Abstract
Salix babylonica L. is a popular ornamental tree species in China and widely cultivated in Asia, Europe, and North America. Anthracnose in S. babylonica poses a serious threat to its growth and reduces its medicinal properties. In 2021, a total of 55 Colletotrichum isolates were isolated from symptomatic leaves in three provinces in China. Phylogenetic analyses using six loci (ITS, ACT, CHS-1, TUB2, CAL, and GAPDH) and a morphological characterization of the 55 isolates showed that they belonged to four species of Colletotrichum, including C. aenigma, C. fructicola, C. gloeosporioides s.s., and C. siamense. Among them, C. siamense was the dominant species, and C. gloeosporioides s.s. was occasionally discovered from the host tissues. Pathogenicity tests revealed that all the isolates of the aforementioned species were pathogenic to the host, and there were significant differences in pathogenicity or virulence among these isolates. The information on the diversity of Colletotrichum spp. that causes S. babylonica anthracnose in China is new.
1. Introduction
Colletotrichum spp. are ones of the most important plant pathogens, saprobes and endophytes genera worldwide [,,]. The fungal genus of Colletotrichum consists of 14 species or species complexes [,,,,,]. Colletotrichum pathogens often cause damage to roots, stems, leaves, flowers, fruits, and seedlings of trees, fruit trees, vegetables, flowers, medicinal plants, and field crops and can lead to plant wilting, anthracnose, fruit rot, leaf lesions, and other symptoms, causing serious economic losses [,]. Many species of Colletotrichum not only affect a wide range of host plants, but also have direct implications for human health [,,]. Therefore, their accurate identification is critical because species differ in pathogenicity, fungicide sensitivity, and other factors affecting disease management in nurseries and seed orchards []. The taxonomy of the Colletotrichum species is quite complex []. The morphological identification of Colletotrichum species has long been difficult due to the plasticity of their morphological characteristics [,]. DNA sequences for identifying fungi are useful []. The internal transcribed spacer (ITS) region has been used as a barcoding locus for identifying fungi [,]. However, erroneous fungal identifications using ITS sequences have occurred [,]. Thus, it is difficult to identify fungi solely by the ITS region [,]. Therefore, in addition to the ITS region, other loci, such as ACT (actin), CAL (calmodulin), CHS-1 (chitin synthase), GAPDH (glyceraldehyde-3-phosphate dehydrogenase), and TUB2 (β-tubulin), have been applied to distinguish Colletotrichum species [,,,]. At present, multi-locus sequence data are widely used in the identification of Colletotrichum species [,,,,,].
Salix babylonica L. (Salicaceae) is distributed mostly in the northern hemisphere []. Since S. babylonica has a high ornamental value with its slender and graceful branches, it is widely planted by rivers and roadsides [,,]. Salix babylonica also possesses a wide range of ecological characteristics, such as being easy to propagate, having a strong adaptability, and absorbing harmful gases, etc. [,,,,]. In terms of utilization, S. babylonica has been increasingly employed in environmental restoration work and has shown promise for biofuel production and the phytoremediation of soil [,,]. In addition, S. babylonica has an important medicinal value, the bark has astringent and tonic properties, and young twigs and catkins are antipyretic [,]. Modern medical research shows that the leaves of S. babylonica have good medicinal properties, such as relieving heat/fever, reducing inflammation, and detoxification []. However, S. babylonica is susceptible to diseases caused by phytopathogenic fungi. Anthracnose is one of the main diseases in S. babylonica. At the early stage of an anthracnos infection, there are small circular black spots on the leaves, which become irregular large spots. Finally, the whole leaf will wither. In 1997, anthracnose in S. babylonica was first reported in Greece []. The disease caused trees to lose their leaves repeatedly and seriously affected the ornamental value of the hosts. However, the morphological characteristics and taxonomy of Colletotrichum pathogens on S. babylonica have not been studied in detail.
From June to October 2021, anthracnose in S. babylonica occurred in three provinces in China. Therefore, this research study aimed to identify the Colletotrichum species causing anthracnose in S. babylonica based on morphological characteristics and multi-locus phylogenetic analyses and to determine the pathogenicity of the isolates with Koch’s postulates.
2. Results
2.1. Field Symptoms and Fungal Isolation
Anthracnose in S. babylonica was usually observed between June and October every year. The symptoms began as dark brown, irregular spots, and the centers were grayish white (Figure 1a–c). The spots gradually enlarged with time. Eventually the leaves withered and defoliated. Orange conidial masses often developed after the leaves were incubated in Petri dishes for 24 h with a high humidity (Figure 1d).

Figure 1.
Symptoms of Salix babylonica anthracnose in the field. (a–c) Diseased leaves infected naturally. (d) Orange conidial masses after the leaves were incubated for 24 h under moist conditions. Scale bars: (c) = 1 cm; (d) = 500 µm.
In this study, a total of six diseased sample batches were collected from six areas in the three provinces of China (Table 1). Thirty leaves were collected for each sample batch. A total of 55 Colletotrichum isolates were isolated according to their colony morphology on PDA and the ITS sequence data. Among these isolates, 12 isolates were from Suzhou, 10 isolates from Zibo, 10 isolates from Wuhan, and 23 isolates from Nanjing. Based on their ITS sequence data and colony characteristics on PDA, the isolates were divided into four types. Of these, 17 representative isolates were selected for further study and were sent to the China Forestry Culture Collection Center (CFCC).

Table 1.
The sample list of Colletotrichum isolates collected from Salix babylonica in China.
2.2. Multi-Locus Phylogenetic Analyses
Seventeen representative isolates of Colletotrichum from different areas were selected for sequencing and analyses. The BLAST result of the ITS sequences showed that the 17 isolates belonged to the C. gloeosporioides species complex. They were analyzed using multi-locus sequences (ITS, ACT, CHS-1, TUB2, CAL, and GAPDH) and compared with 42 isolates of Colletotrichum (23 species), and C. boninense (CBS 123755) was used as the outgroup. A maximum likelihood estimation and Bayesian inference analyses with the concatenated sequences (ITS, ACT, CHS-1, TUB2, CAL, and GAPDH) identified the 17 isolates as C. aenigma, C. fructicola, C. gloeosporioides s.s., and C. siamense (Figure 2). Among these isolates, three isolates (HQ2-1, HQ2-6, and WH2-9) were in the same clade with C. aenigma with a bootstrap support value of 100; two isolates (SD1-6 and SD1-9) were in the same clade with C. fructicola with a bootstrap support value of 99; three isolates (WH2-4, NL1-7, and MXL1-7) were in the same clade with C. gloeosporioides s.s. with a bootstrap support value of 75; and nine isolates (YH2-2, YH2-3, YH2-5, YH2-6, WH2-7, NL1-10, NL1-13, MXL1-1, and MXL1-10) were grouped with C. siamense with high support values (ML/BI = 95/1).
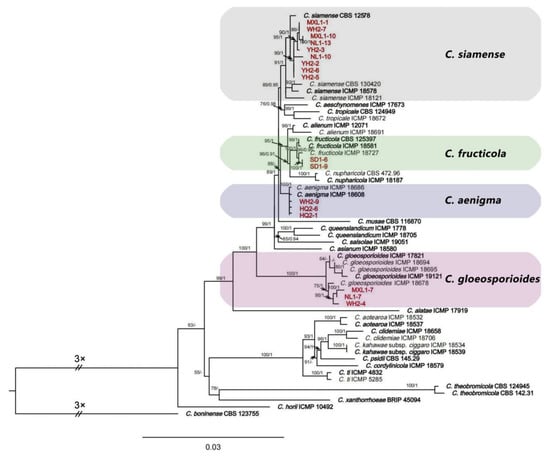
Figure 2.
Phylogenetic relationship of Colletotrichum isolates (YH2-2, YH2-3, YH2-5, YH2-6, HQ2-1, HQ2-6, WH2-4, WH2-7, WH2-9, SD1-6, SD1-9, NL1-7, NL1-10, NL1-13, MXL1-1, MXL1-7, MXL1-10) from Salix babylonica with related taxa derived from the concatenated sequences of ITS, ACT, CHS-1, TUB2, CAL, and GAPDH loci using a maximum likelihood estimation and Bayesian inference analyses. Bootstrap support values (ML ≥ 50) and Bayesian posterior probability (PP ≥ 0.90) are shown at the nodes (ML/PP). Colletotrichum boninense (CBS 123755) is an outgroup. Bar = 0.03 substitutions per nucleotide position. Bold indicates ex-types. The red color text indicates strains of this study.
2.3. Morphological Study
Based on the results of the phylogenetic analyses, the 17 Colletotrichum isolates characterized in this study belonged to four species: C. aenigma (three isolates), C. fructicola (three isolates), C. gloeosporioides (two isolates), and C. siamense (nine isolates). Representative isolates from each Colletotrichum species were selected to carry out detailed morphological descriptions.
2.3.1. Colletotrichum aenigma B. Weir and P.R. Johnst (Figure 3)
The colonies were white, and the aerial mycelium was white, dense, and cottony. In contrast to the colonies, the center was gray, and the margin was white. Orange conidial masses and ascomata were observed in the colonies. The colony growth rate on PDA was 13.2 mm/d. The acervuli were orange, elliptic, numerous, and pale to dark grey at the base. The conidiophores were hyaline to pale brown, smooth, septate, and sometimes branched. The conidiogenous cells were hyaline, cylindrical to ampulliform, smooth, thin-walled, (7.4–) 11.7–21.1 (–24.5) × (3.3–) 3.3–4.1 (–4.7) µm (mean ± SD = 16.4 ± 4.7 × 3.7 ± 0.4 µm (n = 30)), and with an L/W ratio = 4.4. The conidia were hyaline, aseptate, smooth, straight, subcylindrical, (12.6–) 15.1–16.3 (–16.7) × (4.7–) 5.3–6.1 (–6.3) µm (mean ± SD = 15.7 ± 0.6 × 5.7 ± 0.4 µm (n = 50)), with an L/W ratio = 2.7, and with a rounded end. The ascomata were brown to black, globose, and clustered. The asci were hyaline, clavate or fusiform, smooth, eight-spored, (52.5–) 58.1–71.5 (–78.5) × (7.4–) 10.8–13.8 (–16.4) µm (mean ± SD = 64.8 ± 6.7 × 12.3 ± 1.5 µm (n = 30)), and with an L/W ratio = 5.3. The ascospores were hyaline, aseptate, smooth, subcylindrical or ellipsoidal, slightly curved, uniseriate or biseriate, (14.5–) 16.5–20.7 (–20.3) × (3.9–) 4.2–5.2 (–5.4) µm (mean ± SD = 18.6 ± 2.1 × 4.7 ± 0.5 µm (n = 50)), and with an L/W ratio = 4.0. The appressoria were one-celled, ovoid or ellipsoidal, brown or dark brown, smooth, (6.5–) 7.3–9.1 (–9.8) × 4.6–7.0 (–7.0) µm (mean ± SD = 8.2 ± 0.9 × 5.8 ± 1.2 µm (n = 50)), and with an L/W ratio = 1.4.
The specimens examined were as follows: (1) China, Hubei Province: Wuhan City, 30°43′10″ N, 114°31′59″ E, on the leaves of Salix babylonica, October 2021, Mengyu Zhang, culture WH2-9; (2) and China, Jiangsu Province: Suzhou City, 31°20′34″ N, 120°35′18″ E, on the leaves of S. babylonica, June 2021, Mengyu Zhang, cultures HQ2-1 and HQ2-6.
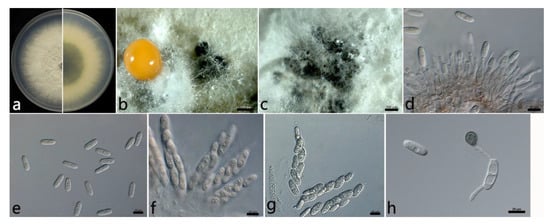
Figure 3.
The morphological characteristics of Colletotrichum aenigma (WH2-9) isolated from anthracnose leaves of Salix babylonica. (a) Colony on PDA from above and below (5 d). (b) Conidial mass and ascomata (on PDA). (c) Ascomata (on PDA). (d) Conidiophores, conidiogenous cells, and conidia. (e) Conidia. (f) Asci and ascospores. (g) Ascospores. (h) Conidia and appressorium. Scale bars: (b,c) = 500 µm; (d–h) = 10 µm.
2.3.2. Colletotrichum fructicola Prihastuti, L. Cai and K.D. Hyde (Figure 4)
The aerial mycelium was white to gray, dense, and cottony. In contrast to the colonies, the center was dark green, and the margin was white. Orange conidial masses and ascomata were observed in the center of the colonies. The colony growth rate on PDA was 13.6 mm/d. The acervuli were orange, elliptic, few, and pale to dark grey at the base. The conidiophores were hyaline to pale brown, smooth, septate, and sometimes branched. The conidiogenous cells were cylindrical to flask-shaped, hyaline, tapering towards the apex, smooth, thin-walled, (10.5–) 12.4–17.6 (–22.5) × (2.6–) 3.1–3.9 (–4.2) µm (mean ± SD = 15.0 ± 2.6 × 3.5 ± 0.4 µm (n = 50)), and with an L/W ratio = 4.3. The conidia were one-celled, aseptate, straight, subcylindrical, hyaline, (10.8–) 12.5–15.7 (–17.2) × (4.6–) 5.5–7.3 (–8.3) µm (mean ± SD = 14.1 ± 1.6 × 6.4 ± 0.9 µm (n = 50)), with an L/W ratio = 2.2, and with a rounded end. The ascomata were brown to black, round, and in clusters. The asci were hyaline, clavate, smooth, eight-spored, (40.3–) 40.5–52.7 (–55.0) × (8.1–) 8.6–11.4 (–13.0) µm (mean ± SD = 46.6 ± 6.1 × 10.0 ± 1.4 µm (n = 30)), and with an L/W ratio = 4.6. The ascospores were hyaline, aseptate, smooth, allantoid or ellipsoidal, curved, biseriate, (14.1–) 16.3–19.7 (–20.6) × (4.1–) 4.4–5.2 (–5.3) µm (mean ± SD = 18.0 ± 1.7 × 4.8 ± 0.4 µm (n = 50)), and with an L/W ratio = 3.8. The appressoria were one-celled, ovoid or ellipsoidal, brown or dark brown, smooth, (6.9–) 7.9–10.7 (–11.6) × (5.5–) 6.0–8.0 (–9.1) µm (mean ± SD = 9.3 ± 1.4 × 7.0 ± 1.0 µm (n = 50)), and with an L/W ratio = 1.3.
The specimens examined were as follows: China, Shandong Province: Zibo City, 36°37′58″ N, 117°53′43″ E, on the leaves of Salix babylonica, September 2021, Mengyu Zhang, cultures SD1-6 and SD1-9.
Notes: In this study, the conidia (12.5–15.7 × 5.5–7.3) and appressoria (7.9–10.7 × 6.0–8.0 (–9.1) µm) of the C. fructicola isolates were larger than those of the ex-type (ICMP 18581: 10.5–12.6 × 3.2–3.9 (–4.3) µm and 6.1–8.6 × 3.6–5.4 µm), respectively. For the sexual stage, the asci (40.5–52.7 × 8.6–11.4 µm) and ascospores (16.3–19.7 × 4.4–5.2 µm) were also larger than those of the ex-type (ICMP 18581: 34.2–48.2 × 7.0–8.2 µm and 10.5–13.3 × 3.0–3.7 (–4.0) µm), respectively. In the study by Prihastuti et al. [], C. fructicola did not develop acervuli in PDA culture, but it developed acervuli in PDA in the present study (Figure 4b). The differences in morphology could be due to different hosts and should be further studied in the future.

Figure 4.
The morphological characteristics of Colletotrichum fructicola (SD1-6) isolated from anthracnose leaves of Salix babylonica. (a) Colony on PDA from above and below (5 d). (b) Conidial mass (on PDA). (c) Ascomata (on PDA). (d) Conidiophores, conidiogenous cells, and conidia. (e) Conidia. (f) Ascus. (g) Ascospores. (h) Conidia and appressoria. Scale bars: (b,c) = 500 µm; (d–h) = 10 µm.
2.3.3. Colletotrichum gloeosporioides s.s. (Penz.) Penz. and Sacc (Figure 5)
The colonies on the PDA were white to grayish white at the center; in contrast, the center was dark green, and the margin was white. The aerial mycelium was white, dense, and cottony with a growth rate of 14.2 mm/d. Orange conidial masses were often observed in the center of the colonies. The acervuli were orange, elliptic, numerous, and pale to dark grey at the base. The conidiophores were hyaline to pale brown, smooth, septate, and rarely branched. The conidiogenous cells were cylindrical to flask-shaped, hyaline, tapering towards the apex, smooth, thin-walled, (6.5–) 9.4–18.8 (–21.0) × (3.3–) 3.4–4.2 (–4.5) µm (mean ± SD = 14.1 ± 4.7 × 3.8 ± 0.4 µm (n = 50)), and with an L/W ratio = 3.7. The conidia were hyaline, one-celled, aseptate, straight, subcylindrical with rounded ends, (12.1–) 14.0–16.0 (–16.9) × (5.7–) 6.2–7.0 (–7.3) µm (mean ± SD = 15.0 ± 1.0 × 6.6 ± 0.4 µm (n = 50)), and with an L/W ratio = 2.3. The appressoria were one-celled, ovoid or ellipsoidal, brown or dark brown, smooth, (7.2–) 7.8–9.6 (–10.7) × (5.9–) 5.8–7.2 (–8.4) µm (mean ± SD = 8.7 ± 0.9 × 6.5 ± 0.7 µm (n = 50)), and with an L/W ratio = 1.3.
The specimens examined were as follows: China, Jiangsu Province: Nanjing City, 32°5′10″ N, 118°49′13″ E and 32°3′2″ N, 118°50′26″ E, on the leaves of Salix babylonica, October 2021, Mengyu Zhang, cultures NL1-7 and MXL1-7; and China, Hubei Province: Wuhan City, 30°43′10″ N, 114°31′59″ E, on the leaves of S. babylonica, October 2021, Mengyu Zhang, culture WH2-4.
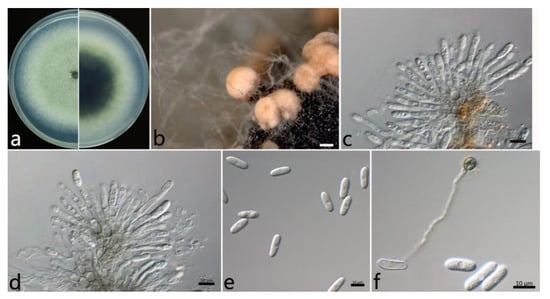
Figure 5.
The morphological characteristics of Colletotrichum gloeosporioides s.s. (NL1-7) isolated from anthracnose leaves of Salix babylonica. (a) Colony on PDA from above and below (5 d). (b) Conidial masses (on PDA). (c,d) Conidiophores, conidiogenous cells, and conidia. (e) Conidia. (f) Conidia and appressorium. Scale bars: (b) = 500 µm; (c,f) = 10 µm.
2.3.4. Colletotrichum siamense Prihastuti, L. Cai and K. D. Hyde (Figure 6)
The colonies on PDA were white to grayish white at the center. The aerial mycelium was abundant and cottony. Orange conidial masses were in the center of the colonies. The colony growth rate on PDA was 14.8 mm/d. The acervuli were orange, spherical or elliptical, numerous, and pale to dark grey at the base. The setae were dark brown, with two to three septates, thick-walled, straight, in groups, tapering toward the apices, and (85.4–) 75.9–111.1 (–117.6) µm (mean ± SD = 93.5 ± 17.6 μm (n = 30)). The conidiophores were hyaline to pale brown, septate, and branched. The conidiogenous cells were phialidic, hyaline, thin-walled, smooth, (9.6–) 10.8–17.4 (–20.0) × (2.3–) 2.9–3.9 (–4.6) µm (mean ± SD = 14.1 ± 3.3 × 3.4 ± 0.5 µm (n = 50)), and with an L/W ratio = 4.2. The conidia were one-celled, straight, subcylindrical, hyaline with a rounded end, (11.5–) 13.8–15.8 (–16.5) × (5.4–) 6.2–7.0 (–7.5) µm (mean ± SD = 14.8 ± 1.0 × 6.6 ± 0.4 µm (n = 50)), and with an L/W ratio = 2.3. The appressoria were one-celled, ovoid or ellipsoidal, brown or dark brown, smooth, (6.7–) 7.1–8.7 (–10.1) × (5.3–) 5.9–6.7 (–7.1) µm (mean ± SD = 7.9 ± 0.8 × 6.3 ± 0.4 µm (n = 50)), and with an L/W ratio = 1.3.
The specimens examined were as follows: China, Jiangsu Province: Suzhou City, 31°20′34″ N, 120°35′18″ E, on the leaves of Salix babylonica, June 2021, Mengyu Zhang, cultures YH2-2, YH2-3, YH2-5, and YH2-6; Nanjing City, 32°5′10″ N, 118°49′13″ E, and 32°3′2″ N, 118°50′26″ E, on the leaves of S. babylonica, October 2021, Mengyu Zhang, cultures NL1-10, NL1-13, MXL1-1, and MXL1-10; and China, Hubei Province: Wuhan City, 30°43′10″ N, 114°31′59″ E, on the leaves of S. babylonica, October 2021, Mengyu Zhang, culture WH2-7.
Notes: The ITS, CHS, and TUB sequences do not separate C. siamense from C. fructicola. However, these species are best distinguished using CAL sequencing and a multi-locus analysis. Colletotrichum siamense was first reported on the berries of Coffea arabica in Thailand []. Most previous studies have had difficulties distinguishing among C. siamense, C. jasmini-sambac, and C. hymenocallidis within the C. gloeosporioides complex [,]. However, later on, C. jasmini-sambac and C. hymenocallidis were demoted as synonyms of C. siamense [].
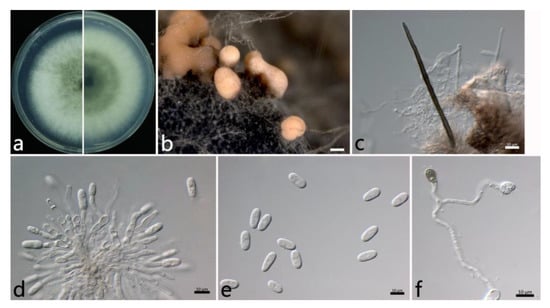
Figure 6.
The morphological characteristics of Colletotrichum siamense (NL1-13) isolated from anthracnose leaves of Salix babylonica. (a) Colony on PDA from above and below (5 d). (b) Conidial masses (on PDA). (c) Seta. (d) Conidiophores, conidiogenous cells, and conidia. (e) Conidia. (f) appressoria. Scale bars: (b) = 200 µm; (c–f) = 10 µm.
2.4. Pathogenicity Tests
At 7 dpi, 17 representative isolates of the four Colletotrichum species developed dark brown lesion symptoms of anthracnose on the leaves of S. babylonica inoculated by a spore suspension. The infection incidence was 100%. No lesions were observed on the leaves of the control plants (Figure 7). However, different isolates had different levels of virulence, resulting in different lesions sizes. Among them, four out of the nine isolates of C. siamense had the most virulence, and C. aenigma had the least virulence (Table 2). The virulence within the same species of C. siamense and C. gloeosporioides s. s. varied significantly. The fungus was re-isolated from the infected tissues, and the morphology of the colony and the ITS sequence data matched the inocula. No fungi were isolated from the control leaves. The re-isolation rate was 100%. Thus, all 17 isolates were pathogens of anthracnose in S. babylonica.
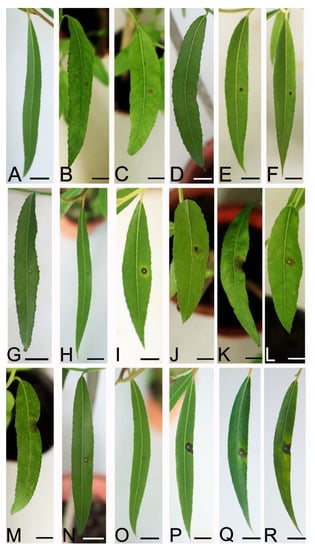
Figure 7.
Symptoms on the leaves of Salix babylonica seedlings on day 7 after inoculation with conidial suspensions. (A) Control. (B–D) isolates HQ2-1, HQ2-6, and WH2-9 (Colletotrichum aenigma). (E,F) isolates SD1-6 and SD1-9 (C. fructicola). (G–I) isolates WH2-4, NL1-7, and MXL1-7 (C. gloeosporioides). (J–R) isolates YH2-2, YH2-3, YH2-5, YH2-6, WH2-7, NL1-10, NL1-13, MXL1-1, and MXL1-10 (C. siamense). Scale bars = 1 cm.

Table 2.
The infection severity of representative Colletotrichum isolates on leaves of Salix babylonica.
3. Discussion
Salix babylonica is endemic in China and has a high ornamental value. Recently, anthracnose in S. babylonica has been discovered, seriously affecting the ecological value of S. babylonica. The identification of fungal pathogens is the most important first step for disease management []. In this study, we collected 55 isolates from six regions in three provinces where S. babylonica is grown and identified four known species of Colletotrichum.
Current identification systems for Colletotrichum species have included traditional morphological features, molecular phylogeny, and other traits []. However, these morphological features show plasticity under different conditions of growth (host, media, temperature, light regime, etc.), and some can be lost or change with repeated subculturing []. The conidia and ascospores developed on S. babylonica in this study are larger than those of the ex-type of C. fructicola (ICMP 18581) from Coffea arabica. Our morphological analyses also showed that the Colletotrichum species had the same sexual state characteristics under the same conditions. For example, C. fructicola and C. aenigma tend to develop asci and ascospores on PDA, resulting in the coexistence of sexual and asexual states. Thus, the identification of fungal pathogens in plants includes not only morphology but also multi-locus phylogenetic analyses [,]. For instance, Wang et al. [] used three DNA sequences of ITS, TUB2, and TEF1-α to confirm a Pestalotiopsis-like species causing gray blight disease in tea plants in China. Poudel et al. [] used ITS sequences to identify Erysiphe fallax causing powdery mildew on phasey beans in the United States. In this study, concatenated sequences of ITS, ACT, CHS-1, TUB2, CAL, and GAPDH were used to construct phylogenetic trees, and we identified the 17 isolates to be C. aenigma, C. fructicola, C. gloeosporioides s.s., and C. siamense.
The pathogenicity tests indicated pathogenic differences among the four species. Colletotrichum siamense had the highest virulence. In this study, C. siamense had the fastest colony growth rate on PDA, and correspondingly, it showed the highest virulence in the pathogenicity test. Secondly, the appressoria of C. siamense germinated easily. Colletotrichum aenigma had the slowest colony growth rate and showed the least virulence. The results indicated that the pathogenicity of the isolates was closely related to the colony growth rate and the appressorial germination rate. Colletotrichum siamense is an important pathogen that can infect many trees and fruits. For instance, C. siamense has been shown to cause anthracnose in pears, a number of host species in Proteaceae, and Cunninghamia lanceolata [,,]. Colletotrichum fructicola was first reported in coffee berries from Thailand [] and was later reported in Pyrus pyrifolia in Japan []. Subsequently, this species was widely recognized as the pathogen that caused pear anthracnose []. However, it can also infect other fruits, for instance, Averrhoa carambola, Prunus sibirica, and Amygdalus persica [,,].
Based on pathogenicity test, C. aenigma, C. fructicola, C. gloeosporioides s.s., and C. siamense were identified as the pathogens of anthracnose in S. babylonica. Of them, C. siamense was the dominant species, and C. gloeosporioides s.s. was occasionally discovered from the host tissues. All of the isolates belong to the C. gloeosporioides species complex. The difference in the dominant species in the six regions may be due to different geographical locations, climates, host varieties, host health conditions, planting methods, and collection times []. Actually, many reports have shown that a host plant can be infected by several different Colletotrichum species. For example, chili is reported to be infected by C. fioriniae, C. fructicola, C. gloeosporioides s.s., C. scovillei, etc. []. Anthracnose in mango is caused by C. asianum, C. fructicola, C. siamense, C. tropicale, etc. []. Therefore, further studies are required to identify the host range and distribution of different Colletotrichum species.
It has been reported that C. siamense, C. gloeosporioides s.s., and C. acutatum can infect S. babylonica [,], but this study proved that C. fructicola and C. aenigma can also infect the leaves of S. babylonica. It is uncertain whether other Colletotrichum species can cause anthracnose in S. babylonica; extensive sampling in all distribution areas is required. In addition, the sensitivity of different Colletotrichum species to fungicides needs to be further studied. This is the first report on the diversity of Colletotrichum species associated with S. babylonica anthracnose worldwide. For controlling S. babylonica anthracnose effectively, these data will help us to select appropriate strategies for managing this disease.
4. Materials and Methods
4.1. Sample Collection and Fungi Isolation
From June to October 2021, the symptoms and pathogenesis of anthracnose in S. babylonica in different areas were assessed. Leaves with typical symptoms of anthracnose were randomly collected from six areas in three provinces (Jiangsu, Shandong, Hubei), China, and the samples (10 leaves/tree) were collected from three trees in each region. The samples were rinsed with running water for 10 min and dried in sterilized Petri dishes []. Small pieces of infected tissue (3–4 mm2) were surface-sterilized in 75% ethanol for 30 s followed by 1% NaClO for 90 s, rinsed three times in sterile water, dried on sterilized filter paper, plated on potato dextrose agar (PDA), and incubated at 25 °C in the dark [,]. Fungal growth was checked daily. Pure cultures were obtained by cutting hyphal tips and the monosporic isolation method []. All isolates were transferred to fresh PDA plates. The representative isolates were selected for further analyses and were sent to the China Forestry Culture Collection Center (CFCC).
4.2. DNA Extraction, PCR Amplification, and Sequencing
In order to obtain the genomic DNA of the strains, mycelium was harvested from colonies of fungal strains grown on PDA after 5 days of incubation at 25℃. Genomic DNA of 55 strains was extracted using the cetyltrimethylammonium bromide (CTAB) protocol []. Polymerase chain reaction (PCR) amplification was carried out on the extracted DNA. The internal transcribed spacer region (ITS), actin (ACT), chitin synthase (CHS-1), β-tubulin 2 (TUB2), calmodulin (CAL), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) loci were amplified using the primer pairs ITS1/ITS4 [], ACT-512F/ACT-783R [], CHS-79F/CHS-354R [], T1/Bt2b [,], CL1C/CL2C [], and GDF1/GDR1 [], respectively (details of primers are given in Table 3). PCR mixture was performed in a total volume of 50 μL, containing 25 μL 2 × Taq Plus Master Mix, 19 μL double-distilled water, 2 μL primer-F, 2 μL primer-R, and 2 μL genomic DNA. The PCR conditions for ITS were 3 min at 94 °C; 30 cycles at 94 °C for 30 s; a 30 s cycle at 55 °C; a 45 s cycle at 72 °C; and then 10 min at 72 °C. The most suitable annealing temperatures differed for the other genes: ACT: 58 °C, CHS-1: 58 °C, TUB2: 55 °C, CAL: 55 °C, and GAPDH: 58 °C. For DNA sequencing, the PCR products were sent to Shanghai Sangon Biotechnology Co., Ltd., Shanghai, China.

Table 3.
PCR primers used for molecular characterization of Colletotrichum isolates.
4.3. Phylogenetic Analyses
The ITS, ACT, CHS-1, TUB2, CAL, and GAPDH sequences with high similarities to the genes/region sequences of Colletotrichum species in GenBank using BLAST were selected, and in total the sequences of 42 Colletotrichum isolates (23 species) were obtained from GenBank for phylogenetic analyses (Table 4). The sequences of Colletotrichum boninense (CBS 123755) were used as an outgroup. Nucleotide sequences of each gene/region of the selected isolates were aligned by the MAFFT ver. 7.313 []. The aligned sequences were edited using BioEdit version 7.0.9.0 []. Six locus sequences (ITS, ACT, CHS-1, TUB2, CAL, and GAPDH) were concatenated by PhyloSuite software []. After selecting the best model with ModelFinder [], phylogenetic relationships were inferred using maximum likelihood (ML) estimation and Bayesian inference (BI). The ML analysis employed IQtree ver. 1.6.8 using the GTR+F+I+G4 model, with the bootstrapping method of 1000 replicates [,]. A bootstrap posed statistical support at ≥50%. BI analysis used the GTR+I+G+F model by MrBayes ver. 3.2.6, including 2 parallel runs and 2,000,000 generations []. Branches that received Bayesian posterior probabilities of 0.90 (BPP) were set as significantly supported. Phylogenetic trees were constructed with FigTree ver. 1.4.4.

Table 4.
A list of isolates of Colletotrichum spp. collected from Salix babylonica leaves in China as well as related taxa/isolates and their sequences used in this study.
4.4. Morphological Study
Morphological examinations focused on the colony characteristics, acervuli, conidiophores, conidiogenous cells, conidia, setae, appressoria, ascomata, asci, and ascospores of representative isolates that were randomly selected from each Colletotrichum species. Mycelial plugs (5 mm diam) from the margin of cultures were transferred to PDA and incubated at 25 °C in the dark. Colony characteristics were photographed with a Canon EOS M50 Mark II camera after 4 d, and colony diameters were measured daily to calculate the mycelial growth rates (mm/d). In order to induce appressorium formation, 10 µL of conidial suspension (106 conidia/mL) was placed on a slide, placed inside plates containing a piece of moistened filter paper with sterile water, and then incubated at 25 °C in dark []. Measurements and morphological descriptions of acervuli, conidiophores, conidiogenous cells, conidia, setae, appressoria, ascomata, asci, and ascospores of the representative isolates were observed using a Zeiss Axio Imager A2m microscope (Carl Zeiss Microscopy, Oberkochen, Germany). Fifty individuals of per structure were measured for each isolate.
4.5. Pathogenicity Tests
Seventeen representative isolates of four Colletotrichum species were used for pathogenicity tests. Healthy 2-yr-old seedlings with 10 leaves per seedling were wound with a sterile needle and inoculated with conidial suspensions (106 conidia/mL) in each leaf. The conidial suspensions were sprayed onto the wound. Control plants were treated with sterile water in the same way. Seedlings were covered with plastic bags after inoculation and maintained in a greenhouse at 25 ± 2 °C and 80% RH for seven days. The experiments were conducted three times, and each treatment had three replicates. Eventually 54 seedlings were used. Seven days after inoculation, the diameter of the lesion on the leaves was measured and the inoculated leaves were used for re-isolation.
Author Contributions
Conceptualization, L.Z.; methodology, M.Z., Y.S. and Y.J.; software, M.Z.; validation, M.Z.; formal analysis, M.Z.; investigation, M.Z. and Y.S.; resources, M.Z.; data curation, D.L.; writing—original draft preparation, M.Z.; writing—review and editing, D.L. and L.Z.; visualization, M.Z.; supervision, L.Z.; project administration, D.L. and L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R & D Program of China (2022YFD1401005), and the National Natural Science Foundation of China (grant number 31971659).
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Acknowledgments
The authors would like to thank those who provided assistance and advice for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum-current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.Z.; Zhang, C.; Liu, F.; Wang, W.Z.; Liu, L.; Cai, L.; Liu, X.L. Colletotrichum species causing anthracnose disease of chili in China. Persoonia 2017, 38, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Woudenberg, J.; Johnston, P.R.; Weir, B.S.; Tan, Y.P.; Shivas, R.G.; Crous, P.W. The Colletotrichum boninense species complex. Stud. Mycol. 2012, 73, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; Cannon, P.F.; Liu, F.; Barreto, R.W.; Guatimosim, E.; Crous, P.W. The Colletotrichum orbiculare species complex: Important pathogens of field crops and weeds. Fungal Divers. 2013, 61, 29–59. [Google Scholar] [CrossRef]
- Damm, U.; O’Connell, R.J.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum destructivum species complex—Hemibiotrophic pathogens of forage and field crops. Stud. Mycol. 2014, 79, 49–84. [Google Scholar] [CrossRef]
- Crouch, J.A. Colletotrichum caudatum s.l. is a species complex. IMA Fungus 2014, 5, 17–30. [Google Scholar] [CrossRef]
- Hyde, K.D.; Cai, L.; Cannon, P.F.; Crouch, J.A.; Crous, P.W.; Damm, U.; Goodwin, P.H.; Chen, H.; Johnston, P.R.; Jones, E.B.G.; et al. Colletotrichum-names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar]
- Cai, L.; Hyde, K.; Taylor, P.; Weir, B.; Waller, J.; Abang, M.; Zhang, J.Z.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y.; et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Liu, L.P.; Gao, J.; Li, Y. Research progress of plant Colletotrichum spp. J. Fungal Res. 2020, 18, 266–281. [Google Scholar]
- Sharma, G.; Shenoy, B.D. Colletotrichum systematics: Past, present and prospects. Mycosphere 2016, 7, 1093–1102. [Google Scholar] [CrossRef]
- Werbel, W.A.; Baroncelli, R.; Shoham, S.; Zhang, S.X. Angioinvasive, cutaneous infection due to Colletotrichum siamense in a stem cell transplant recipient: Report and review of prior cases. Transpl. Infect. Dis. 2019, 21, e13153. [Google Scholar] [CrossRef]
- Marcelino, J.A.; Gouli, S.; Parker, B.L.; Skinner, M.; Giordano, R. Entomopathogenic activity of a variety of the fungus, Colletotrichum acutatum, recovered from the elongate hemlock scale, Fiorinia externa. J. Insect Sci. 2009, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M.J.; Edwards, S.; Inocencio, H.A.; Machado, F.J.; Nuckles, E.M.; Farman, M.; Gauthier, N.A.; Vaillancourt, L.J. Diversity and cross-infection potential of Colletotrichum causing fruit rots in mixed-fruit orchards in Kentucky. Plant Dis. 2021, 105, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Manova, V.; Stoyanova, Z.; Rodeva, R.; Boycheva, I.; Korpelainen, H.; Vesterinen, E.; Wirta, H.; Bonchev, G. Morphological, pathological, and genetic diversity of Colletotrichum species pathogens on solanaceous vegetable crops in Bulgaria. J. Fungi 2022, 8, 1123. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Cai, L.; McKenzie, E.H.C.; Yang, Y.L.; Zhang, J.Z.; Prihastuti, H. Colletotrichum: A catalogue of confusion. Fungal Divers. 2009, 39, 1–17. [Google Scholar]
- Seifert, K.A. Progress towards DNA barcoding of fungi. Mol. Ecol. Resour. 2010, 9 (Suppl. 1), 83–89. [Google Scholar] [CrossRef]
- Mills, P.R.; Sreenivasaprasad, S.; Brown, A.E. Detection and differentiation of Colletotrichum gloeosporioides isolates using PCR. FEMS Microbiol. Lett. 1992, 98, 137–143. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Crouch, J.A.; Clarke, B.B.; Hillman, B.I. What is the value of ITS sequence data in Colletotrichum systematics and species diagnosis? A case study using the falcate-spored graminicolous Colletotrichum group. Mycologia 2009, 101, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Stielow, J.B.; Levesque, C.A.; Seifert, K.A.; Meyer, W.; Irinyi, L.; Smits, D.; Renfurm, R.; Verkley, G.J.M.; Groenewald, M.; Chaduli, D.; et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 2015, 35, 242–263. [Google Scholar] [CrossRef]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; Sato, T.; Alizadeh, A.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud. Mycol. 2019, 92, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Damm, U.; Cai, L.; Crous, P.W. Species of the Colletotrichum gloeosporioides complex associated with anthracnose diseases of Proteaceae. Fungal Divers. 2013, 61, 89–105. [Google Scholar] [CrossRef]
- Liu, F.; Cai, L.; Crous, P.W.; Damm, U. The Colletotrichum gigasporum species complex. Persoonia 2014, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, V.; Groenewald, J.Z.; Polizzi, G.; Crous, P.W. High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia 2017, 39, 32–50. [Google Scholar] [CrossRef]
- Huang, R.; Sun, W.X.; Wang, L.R.; Li, Q.L.; Huang, S.P.; Tang, L.H.; Guo, T.X.; Mo, J.Y.; Hsiang, T. Identification and characterization of Colletotrichum species associated with anthracnose disease of banana. Plant Pathol. 2021, 70, 1827–1837. [Google Scholar] [CrossRef]
- Argus, G.W. Infrageneric classification of Salix (Salicaceae) in the New World. Syst. Bot. Monogr. 1997, 52, 1–121. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.C.; Xie, H.C.; Zhang, S.Y. The effects of the phenol concentrations on photosynthetic parameters of Salix babylonica L. Photosynthetica 2015, 53, 430–435. [Google Scholar] [CrossRef]
- Chen, C.H.; Liu, Y.K.; Chen, G.C.; Shan, Q.H.; Zhang, J.F. Uptake kinetic characteristics of Cu2+ by Salix jiangsuensis CL J-172 and Salix babylonica Linn and the influence of organic acids. Acta Ecol. Sin. 2011, 31, 5255–5263. [Google Scholar]
- Zhang, M.Y.; Li, D.W.; Zhu, L.H. Leaf spots of Salix babylonica caused by Colletotrichum gloeosporioides s.s. and C. siamense newly reported in China. Plant Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Mirck, J.; Isebrands, J.G.; Verwijst, T.; Ledin, S. Development of short-rotation willow coppice system for environmental purposes in Sweden. Biomass Bioenergy 2005, 28, 219–228. [Google Scholar] [CrossRef]
- Yu, X.Z.; Trapp, S.; Zhou, P.H. Phytotoxicity of cyanide to weeping willow trees. Environ. Sci. Pollut. Res. 2005, 12, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Trapp, S.; Pirandello, A. Removal of cyanide by woody plants. Chemosphere 2004, 54, 325–333. [Google Scholar] [CrossRef]
- Yulla, A.K.; Martin, F. Willows beyond wetland: Use of Salix L. species for environmental projects. Water Air Soil Pollut. 2005, 162, 183–204. [Google Scholar]
- Perttu, K.L.; Kowalik, P.J. Salix vegetation filters for purification of water and soils. Biomass Bioenergy 1997, 12, 9–19. [Google Scholar] [CrossRef]
- Pulford, I.D.; Riddell-Black, D.; Stewart, C. Heavy metal uptake by willow clones from sewage sludge-treated soil: The potential for phytoremediation. Int. J. Phytoremed. 2002, 4, 59–72. [Google Scholar] [CrossRef]
- Singh, H.; Raturi, R.; Badoni, P.P. Isolation of secondary metabolites from the roots of Salix babylonica. In Proceedings of the International Conference on Materials, Alloys and Experimental Mechanics (ICMAEM), Secunderabad, India, 3–4 July 2017. [Google Scholar]
- Ganai, A.M.; Ahmad, H.A.; Matto, F.A. Nutritive value of Salix leaves for sheep. Indian Vet. J. 2006, 83, 895–896. [Google Scholar]
- Mu, D.; Liu, Z.K.; Tao, Y.; Zhou, L.Z.; Wang, J.Q.; Xu, W.W.; Luo, G.Q. Analysis of volatile compounds of Salix babylonica by GC-MS. J. Chin. Med. Mater. 2014, 37, 1001–1005. [Google Scholar]
- Tzavella-Klonari, K.; Aggelaki, M.D.; Karadimos, D.A. First report of anthracnose on weeping willow in Greece. Plant Dis. 1997, 81, 960. [Google Scholar] [CrossRef] [PubMed]
- Prihastuti, H.; Cai, L.; Chen, H.; McKenzie, E.H.; Hyde, K.D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009, 39, 89–109. [Google Scholar]
- Yang, Y.L.; Liu, Z.Y.; Cai, L.; Hyde, K.D.; Yu, Z.N.; McKenzie, E.H.C. Colletotrichum anthracnose of Amaryllidaceae. Fungal Divers. 2009, 39, 123–146. [Google Scholar]
- Wikee, S.; Cai, L.; Pairin, N.; McKenzie, E.H.C.; Su, Y.Y.; Chukeatirote, E.; Thi, H.N.; Bahkali, A.H.; Moslem, M.A.; Abdelsalam, K.; et al. Colletotrichum species from jasmine (Jasminum sambac). Fungal Divers. 2011, 46, 171–182. [Google Scholar] [CrossRef]
- Thaochan, N.; Pornsuriya, C.; Chairin, T.; Chomnunti, P.; Sunpapao, A. Morphological and molecular characterization of Calonectria foliicola associated with leaf blight on rubber Tree (Hevea brasiliensis) in Thailand. J. Fungi 2022, 8, 986. [Google Scholar] [CrossRef]
- Silva, D.N.; Talhinhas, P.; Várzea, V.; Cai, L.; Paulo, O.S.; Batista, D. Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: An example from coffee (Coffea spp.) hosts. Mycologia 2012, 104, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Sunpapao, A.; Suwannarach, N.; Kumla, J.; Dumhai, R.; Riangwong, K.; Sanguansub, S.; Wanchana, S.; Arikit, S. Morphological and molecular identification of plant pathogenic fungi associated with dirty panicle disease in coconuts (Cocos nucifera) in Thailand. J. Fungi 2022, 8, 335. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Cheewangkoon, R.; Carnegie, A.J.; Burgess, T.I.; Summerell, B.A.; Edwards, J.; Taylor, P.W.J.; Groenewald, J.Z. Foliar pathogens of eucalypts. Stud. Mycol. 2019, 94, 125–298. [Google Scholar] [CrossRef]
- Wang, Y.C.; Xiong, F.; Lu, Q.H.; Hao, X.Y.; Zheng, M.X.; Wang, L.; Li, N.N.; Ding, C.Q.; Wang, X.C.; Yang, Y.J. Diversity of Pestalotiopsis-like species causing gray blight disease of tea plants (Camellia sinensis) in China, including two novel Pestalotiopsis species, and analysis of their pathogenicity. Plant Dis. 2019, 103, 2548–2558. [Google Scholar] [CrossRef]
- Poudel, B.; Zhang, S. First report of Erysiphe fallax causing powdery mildew on phasey bean (Macroptilium lathyroides) in the United States. Plant Dis. 2019, 20, 35–37. [Google Scholar] [CrossRef]
- Fu, M.; Crous, P.W.; Bai, Q.; Zhang, P.F.; Xiang, J.; Guo, Y.S.; Zhao, F.F.; Yang, M.M.; Hong, N.; Xu, W.X.; et al. Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia 2019, 42, 1–35. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, D.W.; Zhu, Y.N.; Si, Y.Z.; Huang, J.H.; Zhu, L.H.; Ye, J.R.; Huang, L. Diversity and pathogenicity of Colletotrichum species causing anthracnose on Cunninghamia lanceolata. Plant Pathol. 2022, 71, 1757–1773. [Google Scholar] [CrossRef]
- Zhang, P.F.; Zhai, L.F.; Zhang, X.K.; Huang, X.Z.; Hong, N.; Xu, W.X.; Wang, G.P. Characterization of Colletotrichum fructicola, a new causal agent of leaf black spot disease of sandy pear (Pyrus pyrifolia). Eur. J. Plant Pathol. 2015, 143, 651–662. [Google Scholar] [CrossRef]
- Li, S.N.; Zhang, W.M. Post-harvest anthracnose of carambola (Averrhoa carambola) caused by Colletotrichum fructicola in China. Plant Dis. 2022. [Google Scholar] [CrossRef]
- Han, S.; Xu, X.; Jiang, Y.R.; Yuan, H.; Li, S.J.; Liu, Y.; Lin, T.; Qiao, T.; Yang, C.; Li, S.; et al. Colletotrichum fructicola causal agent of shot-hole symptoms on leaves of Prunus sibirica in China. Plant Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Usman, H.M.; Tan, Q.; Karim, M.M.; Adnan, M.; Yin, W.X.; Zhu, F.X.; Luo, C.X. Sensitivity of Colletotrichum fructicola and Colletotrichum siamense of peach in China to multiple classes of fungicides and characterization of pyraclostrobin-resistant isolates. Plant Dis. 2022, 105, 3459–3465. [Google Scholar] [CrossRef]
- Lin, W.L.; Duan, C.H.; Wang, C.L. Identification and virulence of Colletotrichum species causing anthracnose on mango. Plant Pathol. 2023, 72, 623–635. [Google Scholar] [CrossRef]
- Swain, S.V.; Koike, S.T.; Michailides, T.J.; Feng, C.; Correll, J.C. First report of twig canker on willow caused by Colletotrichum acutatum in California. Plant Dis. 2012, 96, 1822–1823. [Google Scholar] [CrossRef]
- Si, Y.Z.; Sun, J.W.; Li, D.W.; Huang, L.; Ju, Y.; Zhu, L.H. Leaf spot of Sapindus mukorossi caused by Diaporthe biconispora in China. Australas. Plant Pathol. 2021, 50, 193–202. [Google Scholar] [CrossRef]
- Sun, J.W.; Si, Y.Z.; Li, D.W.; Jin, G.Q.; Zhu, L.H. First report of leaf blotch of Aesculus chinensis caused by Colletotrichum gloeosporioides and Colletotrichum fructicola in China. Plant Dis. 2020, 104, 3065–3066. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.C.; Zhang, Y.; Li, D.W.; Ye, J.R. Colletotrichum gloeosporioides sensu stricto is a pathogen of leaf anthracnose on Evergreen Spindle tree (Euonymus japonicus). Plant Dis. 2016, 100, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; He, J.; Ding, L.; Kang, J.; Zhang, Q.; Zheng, Q. Single spore strains without producing fruit body isolated from Cordyceps militeris and their RAPD analysis. Southwest China J. Agric. Sci. 2007, 20, 547–550. [Google Scholar]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum gloeosporioides isolates from avocado and almond fruits with molecular and pathogenicity tests. Appl. Environ. Microbiol. 1996, 62, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Templeton, M.D.; Rikkerink, E.H.A.; Solon, S.L.; Crowhurst, R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Luo, C.X.; Wu, H.J.; Peng, B.; Kang, B.S.; Liu, L.M.; Zhang, M.; Gu, Q.S. Colletotrichum species associated with anthracnose disease of watermelon (Citrullus lanatus) in China. J. Fungi 2022, 8, 790. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).