Prediction of the Medicinal Mechanisms of Pinellia ternata Breitenbach, a Traditional Medicine for Gastrointestinal Motility Disorders, through Network Pharmacology
Abstract
:1. Introduction
2. Results
2.1. Information for 366 Targets Derived through Correlation Investigation between Compounds and Targets
2.2. Twenty Active Compounds Met the Criteria for ADME Parameters
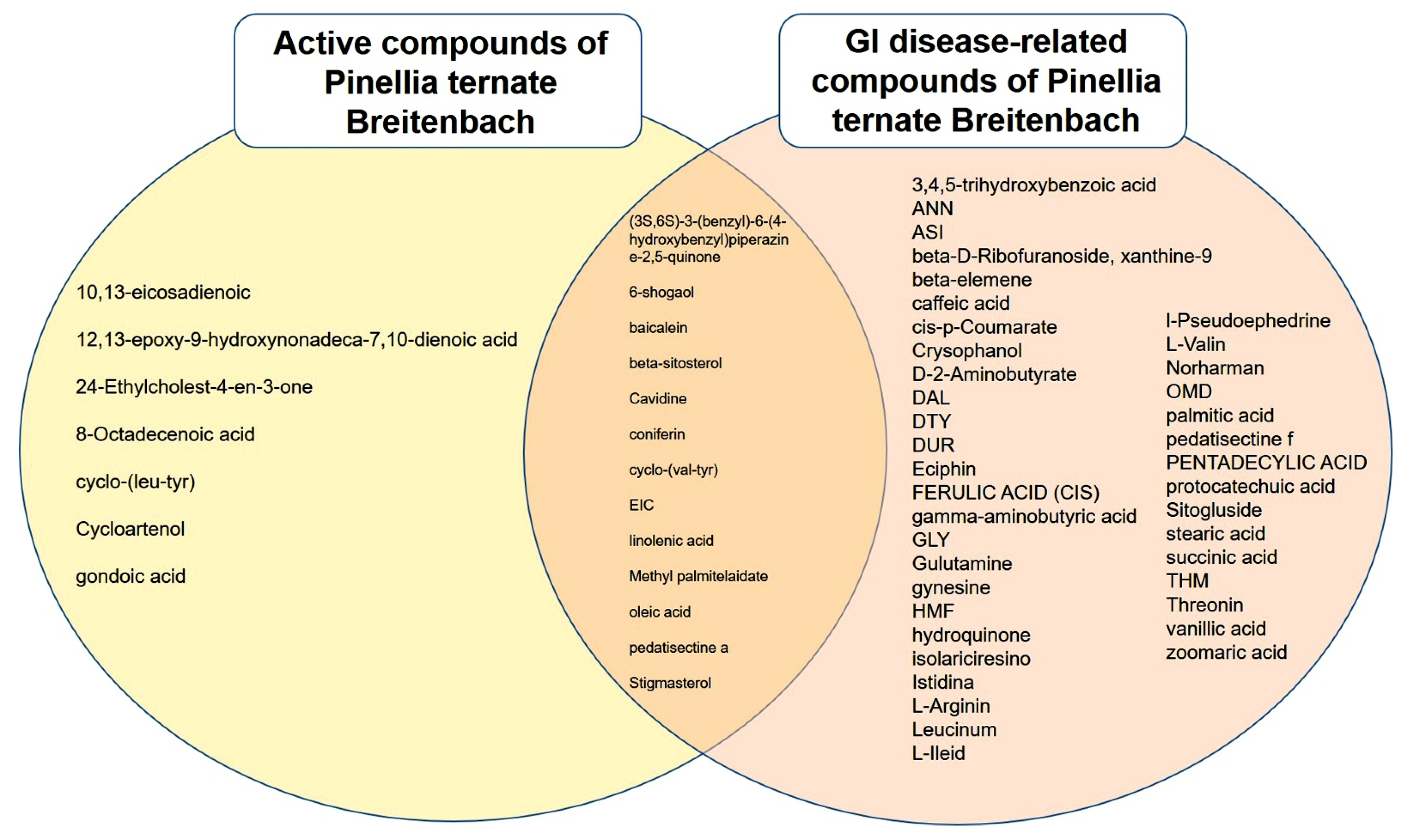
2.3. Identification of 53 Compounds Related to Gastrointestinal (GI) Disease in PTB
2.4. All 52 GI Disease-Related Compounds, Except 6-Shogaolin, in PTB Are Related to GMDs
2.5. The Network of GMD-Related Genes and Compounds for Identifying Molecules of Interest
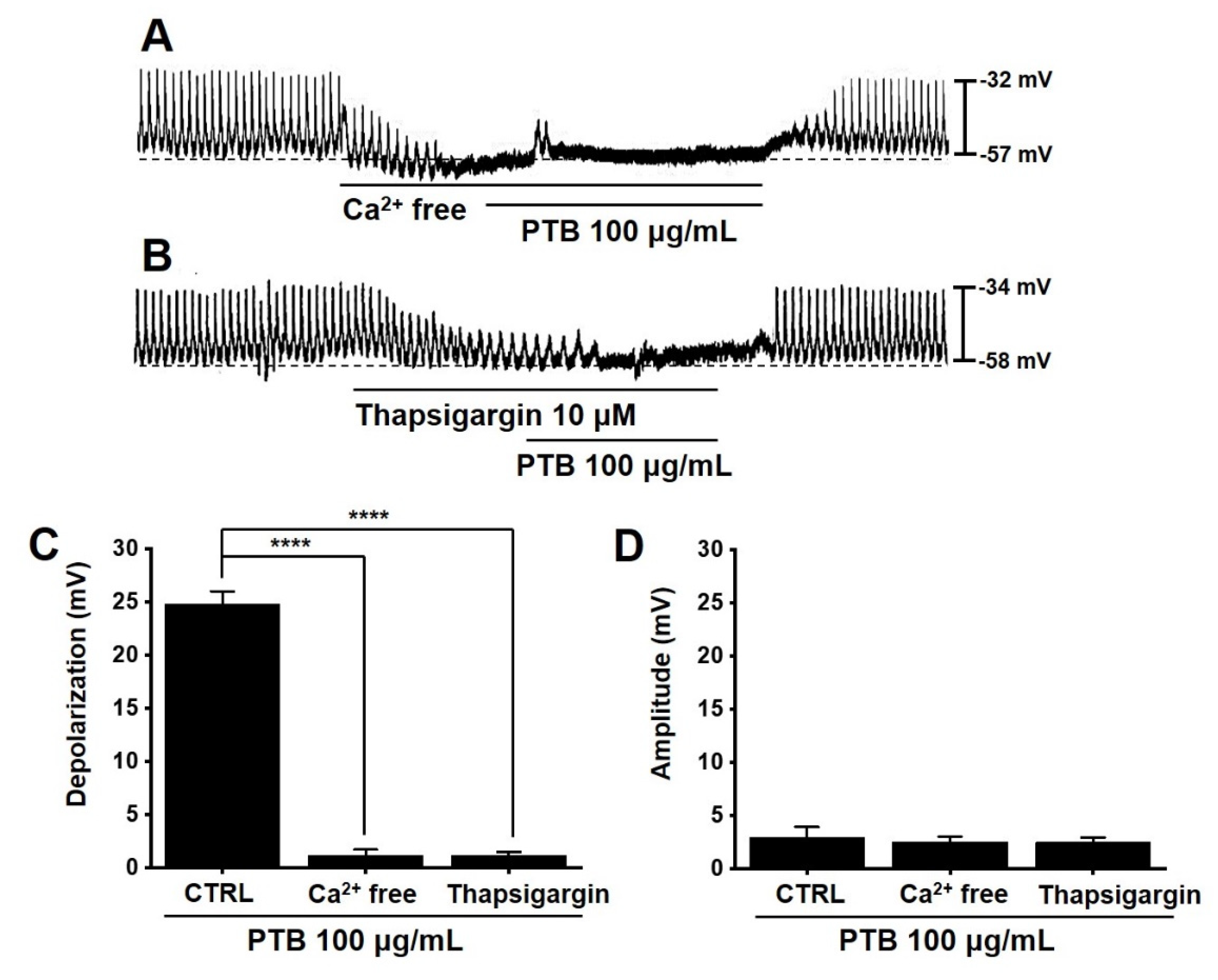
2.6. Effects of PTB Extract on the Pacemaker Potential of ICCs
2.7. Importance of Ca2+ in PTB Extract-Induced Pacemaker Potential Depolarization of ICCs
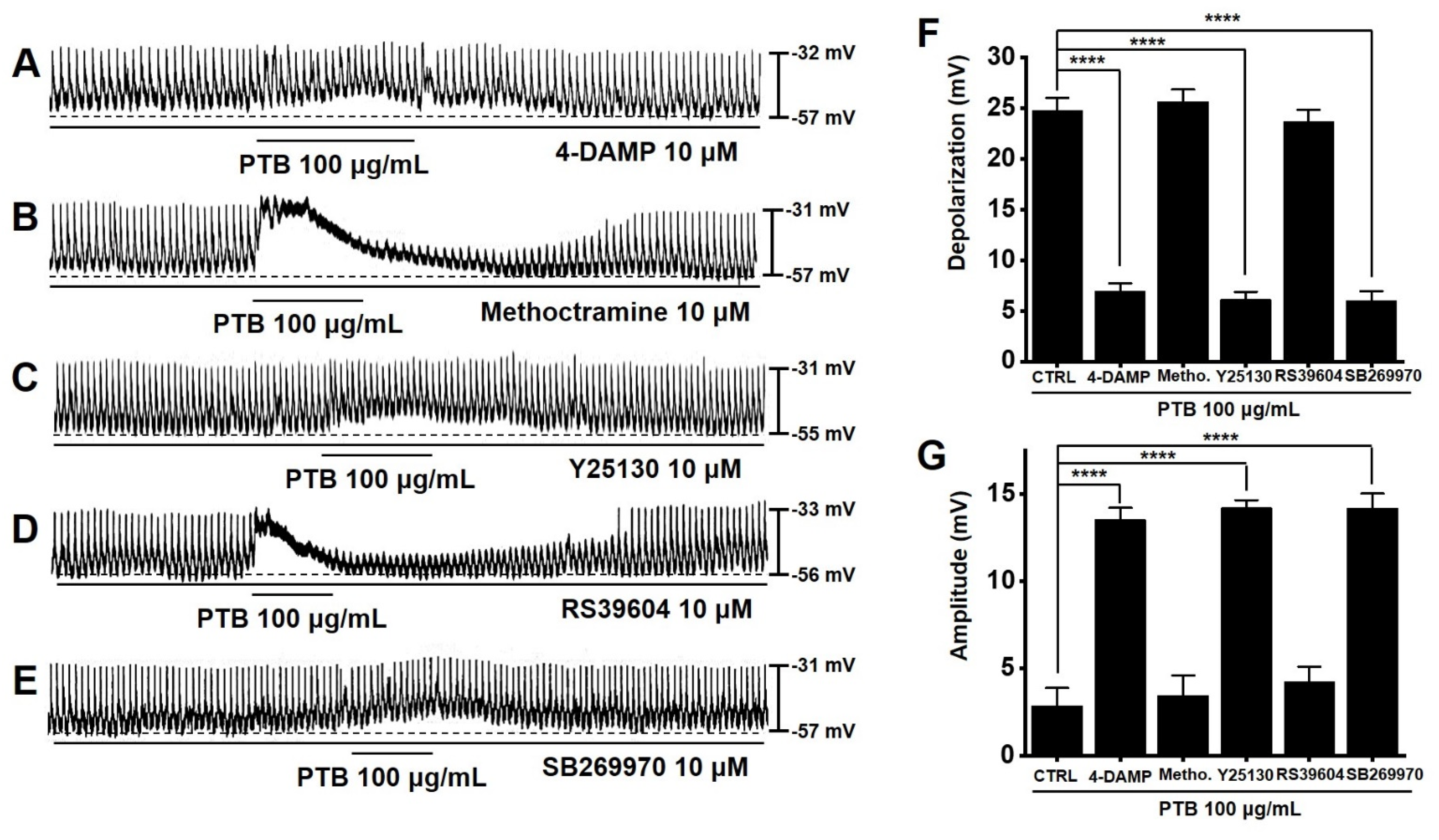
2.8. Importance of Muscarinic M3, 5-HT3, and 5-HT7 Receptors in PTB Extract-Induced Pacemaker Potential Depolarization of ICCs
2.9. Effects of PTB Extract on the ITR
3. Discussion
4. Materials and Methods
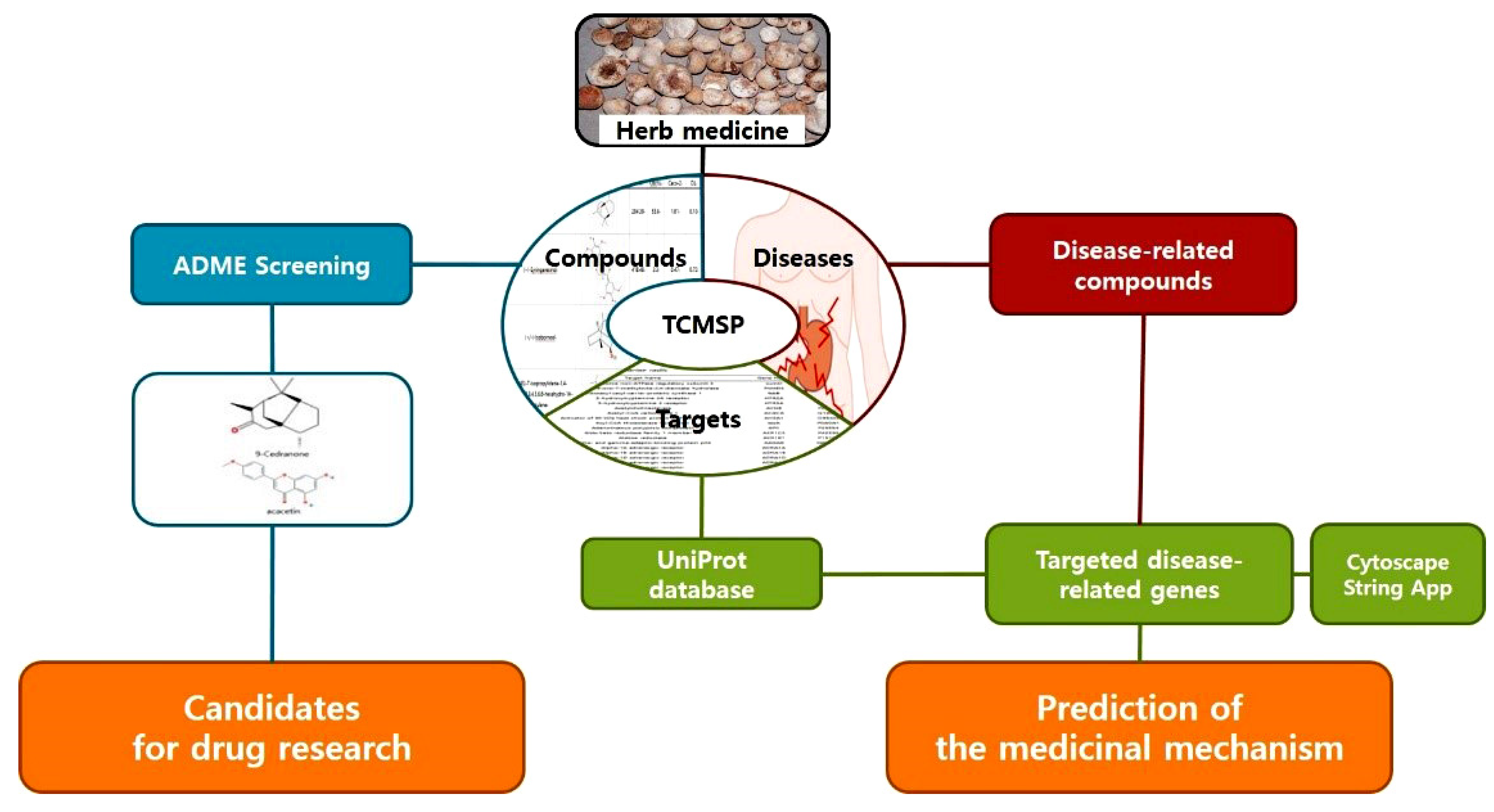
4.1. Network-Based Pharmacological Analysis by PTB
4.1.1. Identification of PTB Compounds
4.1.2. Analysis of Targets
4.1.3. Network Analysis
4.1.4. Active Compound Screening
4.2. Animal Experiments
4.2.1. Preparation of the PTB Extract
4.2.2. Preparation of ICCs
4.2.3. Electrophysiological Experiments
4.2.4. Intestinal Transit Rate (ITR)
4.2.5. GMD Model Mice
4.2.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gralnek, I.M.; Hays, R.D.; Kilbourne, A.; Naliboff, B.; Mayer, E.A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000, 119, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Pare, P.; Ferrazzi, S.; Thompson, W.G.; Irvine, E.J.; Rance, L. An epidemiological survey of constipation in canada: Definitions, rates, demographics, and predictors of health care seeking. Am. J. Gastroenterol. 2001, 96, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Talley, N.J. Health-related quality of life in functional dyspepsia. Aliment. Pharmacol. Ther. 2003, 18, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D. Gastrointestinal peristalsis: Joint action of enteric nerves, smooth muscle, and interstitial cells of Cajal. Microsc. Res. Tech. 1999, 47, 239–247. [Google Scholar] [CrossRef]
- Huizinga, J.D.; Lammers, W.J. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1–G8. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.J.; Rhee, P.L.; Sanders, K.M.; Ward, S.M. The significance of interstitial cells in neurogastroenterology. J. Neurogastroenterol. Motil. 2014, 20, 294–317. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef]
- Streutker, C.J.; Huizinga, J.D.; Driman, D.K.; Riddell, R.H. Interstitial cells of Cajal in health and disease. Part I: Normal ICC structure and function with associated motility disorders. Histopathology 2007, 50, 176–189. [Google Scholar] [CrossRef]
- Maki, T.; Takahashi, K.; Shibata, S. An anti-emetic principle of Pinellia ternata tuber. Planta Med. 1987, 53, 410–414. [Google Scholar] [CrossRef]
- Zhao, L.; Su, X. Comparative analysis of alkaloid components in cultured and planted Pinellia ternata (Thunb.) Breit. China J. Chin. Med. 1990, 15, 146–190. [Google Scholar]
- Wang, R.; Ni, J.M.; Ma, R. Volatile oils of Pinellia ternate. J. Chin. Pharm. 1995, 30, 457–459. [Google Scholar]
- Gonda, R.; Tomoda, M.; Shimizu, N.; Ohara, N.; Takagi, H.; Hoshino, S. Characterization of an acidic polysaccharide with immunological activities from the tuber of Pinellia ternata. Biol. Pharm. Bull. 1994, 17, 1549–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlert, F.J.; Pak, K.J.; Griffin, M.T. Muscarinic agonists and antagonists: Effects on gastrointestinal function. Handb. Exp. Pharmacol. 2012, 208, 343–374. [Google Scholar]
- Mawe, G.M.; Hoffman, J.M. Serotonin Signaling in the Gastrointestinal Tract: Functions, dysfunctions, and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.N.; Ohya, S.; Nishizawa, Y.; Sawamura, K.; Iino, S.; Syed, M.M.; Goto, K.; Imaizumi, Y.; Nakayama, S. Serotonin augments gut pacemaker activity via 5-HT3 receptors. PLoS ONE 2011, 6, e24928. [Google Scholar] [CrossRef] [Green Version]
- Lino, S.; Horiguchi, K. Interstitial Cells of Cajal Are Involved in Neurotransmission in the Gastrointestinal Tract. Acta. Histochem. Cytochem. 2006, 39, 145–153. [Google Scholar]
- Chen, J.H.; Cui, G.Y.; Liu, J.Y.; Tan, R.X. Pinelloside, an antimicrobial cerebroside from Pinellia ternata. Phytochemistry 2003, 64, 903–906. [Google Scholar] [CrossRef]
- Niijima, A.; Okui, Y.; Kubo, M.; Higuchi, M.; Taguchi, H.; Mitsuhashi, H.; Maruno, M. Effect of Pinellia ternata tuber on the efferent activity of the gastric vagus nerve in the rat. Brain Res. Bull. 1993, 32, 103–106. [Google Scholar] [CrossRef]
- Wallace, J.L.; Devchand, P.R. Emerging roles for cyclooxygenase-2 in gastrointestinal mucosal defense. Br. J. Pharmacol. 2005, 145, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Strege, P.R.; Mazzone, A.; Bernard, C.E.; Neshatian, L.; Gibbons, S.J.; Saito, Y.A.; Tester, D.J.; Calvert, M.L.; Mayer, E.A.; Chang, L.; et al. Irritable bowel syndrome patients have SCN5A channelopathies that lead to decreased NaV1.5 current and mechanosensitivity. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G494–G503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galligan, J.J.; Sternini, C. Insights into the Role of Opioid Receptors in the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 363–378. [Google Scholar] [PubMed]
- Kopic, S.; Corradini, S.; Sidani, S.; Murek, M.; Vardanyan, A.; Föller, M.; Ritter, M.; Geibel, J.P. Ethanol inhibits gastric acid secretion in rats through increased AMP-kinase activity. Cell. Physiol. Biochem. 2010, 25, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; van Citters, G.W.; Heimer, F.; Bonorris, G. Slowing of gastrointestinal transit by oleic acid: A preliminary report of a novel, nutrient-based treatment in humans. Dig. Dis. Sci. 2001, 46, 223–229. [Google Scholar] [CrossRef]
- Ding, K.; Tan, Y.Y.; Ding, Y.; Fang, Y.; Yang, X.; Fang, J.; Xu, D.C.; Zhang, H.; Lu, W.; Li, M.; et al. β-Sitosterol improves experimental colitis in mice with a target against pathogenic bacteria. J. Cell. Biochem. 2019, 120, 5687–5694. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, H.; Li, W.; Wang, Y.; Mu, Q.; Wang, X.; He, Z.; Yao, H. Protective effect of cavidine on acetic acid-induced murine colitis via regulating antioxidant, cytokine profile and NF-κB signal transduction pathways. Chem. Biol. Interact. 2015, 239, 34–45. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, H.W.; Lee, G.S.; Choi, S.; Jun, J.Y.; So, I.; Kim, S.J. Poncirus trifoliate fruit modulates pacemaker activity in interstitial cells of Cajal from the murine small intestine. J. Ethnopharmacol. 2013, 149, 668–675. [Google Scholar] [CrossRef]
- Wu, Y.S.; Lu, H.L.; Huang, X.; Liu, D.H.; Meng, X.M.; Guo, X.; Kim, Y.C.; Xu, W.X. Diabetes-induced loss of gastric ICC accompanied by up-regulation of natriuretic peptide signaling pathways in STZ-induced diabetic mice. Peptides 2013, 40, 104–111. [Google Scholar] [CrossRef] [PubMed]





| Molecule Name | Structure | MW | OB (%) * | Caco-2 * | DL * |
|---|---|---|---|---|---|
| (3S,6S)-3-(benzyl)-6-(4-hydroxybenzyl)piperazine-2,5-quinone | 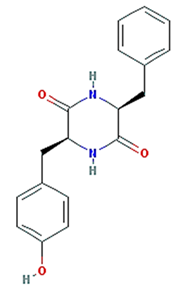 | 310.38 | 46.89 | 0.41 | 0.27 |
| 10,13-eicosadienoic |  | 308.56 | 39.99 | 1.22 | 0.2 |
| 12,13-epoxy-9-hydroxynonadeca-7,10-dienoic acid | 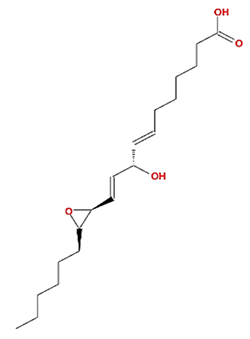 | 324.51 | 42.15 | 0.18 | 0.24 |
| 24-Ethylcholest-4-en-3-one | 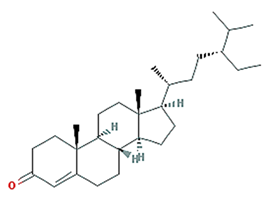 | 412.77 | 36.08 | 1.46 | 0.76 |
| 6-shogaol | 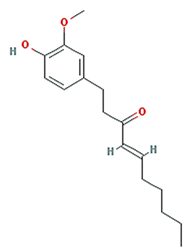 | 276.41 | 31 | 1.07 | 0.14 |
| 8-Octadecenoic acid | 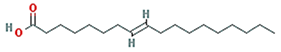 | 282.52 | 33.13 | 1.15 | 0.14 |
| baicalein | 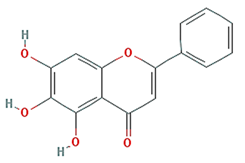 | 270.25 | 33.52 | 0.63 | 0.21 |
| beta-sitosterol |  | 414.79 | 36.91 | 1.32 | 0.75 |
| Cavidine | 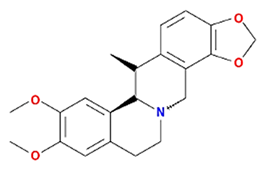 | 353.45 | 35.64 | 1.08 | 0.81 |
| coniferin | 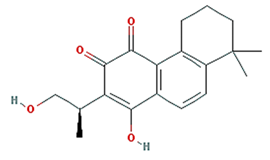 | 314.41 | 31.11 | 0.42 | 0.32 |
| cyclo-(leu-tyr) | 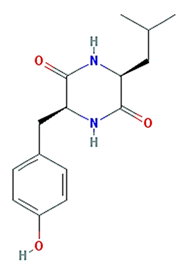 | 276.37 | 111.16 | 0.16 | 0.15 |
| cyclo-(val-tyr) | 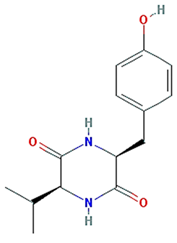 | 262.34 | 122.79 | 0.17 | 0.14 |
| Cycloartenol | 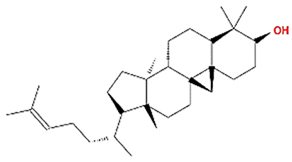 | 426.8 | 38.69 | 1.53 | 0.78 |
| EIC | 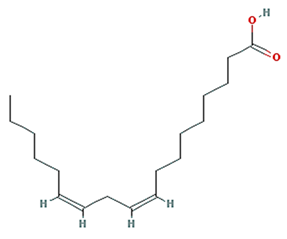 | 280.5 | 41.9 | 1.16 | 0.14 |
| gondoic acid | 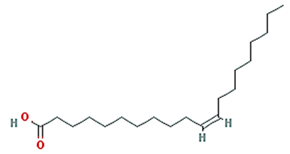 | 310.58 | 30.7 | 1.2 | 0.2 |
| linolenic acid | 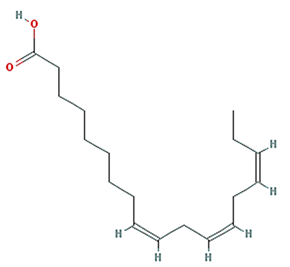 | 278.48 | 45.01 | 1.21 | 0.15 |
| Methyl palmitelaidate |  | 268.49 | 34.61 | 1.4 | 0.12 |
| oleic acid |  | 282.52 | 33.13 | 1.17 | 0.14 |
| pedatisectine a | 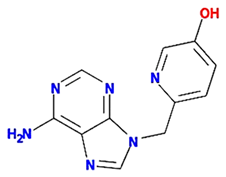 | 242.27 | 64.09 | −0.3 | 0.16 |
| Stigmasterol |  | 412.77 | 43.83 | 1.44 | 0.76 |
| Molecule Name | Gene Name | Disease Name |
|---|---|---|
| (3S,6S)-3-(benzyl)-6-(4-hydroxybenzyl)piperazine-2,5-quinone | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| 3,4,5-trihydroxybenzoic acid | HSP90AA1 | Gastrointestinal stromal tumors (GIST) |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| 6-shogaol | PPARG | Crohn’s disease, unspecified Inflammatory bowel disease Pancreatic cancer Ulcerative colitis |
| ANN | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| ASI | ALOX5 | Gastrointestinal cancers Inflammatory bowel disease Pancreatic cancer Ulcerative colitis |
| NOS1 | GI motility disorder 1 | |
| baicalein | FOS | GI motility disorder 1 |
| HSP90AA1 | Gastrointestinal stromal tumors (GIST) | |
| MPO | GI motility disorder 1 | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| beta-D-Ribofuranoside, xanthine-9 | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| beta-elemene | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| beta-sitosterol | HSP90AA1 | Gastrointestinal stromal tumors (GIST) |
| OPRM1 | GI motility disorder 1 Diarrhea | |
| Opioid-induced bowel dysfunction | ||
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| SCN5A | GI motility disorder 1 | |
| SLC6A4 | GI motility disorder 1 | |
| caffeic acid | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| TNF | GI motility disorder 1 | |
| Crohn’s disease, unspecified | ||
| Cavidine | HSP90AA1 | Gastrointestinal stromal tumors (GIST) |
| HTR3A | Chemotherapy-induced nausea and vomiting Diarrhea Irritable bowel syndrome Postoperative nausea and vomiting | |
| OPRM1 | GI motility disorder 1 Diarrhea Opioid-induced bowel dysfunction | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| SCN5A | GI motility disorder 1 | |
| SLC6A4 | GI motility disorder 1 | |
| cis-p-Coumarate | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| coniferin | CA2 | Pancreatic cancer |
| OPRM1 | GI motility disorder 1 Diarrhea Opioid-induced bowel dysfunction | |
| PPARG | Crohn’s disease, unspecified Inflammatory bowel disease Pancreatic cancer Ulcerative colitis | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| SCN5A | GI motility disorder 1 | |
| Crysophanol | HSP90AA1 | Gastrointestinal stromal tumors (GIST) |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| SCN5A | GI motility disorder 1 | |
| cyclo-(val-tyr) | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| D-2-Aminobutyrate | NOS1 | GI motility disorder 1 |
| DAL | MMP12 | Crohn’s disease, unspecified Gastrointestinal ulcers Ulcerative colitis |
| NOS1 | GI motility disorder 1 | |
| DTY | ACHE | GI motility disorder 1 |
| NOS3 | Colon cancer | |
| PTGS2 | GI motility disorder 1 | |
| Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | ||
| DUR | CA2 | Pancreatic cancer |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| Eciphin | ACHE | GI motility disorder 1 |
| NOS3 | Colon cancer | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| SCN5A | GI motility disorder 1 | |
| SLC6A4 | GI motility disorder 1 | |
| EIC | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| TRPV1 | GI motility disorder 1 | |
| FERULIC ACID (CIS) | LTA4H | Oesophageal cancer |
| NOS3 | Colon cancer | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| gamma-aminobutyric acid | IL6 | GI motility disorder 1 |
| GLY | AMY2A | Pancreatic disease |
| CTNNB1 | Colorectal cancer | |
| LTA4H | Oesophageal cancer | |
| MMP12 | Crohn’s disease, unspecified Gastrointestinal ulcers Ulcerative colitis | |
| NOS1 | GI motility disorder 1 | |
| PTGS2 | GI motility disorder 1 | |
| Adenomatous polyposis | ||
| Colorectal cancer | ||
| Peutz–Jeghers syndrome | ||
| Gulutamine | LTA4H | Oesophageal cancer |
| NOS1 | GI motility disorder 1 | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| gynesine | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| HMF | ACHE | GI motility disorder 1 |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| hydroquinone | TNF | GI motility disorder 1 |
| Crohn’s disease, unspecified | ||
| isolariciresino | CA2 | Pancreatic cancer |
| HSP90AA1 | Gastrointestinal stromal tumors (GIST) | |
| MAPK14 | Crohn’s disease, unspecified | |
| NOS3 | Colon cancer | |
| PPARG | Crohn’s disease, unspecified Inflammatory bowel disease Pancreatic cancer Ulcerative colitis | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| SCN5A | GI motility disorder 1 | |
| Istidina | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| L-Arginin | NOS1 | GI motility disorder 1 |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| Leucinum | NOS1 | GI motility disorder 1 |
| L-Ile | NOS1 | GI motility disorder 1 |
| linolenic acid | ACTB | GI motility disorder 1 |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| TRPV1 | GI motility disorder 1 | |
| l-Pseudoephedrine | NOS3 | Colon cancer |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| SCN5A | GI motility disorder 1 | |
| SLC6A4 | GI motility disorder 1 | |
| L-Valin | NOS1 | GI motility disorder 1 |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| Methyl palmitelaidate | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| Norharman | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| oleic acid | CCK | GI motility disorder 1 |
| CRP | GI motility disorder 1 | |
| GCG | GI motility disorder 1 | |
| INS | GI motility disorder 1 | |
| MPO | GI motility disorder 1 | |
| PPARG | Crohn’s disease, unspecified Inflammatory bowel disease Pancreatic Cancer Ulcerative colitis | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| PYY | GI motility disorder 1 | |
| OMD | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| palmitic acid | IL10 | GI motility disorder 1 |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| TNF | GI motility disorder 1 | |
| Crohn’s disease, unspecified | ||
| pedatisectine a | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| pedatisectine f | ACHE | GI motility disorder 1 |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| PENTADECYLIC ACID | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| protocatechuic acid | ALOX5 | Gastrointestinal cancers Inflammatory bowel disease Pancreatic cancer |
| Ulcerative colitis | ||
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| Sitogluside | HSP90AA1 | Gastrointestinal stromal tumors (GIST) |
| HTR3A | Chemotherapy-induced nausea and vomiting Diarrhea Irritable bowel syndrome Postoperative nausea and vomiting | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| SCN5A | GI motility disorder 1 | |
| stearic acid | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
| Stigmasterol | LTA4H | Oesophageal cancer |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| SCN5A | GI motility disorder 1 | |
| succinic acid | NOS1 | GI motility disorder 1 |
| THM | CA2 | Pancreatic cancer |
| HSP90AA1 | Gastrointestinal stromal tumors (GIST) | |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| Threonin | NOS1 | GI motility disorder 1 |
| PTGS2 | GI motility disorder 1 | |
| Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | ||
| vanillic acid | NOS3 | Colon cancer |
| PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome | |
| zoomaric acid | PTGS2 | GI motility disorder 1 Adenomatous polyposis Colorectal cancer Peutz–Jeghers syndrome |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, N.R.; Park, J.; Ko, S.-J.; Kim, J.N.; Choi, W.; Park, J.-W.; Kim, B.J. Prediction of the Medicinal Mechanisms of Pinellia ternata Breitenbach, a Traditional Medicine for Gastrointestinal Motility Disorders, through Network Pharmacology. Plants 2022, 11, 1348. https://doi.org/10.3390/plants11101348
Choi NR, Park J, Ko S-J, Kim JN, Choi W, Park J-W, Kim BJ. Prediction of the Medicinal Mechanisms of Pinellia ternata Breitenbach, a Traditional Medicine for Gastrointestinal Motility Disorders, through Network Pharmacology. Plants. 2022; 11(10):1348. https://doi.org/10.3390/plants11101348
Chicago/Turabian StyleChoi, Na Ri, Jongwon Park, Seok-Jae Ko, Jeong Nam Kim, Woogyun Choi, Jae-Woo Park, and Byung Joo Kim. 2022. "Prediction of the Medicinal Mechanisms of Pinellia ternata Breitenbach, a Traditional Medicine for Gastrointestinal Motility Disorders, through Network Pharmacology" Plants 11, no. 10: 1348. https://doi.org/10.3390/plants11101348
APA StyleChoi, N. R., Park, J., Ko, S.-J., Kim, J. N., Choi, W., Park, J.-W., & Kim, B. J. (2022). Prediction of the Medicinal Mechanisms of Pinellia ternata Breitenbach, a Traditional Medicine for Gastrointestinal Motility Disorders, through Network Pharmacology. Plants, 11(10), 1348. https://doi.org/10.3390/plants11101348







