Abstract
Triisobutylaluminium-promoted rearrangement of unsaturated glycosides containing electron-donating aglycons, such as C-aryl glycosides, provides direct access to highly functionalised cyclohexane derivatives.
Introduction
The conversion of carbohydrates into carbocycles provides a powerful method for the preparation of highly functionalised enantiomerically pure carbocycles. The pyranose-cyclohexane conversion has received particular attention due to the myriad of bioactive substances such as aminocyclitols, inositols and carbasugars, which are attainable. Among those a more complex one has attracted particular attention: pancratistatine [1,2,3] (Figure 1). It is a polyfunctionalised cyclohexane directly connected to an aromatic ring.
The classical Ferrier-II reaction [2] involving Hg(II) catalysed rearrangement of readily available hex-5-enopyranosides into cyclohexanones has, thus far, been the most widely exploited. In 1997 we reported that the same starting material used for the Ferrier-II reaction, carbohydrate based vinyl acetals (hex-5-enopyranosides) such as 1, undergo smooth reductive rearrangement with triisobutylaluminium (TIBAL) to afford highly functionalized cyclohexanes such as 2 (Scheme 1) [3].
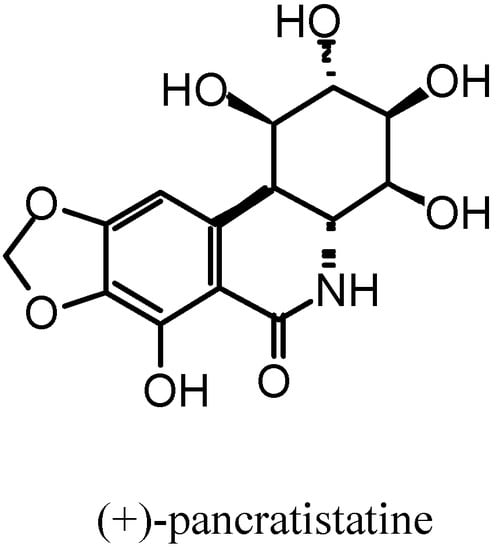
Figure 1.
In sharp contrast with the Ferrier-II reaction, this rearrangement proceeds with retention of the aglycon moiety, due to initial endo-glycosidic bond cleavage leading to a carbacationic intermediate A stabilised by the methoxy group. On the other hand, the original anomeric configuration is preserved.
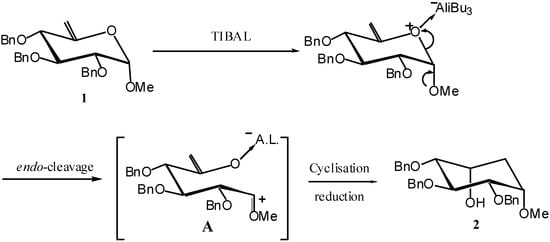
Scheme 1.
We next reasoned that it should be possible to replace the methoxy group by other electron-donating groups (EDG) that would stabilise the analogous carbacationic intermediate B and therefore promote endo cleavage. (Scheme 2) We would like to concentrate in this article on C-glycosides and provide a full experimental account of our preliminary results [4].
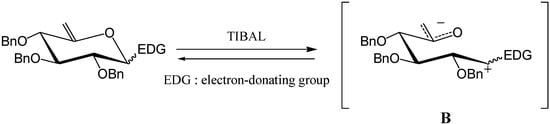
Scheme 2.
Results and Discussion
The C-aryl-glycosides are a family of C-glycosides possessing an electron-donating aglycon, and their carbocyclisation could constitute a new entry into pancratistatine and similar structures. To test this hypothesis we first synthesised the unsaturated C-phenyl glycoside 7. Starting from acetobromoglucose 3 [5] we introduced the phenyl group using a Grignard reagent and then reacetylated to obtain the C-glucoside 4 [6]. Selective iodination, elimination, and protecting group exchange afforded the unsaturated C-phenyl glucoside 7 (Scheme 3).
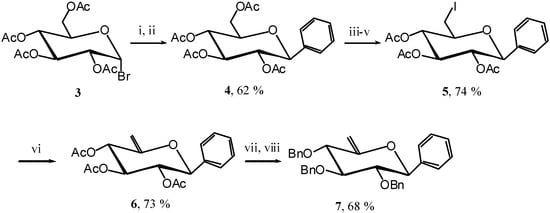
Scheme 3.
Compound 7 was then reacted with TIBAL and gave the desired carbocycle 8 (35 %) along with the de-O-benzylated [7] open-chain product 9 (35 %).
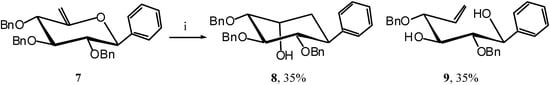
Scheme 4.
Product 9 is the result of an overall reductive cleavage of the endocyclic C5-O bond, a reaction which has already been described in enol ethers [8,9]. A hydro-alumination-elimination mechanism, as shown in Scheme 5, may explain this process.
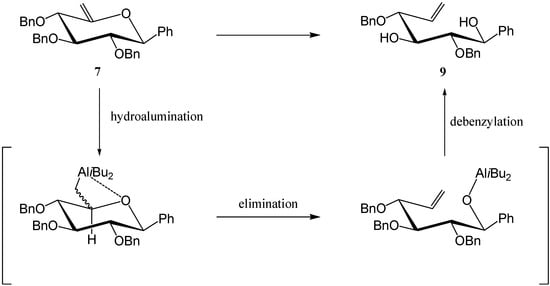
Scheme 5.
The reaction was here slower than usual, indicating that the phenyl might not be a strong enough EDG. We therefore turned our attention to anisole as EDG and synthesised unsaturated compound 13 in the same manner as 7 via the known 10 [10]. (Scheme 6)
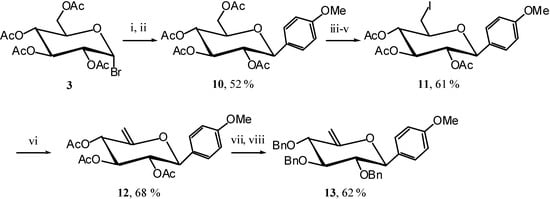
Scheme 6.
Under the action of TIBAL compound 13 only gave 10 % of the open-chain product 16 and 86 % of a mixture of expected carbocycles 14 and 15 in a 4/1 ratio. (Scheme 7)
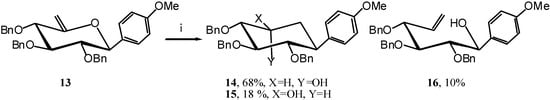
Scheme 7.
To achieve the demonstration that a better EDG gives a better reaction we synthesised compound 20, using again the same methodology, except for the C-glycosylation of trimethoxybenzene to give C-glucoside 17 [11]. (Scheme 8)
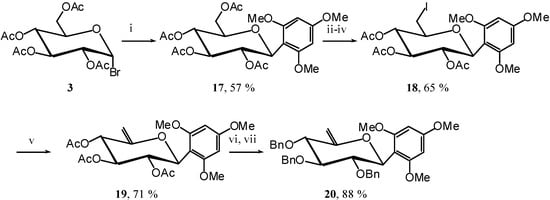
Scheme 8.
Indeed, when compound 20 is submitted to the action of TIBAL, carbocyclic products 21 and 22 are the only ones obtained in a 3:2 molar ratio. (Scheme 9)
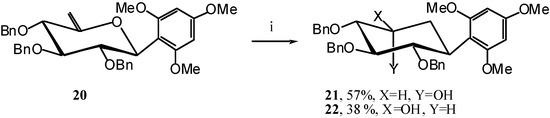
Scheme 9.
To really complete the demonstration, we also need to show that a weak EDG only induces the ring opening. To that end we selected the known C-butyl unsaturated derivative 27 [12]. Its synthesis, shown in Scheme 10, is slightly different from the previous ones and starts from lactone 23 [13] (Scheme 10).
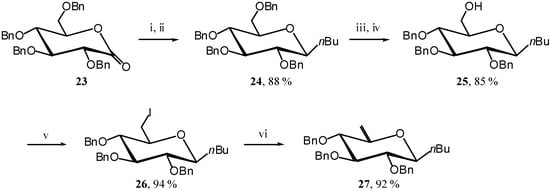
Scheme 10.
Compound 27 reacts with TIBAL to give a single open-chain product 28 [14], hence achieving our demonstration. (Scheme 11)
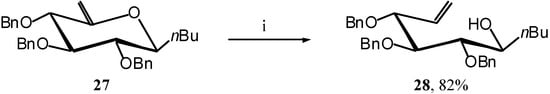
Scheme 11.
Conclusions
We have demonstrated that unsaturated C-glycosides could be carbocyclised by the action of TIBAL, provided that the aglycon is sufficiently electron-donating in nature. Among other applications, this reaction opens a new access to compounds of the pancratistatine family.
Experimental Section
General
Melting points were recorded on a Büchi 510 apparatus and are uncorrected. Optical rotations were measured on a Perkin Elmer 241 digital polarimeter with a path length of 1 dm. Mass spectra were recorded on a Nermag R10-10 spectrometer, using chemical ionisation with ammonia. Elemental analyses were performed by the Service d'Analyse de l'Université Pierre et Marie Curie, 75252 Paris Cedex 05, France. NMR spectra were recorded on a Brüker AM-400 (400 MHz and 100.6 MHz, for 1H and 13C, respectively) or Brüker AC-250 (250 MHz and 63 MHz, for 1H and 13C, respectively) using CDCl3 as solvent and TMS as internal standard. TLC was performed on silica gel 60 F254 (Merck) and developed by charring with conc. H2SO4. Flash column chromatography was performed using silica gel 60 (230-400 mesh, Merck).
(2,3,4-Tri-O-acetyl-6-deoxy-6-iodo-β-D-glucopyranosyl) benzene (5)
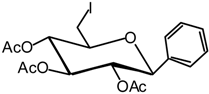
A catalytic amount of sodium is added to a solution 4 (3.74 g, 6.7 mmol) in methanol (40 mL), and the solution is stirred for 5 hours. It is then neutralised using IR 120 (H+) resin, filtered and evaporated in vacuo. The residue is dissolved in anhydrous DMF (15 mL), then PPh3 (4.8 g, 13.4 mmol) is added and the solution is cooled to 0 °C under argon. A solution of diiodine (4.6 g, 13.4 mmol) in anhydrous DMF (5 mL) is then added dropwise and the solution stirred for 45 min at R.T, until the completion of the reaction is detected by TLC (9:1 dichloromethane/MeOH). The solvent is evaporated and the residue is next dissolved in pyridine (20 mL) and acetic anhydride (10 mL). The reaction, monitored by TLC (7:3 cyclohexane/AcOEt), is complete in 4 hours at R.T. The solvent is then removed in vacuo and the residue purified by silica gel flash column chromatography (1:4 AcOEt/cyclohexane) to afford compound 5 (2.9 g, 74 %) as a white solid. = + 6 (c = 1.25, CHCl3); m.p. = 162-163 °C (AcOEt/cyclohexane); 1H-NMR (250 MHz): δ = 7.31-7.20 (5H, H arom.), 5.29 (t, 1H, J2,3 = 9.4 Hz, J3,4 = 9.4 Hz, H-3), 5.06 (dd, 1H, J4,5 = 9.4 Hz, H-4), 5.05 (dd, 1H, J1,2 = 9.7 Hz, H-2), 4.38 (d, 1H, H-1), 3.46 (ddd, 1H, J5,6a = 3.0 Hz, J6b,5 = 5.7 Hz, H-5), 3.34 (dd, 1H, J6b,6a = 11.1 Hz, H-6a), 3.16 (dd, 1H, H-6b), 2.02, 1.94, 1.74 (3 x s, 3 x 3H, 3 x Ac); 13C-NMR (63 MHz): δ = 170.2, 169.2, 168.6 (3 x Ac), 136.1 (C arom. quat.), 128.7, 128.3, 126.9 (5 C arom.), 79.6 (C-1), 76.3 (C-5), 73.7 (C-3), 72.7 (C-2), 72.4 (C-4), 20.6, 20.5, 20.2 (3 x Ac), 3.9 (C-6); MS m/z 494 (M+NH4)+; Anal. calc. C, 45.39; H, 4.44; found. C, 45.51; H, 4.51.
(2,3,4-Tri-O-acetyl-6-desoxy-β-D-xylo-hex-5-enopyranosyl) benzene (6)
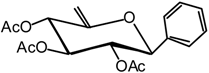
Compound 5 (2.9 g, 6.7 mmol) is dissolved in dry THF (30 mL) and DBU (6.1 mL, 40.6 mmol) is added. The mixture is heated at 70 °C until after 3 hours the end of the reaction is detected by TLC (7:3 cyclohexane/AcOEt). The solvent is then removed in vacuo and the residue purified by silica gel flash column chromatography (1:5 AcOEt/cyclohexane) to afford compound 6 (1.7 g, 73 %) as a white solid. = -53 (c = 1.2, CHCl3); m.p. = 132 °C (AcOEt/cyclohexane); 1H-NMR (250 MHz): δ = 7.32-7.22 (5H, H arom.), 5.59-5.51 (m, 1H, H-4), 5.29-5.12 (m, 2H, H-3, H-2), 4.79 (s, 1H, H-6a), 4.51 (s, 1H, H-6b), 4.49-4.39 (m, 1H, H-1), 2.09, 1.96, 1.74 (3 x s, 3 x 3H, 3 x Ac); 13C-NMR (63 MHz): δ = 170.1, 169.3, 168.8 (3 x Ac), 153.8 (C-5), 135.9 (C arom. quat.), 129.1, 128.5, 127.1 (5 C arom.), 96.5 (C-6), 80.8 (C-1), 73.4 (C-3), 72.7 (C-2), 69.6 (C-4), 20.7, 20.6, 20.3 (3 x Ac); MS: m/z 366 (M+NH4)+; 349 (M+H)+; Anal. calc. C, 62.06; H, 5.79; found. C, 62.05; H, 5.88.
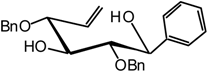
(2,3,4-Tri-O-benzyl-6-desoxy-β-D-xylo-hex-5-enopyranosyl) benzene (7)
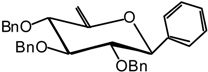
A catalytic amount of sodium is added to a solution 6 (1.1 g, 3.2 mmol) in methanol (10 mL) and the solution is stirred for 12 hours. The solvent is evaporated without neutralisation and the residue dissolved in dry DMF (100 mL) to which benzyl bromide (4.4 mL, 32 mmol) and NaH (0.9 g, 60% in suspension in oil, 19.2 mmol) are added. The mixture is stirred at R.T. until the end of the reaction is detected after 5 hours by TLC (9:1 cyclohexane/AcOEt ). Excess NaH is quenched with methanol, and the mixture is extracted with ether and washed with water. The organic layer is dried (MgSO4), filtered and concentrated. The residue is purified by silica gel flash column chromatography (1:99 AcOEt/cyclohexane) to afford compound 7 (1.05 g, 68 %) as a white solid. = -51 (c = 1.1, CHCl3); m.p. = 71 °C (AcOEt/cyclohexane); 1H-NMR (250 MHz): δ = 7.31-6.92 (20H, H arom.), 4.70 (d, 1H, J = 11.3 Hz, -CHPh), 4.65 (d, 1H, J = 11.7 Hz, -CHPh), 4.63 (s, 1H, H-6a), 4.59 (d, 1H, J = 11.2 Hz, -CHPh), 4.53 (d, 1H, J = 11.5 Hz, -CHPh), 4.53 (s, 1H, H-6b), 4.37 (d, 1H, J1,2 = 9.7 Hz, H-1), 4.19 (d, 1H, J = 10.5 Hz, -CHPh), 3.91 (d, 1H, J4,3 = 7.4 Hz, H-4), 3.64 (d, 1H, J = 11.9 Hz, -CHPh), 3.62 (t, 1H, J2,3 = 7.7 Hz, H-3), 3.47 (dd, 1H, H-2); 13C-NMR (63 MHz): δ = 156.6 (C-5), 138.7, 138.5, 138.0, 137.7 (4 C arom. quat.), 128.6-127.7 (20 C arom.), 95.2 (C-6), 84.7 (C-3), 83.7 (C-2), 81.3 (C-1), 79.3 (C-4), 74.7, 74.6, 73.0 (3 CHPh); MS :m/z 510 (M+NH4)+; Anal. calc. C, 80.46; H, 6.64; found. C, 80.45; H, 6.59.
(1R,2S,3S,4R,5R)-2,3,4-Tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) benzene (8) and 2,4-di-O-benzyl- 5,6-dehydro-1-C(S)-phenyl-D-glucitol (9)
A solution of TIBAL (0.6 mL, 0.6 mmol, 1M in toluene) is added to a solution of 7 (55 mg, 0.11 mmol) in dry toluene (1 mL) at R.T. under argon. The mixture is stirred at 50 °C for 12 hours, until the end of the reaction is detected by TLC (4:1 cyclohexane/AcOEt). The mixture is cooled to R.T. and water (2 mL) is slowly added, and stirred for 15 min. After extraction with AcOEt (3 x 10 mL) and washing with water (10 mL) the organic layer is dried (MgSO4), filtered and the solvent is evaporated. The residue purified by silica gel flash column chromatography (1:9 AcOEt/cyclohexane) to afford two major products 8 (18 mg, 35 %), as a white solid, and 9 (16 mg, 35 %) as an oil.
(1R,2S,3S,4R,5R)-2,3,4-Tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) benzene (8)
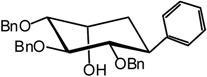
2,4-Di-O-benzyl-5,6-dehydro-1-C(S)-phenyl-D-glucitol (9)
4-(2,3,4-Tetra-O-acetyl-6-desoxy-6-iodo-β-D-glucopyranosyl) anisole (11)
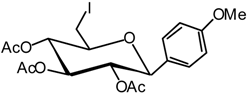
A catalytic amount of sodium is added to a solution 10 (3.7 g, 8.4 mmol) in methanol (50 mL), and the solution is stirred for 12 hours. It is then neutralised using IR 120 (H+) resin, filtered and evaporated in vacuo. The residue is dissolved in anhydrous DMF (20 mL), then PPh3 (4.7 g, 17.9 mmol) is added, and the solution is cooled to 0 °C under argon. A solution of diiodine (4.5 g, 17.9 mmol) in anhydrous DMF (5 mL) is then added dropwise and the solution stirred for 30 min at R.T., until the end of the reaction is detected by TLC (9:1 dichloromethane/MeOH). The solvent is evaporated and the residue is dissolved in pyridine (20 mL) and acetic anhydride (10 mL). The reaction is monitored by TLC (7:3 cyclohexane/AcOEt) for 4 hours at R.T. The solvent is then removed in vacuo and the residue purified by silica gel flash column chromatography (1:4 AcOEt/cyclohexane) to afford compound 11 (2.6 g, 61 %) as a white solid. = -8 (c = 1.8, CHCl3); m.p. = 99 °C (EtOH); 1H-NMR (250 MHz): δ = 7.21 (d, 2H, J = 8.8 Hz, 2 H arom.), 6.81 (d, 2H, J = 8.8 Hz, 2 H arom.), 5.29 (t, 1H, J2,3 = 9.4 Hz, J3,4 = 9.4 Hz, H-3), 5.12-4.98 (m, 2H, H-4 H-2), 4.33 (d, 1H, J1,2 = 9.7 Hz, H-1), 3.73 (s, 3H, OMe), 3.44 (ddd, 1H, J5,6a = 3.0, J6b,5 = 5.7 Hz, J5,4 = 9.3 Hz, H-5), 3.32 (dd, 1H, J6b,6a = 11.2 Hz, H-6a), 3.13 (dd, 1H, H- 6b), 2.03, 1.94, 1.75 (3 x s, 3 x 3H, 3 x Ac); 13C-NMR (63 MHz): δ = 170.3, 169.3, 168.8 (3 x Ac), 159.9 (C arom. quat.), 128.4, 113.8 (5 C arom.), 79.4 (C-1), 76.3 (C-5), 73.8 (C-3), 72.7 (C-2), 72.4 (C- 4), 55.2 (OMe), 20.7, 20.6, 20.4 (3 x Ac), 4.2 (C-6); MS: m/z 524 (M+NH4)+; Anal. calc. C, 45.07; H, 4.58; found. C, 44.98; H, 4.54.
(2,3,4-Tri-O-acetyl-6-desoxy-β-D-xylo-hex-5-enopyranosyl) anisole (12)
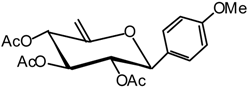
Compound 11 (1.6 g, 3.2 mmol) is dissolved in dry THF (15 mL) and DBU (2.9 mL, 19 mmol) is added. The mixture is heated at 70 °C until the end of the reaction is detected by TLC (7:3 cyclohexane/AcOEt) after 3 hours. The solvent is then removed in vacuo and the residue purified by silica gel flash column chromatography (1:5 AcOEt/cyclohexane) to afford 12 (812 mg, 68 %) as a white solid. = -51 (c = 1.0, CHCl3); m.p. = 94 °C (Et2O/pentane); 1H-NMR (250 MHz): δ = 7.14 (d, 2H, J = 8.8 Hz, 2 H arom.), 6.72 (d, 2H, J = 8.8 Hz, 2 H arom.), 5.47-5.39 (m, 1H, H-4), 5.17-5.04 (m, 2H, H-3, H-2), 4.69 (sl, 1H, H-6a), 4.43 (sl, 1H, H-6b), 4.35-4.22 (m, 1H, H-1), 3.64 (s, 3H, OMe), 1.99, 1.87, 1.65 (3 x s, 3 x 3H, 3 x Ac); 13C-NMR (63 MHz): δ = 169.9, 169.1, 168.6 (3 x Ac), 159.9 (C arom. quat.), 153.6 (C-5), 128.3, 127.7, 113.7 (5 C arom.), 96.1 (C-6), 80.4 (C-1), 73.3 (C-3), 72.4 (C-2), 69.4 (C-4), 55.0 (OMe), 20.5, 20.4, 20.2 (3 x Ac); MS: m/z 396 (M+NH4)+, 379 (M+H)+; Anal. calc. C, 60.31; H, 5.86; found. C, 60.17; H, 5.98.
(2,3,4-Tri-O-benzyl-6-desoxy-β-D-xylo-hex-5-enopyranosyl) anisole (13)
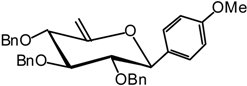
A catalytic amount of sodium is added to a solution 12 (350 mg, 0.92 mmol) in methanol (3 mL), and the solution is stirred for 12 hours. The solvent is evaporated without neutralisation and the residue dissolved in dry DMF (30 mL) to which benzyl bromide (1.1 mL, 9.2 mmol) and (222 mg, 60% in suspension in oil, 5.5 mmol) are added. The mixture is stirred at R.T. until the end of the reaction is detected by TLC (9:1 cyclohexane/AcOEt) after 5 hours. Excess NaH is quenched with methanol, and the mixture is extracted with ether and washed with water. Organic layer is dried (MgSO4), filtered and concentrated. The residue purified by silica gel flash column chromatography (1:99 AcOEt/cyclohexane) to afford 13 (299 g, 62 %) as a white solid. = -53 (c = 1.0, CHCl3); m.p. = 77-78 °C (Et2O/pentane); 1H-NMR (250 MHz): δ = 7.33-6.75 (19H, H arom.), 4.81 (d, 1H, J = 11.3 Hz, -CHPh), 4.54 (d, 1H, J = 12.0 Hz, -CHPh), 4.72 (s, 1H, H-6a), 4.69 (d, 1H, J = 11.3 Hz, -CHPh), 4.64 (d, 1H, J = 11.5 Hz, -CHPh), 4.63 (s, 1H, H-6b), 4.42 (d, 1H, J1,2 = 9.7 Hz, H-1), 4.31 (d, 1H, J = 10.5 Hz, -CHPh), 4.00 (d, 1H, J4,3 = 7.4 Hz, H-4), 3.81 (d, 1H, J = 10.5 Hz, -CHPh), 3.76 (s, 3H, OMe), 3.71 (t, 1H, J2,3 = 7.7 Hz, H-3), 3.53 (dd, 1H, H-2); 13C-NMR (63 MHz): δ = 156.7 (C-5), 159.9, 138.5, 138.0, 137.7 (4 C arom. quat.), 130.9-113.9 (20 C arom.), 95.2 (C-6), 84.7 (C-3), 83.8 (C-2), 81.1 (C-1), 79.4 (C-4), 74.8, 74.6, 73.0 (3 CHPh), 55.5 (OMe); MS: m/z 540 (M+NH4)+; Anal. calc. C, 77.62; H, 6.71; found. C, 77.61; H, 6.67.
1R,2S,3S,4R,5S)-2,3,4-Tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) anisole (14), (1R,2S,3S,4R,5R)- 2,3,4-tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) anisole (15) and 2,3,4-tri-O-benzyl-5,6-dehydro-1- C(S)-anisyl-D-glucitol (16)
A solution of TIBAL (1 mL, 1 mmol, 1M in toluene) is added to a solution of 13 (85 mg, 0.16 mmol) in dry toluene (1 mL) at R.T. under argon. The mixture is stirred at 50 °C for 1 hour, until the end of the reaction is detected by TLC (7:3 cyclohexane/AcOEt). The mixture is cooled to R.T. and water (2 mL) is slowly added, and stirred for 15 min. After extraction with AcOEt (3 x 10 mL) and washing with water (10 mL) the organic layer is dried (MgSO4), filtered and the solvent is evaporated. The residue purified by silica gel flash column chromatography (1/9 AcOEt/cyclohexane) to afford three major products 14 (58 mg, 68 %) as a white solid, 15 (58 mg, 68 %) as a white solid and 16 (9 mg, 10 %) as an oil.
(1R,2S,3S,4R,5R)-2,3,4-Tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) anisole (14)
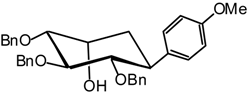
(1R,2S,3S,4R,5S)-2,3,4-Tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) anisole (15)
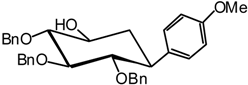
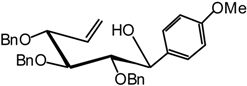
2,3,4-Tri-O-benzyl-5,6-dehydro-1-C(S)-anisyl-D-glucitol (16)
1,3,5-Trimethoxy-2-(2,3,4-tri-O-acetyl-6-desoxy-6-iodo-β-D-glucopyranosyl) benzene (18)
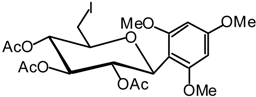
A catalytic amount of sodium is added to a solution 17 (4.5 g, 9.0 mmol) in methanol (50 mL), and the solution is stirred for 5 hours. It is then neutralised using IR 120 (H+) resin, filtered and evaporated in vacuo. The residue is dissolved in anhydrous DMF (20 mL), then PPh3 (4.7 g, 18 mmol) is added, and the solution is cooled to 0 °C under argon. A solution of diiodine (4.6 g, 18 mmol) in anhydrous DMF (20 mL) is then added dropwise and the solution stirred for 45 min at R.T., until the end of the reaction is detected by TLC (9:1 dichloromethane/MeOH). Solvent is evaporated. The residue is next dissolved in pyridine (20 mL) and acetic anhydride (10 mL). The reaction is monitored by TLC (1:1 cyclohexane/AcOEt) for 3 hours at R.T. The solvent is then removed in vacuo and the residue purified by silica gel flash column chromatography (1:4 AcOEt/cyclohexane) to give compound 18 (3.32 g, 65 %) as a white solid. = -9 (c = 1.1, CHCl3); m.p. = 157 °C (iPrOH); 1H-NMR (250 MHz): δ = 6.05-5.96 (m, 2H, 2 H arom.), 5.84 (t, 1H, J1,2 = 9.7 Hz, J2,3 = 9.7 Hz, H-2), 5.30 (t, 1H, J3,4 = 9.4 Hz, H-3), 5.04 (d, 1H, J4,5 = 9.4 Hz, H-4), 4.98 (d, 1H, H-1), 3.79 (s, 3H, OMe), 3.72 (s, 6H, 2 x OMe), 3.39-3.31 (m, 1H, H-5), 3.28 (dd, 1H, J5,6a = 3.2, J6b,6a = 10.8 Hz, H-6a), 3.13 (dd, J6b,5 = 4.8 Hz, 1H, H-6b), 2.02, 1.94, 1.67 (3 x s, 3 x 3H, 3 x Ac); 13C-NMR (63 MHz): δ = 170.4, 169.4, 169.1 (3 x Ac), 161.8, 161.1, 160.1 (4 x C arom. quat.), 91.4, 90.6 (2 C arom.), 76.1 (C-5), 74.8 (C-3), 73.0 (C-4), 71.6 (C-1), 69.8 (C-2), 56.2, 55.3 (3 x OMe), 20.9, 20.8, 20.6 (3 x Ac), 5.0 (C-6) ; MS: m/z 584 (M+NH4)+; Anal. calc. C, 44.53; H, 4.80; found. C, 44.44; H, 4.76
1,3,5-Trimethoxy-2-(2,3,4-tri-O-acetyl-6-desoxy-β-D-xylo-hex-5-enopyranosyl) benzene (19)
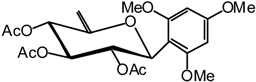
Compound 18 (1.5 g, 2.6 mmol) is dissolved in dry THF (15 mL) and DBU (2.5 mL, 16 mmol) is added. The mixture is heated at 70 °C until the end of the reaction is detected after 2 hours by TLC (7:3 cyclohexane/AcOEt). The solvent is then removed in vacuo and the residue purified by silica gel flash column chromatography (1:5 AcOEt/cyclohexane) to compound 19 (826 mg, 71 %) as a white solid. = -43 (c = 1.2, CHCl3); m.p. = 142 °C (Et2O/pentane); 1H-NMR (250 MHz): δ = 6.03 (s, 2H, 2 H arom.), 5.98 (dd, 1H, J1,2 = 10.2 Hz, J2,3 = 9.6 Hz, H-2), 5.54 (dl, 1H, J4,3 = 9.6 Hz, H-4), 5.17 (t, 1H, H-3), 5.02 (d, 1H, H-1), 4.72 (sl, 1H, H-6a), 4.45 (sl, 1H, H-6b), 3.75, 3.73 (2 x s, 9H, 3 x OMe), 2.08, 1.97, 1.68 (3 x s, 3 x 3H, 3 x Ac); 13C-NMR (63 MHz): δ = 169.9, 169.2, 167.0 (3 x Ac), 161.9, 160.3, 154.1, 103.4, 90.7 (6 C arom. C-5), 95.0 (C-6), 73.9 (C-3), 72.7 (C-1), 69.5 (C-4), 69.3 (C-2), 55.8, 55.0 (3 x OMe), 20.6, 20.2 (3 x Ac); MS: m/z 456 (M+NH4)+; 439 (M+H)+; Anal. calc. C, 57.53; H, 5.98; found. C, 57.55; H, 6.01.
1,3,5-Trimethoxy-2-(2,3,4-tri-O-benzyl-6-desoxy-β-D-xylo-hex-5-enopyranosyl) benzene (20)
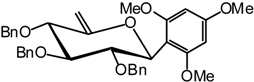
A catalytic amount of sodium is added to a solution 19 (300 mg, 0.68 mmol) in methanol (3 mL), and the solution is stirred for 12 hours. The solvent is evaporated without neutralisation and the residue dissolved in dry DMF (30 mL) to which benzyl bromide (0.8 mL, 6.8 mmol) and NaH (165 mg, 60% in suspension in oil, 4.1 mmol) are added. The mixture is stirred at R.T. until the end of the reaction is detected after 2 hours by TLC (9:1 cyclohexane/AcOEt). Excess NaH is quenched with methanol, and the mixture is extracted with ether and washed with water. Organic layer is dried (MgSO4), filtered and concentrated. The residue purified by silica gel flash column chromatography (1:99 AcOEt/cyclohexane) compound 20 (350 mg, 88 %) as an oil. = -28 (c = 1.4, CHCl3); 1H-NMR (250 MHz): δ = 7.42-6.04 (17H, H arom.), 5.05 (d, 1H, J1,2 = 10.0 Hz, H-1), 4.86 (d, 1H, J = 10.7 Hz, -CHPh), 4.77 (d, 1H, J = 10.3 Hz, -CHPh), 4.73 (d, 1H, J = 10.8 Hz, CHPh), 4.70 (sl, 1H, H-6a), 4.68 (sl, 1H, H-6b), 4.67 (d, 1H, J = 11.4 Hz, -CHPh), 4.45 (d, 1H, J = 10.8 Hz, -CHPh), 4.39 (dd, 1H, J2,3 = 9.9 Hz, H-2), 4.06 (d, 1H, J = 10.7 Hz, -CHPh), 4.02 (dl, 1H, J4,3 = 8.7 Hz, H-4), 3.76, 3.70 (2 s, 9H, 3 x OMe), 3.64 (t, 1H, H-3); 13C-NMR (63 MHz): δ = 161.6, 160.4, 157.5, 138.6, 138.3, 138.2, 128.3- 127.3, 106.4, 91.0 (25 C arom. C-5), 94.2 (C-6), 85.6 (C-3), 79.8 (C-4), 79.2 (C-2), 73.5 (C-1), 75.2, 74.2, 73.4 (3 CHPh), 55.8, 55.2 (3 x OMe); MS: m/z 600 (M+NH4)+, 583 (M+H)+; Anal. calc. C, 74.20; H, 6.57; found. C, 74.56; H, 6.86.
1,3,5-Trimethoxy-2-(1R,2S,3S,4R,5R)-2,3,4-tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) benzene (21) and 1,3,5-trimethoxy-2-(1R,2S,3S,4R,5S)-2,3,4-tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) benzene (22)
A solution of TIBAL (0.9 mL, 0.9 mmol, 1M in toluene) is added to a solution of 20 (100 mg, 0.17 mmol) in dry toluene (1 mL) at R.T. under argon. The mixture is stirred at 50 °C for 30 min, until the end of the reaction is detected by TLC (7:3 cyclohexane/AcOEt). The mixture is cooled to R.T. and water (2 mL) is slowly added, and stirred for 15 min. After extraction with AcOEt (3 x 10 mL) and washing with water (10 mL) the organic layer is dried (MgSO4), filtered and the solvent is evaporated. The residue purified by silica gel flash column chromatography (3:7 AcOEt/cyclohexane) to afford a 2/1 mixture of two major unseparable products 21 and 22 (95 mg, 95 %) as an oil.
1,3,5-Trimethoxy-2-((1R,2S,3S,4R,5R)-2,3,4-tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) benzene (21)
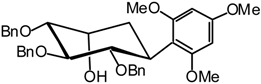
1H-NMR (400 MHz): δ = 7.45-6.92 (15H, arom. H), 6.25 (d, 1H, J = 2.3 Hz, arom. H), 6.12 (d, 1H, J = 2.3 Hz, arom. H), 5.16-4.78 (m, 4H, 4 -CHPh), 4.66 (d, 1H, J = 10.5 Hz, -CHPh), 4.27 (dd, 1H, J1,2 = 10.9 Hz, J2,3 = 9.1 Hz, H-2), 4.26-4.22 (m, 1H, H-5), 4.21 (d, 1H, J = 10.6 Hz, -CHPh), 4.04 (ddd, 1H, J1,6a = 13.2 Hz, J1,6e = 3.9 Hz, H-1), 3.98 (t, 1H, J3,4 = 9.2 Hz, H-3), 3.87 (s, 3H, OMe), 3.84 (s, 3H, OMe), 3.77 (s, 3H, OMe), 3.65 (dd, 1H, J4,5 = 3.3 Hz, H-4), 2.59 (br s, 1H, -OH), 2.36-2.24 (m, 1H, H- 6a), 1.87 (dt, 1H, J6e,6a = 14.0 Hz, J6e,5 = 3.6 Hz, H-6e); 13C-NMR (100 MHz): δ = 160.1-90.7 (24 C arom.), 86.6 (C-4), 83.8 (C-3), 82.2 (C-2), 76.7-72.5 (3 CHPh), 67.0 (C-5), 56.2-54.9 (3 OMe), 31.7 (C-1), 30.7 (C-6); MS: m/z 602 (M+NH4)+; Anal. for the mixture of isomers calc. C, 73.95; H, 6.89; found. C, 73.89; H, 6.91.
1,3,5-Trimethoxy-2-((1R,2S,3S,4R,5S)-2,3,4-tri-O-benzyl-2,3,4,5-tetrahydroxycyclohexyl) benzene (22)
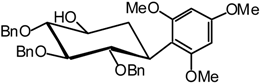
1H-NMR (400 MHz): δ = 7.45-6.92 (15H, arom. H), 6.25 (d, 1H, J = 2.3 Hz, arom. H), 6.15 (d, 1H, J = 2.3 Hz, arom. H), 5.16-4.78 (m, 4H, 4 -CHPh), 4.65 (d, 1H, J = 10.4 Hz, -CHPh), 4.36 (dd, 1H, J1,2 = 10.8 Hz, J2,3 = 9.0 Hz, H-2), 4.19 (d, 1H, J = 10.6 Hz, -CHPh), 3.88 (s, 3H, OMe), 3.83 (s, 3H, OMe), 3.81 (s, 3H, OMe), 3.71 (ddd, 1H, J5,6a = 11.6 Hz, J5,6e = 5 Hz, J5,4 = 9.1 Hz, H-5), 3.66 (t, 1H, J3,4 = 9.2 Hz, H-3), 3.55 (ddd, 1H, J1,6a = 13.2 Hz, J1,6e = 3.9 Hz, H-1), 3.52 (t, 1H, H-4), 2.45 (br s, 1H, -OH), 2.36-2.24 (m, 1H, H-6a), 1.87 (dt, 1H, J6e,6a = 14.0 Hz, J6e,5 = 3.6 Hz, H-6e); 13C-NMR (100 MHz): δ = 160.1-90.7 (24 C arom.), 86.6 (C-4), 83.6 (C-3), 82.4 (C-2), 76.7-72.5 (3 CHPh), 71.6 (C-5), 56.2-54.9 (3 OMe), 34.3 (C-1), 32.2 (C-6); MS: m/z 602 (M+NH4)+; Anal. for the mixture of isomers calc. C, 73.95; H, 6.89; found. C, 73.89; H, 6.91.
2,3,4-Tri-O-benzyl-5,6-dehydro-1-C(S)-butyl-D-glucitol (28)[14].
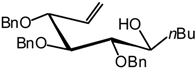
A solution of TIBAL (1.2 mL, 1.2 mmol, 1M in toluene) is added to a solution of 27 (110 mg, 0.23 mmol) in dry toluene (1 mL) at R.T. under argon. The mixture is stirred at 50 °C for 2 hours 30 min, until the end of the reaction is detected by TLC (7:3 cyclohexane/AcOEt). The mixture is cooled to R.T. and water (2 mL) is slowly added, and stirred for 15 min. After extraction with AcOEt (3 x 10 mL) and washing with water (10 mL) the organic layer is dried (MgSO4), filtered and the solvent is evaporated. The residue purified by silica gel flash column chromatography (3:7 AcOEt/cyclohexane) to afford a major product 28 (90 mg, 82 %) as an oil. = -17 (c = 1.8, CHCl3), lit.[14] = -21 (c = 18.7, CHCl3); 1H-NMR (250 MHz): δ = 7.51-7.12 (15H, arom. H), 5.85 (ddd, 1H, J5,6a = 11.5 Hz, J5,6b = 16.4 Hz, J5,4 = 7.6 Hz, H-5), 5.31 (dd, 1H, J6a,6b = 1.5 Hz, H-6a), 5.28 (dd, 1H, H-6b), 4.73 (d, 1H, J = 11.3 Hz, -CHPh), 4.65 (d, 1H, J = 11.2 Hz, -CHPh), 4.56 (d, 2H, J = 11.6 Hz, 2 -CHPh), 4.42 (d, 1H, J = 11.4 Hz, -CHPh), 4.31 (d, 1H, J = 11.5 Hz, -CHPh), 4.18 (dd, 1H, J4,3 = 5.7 Hz, H-4), 3.85-3.75 (m, 1H, H-1), 3.74 (dd, J3,2 = 4.0 Hz, H-3), 3.47 (dd, J2,1 = 5.4 Hz, H-2), 2.86 (d, 1H, J = 6.1 Hz, -OH), 1.6-0.7 (m, 9H, butyl); MS :m/z 492 (M+NH4)+
References
- Danishefsky, S.; Lee, J. L. J. Am. Chem. Soc. 1989, 111, 4829–4837. [CrossRef]
- Ferrier, R. J. J. Chem. Soc. Perkin Trans. 1 1979, 1455–1458. [CrossRef]
- Das, S. K.; Mallet, J.-M.; Sinaÿ, P. Angew. Chem., Int. Ed. Engl. 1997, 36, 493–496, [Angew. Chem. 1997, 109, 513–516].
- Sollogoub, M.; Mallet, J.-M.; Sinaÿ, P. Angew. Chem. Int. Ed. Engl. 2000, 39, 362–364. [CrossRef]
- Capon, B.; Collins, P. M.; Levy, A. A.; Overend, W. G. J. Chem. Soc. 1964, 4, 3242–3254. [CrossRef]
- Hurd, C. D.; Holysz, R. P. J. Am. Chem. Soc. 1950, 72, 1732–1735. [CrossRef]
- Lecourt, T.; Hérault, A.; Pearce, A.J.; Sollogoub, M.; Sinaÿ, P. Chem. Eur. J. 2004, 12, 2960–2971. [CrossRef] [PubMed]
- Pino, P.; Lorenzi, P. J. Org. Chem. 1966, 31, 329–331. [CrossRef]
- Paquette, L. A.; Friedrich, D.; Rogers, R. D. J. Org. Chem. 1991, 56, 3841–3849. [CrossRef]
- Allevi, P.; Anastasia, M.; Ciuffreda, P.; Fiecchi, A.; Scala, A. J. Chem. Soc., Chem. Commun. 1987, 16, 1245–1246. [CrossRef]
- Eade, R. A.; Pham, H.-P. Aust. J. Chem. 1979, 32, 2483–2493.
- Rouzaud, D. Thèse de troisième cycle; Université de Paris-Sud Orsay, 1985. [Google Scholar]
- Kuzuhara, H.; Fletcher, H. G., Jr. J. Org. Chem. 1967, 32, 2531–2534. [CrossRef]
- Sample availability: Not available.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.