectra, see Table 1. MS m/z (rel. int.): 460 [M+] (39), 445 (100), 377 (89), 361 (10), 347 (11), 323 (12), 203 (8), 181 (61), 91 (6), 77 (5), 69 (27), 55 (9).
Mesuasinone (2). Yellow solid. m.p. 118-119 °C. UV nm (log ε): 208 (3.82), 254 (3.96), 274.0 (3.93), 334 (3.53). IR νmax (cm-1): 3229, 2925, 2865, 1640, 1576, 1496. For 1H- and 13C-NMR spectra, see Table 1. MS m/z (rel. int.): 446 [M+] (14), 431 (4), 391 (8), 363 (100), 307 (21), 154 (12), 69 (10).
4-Methoxy-1,3,5-trihydroxyanthraquinone (3). Orange solid. UV nm: 279, 320, 425, 470, 485. IR νmax (cm-1): 3420, 2920, 2860, 1720, 1630, 1470. MS m/z (rel. int.): 286 [M+] (100), 268 (87), 257 (10), 243 (38), 212 (27), 180 (30). The 1H- and 13C-NMR spectral data are consistent with published data [10].
2,5-Dihydroxy-1,3,4-trimethoxyanthraquinone (4). Orange solid. UV nm: 218,276,410. IR νmax (cm-1): 3400, 2920, 2840, 1660, 1630,1540. MS m/z (rel. int.): 330 [M+] (100), 315 (60), 312 (5), 297 (20), 287 (22), 272 (24), 227 (20), 58 (23). The 1H- and 13C-NMR spectral data are consistent with published data [10].
Mammea A/AB (5). Colourless solid. UV nm: 283, 337. MS m/z (rel. int.): 406 [M+] (2), 392 (18), 377 (10), 349 (100), 293 (10). The 1H- and 13C-NMR (CDCl3) spectral data are consistent with the published data [11].
Stigmasterol (6). White needles. m.p. 155-156 °C (Lit. 168-169 °C) [12]. IR νmax (cm-1): 3399, 2939, 1457, 1374. MS m/z (rel. int.): 412 [M+] (60), 394 (7), 369 (7), 351 (15), 300 (26), 271 (38), 255 (40), 159 (41), 145 (42), 133 (45), 123 (31), 105 (42), 95 (44), 83 (77), 69 (62), 55 (100). The 1H- and 13C- NMR (CDCl3) spectral data are consistent with literature [13].
Friedelin (7). White needles. m.p. 245-246 °C (Lit. 260-263 °C) [14]. IR νmax (cm-1): 1714, 1457, 1380. MS m/z (rel. int.): 426 [M+] (12), 411 (6), 302 (11), 273 (16), 246 (17), 231 (16), 218 (19), 205 (20), 191 (18), 179 (19), 163 (27), 149 (18), 137 (24), 123 (60), 109 (64), 95 (76), 81 (63), 69 (100), 55 (75), 41 (44). The 1H- and 13C-NMR spectral data are consistent with literature [13].
Betulinic acid (8). White solid. m.p. 290-291 °C (Lit. 291-292 °C) [15]. IR νmax (cm-1): 3460, 2939, 1687, 1453, 1375. MS m/z (rel. int.): 456 [M+] (7), 438 (4), 248 (40), 207 (48), 189 (100), 69 (17). The 1H- and 13C-NMR spectral data are consistent with published data [16,17].
4. Conclusions
Two new pyranoxanthones, mesuarianone (1) and mesuasinone (2), along with two anthraquinones, a coumarin, and three triterpenes were isolated from the stem bark of Mesua beccariana. Future work on the biological activities such as anti-cancer and anti-oxidant properties will be carried out in due course.
Acknowledgments
We would like to thank Jegak Uli for collection of the plant samples and Ms Shareena Safiai for NMR spectra. Financial support from UPM under the RUGS research fund is gratefully acknowledged.
References and Notes
- Ee, G.C.L.; Lim, C.K.; Rahmat, A.; Lee, H.L. Cytotoxic activities of chemical constituents from Mesua daphnifolia. Trop. Biomed. 2005, 22, 99–102. [Google Scholar] [PubMed]
- Walia, S.; Mukerjee, S.K. Ferrxanthone, a 1,3,5,6-tetraoxygenated xanthone from Mesua ferrea. Phytochemistry 1984, 23, 1816–1817. [Google Scholar] [CrossRef]
- Raju, M.S.; Srimannarayan, G.; Rao, N.V.S. Structure of mesuaferrone-B a new biflavonone from the stamens of Mesua ferrea Linn. Tetrahedron Lett. 1976, 49, 4509–4512. [Google Scholar] [CrossRef]
- Chow, Y.L.; Quon, H.H. Chemical constituents of the heartwood of Mesua ferrea. Phytochemistry 1968, 7, 1871–1874. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Pai, B.R.; Subramaniam, P.S.; Rao, U.R.; Muthukumaraswa, N. Constituents of Mesua ferrea L.-mesuaxanthone A and mesuaxanthone B. Tetrahedron 1967, 23, 243–248. [Google Scholar] [CrossRef]
- Morel, C.; Guilet, D.; Oger, J.M.; Seraphin, D.; Sevenet, T.; Wiart, C.; Hadi, A.H.A.; Richomme, P.; Bruneton, J. 6-Acylcoumarins from Mesua racemosa. Phytochemistry 1999, 50, 1243–1247. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Selliah, S.S.; Sultanbawa, M.U.S.; Games, D.E. Xanthones and 4-phenylcoumarins of Mesua thwaitesii. Phytochemistry 1975, 14, 265–269. [Google Scholar] [CrossRef]
- Ee, G.C.L.; Lim, C.K.; Cheow, Y.L.; Sukari, M.A. Xanthones and triterpenoids from Mesua daphnifolia and Garcinia maingayi. Malaysian J. Sci. 2005, 24, 183–185. [Google Scholar]
- Ee, G.C.L.; Lim, C.K.; Ong, G.P.; Sukari, M.A.; Lee, H.L. Daphnifolin, a new xanthone from Mesua daphnifolia. J. Asian Nat. Prod. Res. 2006, 8, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Wijnsma, R.; Verpoorte, R.; Mulder-Krieger, T.; Svendsen, A.B. Anthraquinones in callus cultures of Cinchona ledgeriana. Phytochemistry 1984, 23, 2307–2311. [Google Scholar] [CrossRef]
- Verotta, L.; Lovaglio, E.; Vidari, G.; Finzi, P.V.; Neri, M.G.; Raimondi, A.; Parapini, S.; Taramelli, D.; Riva, A.; Bombardelli, E. 4-Alkyl- and 4-phenylcoumarins from Mesua ferrea as promising multidrug resistant antibacterials. Phytochemistry 2004, 65, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, F.; Mohamed, S.; Lajis, M.N. Hypoglycaemic effect of Parkia speciosa seeds due to the synergistic action of [beta]-sitosterol and stigmasterol. Food Chem. 1994, 49, 339–345. [Google Scholar] [CrossRef]
- Ee, G.C.L.; Kua, A.S.M.; Lim, C.K.; Jong, V.; Lee, H.L. Inophyllin A, a new pyranoxanthone from Calophyllum inophyllum (Guttiferae). Nat. Prod. Res.: Formerly Nat. Prod. Lett. 2006, 20, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Klass, J.; Tinto, W.F.; McLean, S.; Reynolds, W.F. Friedeland Triterpenoids from Peritassa compta: Complete 1H and 13C Assignments by 2D nmr Spectroscopy. J. Nat. Prod. 1992, 55, 1626–1630. [Google Scholar] [CrossRef]
- Kim, D.S.H.L.; Chen, Z.; Nguyen, V.T.; Pezzuto, J.M.; Qiu, S.; Lu, Z.-Z. A Concise Semi-Synthetic Approach to Betulinic Acid from Betulin. Synth. Commun. 1997, 27, 1607–1612. [Google Scholar] [CrossRef]
- Tapondjou, A.L.; Miyamoto, T.; Lacaille-Dubois, M.-A. Glucuronide triterpene saponins from Bersama engleriana. Phytochemistry 2006, 67, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Galgon, T.; Höke, D.; Dräger, B. Identification and quantification of betulinic acid. Phytochem. Anal. 1999, 10, 187–190. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 1–8 are available from the authors. |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).