Abstract
Bromination of 5,6-dimethoxyindan-1-one with Br2 in acetic acid at room temperature produced exclusively the corresponding 2,4-dibromo compound in 95% yield. Reaction of 5,6-dimethoxyindan-1-one with Br2 in the presence of KOH, K2CO3 or Cs2CO3 at ~0°C gave the monobrominated product 4-bromo-5,6-dimethoxyindan-3-one in 79%, 81% and 67% yield, respectively. 5,6-Dihydroxyindan-1-one was dibrominated on the aromatic ring affording 4,7-dibromo-5,6-dihydroxyindan-1-one both in acetic acid at room temperature and in the presence of KOH at ~0°C. 5,6-Difluoroindan-1-one and 1-indanone were α-monobrominated in acetic acid and α,α-dibrominated under KOH conditions at room temperature.
Introduction
Bromoaromatics are widely used as intermediates in the manufacture of pharmaceuticals, agrochemicals and specialty chemical products [1]. Recently, bromoarenes have assumed increasing importance in organic synthesis as useful reagents for functionalization through carbon-carbon bond formation of diarenes, ethylenic, or acetylenic condensation via metal catalyzed cross coupling reactions such as the Suzuki, Negishi, and Sonogashira reactions [2]. Various reagents and reaction conditions have been developed and reported for the bromination of aromatic systems using different brominating agents, including Br2/SbF3/HF [3], NBS/H2SO4/CF3COOH [4], NBS/NaOH [5], NBS/ FeCl3 [6], HBr/tert-BuOOH, HBr/H2O2, and HBr/DMSO [7], NH4Br/H2O2/CH3COOH [8], CuBr/tert-BuONO [9], Br2/SO2Cl2 over microporous catalyst [10], 3-methylimidazolium tribromide [11], NaBr/Oxone®, KBr/Oxone®, and KBr/H2O2/Oxone® [12], Br2/tetrabutylammonium peroxydisulfate [13], quaternary ammonium tribromide [14] and quinolinium bromochromate in glacial acetic acid [15].
On the other hand, selective α-bromination of carbonyl compounds is another important transformation, as the resulting α-brominated products are also versatile synthetic intermediates. Some of the reported methods involve the use of NBS/NH4OAc [16], hypervalent iodine sulfonate/magnesium halides under microwaves [17], N-halosuccinimide/TsOH/CH3CN [18], Br2/C2-symmetric diphenylpyrrolidine catalyst [19], nonselective dibromination/selective debromination [20], N-methylpyrrolidin-2-one hydrotribromide complex (MPHT) [21].
We report herein our attempts to find a simple, regioselective and high-yielding monobromination method for the aromatic bromination of 5,6-dimethoxy-, 5,6-dihydroxy-, 5,6-difluoroindan-1-one and indan-1-one, which all possess both an aromatic ring and an unprotected carbonyl group. The reaction conditions studied involved the use of Br2 under neutral, acidic and basic conditions and a variety of well known brominating reagents such as KBr, NH4Br and pyridinium bromochromate.
Results and Discussion
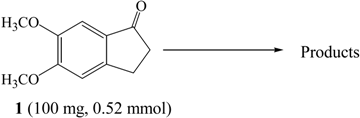
A number of different bromination methods were examined using as a model compound 5,6-dimethoxyindan-1-one (1), which was synthesized according to the previously reported procedure [22]. The results are summarized in Table 1.
When compound 1 was reacted with Br2 in CCl4 (entry I) or in CHCl3 (entry III) for 2 hrs at room temperature, 2,2-dibromo-5,6-dimethoxyindan-1-one (2) was obtained in 44% or 22% yield as the major product and 2,2,4-tribromo-5,6-dimethoxyindan-1-one (3) was obtained in 5% or 2% yield, respectively, along with recovered starting material. In the 1H-NMR spectrum of 2 the two C3 proton peaks were identified as a singlet at 4.22 ppm and the two aromatic hydrogens were observed at 6.79 and 7.30 ppm. The molecular ion peak at m/z 350 corresponding to the dibrominated product was also observed in its mass spectrum. In the 1H-NMR spectrum of 3, the two proton peaks of C3 appeared as a singlet at 4.16 ppm and one aromatic hydrogen was seen at 7.37 ppm. The Br-substituted position of the aromatic ring was identified from the NOESY spectrum. If the C4 hydrogen were not substituted with bromine, a correlation should be observed between the C4 and C3 hydrogens in the NOESY spectrum, but we did not observe such a cross peak, therefore we conclude that the 4-bromo derivative was synthesized. The molecular ion peak at m/z 428 which confirmed the formation of a tribrominated product was noted in the mass spectrum of compound 3.
Under ice bath conditions, 2-bromo-5,6-dimethoxyindan-1-one (4) and 4-bromo-5,6-dimethoxy-indan-1-one (5) were formed in 35% and 14% yield using CCl4 as solvent, and in 14% and 5% yield in CHCl3 (entries II, IV).

Table 1.
Bromination of 5,6-dimethoxyindan-1-one (1).
 | |||
|---|---|---|---|
| Entry | Reaction conditions | Products (Yield) b | |
| I | Br2a (0.52 mmol)/CCl4/rt/2hrs | 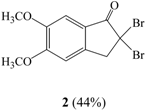 | 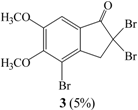 |
| III | Br2 (0.52 mmol)/CCl4/ice bath/2 hrs | 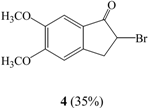 | 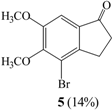 |
| II | Br2 (0.52 mmol)/CHCl3/rt/2 hrs | 2 (22%) | 3 (2%) |
| IV | Br2 (0.52 mmol)/CHCl3/ice bath/2 hrs | 4 (14%) | 5 (5%) |
| V | Br2 (0.52 mmol)/I2, Fe/CCl4/ice bath/6 hrs | 4 (32%) | |
| VI | Br2 (0.52 mmol)/AlCl3 (1.04 mmol)/CH2Cl2/ice bath/2 hrs | 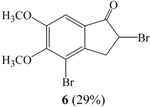 | 5 (8%) |
| VII | KBr (0.57 mmol)/Oxone® (0.57 mmol)/MeOH/0→5°C/2 hrs | 4 (46%) | |
| VIII | NBS (0.57 mmol)/THF/rt/1 hr | No reaction | |
| IX | NH4Br (0.57 mmol)/H2O2 (0.57 mmol)/AcOH/rt/2 hrs | No reaction | |
| X | Pyridinium bromochromate (0.52 mmol)/glacial AcOH/30-40°C/ 20 min. | No reaction | |
a When Br2 was used the reaction was carried out with exclusion of light;b Yields refer to pure isolated products. The recovered starting material and traces of other product(s) are not listed.
In the 1H-NMR spectrum of 4, the single C2 proton appeared as two double doublets at 4.66 ppm, two C3 proton peaks were observed as double doublets at 3.34 and 3.77 ppm and two aromatic hydrogens were seen at 6.85 and 7.23 ppm. The molecular ion peak at m/z 271 in the mass spectrum of compound 4 indicated the formation of a monobrominated product. In the 1H-NMR spectrum of 5, two proton peaks of C2 and C3 were identified as triplets at 2.71 and 3.01 ppm and an aromatic proton was observed at 7.21 ppm. The mass spectrum of 5 was similar to that of compound 4.
The reaction of Br2, I2 and Fe with 1 in CCl4 for 6 hrs in an ice bath gave compound 4 (entry V), while in the presence of AlCl3, the reaction of 1 and Br2 in CH2Cl2 for 2 hrs in an ice bath afforded 2,4-dibromo-5,6-dimethoxyindan-1-one (6) and 5 in yields of 25% and 9 %, respectively (entry VI). In the 1H-NMR spectrum of 6, the one proton peak of C2 was observed as a multiplet at 4.62-4.69 ppm and the two proton peaks of C3 were observed as a double triplet at 3.29 ppm and as two double doublets at 3.72 ppm. One aromatic proton was observed at 7.28 ppm. The molecular ion peak at m/z 350 in the mass spectrum of 6 confirmed the dibrominated nature of the product.
KBr and Oxone® (2K2HSO5ּKHSO4ּK2SO4) is an oxybromination reagent used in high para- selective brominations of aromatic compounds [12]. We treated compound 1 with Oxone® and KBr in methanol for 2 hr to obtain 4 in 46% yield (entry VII). No reactions were observed between 1 and NBS in tetrahydrofuran (entry VIII), NH4Br/H2O2/AcOH (entry IX) or quinolium bromochromate in glacial acetic acid (entry X).
In order to compare the reactivity and regioselectivity of different 5,6-disubstituted indan-1-ones in the aromatic bromination, reactions of 5,6-dimethoxy- and 5,6-dihydroxyindan-1-one (1 and 8), indan-1-one (10) and 5,6-difluoroindan-1-one (13) were also studied carried out under acidic and basic conditions. The results are shown in Table 2.
Bromination of 1 in the presence of acetic acid at room temperature formed 6 exclusively in 95% yield (entry XI). Under acidic conditions, the carbonyl group is easily converted to the corresponding enol form, which reacts preferentially with molecular bromine affording the α-brominated product.
We next examined the use of solid K2CO3, KOH or Cs2CO3 as bases. The reaction of 1 and Br2/ K2CO3 in CH2Cl2 at room temperature for 1 hr gave compound 6 as the major product and 5 as a minor one (entry XII), but under ice bath condition, 5 and 4,7-dibromo-5,6-dimethoxyindan-1-one (7) were obtained in 79% and 9% yields (entry XIII). In the 1H-NMR spectrum of 7, no aromatic proton could be seen and the molecular ion peak at m/z 350 in the mass spectrum indicated formation of a dibrominated product. When KOH or Cs2CO3 were used as base, compounds 5 and 7 were obtained in 81% and 7% yield with the former (entry XIV) and 67% and 20% yield with Cs2CO3 (entry XV), respectively. Under basic and cold conditions, the rate of electrophilic aromatic substitution of Br2 can be assumed to be faster than that of the enolate formation in 1, but if the reaction time is extended beyond 1 hr, then the dibrominated derivative 7 is formed as the major product and the aromatic and α-brominated product 6 is a minor product.
To apply to the other substrates the same reaction conditions that showed the best reactivity and regioselectivity in the bromination of compound 1, we selected 1.1 equivalent of Br2 under acetic acid conditions and 2 equivalents of Br2 under conditions containing KOH. Compound 8 was treated with Br2 in acetic acid and using KOH to give 4,7-dibromo-5,6-dihydroxyindan-1-one (9) in 47% and 55% yields, respectively (entry XVI). The reactions of indan-1-one (10), synthesized from 1 by a reported method [23], with Br2 under acidic and basic conditions at room temperature gave the α-brominated product 2-bromoindan-1-one (11) in 84% yield and the α,α-dibrominated product 2,2-dibromoindan-1-one (12) in 67% yield, respectively (entry XVII). The reaction of 5,6-difluoroindan-1-one (13) with Br2 in acetic acid yielded 2-bromo-5,6-difluoroindan-1-one (14) in 65% yield and with Br2 in KOH it yielded 2,2-dibromo-5,6-difluoroindan-1-one (15) regioselectively in 84% yield (entry XVIII).
The bromination of compound 8, which possesses a strong activating group, showed exclusively aromatic direction under acidic conditions at room temperature and KOH conditions at 0°C, and the bromination of compound 1, with a moderately activating group, led to α-mono- and monoaromatic substitution under acidic conditions at room temperature and monoaromatic direction under basic conditions at 0°C, respectively. Compounds 10 and 13, having deactivating groups, were α-monobrominated in acetic acid and α,α-dibrominated under KOH conditions at room temperature.

Table 2.
Bromination of 5,6-dimethoxy-, 5,6-dihydroxy-, 5,6-difluoro-indan-1-one and indan-1-one (1, 8, 13 and 10) under acidic and basic conditions.
| Entry | Starting materials | Reaction conditions | Products (Yield) b | |
|---|---|---|---|---|
| XI | 1 (100 mg, 0.52 mmol) | Br2a (0.57 mmol)/AcOH/rt/2 hrs | 6 (95%) | |
| XII | Br2 (1.04 mmol)/K2CO3 (1.56 mmol)/CH2Cl2/ rt/1hr | 6 (44%) | 5 (23%) | |
| XIII | Br2 (1.04 mmol)/K2CO3 (1.56 mmol)/CH2Cl2/ ice bath/1hr | 5 (79%) |  | |
| XIV | Br2 (1.04 mmol)/KOH (1.56 mmol)/CH2Cl2/ ice bath/1hr | 5 (81%) | 7 (7%) | |
| XV | Br2 (1.04 mmol)/Cs2CO3 (1.56 mmol)/CH2Cl2/ ice bath/1hr | 5 (67%) | 7 (20%) | |
| XVI | 8 (100 mg, 0.61 mmol) | Br2 a (0.67 mmol)/AcOH/rt/3.5 hrs |  | |
| Br2 (1.22 mmol)/KOH (1.83 mmol)/CH2Cl2/ice bath/0.5 hrs | 9 (55%) | |||
| XVII | 10 (100 mg, 0.76 mol) | Br2 (0.84 mmol)/AcOH/rt/2.5 hrs |  | |
| Br2 (1.52 mmol)/KOH (2.28 mmol)/CH2Cl2/rt/ 20 hrs |  | |||
| XVIII | 13 (100 mg, 0.60 mol) | Br2 (0.66 mmol)/AcOH/rt/1 hr |  | |
| Br2 (1.20 mmol)/KOH (1.80 mmol)/CH2Cl2/rt/ 20 hrs |  | |||
a When Br2 was used, the reactions were carried out with exclusion of light.b Yields refer to pure isolated products. The recovered starting material and traces of other product(s) are not listed.
Conclusions
We have presented a simple and regioselective method for the aromatic monobromination without the protection of the carbonyl group of 5,6-dimethoxyindan-1-one (1), using Br2 under acidic or basic conditions. An aromatic monobrominated product, 4-bromo-5,6-dimethoxy-indan-1-one (5) was regioselectively obtained in 81% yield under KOH conditions at 0°C. Compound 8, having the strong OH activating group produced the aromatic dibrominated compound 9 under both acidic and basic (KOH) conditions at 0°C. The aromatic bromination of 10 and 13 did not occur under acidic and KOH conditions at 0°C, but α-monobromo derivatives were obtained under acidic conditions and α,α-dibromo derivatives were obtained under KOH conditions at room temperature, respectively.
Experimental
General
All non-aqueous reactions were performed under a dry atmosphere of nitrogen. Commercial reagents, including 1-indanone, were purchased from Aldrich, Fluka, or Sigma. 5,6-Dimethoxy-1-indanone [22], 5,6-dihydroxy- [23] and 5,6-difluoro-1-indanone [24] were synthesized according to known procedures. Solvents were purified and dried prior to use. Melting points were measured on a Thomas-Hoover melting point apparatus and are not corrected. Unless stated otherwise 1H- (400 MHz), 13C-NMR (100 MHz), and NOESY spectra were recorded in CDCl3 on a Varian 400 MHz spectrometer. Chemical shifts (δ) are in parts per million (ppm) relative to tetramethylsilane, and coupling constants (J) are given in Hertz. IR spectra were recorded on a Jasco FT-IR 300E spectrometer as KBr pellets. Low FAB mass spectra were obtained on a Tandem mass spectrometer. Analytical TLC was performed on pre-coated silica gel 60 F254 plates (Merck). Solvent systems used for TLC were ethyl acetate/n-hexane mixtures and 10% MeOH in methylene chloride. Column chromatography was carried out on Merck silica gel 9385 (230-400 mesh), eluting with ethyl acetate/n-hexane mixtures.
1. Bromination of 5,6-dimethoxyindan-1-one (1).
1.1. Bromination with bromine/CCl4 at room temperature.
Bromine (1.3 mL, 2.60 mmol) was added with syringe to a solution of 1 (500 mg, 2.60 mmol) in CCl4 (35 mL) at room temperature. Light was excluded from the flask and the reaction mixture was stirred for 2 hrs. Then excess bromine and CCl4 were removed by simple distillation. The residue was neutralized with 10% NaOH and extracted with methylene chloride, which was dried over anhydrous MgSO4, filtered and concentrated. The residue was purified by column chromatography to give 2,2-dibromo-5,6-dimethoxyindan-1-one (2) in 44% yield and 2,2,4-tribromo-5,6-dimethoxyindan-1-one (3) in 5% yield. Compound 2: m.p.: 112-113°C; 1H-NMR δ: 3.94 (3H, s, CH3O-), 4.00 (3H, s, CH3O-), 4.22 (2H, s, H-3), 6.79 (1H, s, Ar-H), 7.30 (1H, s, Ar-H); 13C-NMR δ: 52.4, 56.4, 56.6, 57.3, 106.3, 106.7, 121.4, 142.6, 150.5, 157.5, 191.7; GC-MS (EI, M+) m/z: 350; IR cm-1: 1709 (C=O). Compound 3: m.p.: 170-171°C; 1H-NMR δ: 3.94 (3H, s, CH3O-), 4.00 (3H, s, CH3O-), 4.16 (2H, s, H-3), 7.37 (1H, s, Ar-H); 13C-NMR δ: 53.0, 55.7, 56.6, 61.2, 107.2, 115.3, 125.4, 141.3, 154.4, 154.6, 191.8; GC-MS (EI, M+) m/z: 428; IR cm-1: 1723 (C=O).
1.2. Bromination with bromine/CCl4 in an ice bath.
The experimental method described above in 1.1. was followed, with the exception that the reaction mixture was placed in an ice bath, to yield 2-bromo-5,6-dimethoxyindan-1-one (4) in 35% yield and 4-bromo-5,6-dimethoxyindan-1-one (5) in 14% yield, respectively. Compound 4: m.p.: 160-161°C (lit. 159-160°C [25]). Compound 5: m.p.: 103-106°C; 1H-NMR δ: 2.71 (2H, t, J=5.4, H-3), 3.01 (2H, t, J=5.4, H-2), 3.90 (3H, s, CH3O-), 3.94 (3H, s, CH3O-), 7.21 (1H, s, Ar-H); 13C-NMR δ: 26.7, 36.5, 56.4, 60.9, 104.9, 116.5, 133.7, 149.0, 152.4, 153.8, 205.6; GC-MS (EI, M+) m/z: 271; IR cm-1: 1696 (C=O).
1.3. Bromination with bromine/CHCl3 at room temperature.
The experimental method described in 1.1 was followed, with CHCl3 as solvent, to give 2 in 22% yield and 3 in 2% yield.
1.4. Bromination with bromine/CHCl3 in an ice bath.
The experimental method described in 1.2 was followed, using CHCl3 as solvent, to give 4 in 14% yield and 5 in 5% yield, respectively.
1.5. Bromination with bromine/I2, Fe in an ice bath.
A crystal of iodine and a pinch of iron powder were added to a solution of 1 (100 mg, 0.52 mmol) in CCl4 (7 mL) and the reaction mixture was cooled to 0°C. Light was excluded from the flask and bromine (0.26 mL, 0.52 mmol) was added with stirring over a period of 6 hrs, without allowing the temperature to rise above 5°C. Bromine and CCl4were removed by distillation and the residue was washed thoroughly with 10 % NaOH solution, extracted with CH2Cl2 and the organic phase was dried over anhydrous MgSO4, filtered and concentrated. The residue was purified by column chromatography to give 4 in 32% yield.
1.6. Bromination with bromine/AlCl3 in an ice bath.
A dried flask was loaded with AlCl3 (693.2 mg, 5.20 mmol) in anhydrous CH2Cl2 (60 mL). A solution of 1 (500 mg, 2.60 mmol) in anhydrous CH2Cl2 (20 mL) was then added dropwise to the reaction mixture, which was cooled in an ice bath and bromine (1.95 mL, 1.14 mmol) was then added with exclusion of light. After 2 hrs, the reaction mixture was poured into water, treated with 10 % NaOH, and extracted with CH2Cl2. The organic extract was dried over anhydrous MgSO4 and the solvent evaporated under reduced pressure. The products were purified by column chromatography over silica gel to give 2,4-dibromo-5,6-dimethoxyindan-1-one (6) in 29% yield and 5 in 8% yield. Compound 6: m.p.: 120-122°C; 1H-NMR δ: 3.29 (1H, dt, J=18.6, 3.1, H-3a), 3.72 (1H, tdd, J=18.2, 7.4, 3.0, H-3b), 3.92 (3H, s, CH3O-), 3.99 (3H, s, CH3O-), 4.62-4.69 (1H, m, H-2), 7.28 (1H, s, Ar-H); 13C-NMR δ: 38.7, 43.6, 56.6, 61.1, 106.0, 116.0, 130.2, 145.3, 154.4, 198.5; GC-MS (EI, M+) m/z: 350; IR cm-1: 1715 (C=O).
1.7. Bromination with potassium bromide/oxone℘/MeOH at room temperature.
Oxone℘ (703.3 mg, 1.14 mmol) was added to a stirred solution of KBr (136.1 mg, 1.14 mmol) and 1 (200 mg, 1.04 mmol) in methanol (10 mL) and the reaction mixture was stirred 2 hrs at room temperature, then the mixture was filtered and the solvent evaporated under reduced pressure. The products were purified by column chromatography over silica gel to give 4 in 46% yield.
1.8. Bromination with bromine/AcOH at room temperature.
Bromine (1.43 mL, 2.86 mmol) was added dropwise to solution of 1 (500 mg, 2.60 mmol) in acetic acid (40 mL). The solution was stirred 2 hrs at room temperature. The reaction mixture was poured into water and treated with 5 % sodium bisulfite solution to remove the excess bromine. The solid was filtered, washed with water and recrystallized from methanol to give compound 6 in 95% yield.
1.9. Bromination with bromine/K2CO3 at room temperature.
Bromine (1.52 mL, 1.04 mmol) was added with exclusion of light to a solution of 1 (100 mg, 0.52 mmol) and K2CO3 (215 mg, 1.56 mmol) in CH2Cl2 (20 mL) at room temperature. After 1 hr the reaction was quenched with 1 M Na2S2O3 and extracted with CH2Cl2, dried over anhydrous MgSO4 and evaporated. The products were purified by column chromatography over silica gel to give 6 in 44% yield and 5 in 23% yield, respectively.
1.10. Bromination with bromine/K2CO3 in an ice bath.
The experimental method described in 1.9 was followed, but with cooling in an ice bath, to yield 5 in 79% yield and 4,7-dibromo-5,6-dimethoxyindan-1-one (7) in 9% yield. Compound 7: m.p.: 107-109°C; 1H-NMR δ: 2.77 (2H, t, J=5.6, H-3), 2.77 (2H, t, J=5.6, H-2), 3.91 (3H, s, CH3O-), 4.02 (3H, s, CH3O-); 13C-NMR δ: 26.3, 37.4, 53.1, 56.6, 61.2, 61.4, 107.2, 114.6, 115.7, 153.4, 202.6; GC-MS (EI, M+) m/z: 350; IR cm-1: 1708 (C=O).
1.11. Bromination with bromine/KOH in an ice bath
The experimental method described in 1.9 was followed to yield 5 in 81% yield and 7 in 7% yield.
1.12. Bromination with bromine/Cs2CO3 / in an ice bath
The method described in 1.9 was followed to yield 5 in 67% yield and 7 in 20% yield.
2. Bromination of 5,6-dihydroxyindan-1-one (8): preparation of the starting material.
Compound 1 (1 g, 5.21 mmol) was held at reflux in 48% HBr (50 mL; freshly distilled) for 2 hrs under a N2 atmosphere. The reaction mixture was allowed to cool to room temperature and evaporated to dryness. The residue was diluted with H2O and extracted with ethyl acetate. The organic layer was dried (anhydrous MgSO4), filtered and concentrated. The resulting residue was purified by column chromatography (ethyl acetate-n-hexane = 1:5) to give 5,6-dihydroxyindan-1-one (8, 500 mg, 59 %); m.p.: 107-109°C; 1H-NMR (DMSO-d6) δ: 2.40 (2H, t, J=6.8, C3-H), 2.80 (2H, t, J=5.4, C2-H), 6.75 (1H, s, Ar-H), 6.84 (1H, s, Ar-H); 13C-NMR (DMSO) δ: 25.3, 36.7, 108.3, 112.4, 129.1, 146.2, 149.4, 153.6, 205.0; GC-MS (EI, M+) m/z: 164.
2.1. Bromination with bromine/AcOH at room temperature.
Bromine (0.34 mL, 0.67 mmol) was added dropwise to solution of 8 (100 mg, 0.61 mmol) in acetic acid (10 mL). The reaction mixture was stirred 3.5 hrs at room temperature and worked up as described for 1.8 to yield 4,7-dibromo-5,6-dihydroxyindan-1-one (9) in 47% yield; m.p.: 179-182°C; 1H-NMR (DMSO-d6) δ: 2.59 (2H, t, J=5.6, H-3), 2.81 (2H, t, J=5.6, H-2); 13C-NMR (DMSO-d6) δ: 26.9, 36.8, 98.6, 132.8, 144.8, 146.8, 152.0, 205.8; GC-MS (EI, M+) m/z: 322; IR cm-1: 3438 (OH), 1664 (C=O).
2.2. Bromination with bromine/KOH in an ice bath.
Bromine (1.78 mL, 1.22 mmol) was added with exclusion of light to a solution of 8 (100 mg, 0.61mmol) and KOH (252 mg, 1.83 mmol) in CH2Cl2 (20 mL) at room temperature. After 30 min in an ice bath, the reaction mixture was worked up as described for 1.9 to give 9 in 55% yield.
3. Bromination of indan-1-one (10)
3.1. Bromination with bromine/AcOH at room temperature
Bromine (0.43 mL, 0.84 mmol) was added dropwise to a solution of 10 (100 mg, 0.61 mmol) in acetic acid (10 mL). The reaction mixture was stirred 2.5 hrs at room temperature and worked up as described for 1.8 to yield 2-bromoindan-1-one (11) in 84% yield. Compound 11: m.p.: 134-136°C (lit. 132-134°C [26])
3.2. Bromination with bromine/KOH at room temperature
Bromine (2.22 mL, 1.52 mmol) was added with exclusion of light to a solution of 10 (100 mg, 0.61 mmol) and KOH (314 mg, 2.28 mmol) in CH2Cl2 (20 mL) at room temperature. After 20 hrs at this temperature the reaction mixture was worked up as described for 1.9 to give 2,2-dibromoindan-1-one (12) in 67% yield. Compound 12: m.p.: 132-133°C (lit. 134°C [27]).
4. Bromination of 5,6-difluoroindan-1-one (13)
4.1. Bromination with bromine/AcOH at room temperature
Bromine (0.34 mL, 0.66 mmol) was added a dropwise to a solution of 13 (100 mg, 0.60 mmol) in acetic acid (10 mL). The reaction mixture was stirred 1 hr at room temperature and worked up as described for 1.8 to yield 2-bromo-5,6-difluoroindan-1-one (14) in 65% yield. Compound 14: m.p.: 68-70°C; 1H-NMR δ: 3.39 (1H, d, J=18.0, H-3a), 3.81 (1H, t, J=22.0, 7.6, H-3b), 4.66 (1H, dd, J=7.4, 3.2, H-2), 7.27 (1H, t, J=8.0, Ar-H), 7.64 (1H, t, J=8.0, Ar-H); 13C-NMR δ: 41.9, 47.7, 117.5, 117.7, 119.3, 119.5, 152.3, 154.2, 201.9; GC-MS (EI, M+) m/z: 247; IR cm-1: 1720 (C=O).
4.2. Bromination with bromine/KOH at room temperature
Bromine (1.75 mL, 1.20 mmol) was added with exclusion of light to a solution of 13 (100 mg, 0.60 mmol) and KOH (248 mg, 1.80 mmol) in CH2Cl2 (20 mL) at room temperature. After 20 hrs at this temperature, the reaction mixture was worked up as described for 1.9 to give 2,2-dibromo-5,6-difluoroindan-1-one (15) in 84% yield. Compound 15: m.p.: 124-126°C; 1H-NMR δ: 4.24 (2H, s, H-3), 7.23 (1H, t, J=7.6, Ar-H), 7.74 (1H, t, J= 8.0, Ar-H); 13C-NMR δ: 52.0, 55.4, 115.1, 144.3, 150.4, 152.8, 155.5, 158.0, 191.0; GC-MS (EI, M+) m/z: 326; IR cm-1: 1720 (C=O).
Acknowledgements
We thank the Catholic University of Daegu for financial support.
References
- Taylor, R. (Ed.) Electrophilic aromatic substitution; John Wiley & Sons: Chichester, 1990.
- Diederich, F.; Stang, P.J. Metal-catalyzed cross-coupling reactions; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Jacquesy, J.; Jouannetaud, M.; Makani, S. meta-Bromination of phenols in superacids. J. Chem. Soc. Commun. 1980, 110–111. [Google Scholar]
- Duan, J.; Zhang, L.H.; Dolbier, W.R., Jr. A convenient new methods for the bromination of deactivated aromatic compounds. Synlett. 1999, 1245–1246. [Google Scholar] [CrossRef]
- Auerbach, J.; Weissman, S.A.; Blacklock, T.J. N-Bromosuccinimide/dibromodimethylhydantoin in aqueous base: A practical method for the bromination of activated benzoic acids. Tetrahedron Lett. 1993, 34, 931–934. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. Halogenation of aromatic compounds by N-chloro, N-bromo, and N-iodosuccinimide. Chem. Lett. 2003, 32, 932–933. [Google Scholar] [CrossRef]
- Barhate, N.B.; Gajare, A.S.; Wakharkar, R.D.; Bedekar, A.V. Simple and efficient chlorination and bromination of aromatic compounds using aqueous TBHP (or H2O2) and a hydrohalic acid. Tetrahedron Lett. 1998, 39, 6349–6350. [Google Scholar]
- Majetich, G.; Hicks, R.; Reister, S. Electrophilic aromatic bromination using bromodimethyl-sulfonium bromide generated in situ. J. Org. Chem. 1997, 62, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.V.V.K.; Narender, N.; Srinivasu, P.; Kulkarni, S.J.; Ragavan, K.V. Novel bromination method for aniline and anisole using NH4Br/H2O2 in CH3COOH. Synth. Commun. 2004, 34, 2143–2152. [Google Scholar] [CrossRef]
- Gnaim, J.M.; Sheldon, R.A. Regioselective bromination of aromatic compounds with Br2/SO2Cl2 over microporous catalysts. Tetrahedron Lett. 2005, 46, 4465–4468. [Google Scholar] [CrossRef]
- Chiappe, C.; Leandri, E.; Pieraccini, D. Highly efficient bromination of aromatic compounds using 3-methylimidazolium tribromide as reagent/solvent. Chem. Commun. 2004, 2536–2537. [Google Scholar]
- Narender, N.; Sriniva, P.; Ramakrishna, M.; Kilkarmi, S.J.; Reghaven, K.V. An efficient and regioselective oxybromination of aromatic compounds using potassium bromide and Oxone®. Synth. Commun. 2002, 32, 2313–2318. [Google Scholar] [CrossRef]
- Doyle, M.P.; Van Lente, M.A.; Mowat, R.; Fobare, W.F. Alkyl nitrite-metal halide deamination reactions. 7. Synthetic coupling of elctrophilic bromination with substitutive deamination 7. J. Org. Chem. 1980, 45, 2570–2575. [Google Scholar]
- Park, M.Y.; Yang, S.G.; Jadhav, V.; Kim, Y.H. Practical and regioselective brominations of aromatic compounds using tetrabutylammonium peroxydisulfate. Tetrahedron Lett. 2004, 45, 4887–4890. [Google Scholar] [CrossRef]
- Ozgun, B.; Degirmenbasi, N. Quinolium bromochromate − A new reagent for bromination and oxidation. Synth. Commun. 1996, 26, 3601–3601. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. A mild and efficient procedure for alpha-bromination of ketones using N-bromosuccinimide catalysed by ammonium acetate. Chem. commun. 2004, 470–471. [Google Scholar] [CrossRef]
- Lee, J.C.; Park, J.Y.; Yoon, S.Y.; Bae, Y.H.; Bae, Y.H.; Lee, S.J. Efficient microwave induced direct α-bromination of carbonyl compounds. Tetrahedron Lett. 2004, 45, 191–193. [Google Scholar] [CrossRef]
- Lee, J.C.; Bae, Y.H.; Chang, S.-K. Efficient α-halogenation of carbonyl compounds by N-bromosuccinimide and N-chlorosuccinimides. Bull. Korea Chem. Soc. 2003, 24, 407–408. [Google Scholar] [CrossRef]
- Bertelsen, S.; Halland, N.; Bachmann, S.; Marigo, M.; Braunton, A.; Jorgensen, K.A. Organocatalytic asymmetric α-bromination of aldehydes and ketones. Chem Commun. 2005, 4821–4823. [Google Scholar] [CrossRef]
- Choi, H.Y.; Chi, D.Y. Nonselective bromination-selective debromination strategy: Selective bromination of unsymmetrical ketones on singly activated carbon against doubly activated carbon. Org. Lett. 2003, 5, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, A.; Provot, O.; Rasolojaona, O.; Alami, M.; Brion, J.-D. N-Methylpyrrolidin-2-one hydrobromide (MPHT) a mild reagent for selective bromination of carbonyl compounds : Synthesis of substituted 2-bromo-1-naphthols. Tetrahedron Lett. 2005, 46, 4187–4191. [Google Scholar] [CrossRef]
- Jung, W.; Ma, E. Synthesis of 2-(5,6-dimethoxy-1-indenyl)ethylamine. Yakhak Hoeji 2003, 47, 1–4. [Google Scholar]
- Sonesson, C.; Barf, T.; Nilsson, J.; Dijkstra, D.; Carlsson, A.; Svensson, K.; Smith, M.W.; Martin, I.J.; Duncan, J.N.; King, L.J.; Wikstrom, H. Synthesis and evaluation of pharmacological and pharmacokinetic properties of monopropyl analogs of 5-, 7-, and 8-[[(trifluoromethyl)-sulfonyl]oxy]-2-aminotetralines: Central dopamine and serotonin receptor activity. J. Med. Chem. 1995, 38, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Ma, E. Synthesis of 2-amino-5,6-difluoroindanּHCl. Yakhak Hoeji 1999, 43, 751–755. [Google Scholar]
- Logan, R.T.; Redpath, J.; Roy, R.G. Indene and naphthalene derivatives. US Pat. 4705782, 1987. [Google Scholar]
- Johnson, W.S.; Shelberg, W.E. A plan for distinguishing between some five- and six-membered ring ketones. J. Am. Chem. Soc. 1945, 67, 1745–1754. [Google Scholar] [CrossRef]
- House, H.O.; McDaniel, W.C. Perhydroindan derivatives. 18. The use of indenone ketals as dienophiles. J. Org. Chem. 1977, 42, 2155–2163. [Google Scholar]
- Sample Availability: Samples of the compounds are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.