Abstract
CaMoO4 nanocakes with uniform size and morphology were prepared on a large scale via a room temperature reverse-microemulsion method. The products were characterized in detail by X-ray powder diffraction, field-emission scanning electron microscopy, transmission electron microscopy, and high-resolution transmission electron microscopy. By establishing the relations between the thermodynamic functions of nano-CaMoO4 and bulk-CaMoO4 reaction systems, the equations for calculating the surface thermodynamic functions of nano-CaMoO4 were derived. Then, combined with in-situ microcalorimetry, the molar surface enthalpy, molar surface Gibbs free energy, and molar surface entropy of the prepared CaMoO4 nanocakes at 298.15 K were successfully obtained as (19.674 ± 0.017) kJ·mol−1, (619.704 ± 0.016) J·mol−1, and (63.908 ± 0.057) J·mol−1·K−1, respectively.
1. Introduction
The thermodynamic properties are some of the most important attributes of nanomaterials. In 2001, Hill [1–3] started to work on the nanothermodynamics area, and his research has opened up the door for the development of the discipline of nanothermodynamics. Surface effect is a basic effect of nanomaterials and many unique properties of nanomaterials are derived from it [4]. Surface thermodynamic properties, including surface Gibbs free energy, surface enthalpy and surface entropy, are an important expression of surface effects, and they have a direct relationship with the chemical thermodynamics [5], chemical kinetics [6], catalysis [7,8], sense [9], adsorption [10], phase transition [11], electrochemistry [12] of nanomaterials. The study of the surface thermodynamic properties of nanomaterials and their structure-function relationships with particle size, morphology and structure is valuable to understand the nature of chemical reactions, but, till now, the development of the surface thermodynamics of nanomaterials is quite poor [13]. How to acquire surface thermodynamic functions of nanomaterials such as molar surface Gibbs free energy, molar surface enthalpy and molar surface entropy is still a great challenge.
Calcium molybdate (CaMoO4) with scheelite type structure has attracted broad interest due to its interesting potential applications in various realms such as photoluminescence [14,15], scintillation detectors [16], optical fibers [17], catalysts [18], microwave applications [19], energy storage [20], etc. As we know, nanostructured materials possess many unique and interesting properties which differ from their bulk versions. These properties depend closely on their uniform shape and narrow size distribution. So far, various properties of CaMoO4 nanostructures have been well studied, but, to the best of our knowledge, very few reports on their surface thermodynamic properties are available. Studying the surface thermodynamic properties of CaMoO4 nanostructures is of great significance to understand their reaction mechanism. Besides, the obtained values will also play an important role in their theoretical study and industrial production.
In this paper, we report a simple and facile route for the large scale synthesis of uniform, single-crystalline and well-defined CaMoO4 nanocakes, and presented an effective and general route for acquiring the surface thermodynamic functions of nanomaterials by in-situ microcalorimetry.
2. Results and Discussion
A typical X-ray powder diffraction (XRD) pattern of the prepared products is shown in Figure 1a. All of the diffraction peaks of the products can be indexed to the pure tetragonal phase of CaMoO4 with the cell parameters a = 5.19 Å and c = 11.25 Å (JCPDS Card No. 029-0351). No impurity peaks were detected, indicating the formation of pure products. The sharp and narrow diffraction peaks suggest that the products are highly crystalline. Figure 1b presents a field-emission scanning electron microscopy (FE-SEM) image of the prepared products. The FE-SEM image reveals that a large number of cake-like structures with uniform size and morphology have been achieved. Figure 1c is the transmission electron microscopy (TEM) image of the products. It further proves that the obtained products are cake-like structures, and each cake is about 300 nm in diameter and 60 nm in thickness. To obtain further information about the prepared CaMoO4 nanocakes, high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) analysis of a randomly chosen single CaMoO4 nanocake were performed. Figure 1d is the TEM image of a single CaMoO4 nanocake of about 300 nm in diameter. Figure 1e is the SAED pattern of the single CaMoO4 nanocake shown in Figure 1d. The presence of sharp diffraction spots rather than an amorphous ring is suggestive of the predicted formation of a single-crystal form of CaMoO4. The SAED pattern can be indexed to pure CaMoO4 crystals with a tetragonal scheelite structure. It also demonstrates that the nanocake is a single crystal with the (001) plane as the 2D exposed surface. The HRTEM image shown in Figure 1f further indicates that the observed CaMoO4 nanocakes are single crystals with no defects or dislocations. The lattice fringes reveal that the single crystal CaMoO4 nanocakes possess interplanar spacing of about 0.263 nm, corresponding to the (200) and (020) planes of tetragonal CaMoO4.
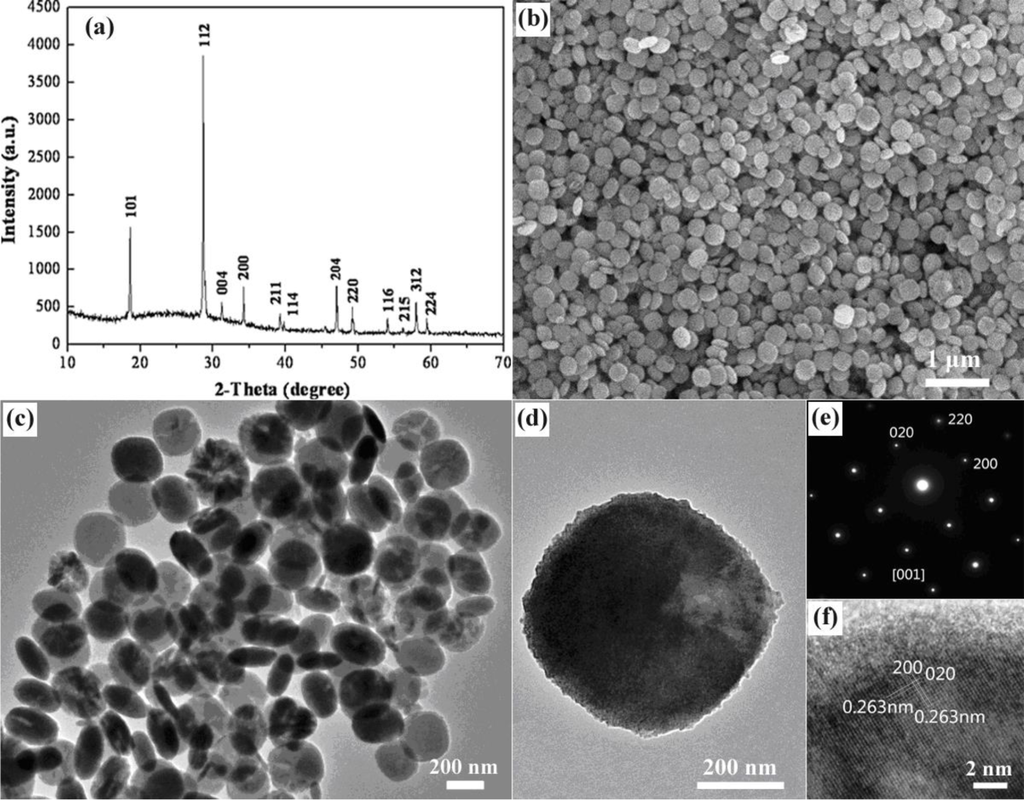
Figure 1.
(a, b, c) XRD pattern, SEM image and TEM image of the prepared CaMoO4 nanocakes, (d) TEM image of a single prepared CaMoO4 nanocake, (e, f) SAED pattern and HRTEM image corresponding to the single prepared CaMoO4 nanocake in (d).
The RD496-III type microcalorimeter (Mianyang CP Thermal Analysis Instrument Co., Ltd., Mianyang, China), based on thermokinetic theory, can simultaneously output both in-situ thermodynamic and kinetic information [21]. Briefly, equivalent amounts of the prepared nano-CaMoO4 and bulk-CaMoO4, respectively, were reacted with 1.0 mL of 0.1 M HCl in the microcalorimeter at 298.15 K and standard pressure. The microcalorimetric experiment of each reaction system was repeated five times, and the molar enthalpy of reaction and rate constants obtained are listed in Table 1.

Table 1.
Molar enthalpies of reaction and rate constants for the bulk CaMoO4 and nano CaMoO4 reaction systems.
Applying Equations (6), (11) and (12), the molar surface enthalpy, molar surface Gibbs free energy and molar surface entropy of the prepared CaMoO4 nanocakes were respectively calculated as below:
3. Experimental Section
The typical synthesis process for CaMoO4 nanocakes is as follows: two microemulsion solutions were respectively prepared by adding at room temperature 1.76 mL of 0.64 M Na2MoO4 and 1.76 mL of 0.08 M CaCl2 aqueous solution into a 13 mL TritonX-100/n-octanol/cyclohexane system (according to the mass ratio 3:2:8). After 10 min of stirring, the two microemulsion solutions were slowly mixed and stirred for another 20 min. The resulting mixture was aged without stirring for 48 h at room temperature. The white precipitates formed were separated by centrifugation, washed successively with acetone, deionized water and absolute ethanol, and then dried under vacuum at room temperature. Finally, the products were harvested. The products were characterized by X-ray powder diffraction (XRD, Philips PW 1710, The Netherlands, with Cu Kα radiation, λ = 1.5406 Å), field-emission scanning electron microscopy (FE-SEM, JSM-6700F, JEOL, Japan), transmission electron microscopy (TEM, JEOL JEM-200CX, 200 KV, Japan), and high-resolution transmission electron microscopy (HRTEM, JEOL JEM-2010, 200 KV, Japan).
4. Methodology
When the dimension of a material reaches nanometer level, the proportion of surface activated atoms is significantly increased, so the surface thermodynamic properties can no longer be ignored. Accordingly, the thermodynamic functions of nanomaterials should consist of two parts: bulk phase and surface phase. The molar Gibbs free energy for a chemical reaction in nanosystem can be expressed as [22]:
where superscript n denotes nanosystem, b denotes bulk phase, and s denotes surface phase.
In the same way, the standard molar enthalpy for a chemical reaction in nanosystem can be written as:
In order to clarify the specific meaning of ΔrGms, the chemical reaction equations for ΔrGmn and ΔrGmb should be expresses first. In this work, we used CaMoO4 (nano or bulk) and HCl as a reaction system. Thus the chemical reaction equations for ΔrGmn and ΔrGmb are as follows:
Thus, based on the state function characteristics, the chemical equations for ΔrGms should be written as: reaction (A) – reaction (B). Namely, the chemical equation for ΔrGms is as below:
According to the definition of surface Gibbs free energy of a material [23], the chemical equation for molar surface Gibbs free energy of nano CaMoO4 is as follows:
Combining reaction (C) with reaction (D), the molar surface Gibbs free energy of nano CaMoO4 can be expressed as:
Coupling Equation (3) with Equation (4), the molar surface Gibbs free energy of nano CaMoO4 can also be written as:
In the same way, the molar surface enthalpy of nano CaMoO4 can be expressed as:
Since the standard molar enthalpy for a chemical reaction can be measured directly by in-situ microcalorimetry (namely, ΔrHmb and ΔrHmn can be measured directly), the molar surface enthalpy of nano-CaMoO4 can be calculated by employing Equation (6).
As we know, a chemical reaction occurs on the molecular or atom level and has nothing to do with the size of a material. Thus, the nano-CaMoO4 reaction system and its corresponding bulk-CaMoO4 reaction system possess the same transition states and final states, and the only difference is their initial states [24,25]. The Gibbs free energies for both the nano and its corresponding bulk reaction system are depicted in Figure 2.
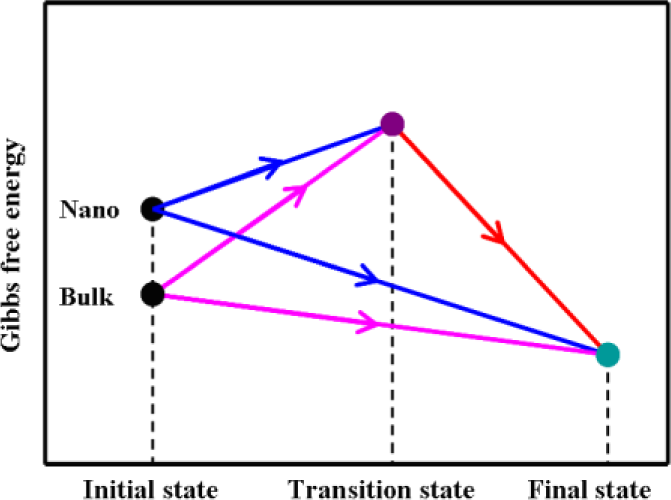
Figure 2.
Schematic diagram for the Gibbs free energies of nano-CaMoO4 and its corresponding bulk-CaMoO4 reaction systems.
From Figure 2, the following equations can be obtained on the basis of characteristics of state function.
Combining Equations (5), (7) and (8), the molar surface Gibbs free energy of nano CaMoO4 can be expressed as:
According to the transition state theory proposed by Eyring et al., the relation between rate constant and Gibbs free energy of activation can be expressed as:
where kB represents Boltzmann constant, and h represents Planck constant.
Since the rate constant can be acquired through regression analysis of the thermokinetic data recorded by in-situ microcalorimetry [26], the molar surface Gibbs free energy of nano-CaMoO4 can be acquired by using Equation (11).
In accordance with the formula G = H-TS, the molar surface entropy Sms of nano-CaMoO4 can be obtained as:
Thus, the molar surface enthalpy, molar surface Gibbs free energy, and molar surface entropy of nano CaMoO4 can be acquired from Equations (6), (11) and (12), respectively.
5. Conclusions
In summary, large-scale uniform CaMoO4 nanocakes were obtained by a simple and facile room temperature reverse-microemulsion method. XRD, FESEM, TEM and HRTEM were employed to demonstrate the high purity and good crystallinity of the CaMoO4 nanocakes. The equations for calculating the surface thermodynamic functions of nano-CaMoO4 were derived by building the relationships between the thermodynamic functions of the nano-CaMoO4 and bulk-CaMoO4 reaction systems. Then, coupled with in-situ microcalorimetry, the molar surface enthalpy, molar surface Gibbs free energy and molar surface entropy of the prepared CaMoO4 nanocakes were successfully acquired. Moreover, this is an effective and general route for acquiring the surface thermodynamic functions of other nanomaterials.
Acknowledgments
This work is financially supported by the Natural Science Foundation of China (No. 21273050), and Guangxi Natural Science Foundation of China (No. 0991001z, No. 0991085).
Author Contributions
Gaochao Fan and Zaiyin Huang conceived the idea; Xingxing Li did the experiment and wrote the paper. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hill, T.L. Perspective: Nanothermodynamics. Nano Lett. 2001, 1, 111–112. [Google Scholar]
- Hill, T.L. Extension of Nanothermodynamics to Include a One-dimensional Surface Excess. Nano Lett. 2001, 1, 159–160. [Google Scholar]
- Hill, T.L. A Different Approach to Nanothermodynamics. Nano Lett. 2001, 1, 273–275. [Google Scholar]
- Ball, P.; Garwin, L. Science at the Atomic Scale. Nature 1992, 355, 761–765. [Google Scholar]
- Xue, Y.Q.; Gao, B.J.; Gao, J.F. The Theory of Thermodynamics for Chemical Reactions in Dispersed Heterogeneous Systems. J. Colloid Interf. Sci. 1997, 191, 81–85. [Google Scholar]
- Du, J.P.; Wang, H.Y.; Zhao, R.H. Size-dependent Thermodynamic Properties and Equilibrium Constant of Chemical Reaction in Nanosystem: An Experimental Study. J. Chem. Thermodyn. 2013, 65, 29–33. [Google Scholar]
- Kuang, Q.; Wang, X.; Jiang, Z.; Xie, Z.; Zheng, L. High-energy-surface Engineered Metal Oxide Micro-and Nanocrystallites and their Applications. Acc. Chem. Res. 2013, 47, 308–318. [Google Scholar]
- Hu, L.H.; Peng, Q.; Li, Y. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar]
- Zhou, Z.Y.; Tian, N.; Li, J.T.; Broadwell, I.; Sun, S.G. Nanomaterials of High Surface Energy with Exceptional Properties in Catalysis and Energy Storage. Chem. Soc. Rev. 2011, 40, 4167–4185. [Google Scholar]
- Wen, Y.Z.; Xue, Y.Q.; Cui, Z.X.; Wang, Y. Thermodynamics of Nanoadsorption from Solution: Theoretical and Experimental Research. J. Chem. Thermodyn. 2015, 80, 112–118. [Google Scholar]
- Cui, Z.X.; Zhao, M.Z.; Lai, W.P.; Xue, Y.Q. Thermodynamics of Size Effect on Phase Transition Temperatures of Dispersed Phases. J. Phys. Chem. C 2011, 115, 22796–22803. [Google Scholar]
- Yang, Y.F.; Xue, Y.Q.; Cui, Z.X.; Zhao, M.Z. Effect of Particle Size on Electrode Potential and Thermodynamics of Nanoparticles Electrode in Theory and Experiment. Electrochim. Acta. 2014, 136, 565–571. [Google Scholar]
- Jiang, J.Y.; Huang, Z.Y.; Mi, Y.; Li, Y.F.; Yuan, A.Q. Development and Prospects for Thermodynamics of Nanomaterials. Prog. Chem. 2010, 22, 1058–1067. [Google Scholar]
- Gong, Q.; Qian, X.F.; Ma, X.D.; Zhu, Z.K. Large-scale Fabrication of Novel Hierarchical 3D CaMoO4 and SrMoO4 Mesocrystals via a Microemulsion-mediated Route. Cryst. Growth Des. 2006, 6, 1821–1825. [Google Scholar]
- Singh, B.P.; Parchur, A.K.; Ningthoujam, R.S.; Ansari, A.A.; Singha, P.; Rai, S.B. Influence of Gd3+ co-doping on structural property of CaMoO4:Eu nanoparticles. Dalton T. 2014, 43, 4770–4778. [Google Scholar]
- Kwan, S.; Kim, F.; Akana, J.; Yang, P.D. Synthesis and assembly of BaWO4 nanorods. Chem. Commun. 2001, 5, 447–448. [Google Scholar]
- Tanaka, K.; Miyajima, T.; Shirai, N.; Zhang, Q.; Nakata, R. Laser photochemical ablation of CdWO4 studied with the time-of-flight mass spectrometric technique. J. Appl. Phys. 1995, 77, 6581–6587. [Google Scholar]
- Sen, A.; Pramanik, P. Low-temperature Synthesis of Nano-sized Metal Molybdate Powders. Mater. Lett. 2001, 50, 287–294. [Google Scholar]
- Choi, G.K.; Cho, S.Y.; An, J.S.; Hong, K.S. Microwave dielectric properties and sintering behaviors of scheelite compound CaMoO4. J. Eur. Ceram. Soc. 2006, 26, 2011–2015. [Google Scholar]
- Sharma, N.; Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R.; Dong, Z.L.; White, T.J. Carbon-coated nanophase CaMoO4 as anode material for Li ion batteries. Chem. Mater. 2004, 16, 504–512. [Google Scholar]
- Fan, G.C.; Huang, Z.Y.; Jiang, J.Y.; Sun, L. Standard molar enthalpy of formation of the ZnO nanosheets. J. Therm. Anal. Calorim. 2012, 110, 1471–1474. [Google Scholar]
- Du, J.P.; Zhao, R.H.; Xue, Y.Q. Effects of Sizes of Nano-copper Oxide on the Equilibrium Constant and Thermodynamic Properties for the Reaction in Nanosystem. J. Chem. Thermodyn. 2012, 45, 48–52. [Google Scholar]
- Fu, X.C.; Shen, W.X.; Yao, T.Y.; Hou, W.H. Physical chemistry, 5th ed; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Fan, G.C.; Sun, L.; Huang, Z.Y.; Jiang, J.Y.; Li, Y.F. Thermodynamic Functions of the Grain-like ZnO Nanostructures. Mater. Lett. 2011, 65, 2783–2785. [Google Scholar]
- Fan, G.C.; Jiang, J.Y.; Li, Y.F.; Huang, Z.Y. Thermodynamic Functions of the ZnO Nanoweeds. Mater. Chem. Phys. 2011, 130, 839–842. [Google Scholar]
- Gao, S.L.; Chen, S.P.; Hu, R.Z.; Li, H.Y.; Shi, Q.Z. Derivation and Application of Thermodynamic Equations. Chin. J. Inorg. Chem. 2002, 18, 362–366. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).