Abstract
The fatal acute respiratory coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since COVID-19 was declared a pandemic by the World Health Organization in March 2020, infection and mortality rates have been rising steadily worldwide. The lack of a vaccine, as well as preventive and therapeutic strategies, emphasize the need to develop new strategies to mitigate SARS-CoV-2 transmission and pathogenesis. Since mouse hepatitis virus (MHV), severe acute respiratory syndrome coronavirus (SARS-CoV), and SARS-CoV-2 share a common genus, lessons learnt from MHV and SARS-CoV could offer mechanistic insights into SARS-CoV-2. This review provides a comprehensive review of MHV in mice and SARS-CoV-2 in humans, thereby highlighting further translational avenues in the development of innovative strategies in controlling the detrimental course of SARS-CoV-2. Specifically, we have focused on various aspects, including host species, organotropism, transmission, clinical disease, pathogenesis, control and therapy, MHV as a model for SARS-CoV and SARS-CoV-2 as well as mouse models for infection with SARS-CoV and SARS-CoV-2. While MHV in mice and SARS-CoV-2 in humans share various similarities, there are also differences that need to be addressed when studying murine models. Translational approaches, such as humanized mouse models are pivotal in studying the clinical course and pathology observed in COVID-19 patients. Lessons from prior murine studies on coronavirus, coupled with novel murine models could offer new promising avenues for treatment of COVID-19.
1. Introduction
In December 2019, a newly identified β-coronavirus infected thousands of people in the Wubei province, China, causing the acute respiratory coronavirus disease 2019 (COVID-19) (https://globalbiodefense.com/novel-coronavirus-covid-19-portal/). COVID-19 is a highly transmittable and potentially fatal viral infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The outbreak originating in China spread worldwide, caused major socio-economic and health consequences and was declared a pandemic by the World Health Organization (WHO) on 11 March 2020 []. This pandemic has, in particular, exposed vulnerable populations to a global health crisis. As of 30 June 2020, over 10 million people were tested positive for SARS-CoV-2, which pushed the health system in various countries to its limits and resulted in more than 500,000 deaths worldwide []. To date, there are neither proven options for prophylaxis nor for therapy. The steadily increasing numbers of infected persons are alarming and urge deciphering the pathomechanisms of COVID-19 to define new tools for risk stratification and development of novel treatment strategies. Comprehensive studies, including clinical and experimental approaches are of paramount importance.
For decades, the mouse has served as an excellent model not only to investigate inflammation, immune response, and infections including those of a viral nature, but also to develop new diagnostic, preventive, and therapeutic approaches. Infection models comprise various viruses including the respiratory or enterotropic mouse hepatitis virus (MHV), which belongs to the coronavirus family of enveloped positive-strand RNA viruses. Since SARS-CoV-2 is a coronavirus, the murine infection with MHV amongst others could serve as an experimental model to study principles of COVID-19. The present review provides a comprehensive overview of coronavirus in mice and the newly discovered SARS-CoV-2, putting these viruses into relation to other coronaviruses. Integration of murine expertise in viral infection could offer the opportunity to derive new strategies to rapidly decipher the pathomechanisms of COVID-19. Here, we focused on major topics that comprise a description of coronaviruses, host species as well as organotropism, transmission, clinical disease, pathogenesis, therapy, and control of MHV and COVID-19. We also provide information on the relevance of MHV and mice as models for widening the knowledge of the pathogenesis and therapeutic approaches for the human coronaviruses with the emphasis on SARS-CoV-2.
5. MHV as a Model for SARS-CoV and SARS-CoV-2
Seven coronaviruses cause human infection. Typically mild disease with common cold symptoms is observed in four of these, namely HCoV 229E, HCoV NL63, HCoV HKU1, and HCoV OC43. In contrast, MERS-CoV, SARS-CoV, and SARS-CoV-2 are zoonotic and, in addition to mild disease, also lead to severe respiratory illness and fatalities. The underlying immune mechanisms that contribute to the development of COVID-19 remain poorly understood partly because the initial outbreak is recent. The most intensely studied animal coronavirus is MHV, which induces a variety of conditions in mice, including respiratory, enteric, hepatic, and neurologic infections. Obtaining epidemiological information and molecular profiles of mice and humans, with MHV, or SARS-CoV-2, respectively, can help us in understanding the pathomechanisms of SARS-CoV-2, and in developing preventive strategies or clinical interventions. The present review provides comprehensive information pertaining to MHV and SARS-CoV-2, and the main points are summarized in Figure 1. One main focus of this work was to present information on MHV as a model for SARS-CoV-2 and the use of mouse models for elucidating the pathomechanisms of SARS-CoV-2.
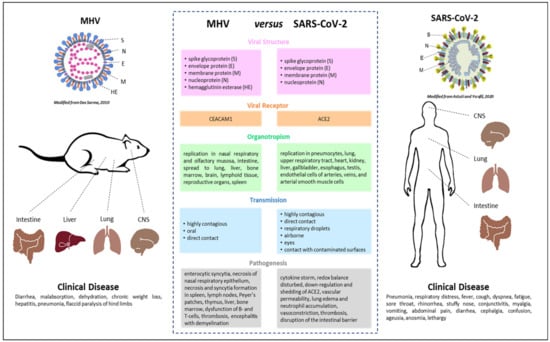
Figure 1.
Summary of the main similarities and differences between the coronaviruses mouse hepatitis virus (MHV) and severe acute respiratory syndrome coronaviruses 2 (SARS-CoV-2). The images for MHV [] and SARS-CoV-2 [] in this figure were modified.
MHV is an excellent model for studying the pathogenesis including tropism and virulence, as well as immune response to coronaviruses and was used as a model for SARS-CoV. Work with MHV only requires biosafety level 2 containment as opposed to biosafety level 3 containment when working with SARS-CoV and SARS-CoV-2. Interestingly, even though some coronaviruses are antigenically closely related they are biologically different [], and pulmonary response in mice is mouse and virus strain-dependent [,]. Intranasal inoculation of BALB/c mice with MHV-3, MHV-A59, MHV-1, MHV-S, and MHV-JHM revealed that MHV-1, which is primarily pneumovirulent, produced a transient lung pathology most similar to SARS, which completely resolved by day 21. While C57BL/6J mice exhibited only a mild pulmonary disease, A/J mice developed severe progressive pulmonary disease by day 2 post-MHV-1 infection with 100% mortality within 7 to 10 days of infection. On the other hand, C3H/St mice developed intermediate susceptibility with 40% mortality by day 28. Similarly, after intranasal inoculation of susceptible A/J, C3H/HeJ, and BALB/c as well as resistant C57BL/6 strains of mice with MHV-1 both A/J and C3H/HeJ mice exhibited enhanced weight loss and clinical illness compared to BALB/c and C57BL/6 mice []. C3H/HeJ mice, which naturally lack the toll-like receptor 4 [], showed increased morbidity, mortality, and severe pulmonary disease compared to C3H/HeN mice []. Intranasal inoculation with MHV-1 produced lethal pneumonitis in A/J mice [] and acute lung injury in 129/SvEv/C57BL6/J mice [].
Another report showed that MHV-A59 replicated in the lung and induced acute pneumonia and severe lung injuries in both young and old C57BL/6 mice, which closely mimicked ARDS by SARS-CoV and MERS-CoV in human lungs []. Also, since SARS-CoV-2 infection may produce neurological features including central nervous system (CNS) injuries [,] MHV-A59 and MHV-JHM may be used as models to decipher the mechanisms of virus entry into the CNS and the resulting immune response.
Notably, MHV-1 replicated well to similar levels in the lung in all strains of mice inoculated [,]. Tissue destruction occurs due to viral replication but severe lung damage is mainly immunopathological in nature, correlating better to the elevated inflammatory responses than to viral replication in the lung []. Therefore, inhibition of inflammatory responses is important in protecting the lung from injury.
Very small changes in coronavirus proteins can greatly affect tropism and virulence, which do not depend only on the spike protein [], but also on a combination of the structural protein M and the nonstructural replicase-associated proteins nsp1 and nsp13, these proteins being conserved among β-coronaviruses []. Less type 1 IFN was produced by A/J mice than in resistant strains of mice [,]. A previous report show that the lack of signaling by the CD200 receptor, a myeloid receptor [] which is expressed on macrophages, granulocytes, dendritic cells, T cells, B cells, and NK cells [,], strongly enhances type I IFN production and viral clearance, thereby improving the outcome of MHV infection, particularly in female mice. MHV clearance is dependent on TLR7-mediated type I IFN production. Sex differences in TLR7 responses were reported for humans []. The total number of IFNγ-producing CD4 T cells was significantly increased in C3H/HeJ mice compared to C3H/HeN mice. Both CD4 and CD8 T cells contribute to the MHV-1-induced disease [,] since depletion of either subset ameliorates morbidity and mortality. Also, a significant increase in the total number and frequency of T regulatory cells may aid in modulating the CD4 and CD8 T cells []. Serum cytokines and chemokines were markedly elevated in susceptible A/J mice [].
6. Mouse Models for Infection with SARS-CoV and SARS-CoV-2
Different animal models including mice, ferrets, Syrian hamsters, and primates [,,,,,] are used to study SARS-CoV-2. An ideal animal model should reproduce viral replication, clinical signs and symptoms, pathogenesis, and immune response. Due to the interspecies differences, no single animal model for SARS perfectly recapitulates the phenotype. Being able to conduct research with small animal models is desirable, since it can help us understand the pathogenesis and speed up development of new therapies and vaccines. There are various advantages of mice, including their small size, rapid reproduction, accessible technology for genetic modification, and comparability based on genetically identical cohorts. Notably, the use of animal models for SARS-CoV-2 infection is challenged by the requirements of biosafety level 3 containment and restricted license to conduct effective research. Since SARS-CoV-2 does not bind to the murine ACE2 receptor there are basically two ways of developing a mouse model; either by altering the host or the virus.
In contrast to SARS-CoV-2, SARS-CoV is able to infect wild type mice. After nasal inoculation of 4- to 6-week-old BALB/c mice the virus replicated in the respiratory tract and was cleared within one week. Neutralizing antibodies were produced and prevented viral replication in naïve mice by prior transfer of immune serum from convalescent mice. Notably, mice did not show any symptoms. Furthermore, pathological lung changes were restricted to mild and focal peribronchiolar mononuclear inflammatory infiltrates. Viral antigens and nucleic acids were only located in bronchiolar epithelial cells []. Also, five- to six-week-old C57BL/6 mice supported transient nonfatal systemic infection with SARS-CoV in the lung, which disseminated to the brain. It was suggested that a highly effective innate antiviral response in the lung was primarily responsible for viral clearance. In contrast, adaptive cellular immunity and natural killer cells played only a minor role []. Another study using 129SvEv mice showed that SARS-CoV infection resulted in self-limited bronchiolitis but progressed to severe pulmonary inflammation in Signal Transducer and Activator of Transcription 1 (STAT1) knockout mice, which are resistant to the effect of interferons, undermining the importance of the interferon response []. Taken together, due to the differences in pathogenesis to human disease the use of wild type mice in SARS-CoV research was limited.
Mouse models have been widely used to investigate determinants of lung development, aberrant alveolarization, chronic lung diseases, and inflammatory response [,]. Human and mouse lung development progresses through five successive stages. While the sequence of the stages are identical in both species, the timing during these periods varies. For example, lungs of term infants are in the alveolar phase of lung development at birth, whereas mice are in the saccular stage [,,]. This aspect is important when attempting to model age-dependent lung diseases [,,]. To overcome this limitation of mouse models, novel methodologies need to be developed using humanized mouse models such as models with natural human target cells [].
The fact that the prevalence of COVID-19 is much higher in adults than in children indicates that aging-related processes in the lung increase the susceptibility for an infection with SARS-CoV-2. This is most likely due to immunosenescence. The concept of aging processes in the immune system coupled with changes in cytokine responses has also been referred to as inflammaging and could adversely modify and exaggerate immunological responses following viral infection [,]. Notably, the majority of murine studies investigating immune response, pathomechanisms of viral infection, as well as acute and chronic lung diseases use animals that are rarely older than six months. A recent study [] investigated the lung cell-specific changes in young and old mice and proposed a deregulated control of epigenetic processes and cell metabolism in lung cells, as well as lung matrix remodeling in aging lungs. These age-related dynamics could facilitate virus adhesion and entry in the respiratory system in older humans and mice. Therefore, the age-dependent dynamic of organ physiology, matrix remodeling, cell homeostasis, and immune response need to be considered in COVID-19 research.
In contrast to the mild pathological changes seen in SARS-CoV infection in young wild type mice, infection of 12- to 14-month-old BALB/c mice was associated with increased viral replication in the lungs, infection of pneumocytes, interstitial pneumonia, clinical illness, and weight loss []. Thus, several models of SARS employing aged mice were established. Compared to young mice, aged BALB/c mice showed elevated levels of IFN-α, IFN-γ, and TNF-α early in infection []. Another study with 12- to 14-month-old BALB/c mice showed that depletion of CD4 T cells lead to an aggravated immune-mediated interstitial pneumonia, delayed clearance of SARS-CoV, reduced neutralizing antibody and cytokine production, as well as reduced pulmonary recruitment of lymphocytes []. The depletion of CD8 cells had no effect. This result points to an important role of CD4 cells in viral clearance in aged mice.
Various genetically engineered mouse lines were developed for studies with SARS [,,]. Mice transgenic for the expression of hACE2 (hACE2-transgenic mice) were infected by SARS-CoV and replication occurred in the lung []. However, SARS-CoV also spread to the brain and the infection finally resulted in death due to CNS failure and not as a result of SARS []. Another study also found high virus titers in the lungs and in the brains []. Viremia occurred and lower virus titers were also detected in other organs. Mortality was 100%, most likely due to CNS failure. Notably, the same study used a second transgenic line with less abundant expression of hACE, which did not develop relevant CNS infection and recovered completely. Nevertheless, due to a different pathomechanism, leading to a fatal outcome, hACE2-transgenic mice are generally not regarded as an optimal model for the study of SARS. Recently, transgenic mice were also generated for SARS-CoV-2 research []. SARS-CoV-2 infection lead to interstitial pneumonia with diffuse lesions. However, the pathogenicity of SARS-CoV-2 compared to SARS-CoV was mild in these transgenic mice. Another mouse model expressing human ACE2 (hACE2) was created by using CRISPR/ Cas9 knockin technology. Both young and aged hACE2 mice showed high viral loads in lung, trachea, and brain. The viral loads in the lung were higher than in the previously described model. Interstitial pneumonia and elevated cytokines were aggravated in aged hACE2 mice. Nevertheless, there were no fatalities [].
A different approach to alter the host is based on the transduction with an adenoviral vector, which leads to transient expression of hACE2 [,]. Prior to infection with SARS-CoV-2, mice were inoculated with a replication-deficient adenovirus (Ad5-hACE2). This approach is faster than the generation of transgenic mice and limits the expression of hACE2 to the respiratory system. Ad5-hACE2-sensitized mice infected by SARS-CoV-2 showed weight loss, high viral replication in the lungs, and severe pulmonary pathology [,]. Cytokine and chemokine responses to SARS-CoV-2 infection were similar to those observed in humans []. However, no lethalities were reported. Some therapeutic options were evaluated including patient-derived convalescent plasma, neutralizing monoclonal antibodies, the interferon I inducer Poly I:C, and the antiviral remdesivir, which were effective in these mice [,].
As already mentioned, the infection of young mice results only in mild disease. By serial passage in the murine respiratory tract it was possible to develop mouse-adapted virus strains that lead to severe disease. Mouse-adapted SARS-CoVs were created by several groups and have been used broadly [,,]. Infection with the mouse-adapted virus MA15 rapidly lead to high viral replication in lungs, viremia, and extrapulmonary manifestations []. Lymphopenia, neutrophilia, and pathological changes in the lungs were observed. The mice eventually died from the systemic viral infection coupled with extensive destruction of pneumocytes and ciliated epithelial cells. Notably, the pulmonary damage does not comprise alveolar edema and hyaline membrane formation, as observed in patients and older mice infected with SARS-CoV. It was suggested that the mice did not survive long enough for development of diffuse alveolar damage. Therefore, the mechanisms leading to death during an infection with MA15 differs from SARS-CoV. It is mainly based on the rapid progression of the infection, which results in 100% mortality, and was also lethal for young mice. To evaluate the reasons for the severe disease in young mice, MA15 mutations were further analyzed [], showing that foremost mutations in the S protein and partly in the replicase nonstructural protein nsp9 were essential to enable infection of young mice. A similiar observation was made with the mouse-adapted virus v2163 []. It was suggested that mutations in the S protein led to changes in binding properties and increased virulence in young mice. Therefore, mutations in the RBD of the S protein contribute to age-related disease severity.
A different mouse-adapted virus based on a mouse-passaged Frankfurt 1 isolate of SARS-CoV maintained age-dependent severity. Infection lead to severe respiratory illness in all adult (6-month-old) mice with a mortality rate of 30 to 50% []. In contrast, young mice (4-week-old) did not develop severe disease. Moreover, severely ill adult mice developed pulmonary edema and diffuse alveolar damage. As observed in humans, a cytokine storm with macrophage and neutrophil infiltration preceded these pathological changes. Adult mice showed higher levels of IL-1α, IL-1β, TNF-α, and IL-6 than young mice, the latter having higher levels of IFN-γ, IL-2, IL-10, and IL-13. This different cytokine response is consistent with the human disease and highlights it as a key driver for age-dependent severity, the insufficient release of IFN-γ in adult mice being the main difference. Treatment of adult mice with intraperitoneal injection of IFN-γ resulted in milder histopathological changes and protected adult mice from a fatal outcome.
Recently, a mouse-adapted strain of SARS-CoV-2 was created [] by remodeling the S and mACE2 binding interface via reverse genetics, a technology that was first developed for targeted recombination of MHV-A59 [], resulting in a recombinant virus (SARS-CoV-2 MA) that utilizes mACE2 for cell entry. SARS-CoV-2 MA is able to replicate in the upper and lower airways of young adult and aged BALB/c mice. As in the previously described models, disease is more severe in aged mice. In contrast to the human disease, the extent of viral replication in the upper airways is less than that observed in the lung. Prophylactic and therapeutic administration of IFN-λ-1a resulted in diminished replication of SARS-CoV-2 MA in mice. Furthermore, serum from S vaccinated mice was able to neutralize SARS-CoV-2 MA.
7. Conclusions and Future Directions
This review provides a comprehensive overview of MHV and SARS-CoV as possible murine surrogate models to understand, decipher, and ultimately use the pathomechanisms and viral characteristics of SARS-CoV-2 as therapeutic approaches. For this purpose, we first described MHV and SARS-CoV-2 in detail with respect to viral strains, host specificity, organotropism, transmission, pathogenesis, as well as clinical disease, mortality, therapy and control in order to clearly highlight the similarities and differences. The question arose concerning the extent to which murine studies using MHV may serve as surrogate models for SARS-CoV-2 to understand the viral biology and to decipher new preventive and therapeutic strategies for COVID-19, a new devastating disease. Based on the diversity of coronaviruses, we also provide information on SARS-CoV and MERS-CoV.
Various lessons can be learnt from these well-established and characterized murine models. Similarities between murine infection with MHV and human infection with SARS CoV-2 include their affinity for the olfactory system (MHV-A59) and pulmonary system (MHV-1), their spread into the brain causing neurologic symptoms (MHV-A59, MHV-JHM), development of microthrombi in the liver (MHV) or the brain (SARS-CoV-2), their high virulence associated with an inability to induce a robust T-cell response, their ability to modify immune response, RNA persistence in the gastrointestinal tract (MHV, SARS-CoV-2) and, depending on the strain, also in the brain (MHV, SARS-CoV). Major differences between MHV and SARS-CoV-2 include the viral receptor (CEACAM1a without co-receptors vs. ACE2 with co-receptors, respectively) as well as some aspects of organotropism and clinical disease. MHV infects only mice while SARS-CoV-2 infects different species including humans, hamsters, ferrets, cats, nonhuman primates.
The extensive comparison between MHV and SARS-CoV-2 emphasizes the fact that while MHV can provide some insight into the viral biology of SARS-CoV-2, it does not fully recapitulate the complexity of this new virus, for example, in terms of virus entry or recognition. Therefore, MHV-infected mice may not offer a perfect model for COVID-19 but definitely a surrogate model. These findings show the enormous importance of the precise characterization and understanding of murine virus models.
Further key disease modifiers need to be taken into account when investigating the biology of MHV and SARS-CoV-2. The investigation of mechanisms which determine heterogenic organotropism of various MHV strains and which protect certain mouse strains from clinical manifestation could provide new and important insights into virus-host interaction. This knowledge may eventually be transferred to SARS-CoV-2. The fact that COVID-19 particularly affects older people, while children and adolescents are more protected, highlights that age is a factor in the susceptibility of the host to SARS-CoV-2. Notably, this review provides strong evidence that both gender and age are central in the manifestation of clinical signs, course of the disease, and mortality in mice and humans. It is crucial to decipher the mechanisms which protect younger people to ultimately develop new therapeutic strategies. Currently, the mechanisms directing the processes of the aging immune system and determinants of susceptibility to SARS-CoV-2 are elusive. Similar differences in cytokine response to SARS-CoV and SARS-CoV-2 infections in mice and humans highlight the important pathomechanistic role of the innate immune system in determining the severity of the disease. However, the exact pathways have yet to be unveiled.
As depicted in this review, a lot of progress has been made by conducting research with wild type viruses and wild type mice. In recent years, new strategies comprising the modification of the host and virus strains by genetic engineering and other techniques lead to new insights into virus-host interaction. Regarding the COVID-19 pandemic, we observe an increased speed of knowledge generation and certainly mouse models will continue to play an important role in this fast paced scientific world. While the advantages and important insights into virus biology using murine MHV models have been recognized, the limitations of this surrogate system require new strategies. To this end, further basic research on coronaviruses and new models including humanized mouse models and mouse-adapted viruses are urgently needed to elucidate the pathomechanism of SARS-CoV-2 infections, enabling the development of new vaccines and therapies.
Funding
This work received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 11 March 2020).
- WHO. Coronavirus Disease (COVID-19), Situation Report-162; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- International Committee on Taxonomy of Viruses. Virus Taxonomy: 2019 Release, EC 51. Available online: https://talk.ictvonline.org/taxonomy (accessed on 7 July 2020).
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, N.J.D.E. Coronaviridae. In Fenner’s Veterinary Virology, 5th ed.; Academic Press: London, UK, 2017; pp. 435–461. [Google Scholar] [CrossRef]
- Liu, D.X.; Fung, T.S.; Chong, K.K.; Shukla, A.; Hilgenfeld, R. Accessory proteins of SARS-CoV and other coronaviruses. Antivir. Res. 2014, 109, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Wang, W.; Zhao, X.; Zai, J.; Li, X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020, 92, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Wu, C.C.; Li, X.; Song, Y.H.; Yao, X.M.; Wu, X.K.; Duan, Y.G.; Zhang, H.; Wang, Y.R.; Qian, Z.H.; et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020, 7, 1012–1023. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Williams, R.K.; Jiang, G.S.; Holmes, K.V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc. Natl. Acad. Sci. USA 1991, 88, 5533–5536. [Google Scholar] [CrossRef]
- Lu, H.; Stratton, C.W.; Tang, Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.T.; Jia, N.; Zhang, Y.W.; Shum, M.H.; Jiang, J.F.; Zhu, H.C.; Tong, Y.G.; Shi, Y.X.; Ni, X.B.; Liao, Y.S.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Cheever, F.S.; Daniels, J.B.; Pappenheimer, A.M.; Bailey, O.T. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. J. Exp. Med. 1949, 90, 181–210. [Google Scholar] [CrossRef] [PubMed]
- MacPhee, P.J.; Dindzans, V.J.; Fung, L.S.; Levy, G.A. Acute and chronic changes in the microcirculation of the liver in inbred strains of mice following infection with mouse hepatitis virus type 3. Hepatology 1985, 5, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Khan, R.S.; Dine, K.; Das Sarma, J.; Shindler, K.S. Intracranial Inoculation Is More Potent Than Intranasal Inoculation for Inducing Optic Neuritis in the Mouse Hepatitis Virus-Induced Model of Multiple Sclerosis. Front. Cell. Infect. Microbiol. 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.W.; Smith, A.L. Mouse hepatitis virus S in weanling Swiss mice following intranasal inoculation. Lab. Anim. Sci. 1983, 33, 355–360. [Google Scholar]
- Barthold, S.W.; Smith, A.L. Mouse hepatitis virus strain—Related patterns of tissue tropism in suckling mice. Arch. Virol. 1984, 81, 103–112. [Google Scholar] [CrossRef]
- Barthold, S.W.; Smith, A.L. Response of genetically susceptible and resistant mice to intranasal inoculation with mouse hepatitis virus JHM. Virus Res. 1987, 7, 225–239. [Google Scholar] [CrossRef]
- Barthold, S.W.; Smith, A.L. Viremic dissemination of mouse hepatitis virus-JHM following intranasal inoculation of mice. Arch. Virol. 1992, 122, 35–44. [Google Scholar] [CrossRef]
- Barthold, S.W.; Beck, D.S.; Smith, A.L. Enterotropic coronavirus (mouse hepatitis virus) in mice: Influence of host age and strain on infection and disease. Lab. Anim. Sci. 1993, 43, 276–284. [Google Scholar]
- Homberger, F.R.; Zhang, L.; Barthold, S.W. Prevalence of enterotropic and polytropic mouse hepatitis virus in enzootically infected mouse colonies. Lab. Anim. Sci. 1998, 48, 50–54. [Google Scholar]
- Homberger, F.R. Enterotropic mouse hepatitis virus. Lab. Anim. 1997, 31, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.W.; Smith, A.L. Mouse Hepatitis Virus. In The Mouse in Biomedical Research, 2nd ed.; Fox, J.G., Davisson, M.T., Quimby, F.W., Barthold, S.W., Newcomer, C.E., Smith, A.L., Eds.; Elsevier: Burlington, MA, USA, 2007; Volume 2, pp. 141–178. [Google Scholar]
- Barthold, S.W.; Smith, A.L. Duration of mouse hepatitis virus infection: Studies in immunocompetent and chemically immunosuppressed mice. Lab. Anim. Sci 1990, 40, 133–137. [Google Scholar]
- Rehg, J.E.; Blackman, M.A.; Toth, L.A. Persistent transmission of mouse hepatitis virus by transgenic mice. Comp. Med. 2001, 51, 369–374. [Google Scholar] [PubMed]
- Barthold, S.W.; de Souza, M.S.; Smith, A.L. Susceptibility of laboratory mice to intranasal and contact infection with coronaviruses of other species. Lab. Anim. Sci. 1990, 40, 481–485. [Google Scholar] [PubMed]
- Barthold, S.W.; Beck, D.S.; Smith, A.L. Mouse hepatitis virus and host determinants of vertical transmission and maternally-derived passive immunity in mice. Arch. Virol. 1988, 100, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Lavi, E.; Gilden, D.H.; Highkin, M.K.; Weiss, S.R. MHV-A59 pathogenesis in mice. Adv. Exp. Med. Biol. 1984, 173, 237–245. [Google Scholar] [CrossRef]
- Lavi, E.; Gilden, D.H.; Wroblewska, Z.; Rorke, L.B.; Weiss, S.R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology 1984, 34, 597–603. [Google Scholar] [CrossRef]
- Buchmeier, M.J.; Lewicki, H.A.; Talbot, P.J.; Knobler, R.L. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology 1984, 132, 261–270. [Google Scholar] [CrossRef]
- Kyuwa, S.; Yamaguchi, K.; Toyoda, Y.; Fujiwara, K.; Hilgers, J. Acute and late disease induced by murine coronavirus, strain JHM, in a series of recombinant inbred strains between BALB/cHeA and STS/A mice. Microb. Pathog. 1992, 12, 95–104. [Google Scholar] [CrossRef]
- Barthold, S.W.; Smith, A.L.; Lord, P.F.; Bhatt, P.N.; Jacoby, R.O.; Main, A.J. Epizootic coronaviral typhlocolitis in suckling mice. Lab. Anim. Sci. 1982, 32, 376–383. [Google Scholar] [PubMed]
- Ding, J.W.; Ning, Q.; Liu, M.F.; Lai, A.; Leibowitz, J.; Peltekian, K.M.; Cole, E.H.; Fung, L.S.; Holloway, C.; Marsden, P.A.; et al. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: Tissue-specific expression of a novel fgl2 prothrombinase. J. Virol. 1997, 71, 9223–9230. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M. Experimental Viral Hepatitis; Charles C. Thomas: Springfield, IL, USA, 1969. [Google Scholar]
- Lucchiari, M.A.; Pereira, C.A.; Kuhn, L.; Lefkovits, I. The pattern of proteins synthesized in the liver is profoundly modified upon infection of susceptible mice with mouse hepatitis virus 3. Res. Virol. 1992, 143, 231–240. [Google Scholar] [CrossRef]
- Tiensiwakul, P.; Husain, S.S. Effect of mouse hepatitis virus infection on iron retention in the mouse liver. Br. J. Exp. Pathol. 1979, 60, 161–166. [Google Scholar] [PubMed]
- Levy, G.A.; Leibowitz, J.L.; Edgington, T.S. Induction of monocyte procoagulant activity by murine hepatitis virus type 3 parallels disease susceptibility in mice. J. Exp. Med. 1981, 154, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Virelizier, J.L.; Allison, A.C. Correlation of persistent mouse hepatitis virus (MHV-3) infection with its effect on mouse macrophage cultures. Arch. Virol. 1976, 50, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Bang, F.B.; Warwick, A. Mouse Macrophages as Host Cells for the Mouse Hepatitis Virus and the Genetic Basis of Their Susceptibility. Proc. Natl. Acad. Sci. USA 1960, 46, 1065–1075. [Google Scholar] [CrossRef]
- Lamontagne, L.; Descoteaux, J.P.; Jolicoeur, P. Mouse hepatitis virus 3 replication in T and B lymphocytes correlate with viral pathogenicity. J. Immunol. 1989, 142, 4458–4465. [Google Scholar]
- De Souza, M.S.; Smith, A.L. Characterization of accessory cell function during acute infection of BALB/cByJ mice with mouse hepatitis virus (MHV), strain JHM. Lab. Anim. Sci. 1991, 41, 112–118. [Google Scholar]
- Boorman, G.A.; Luster, M.I.; Dean, J.H.; Campbell, M.L.; Lauer, L.A.; Talley, F.A.; Wilson, R.E.; Collins, M.J. Peritoneal macrophage alterations caused by naturally occurring mouse hepatitis virus. Am. J. Pathol. 1982, 106, 110–117. [Google Scholar]
- Dempsey, W.L.; Smith, A.L.; Morahan, P.S. Effect of inapparent murine hepatitis virus infections on macrophages and host resistance. J. Leukoc. Biol. 1986, 39, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Casebolt, D.B.; Spalding, D.M.; Schoeb, T.R.; Lindsey, J.R. Suppression of immune response induction in Peyer’s patch lymphoid cells from mice infected with mouse hepatitis virus. Cell. Immunol. 1987, 109, 97–103. [Google Scholar] [CrossRef]
- De Souza, M.S.; Smith, A.L.; Bottomly, K. Infection of BALB/cByJ mice with the JHM strain of mouse hepatitis virus alters in vitro splenic T cell proliferation and cytokine production. Lab. Anim. Sci. 1991, 41, 99–105. [Google Scholar] [PubMed]
- Cook-Mills, J.M.; Munshi, H.G.; Perlman, R.L.; Chambers, D.A. Mouse hepatitis virus infection suppresses modulation of mouse spleen T-cell activation. Immunology 1992, 75, 542–545. [Google Scholar]
- Smith, A.L.; de Souza, M.S.; Finzi, D.; Barthold, S.W. Responses of mice to murine coronavirus immunization. Arch. Virol. 1992, 125, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Winograd, D.F.; de Souza, M.S. In vitro splenic T cell responses of diverse mouse genotypes after oronasal exposure to mouse hepatitis virus, strain JHM. Lab. Anim. Sci. 1991, 41, 106–111. [Google Scholar]
- Schindler, L.; Engler, H.; Kirchner, H. Activation of natural killer cells and induction of interferon after injection of mouse hepatitis virus type 3 in mice. Infect. Immun. 1982, 35, 869–873. [Google Scholar] [CrossRef]
- Cray, C.; Mateo, M.O.; Altman, N.H. In vitro and long-term in vivo immune dysfunction after infection of BALB/c mice with mouse hepatitis virus strain A59. Lab. Anim. Sci. 1993, 43, 169–174. [Google Scholar]
- Lardans, V.; Godfraind, C.; van der Logt, J.T.; Heessen, W.A.; Gonzalez, M.D.; Coutelier, J.P. Polyclonal B lymphocyte activation induced by mouse hepatitis virus A59 infection. J. Gen. Virol. 1996, 77, 1005–1009. [Google Scholar] [CrossRef]
- Sun, N.; Grzybicki, D.; Castro, R.F.; Murphy, S.; Perlman, S. Activation of astrocytes in the spinal cord of mice chronically infected with a neurotropic coronavirus. Virology 1995, 213, 482–493. [Google Scholar] [CrossRef]
- Lane, T.E.; Asensio, V.C.; Yu, N.; Paoletti, A.D.; Campbell, I.L.; Buchmeier, M.J. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 1998, 160, 970–978. [Google Scholar] [PubMed]
- Savarin, C.; Bergmann, C.C. Fine Tuning the Cytokine Storm by IFN and IL-10 Following Neurotropic Coronavirus Encephalomyelitis. Front. Immunol. 2018, 9, 3022. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.T.; Pan, X.Q.; Smith, A.L.; Newman, D.K.; Weiss, S.R.; Ruggieri, M.R., Sr.; Malykhina, A.P. Coronavirus-induced demyelination of neural pathways triggers neurogenic bladder overactivity in a mouse model of multiple sclerosis. Am. J. Physiol.-Ren. Physiol. 2014, 307, F612–F622. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Schelper, R.; Bolger, E.; Ries, D. Late onset, symptomatic, demyelinating encephalomyelitis in mice infected with MHV-JHM in the presence of maternal antibody. Microb. Pathog. 1987, 2, 185–194. [Google Scholar] [CrossRef]
- Haring, J.; Perlman, S. Mouse hepatitis virus. Curr. Opin. Microbiol. 2001, 4, 462–466. [Google Scholar] [CrossRef]
- Woyciechowska, J.L.; Trapp, B.D.; Patrick, D.H.; Shekarchi, I.C.; Leinikki, P.O.; Sever, J.L.; Holmes, K.V. Acute and subacute demyelination induced by mouse hepatitis virus strain A59 in C3H mice. J. Exp. Pathol. 1984, 1, 295–306. [Google Scholar]
- Homberger, F.R.; Thomann, P.E. Transmission of murine viruses and mycoplasma in laboratory mouse colonies with respect to housing conditions. Lab. Anim. 1994, 28, 113–120. [Google Scholar] [CrossRef]
- Homberger, F.R. Maternally-derived passive immunity to enterotropic mouse hepatitis virus. Arch. Virol. 1992, 122, 133–141. [Google Scholar] [CrossRef]
- Barthold, S.W.; Smith, A.L. Duration of challenge immunity to coronavirus JHM in mice. Arch. Virol. 1989, 107, 171–177. [Google Scholar] [CrossRef]
- Barthold, S.W.; Smith, A.L. Virus strain specificity of challenge immunity to coronavirus. Arch. Virol. 1989, 104, 187–196. [Google Scholar] [CrossRef]
- Homberger, F.R.; Barthold, S.W.; Smith, A.L. Duration and strain-specificity of immunity to enterotropic mouse hepatitis virus. Lab. Anim. Sci. 1992, 42, 347–351. [Google Scholar] [PubMed]
- Weir, E.C.; Bhatt, P.N.; Barthold, S.W.; Cameron, G.A.; Simack, P.A. Elimination of mouse hepatitis virus from a breeding colony by temporary cessation of breeding. Lab. Anim. Sci. 1987, 37, 455–458. [Google Scholar] [PubMed]
- Mahabir, E.; Bulian, D.; Needham, J.; Mayer, A.; Mateusen, B.; Van Soom, A.; Nauwynck, H.; Schmidt, J. Transmission of mouse minute virus (MMV) but not mouse hepatitis virus (MHV) following embryo transfer with experimentally exposed in vivo-derived embryos. Biol. Reprod. 2007, 76, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.; Pyrc, K.; van der Hoek, L.; Geier, M.; Berkhout, B.; Pohlmann, S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H., III; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e14. [Google Scholar] [CrossRef]
- Li, W.; Wong, S.K.; Li, F.; Kuhn, J.H.; Huang, I.C.; Choe, H.; Farzan, M. Animal origins of the severe acute respiratory syndrome coronavirus: Insight from ACE2-S-protein interactions. J. Virol. 2006, 80, 4211–4219. [Google Scholar] [CrossRef]
- Chen, J.; Subbarao, K. The Immunobiology of SARS. Annu. Rev. Immunol. 2007, 25, 443–472. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Hong, N.; Yu, W.; Xia, J.; Shen, Y.; Yap, M.; Han, W. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol. 2020, 98, e649–e655. [Google Scholar] [CrossRef]
- Tang, J.W.; To, K.F.; Lo, A.W.; Sung, J.J.; Ng, H.K.; Chan, P.K. Quantitative temporal-spatial distribution of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) in post-mortem tissues. J. Med. Virol. 2007, 79, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.; Wang, Y.; Yuen, T.T.; Chai, Y.; Hou, Y.; Shuai, H.; Yang, D.; Hu, B.; Huang, X.; et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: An ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Muller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef]
- Amraie, R.; Napoleon, M.A.; Yin, W.; Berrigan, J.; Suder, E.; Zhao, G.; Olejnik, J.; Gummuluru, S.; Muhlberger, E.; Chitalia, V.; et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2 and are differentially expressed in lung and kidney epithelial and endothelial cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cell 2020, 9, 1652. [Google Scholar] [CrossRef]
- Aguiar, J.A.; Tremblay, B.J.; Mansfield, M.J.; Woody, O.; Lobb, B.; Banerjee, A.; Chandiramohan, A.; Tiessen, N.; Cao, Q.; Dvorkin-Gheva, A.; et al. Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. Eur. Respir. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Gallagher, T.; Weiss, S.R. Neurovirulent Murine Coronavirus JHM.SD Uses Cellular Zinc Metalloproteases for Virus Entry and Cell-Cell Fusion. J. Virol. 2017, 91, e01564-16. [Google Scholar] [CrossRef]
- Tang, J.W.; Li, Y.; Eames, I.; Chan, P.K.; Ridgway, G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006, 64, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.M.; Norton, A.; Young, F.P.; Collins, D.W. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: A narrative review. Anaesthesia 2020, 75, 1086–1095. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Che Mat, N.F.; Edinur, H.A.; Abdul Razab, M.K.A.; Safuan, S. A single mass gathering resulted in massive transmission of COVID-19 infections in Malaysia with further international spread. J. Travel Med. 2020, 27, taaa059. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Luca, F.; Xu, Y.; Alazizi, A.; Leng, Y.; Hsu, C.D.; Gomez-Lopez, N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife 2020, 9, e58716. [Google Scholar] [CrossRef]
- Delamater, P.L.; Street, E.J.; Leslie, T.F.; Yang, Y.T.; Jacobsen, K.H. Complexity of the Basic Reproduction Number (R0). Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutierrez-Ocampo, E.; Villamizar-Pena, R.; Holguin-Rivera, Y.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Bonilla-Aldana, D.K.; Franco-Paredes, C.; Henao-Martinez, A.F.; et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 34, 101623. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020, 25, 2000180. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr. Infect. Dis. J. 2020, 39, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.J. Coronavirus disease 2019 in children: Current status. J. Chin. Med. Assoc. 2020, 83, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, J.; Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020, 92, 441–447. [Google Scholar] [CrossRef] [PubMed]
- WHO. China Joint Mission on Coronavirus Disease 2019 (COVID-19); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Robert Koch Institute. Taeglicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19); Robert Koch Institute: Berlin, Germany, 2020. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/2020-06-30-de.pdf (accessed on 30 June 2020).
- Center for Disease Control and Prevention. Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2018–2019 Influenza Season; Center for Disease Control and Prevention: Atlanta, GA, USA, 2020. Available online: https://www.cdc.gov/flu/about/burden/2018-2019.html (accessed on 30 June 2020).
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef]
- Rivellese, F.; Prediletto, E. ACE2 at the centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun. Rev. 2020, 19, 102536. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Oude Munnink, B.B.; de Meulder, D.; van Amerongen, G.; van den Brand, J.; Okba, N.M.A.; et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020, 368, 1012–1015. [Google Scholar] [CrossRef]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Sung, B.; Jung, K.J.; Zou, Y.; Yu, B.P. The molecular inflammatory process in aging. Antioxid. Redox Signal. 2006, 8, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Smits, S.L.; de Lang, A.; van den Brand, J.M.; Leijten, L.M.; van Ijcken, W.F.; Eijkemans, M.J.; van Amerongen, G.; Kuiken, T.; Andeweg, A.C.; Osterhaus, A.D.; et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010, 6, e1000756. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Takada, A.; Kawaoka, Y. Antibody-dependent enhancement of viral infection: Molecular mechanisms and in vivo implications. Rev. Med. Virol. 2003, 13, 387–398. [Google Scholar] [CrossRef]
- Meyer, N.J.; Christie, J.D. Genetic heterogeneity and risk of acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2013, 34, 459–474. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Alifano, M.; Alifano, P.; Forgez, P.; Iannelli, A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie 2020, 174, 30–33. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Khan, Z.; Giani, J.F.; Cao, D.Y.; Bernstein, E.A.; Shen, X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 2018, 14, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020, 35, 266–271. [Google Scholar] [CrossRef]
- D’Amico, F.; Baumgart, D.C.; Danese, S.; Peyrin-Biroulet, L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 2020, 18, 1663–1672. [Google Scholar] [CrossRef]
- Li, L.Y.; Wu, W.; Chen, S.; Gu, J.W.; Li, X.L.; Song, H.J.; Du, F.; Wang, G.; Zhong, C.Q.; Wang, X.Y.; et al. Digestive system involvement of novel coronavirus infection: Prevention and control infection from a gastroenterology perspective. J. Dig. Dis. 2020, 21, 199–204. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Allergy and Infectious Diseases. NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19; National Institute of Allergy and Infectious Diseases: Bethesda, MD, USA, 2020. Available online: https://www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19 (accessed on 29 April 2020).
- Takeda. Takeda Initiates Development of a Plasma-Derived Therapy for COVID-19; Takeda: Nagasaki, Japan, 2020; Available online: https://www.takeda.com/newsroom/newsreleases/2020/takeda-initiates-development-of-a-plasma-derived-therapy-for-covid-19/ (accessed on 29 April 2020).
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.K.; Kubin, C.; Barr, R.G.; et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 382, 2411–2418. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tan, L.; Wang, X.; Liu, W.; Lu, Y.; Cheng, L.; Sun, Z. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int. J. Infect. Dis. 2020, 94, 107–109. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, N.; Baig, E.; Ma, X.; Zhang, J.; He, W.; Rowe, A.; Habal, M.; Liu, M.; Shalev, I.; Downey, G.P.; et al. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J. Virol. 2006, 80, 10382–10394. [Google Scholar] [CrossRef] [PubMed]
- Will, J.P.; Hirani, D.; Thielen, F.; Klein, F.; Vohlen, C.; Dinger, K.; Dotsch, J.; Alejandre Alcazar, M.A. Strain-dependent effects on lung structure, matrix remodeling, and Stat3/Smad2 signaling in C57BL/6N and C57BL/6J mice after neonatal hyperoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R169–R181. [Google Scholar] [CrossRef] [PubMed]
- Khanolkar, A.; Hartwig, S.M.; Haag, B.A.; Meyerholz, D.K.; Harty, J.T.; Varga, S.M. Toll-like receptor 4 deficiency increases disease and mortality after mouse hepatitis virus type 1 infection of susceptible C3H mice. J. Virol. 2009, 83, 8946–8956. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Ma, X.Z.; Bartczak, A.; Zhang, J.; Khattar, R.; Chen, L.; Liu, M.F.; Edwards, A.; Levy, G.; McGilvray, I.D. Proteasome inhibition in vivo promotes survival in a lethal murine model of severe acute respiratory syndrome. J. Virol. 2010, 84, 12419–12428. [Google Scholar] [CrossRef]
- Han, B.; Ma, X.; Zhang, J.; Zhang, Y.; Bai, X.; Hwang, D.M.; Keshavjee, S.; Levy, G.A.; McGilvray, I.; Liu, M. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab. Investig. 2012, 92, 1285–1296. [Google Scholar] [CrossRef]
- Yang, Z.; Du, J.; Chen, G.; Zhao, J.; Yang, X.; Su, L.; Cheng, G.; Tang, H. Coronavirus MHV-A59 infects the lung and causes severe pneumonia in C57BL/6 mice. Virol. Sin. 2014, 29, 393–402. [Google Scholar] [CrossRef]
- Palao, M.; Fernandez-Diaz, E.; Gracia-Gil, J.; Romero-Sanchez, C.M.; Diaz-Maroto, I.; Segura, T. Multiple sclerosis following SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2020, 45, 102377. [Google Scholar] [CrossRef] [PubMed]
- Matias-Guiu, J.; Gomez-Pinedo, U.; Montero-Escribano, P.; Gomez-Iglesias, P.; Porta-Etessam, J.; Matias-Guiu, J.A. Should we expect neurological symptoms in the SARS-CoV-2 epidemic? Neurologia 2020, 35, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, J.L.; Srinivasa, R.; Williamson, S.T.; Chua, M.M.; Liu, M.; Wu, S.; Kang, H.; Ma, X.Z.; Zhang, J.; Shalev, I.; et al. Genetic determinants of mouse hepatitis virus strain 1 pneumovirulence. J. Virol. 2010, 84, 9278–9291. [Google Scholar] [CrossRef][Green Version]
- Das Sarma, J. A Mechanism of Virus-Induced Demyelination. Interdiscip. Perspect. Infect. Dis. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Indwiani Astuti, Y. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Rempel, J.D.; Murray, S.J.; Meisner, J.; Buchmeier, M.J. Mouse hepatitis virus neurovirulence: Evidence of a linkage between S glycoprotein expression and immunopathology. Virology 2004, 318, 45–54. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Cowley, T.J.; Steinbrenner, A.D.; Phillips, J.M.; Yount, B.L.; Baric, R.S.; Weiss, S.R. The nsp1, nsp13, and M proteins contribute to the hepatotropism of murine coronavirus JHM.WU. J. Virol. 2015, 89, 3598–3609. [Google Scholar] [CrossRef]
- Khanolkar, A.; Hartwig, S.M.; Haag, B.A.; Meyerholz, D.K.; Epping, L.L.; Haring, J.S.; Varga, S.M.; Harty, J.T. Protective and pathologic roles of the immune response to mouse hepatitis virus type 1: Implications for severe acute respiratory syndrome. J. Virol. 2009, 83, 9258–9272. [Google Scholar] [CrossRef]
- Preston, S.; Wright, G.J.; Starr, K.; Barclay, A.N.; Brown, M.H. The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur. J. Immunol. 1997, 27, 1911–1918. [Google Scholar] [CrossRef]
- Wright, G.J.; Cherwinski, H.; Foster-Cuevas, M.; Brooke, G.; Puklavec, M.J.; Bigler, M.; Song, Y.; Jenmalm, M.; Gorman, D.; McClanahan, T.; et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 2003, 171, 3034–3046. [Google Scholar] [CrossRef]
- Rijkers, E.S.; de Ruiter, T.; Baridi, A.; Veninga, H.; Hoek, R.M.; Meyaard, L. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol. Immunol. 2008, 45, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Karnam, G.; Rygiel, T.P.; Raaben, M.; Grinwis, G.C.; Coenjaerts, F.E.; Ressing, M.E.; Rottier, P.J.; de Haan, C.A.; Meyaard, L. CD200 receptor controls sex-specific TLR7 responses to viral infection. PLoS Pathog. 2012, 8, e1002710. [Google Scholar] [CrossRef] [PubMed]
- Khanolkar, A.; Fulton, R.B.; Epping, L.L.; Pham, N.L.; Tifrea, D.; Varga, S.M.; Harty, J.T. T cell epitope specificity and pathogenesis of mouse hepatitis virus-1-induced disease in susceptible and resistant hosts. J. Immunol. 2010, 185, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, S.M.; Holman, K.M.; Varga, S.M. Depletion of alveolar macrophages ameliorates virus-induced disease following a pulmonary coronavirus infection. PLoS ONE 2014, 9, e90720. [Google Scholar] [CrossRef]
- Lutz, C.; Maher, L.; Lee, C.; Kang, W. COVID-19 preclinical models: Human angiotensin-converting enzyme 2 transgenic mice. Hum. Genom. 2020, 14, 20. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, S.G.; Kim, S.M.; Kim, E.H.; Park, S.J.; Yu, K.M.; Chang, J.H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709.e2. [Google Scholar] [CrossRef]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef]
- Chan, J.F.; Zhang, A.J.; Yuan, S.; Poon, V.K.; Chan, C.C.; Lee, A.C.; Chan, W.M.; Fan, Z.; Tsoi, H.W.; Wen, L.; et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: Implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Woolsey, C.; Borisevich, V.; Prasad, A.N.; Agans, K.N.; Deer, D.J.; Dobias, N.S.; Heymann, J.C.; Foster, S.L.; Levine, C.B.; Medina, L.; et al. Establishment of an African green monkey model for COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yu, P.; Qi, F.; Xu, Y.; Li, F.; Liu, P.; Liu, J.; Bao, L.; Deng, W.; Gao, H.; Xiang, Z.; et al. Age-related rhesus macaque models of COVID-19. Anim. Model. Exp. Med. 2020, 3, 93–97. [Google Scholar] [CrossRef]
- Subbarao, K.; McAuliffe, J.; Vogel, L.; Fahle, G.; Fischer, S.; Tatti, K.; Packard, M.; Shieh, W.J.; Zaki, S.; Murphy, B. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 2004, 78, 3572–3577. [Google Scholar] [CrossRef] [PubMed]
- Glass, W.G.; Subbarao, K.; Murphy, B.; Murphy, P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 2004, 173, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Hogan, R.J.; Gao, G.; Rowe, T.; Bell, P.; Flieder, D.; Paragas, J.; Kobinger, G.P.; Wivel, N.A.; Crystal, R.G.; Boyer, J.; et al. Resolution of primary severe acute respiratory syndrome-associated coronavirus infection requires Stat1. J. Virol. 2004, 78, 11416–11421. [Google Scholar] [CrossRef] [PubMed]
- Herriges, M.; Morrisey, E.E. Lung development: Orchestrating the generation and regeneration of a complex organ. Development 2014, 141, 502–513. [Google Scholar] [CrossRef]
- Morrisey, E.E.; Cardoso, W.V.; Lane, R.H.; Rabinovitch, M.; Abman, S.H.; Ai, X.; Albertine, K.H.; Bland, R.D.; Chapman, H.A.; Checkley, W.; et al. Molecular determinants of lung development. Ann. Am. Thorac. Soc. 2013, 10, S12–S16. [Google Scholar] [CrossRef]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef]
- Copland, I.; Post, M. Lung development and fetal lung growth. Paediatr. Respir. Rev. 2004, 5, S259–S264. [Google Scholar] [CrossRef]
- Alejandre-Alcazar, M.A.; Kwapiszewska, G.; Reiss, I.; Amarie, O.V.; Marsh, L.M.; Sevilla-Perez, J.; Wygrecka, M.; Eul, B.; Kobrich, S.; Hesse, M.; et al. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L537–L549. [Google Scholar] [CrossRef]
- Alejandre Alcazar, M.A.; Kaschwich, M.; Ertsey, R.; Preuss, S.; Milla, C.; Mujahid, S.; Masumi, J.; Khan, S.; Mokres, L.M.; Tian, L.; et al. Elafin Treatment Rescues EGFR-Klf4 Signaling and Lung Cell Survival in Ventilated Newborn Mice. Am. J. Respir. Cell. Mol. Biol. 2018, 59, 623–634. [Google Scholar] [CrossRef]
- Domm, W.; Misra, R.S.; O’Reilly, M.A. Affect of Early Life Oxygen Exposure on Proper Lung Development and Response to Respiratory Viral Infections. Front. Med. (Lausanne) 2015, 2, 55. [Google Scholar] [CrossRef]
- Wahl, A.; De, C.; Abad Fernandez, M.; Lenarcic, E.M.; Xu, Y.; Cockrell, A.S.; Cleary, R.A.; Johnson, C.E.; Schramm, N.J.; Rank, L.M.; et al. Precision mouse models with expanded tropism for human pathogens. Nat. Biotechnol. 2019, 37, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Angelidis, I.; Simon, L.M.; Fernandez, I.E.; Strunz, M.; Mayr, C.H.; Greiffo, F.R.; Tsitsiridis, G.; Ansari, M.; Graf, E.; Strom, T.M.; et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 2019, 10, 963. [Google Scholar] [CrossRef]
- Roberts, A.; Paddock, C.; Vogel, L.; Butler, E.; Zaki, S.; Subbarao, K. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J. Virol. 2005, 79, 5833–5838. [Google Scholar] [CrossRef]
- Chen, J.; Lau, Y.F.; Lamirande, E.W.; Paddock, C.D.; Bartlett, J.H.; Zaki, S.R.; Subbarao, K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010, 84, 1289–1301. [Google Scholar] [CrossRef]
- McCray, P.B., Jr.; Pewe, L.; Wohlford-Lenane, C.; Hickey, M.; Manzel, L.; Shi, L.; Netland, J.; Jia, H.P.; Halabi, C.; Sigmund, C.D.; et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007, 81, 813–821. [Google Scholar] [CrossRef]
- Yang, X.H.; Deng, W.; Tong, Z.; Liu, Y.X.; Zhang, L.F.; Zhu, H.; Gao, H.; Huang, L.; Liu, Y.L.; Ma, C.M.; et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp. Med. 2007, 57, 450–459. [Google Scholar]
- Yoshikawa, N.; Yoshikawa, T.; Hill, T.; Huang, C.; Watts, D.M.; Makino, S.; Milligan, G.; Chan, T.; Peters, C.J.; Tseng, C.T. Differential virological and immunological outcome of severe acute respiratory syndrome coronavirus infection in susceptible and resistant transgenic mice expressing human angiotensin-converting enzyme 2. J. Virol. 2009, 83, 5451–5465. [Google Scholar] [CrossRef]
- Tseng, C.T.; Huang, C.; Newman, P.; Wang, N.; Narayanan, K.; Watts, D.M.; Makino, S.; Packard, M.M.; Zaki, S.R.; Chan, T.S.; et al. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. J. Virol. 2007, 81, 1162–1173. [Google Scholar] [CrossRef]
- Bao, L.; Deng, W.; Huang, B.; Gao, H.; Liu, J.; Ren, L.; Wei, Q.; Yu, P.; Xu, Y.; Qi, F.; et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 2020, 583, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Chen, Q.; Gu, H.J.; Yang, G.; Wang, Y.X.; Huang, X.Y.; Liu, S.S.; Zhang, N.N.; Li, X.F.; Xiong, R.; et al. A Mouse Model of SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe 2020, 28, 124–133.e4. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Case, J.B.; Winkler, E.S.; Thackray, L.B.; Kafai, N.M.; Bailey, A.L.; McCune, B.T.; Fox, J.M.; Chen, R.E.; Alsoussi, W.B.; et al. A SARS-CoV-2 Infection Model in Mice Demonstrates Protection by Neutralizing Antibodies. Cell 2020, 182, 744–753.e4. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhuang, Z.; Zheng, J.; Li, K.; Wong, R.L.; Liu, D.; Huang, J.; He, J.; Zhu, A.; Zhao, J.; et al. Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment. Cell 2020, 182, 734–743.e5. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Deming, D.; Paddock, C.D.; Cheng, A.; Yount, B.; Vogel, L.; Herman, B.D.; Sheahan, T.; Heise, M.; Genrich, G.L.; et al. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007, 3, e5. [Google Scholar] [CrossRef]
- Nagata, N.; Iwata, N.; Hasegawa, H.; Fukushi, S.; Harashima, A.; Sato, Y.; Saijo, M.; Taguchi, F.; Morikawa, S.; Sata, T. Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am. J. Pathol. 2008, 172, 1625–1637. [Google Scholar] [CrossRef]
- Day, C.W.; Baric, R.; Cai, S.X.; Frieman, M.; Kumaki, Y.; Morrey, J.D.; Smee, D.F.; Barnard, D.L. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology 2009, 395, 210–222. [Google Scholar] [CrossRef]
- Frieman, M.; Yount, B.; Agnihothram, S.; Page, C.; Donaldson, E.; Roberts, A.; Vogel, L.; Woodruff, B.; Scorpio, D.; Subbarao, K.; et al. Molecular determinants of severe acute respiratory syndrome coronavirus pathogenesis and virulence in young and aged mouse models of human disease. J. Virol. 2012, 86, 884–897. [Google Scholar] [CrossRef]
- Dinnon, K.H.; Leist, S.R.; Schafer, A.; Edwards, C.E.; Martinez, D.R.; Montgomery, S.A.; West, A.; Yount, B.L.; Hou, Y.J.; Adams, L.E.; et al. A mouse-adapted SARS-CoV-2 model for the evaluation of COVID-19 medical countermeasures. bioRxiv 2020. [Google Scholar] [CrossRef]
- Masters, P.S.; Koetzner, C.A.; Kerr, C.A.; Heo, Y. Optimization of targeted RNA recombination and mapping of a novel nucleocapsid gene mutation in the coronavirus mouse hepatitis virus. J. Virol. 1994, 68, 328–337. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).