The Role of Iron and Zinc in the Treatment of ADHD among Children and Adolescents: A Systematic Review of Randomized Clinical Trials
Abstract
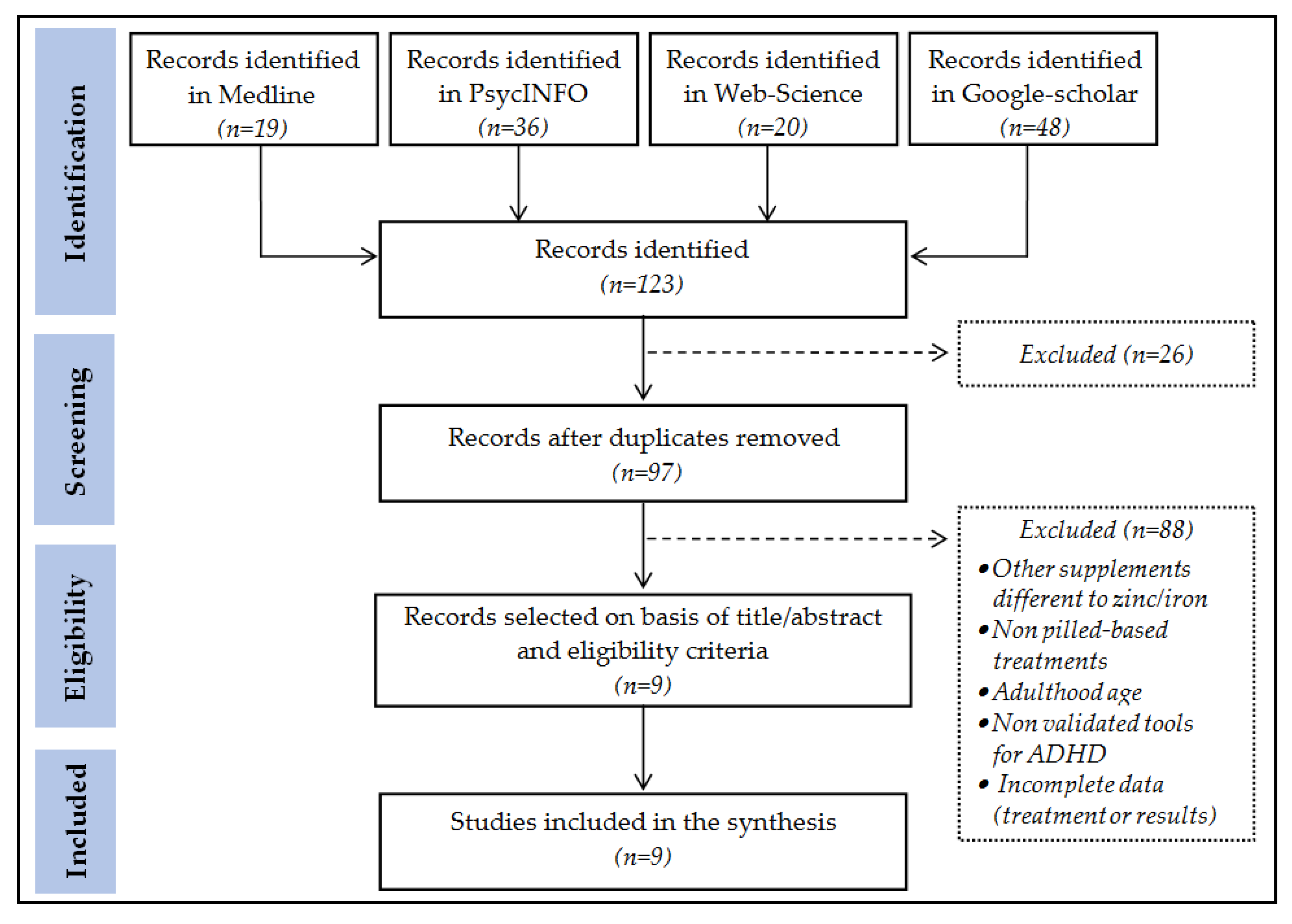
:1. Introduction
2. Materials and Methods
2.1. Procedure
2.2. Inclusion and Exclusion Criteria
2.3. Research and Selection of Studies
- Medline: search = [((zinc OR iron) AND (ADHD)) AND ((treatment or therapy))]. The next filters were employed: (a) type of publication: (Clinical Trial) and (Randomized Controlled Trial); and (b) Date of publication: (From 1 January 2000–31 July 2021).
- PsycINFO: search = [((zinc OR iron) AND (ADHD)) AND ((treatment or therapy))]. The next filters were defined: (a) Type of publication: [Peer Reviewed Journal]; and (b) Date of publication: [1 January 2000–31 July 2021];
- Web of Science: search = [(((zinc OR iron OR ferritin) AND (ADHD)) AND ((treatment or therapy))))]. The next filters were defined: (a) Document Types: [Clinical Trial], and (b) Publication years: [1 January 2000–31 July 2021];
- Google Scholar: search = [(zinc OR iron) AND (supplement) AND (ADHD) AND (treatment or therapy) AND (randomized or “clinical trial”) AND (children or adolescent) AND (“Peer Reviewed Journal”)].
2.4. Study Quality Assessment
3. Results
3.1. Descriptive for the Selected Studies
3.2. Assessment of the Methodological Quality, Adherence and Competence
3.3. Assessment of the Risk of Bias of the Included Studies
3.4. Efficacy of Zinc for ADHD Treatment
3.5. Efficacy of Iron for ADHD Treatment
3.6. Efficacy of Including Simultaneously Iron and Zinc for ADHD Treatment
3.7. Tolerability Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Di Lorenzo, R.; Balducci, J.; Poppi, C.; Arcolin, E.; Cutino, A.; Ferri, P.; D’Amico, R.; Filippini, T. Children and adolescents with ADHD followed up to adulthood: A systematic review of long-term outcomes. Acta Neuropsychiatr. 2021, 1–42. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Chan, M.F.; Al Balushi, R.; Al Falahi, M.; Mahadevan, S.; Al Saadoon, M.; Al-Adawi, S. Child and adolescent mental health disorders in the GCC: A systematic review and meta-analysis. Int. J. Pediatr. Adolesc. Med. 2021, 8, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef]
- Sayal, K.; Prasad, V.; Daley, D.; Ford, T.; Coghill, D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry 2018, 5, 175–186. [Google Scholar] [CrossRef]
- Willis, R.; Dhakras, S.; Cortese, S. Attention-Deficit/Hyperactivity Disorder in Looked-After Children: A Systematic Review of the Literature. Curr. Dev. Disord. Rep. 2017, 4, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrosavljevic, M.; Solares, C.; Cortese, S.; Andershed, H.; Larsson, H. Prevalence of attention-deficit/hyperactivity disorder in older adults: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 282–289. [Google Scholar] [CrossRef]
- Song, P.; Zha, M.; Yang, Q.; Zhang, Y.; Li, X.; Rudan, I. The prevalence of adult attention-deficit hyperactivity disorder: A global systematic review and meta-analysis. J. Glob. Health 2021, 11, 04009. [Google Scholar] [CrossRef]
- Lynch, S.J.; Sunderland, M.; Newton, N.C.; Chapman, C. A systematic review of transdiagnostic risk and protective factors for general and specific psychopathology in young people. Clin. Psychol. Rev. 2021, 87, 102036. [Google Scholar] [CrossRef]
- Bruxel, E.M.; Moreira-Maia, C.R.; Akutagava-Martins, G.C.; Quinn, T.P.; Klein, M.; Franke, B.; Ribasés, M.; Rovira, P.; Sánchez-Mora, C.; Kappel, D.B.; et al. Meta-analysis and systematic review of ADGRL3 (LPHN3) polymorphisms in ADHD susceptibility. Mol. Psychiatry 2020, 26, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Luo, L.; Peng, X.; Wang, Y. Association of Val158Met polymorphism in COMT gene with attention-deficit hyperactive disorder: An updated meta-analysis. Medicine 2020, 99, e23400. [Google Scholar] [CrossRef]
- McNeill, R.V.; Ziegler, G.C.; Radtke, F.; Nieberler, M.; Lesch, K.-P.; Kittel-Schneider, S. Mental health dished up—the use of iPSC models in neuropsychiatric research. J. Neural Transm. 2020, 127, 1547–1568. [Google Scholar] [CrossRef]
- Ronald, A.; de Bode, N.; Polderman, T.J. Systematic Review: How the Attention-Deficit/Hyperactivity Disorder Polygenic Risk Score Adds to Our Understanding of ADHD and Associated Traits. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 1234–1277. [Google Scholar] [CrossRef]
- Schuch, V.; Utsumi, D.A.; Costa, T.V.M.M.; Kulikowski, L.D.; Muszkat, M. Attention Deficit Hyperactivity Disorder in the Light of the Epigenetic Paradigm. Front. Psychiatry 2015, 6, 126. [Google Scholar] [CrossRef] [Green Version]
- Walton, E.; Pingault, J.-B.; Cecil, C.A.; Gaunt, T.R.; Relton, C.C.; Mill, J.; Barker, E.D. Epigenetic profiling of ADHD symptoms trajectories: A prospective, methylome-wide study. Mol. Psychiatry 2016, 22, 250–256. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Li, L.; Lagerberg, T.; Chang, Z.; Cortese, S.; Rosenqvist, M.A.; Almqvist, C.; D’Onofrio, B.M.; Hegvik, T.-A.; Hartman, C.; Chen, Q.; et al. Maternal pre-pregnancy overweight/obesity and the risk of attention-deficit/hyperactivity disorder in offspring: A systematic review, meta-analysis and quasi-experimental family-based study. Int. J. Epidemiol. 2020, 49, 857–875. [Google Scholar] [CrossRef]
- Qu, A.; Cao, T.; Li, Z.; Wang, W.; Liu, R.; Wang, X.; Nie, Y.; Sun, S.; Liu, X.; Zhang, X. The association between maternal perfluoroalkyl substances exposure and early attention deficit hyperactivity disorder in children: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Bogičević, L.; Verhoeven, M.; Van Baar, A.L. Distinct Profiles of Attention in Children Born Moderate-to-Late Preterm at 6 Years. J. Pediatr. Psychol. 2020, 45, 685–694. [Google Scholar] [CrossRef]
- Walczak-Kozłowska, T.; Mańkowska, A.; Chrzan-Dętkoś, M.; Harciarek, M. Attentional system of very prematurely born preschoolers. Dev. Psychol. 2020, 56, 251–260. [Google Scholar] [CrossRef]
- Carlsson, T.; Molander, F.; Taylor, M.J.; Jonsson, U.; Bölte, S. Early environmental risk factors for neurodevelopmental disorders—A systematic review of twin and sibling studies. Dev. Psychopathol. 2020, 33, 1448–1495. [Google Scholar] [CrossRef]
- Nielsen, T.M.; Pedersen, M.V.; Milidou, I.; Glavind, J.; Henriksen, T.B. Long-term cognition and behavior in children born at early term gestation: A systematic review. Acta Obstet. Et Gynecol. Scand. 2019, 98, 1227–1234. [Google Scholar] [CrossRef]
- Rivollier, F.; Krebs, M.-O.; Kebir, O. Perinatal Exposure to Environmental Endocrine Disruptors in the Emergence of Neurodevelopmental Psychiatric Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1318. [Google Scholar] [CrossRef] [Green Version]
- Serati, M.; Barkin, J.L.; Orsenigo, G.; Altamura, A.C.; Buoli, M. Research Review: The role of obstetric and neonatal complications in childhood attention deficit and hyperactivity disorder—A systematic review. J. Child Psychol. Psychiatry 2017, 58, 1290–1300. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Jaffarzadeh, N.; Rezapour, M.; Arani, M.H. Association between exposure to polycyclic aromatic hydrocarbons and attention deficit hyperactivity disorder in children: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2020, 27, 11531–11540. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.W.; Andersen, A.-M.N.; Hvolby, A.; Garne, E.; Andersen, P.K.; Berg-Beckhoff, G. Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. J. Child Psychol. Psychiatry 2015, 57, 540–548. [Google Scholar] [CrossRef]
- Nikolas, M.A.; Nigg, J.T. Moderators of Neuropsychological Mechanism in Attention-Deficit Hyperactivity Disorder. J. Abnorm. Child Psychol. 2014, 43, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirjak, D.; Meyer-Lindenberg, A.; Fritze, S.; Sambataro, F.; Kubera, K.M.; Wolf, R.C. Motor dysfunction as research domain across bipolar, obsessive-compulsive and neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2018, 95, 315–335. [Google Scholar] [CrossRef]
- Schulze, M.; Coghill, D.; Lux, S.; Philipsen, A. Disentangling ADHD’s Presentation-Related Decision-Making—A Meta-Analytic Approach on Predominant Presentations. Front. Psychiatry 2021, 12, 519840. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.T.; Patel, K. Nutritional and Environmental Approaches to Preventing and Treating Autism and Attention Deficit Hyperactivity Disorder (ADHD): A Review. J. Altern. Complement. Med. 2008, 14, 79–85. [Google Scholar] [CrossRef]
- Azadbakht, L.; Hariri, M. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: A systematic review on the recent literature. Int. J. Prev. Med. 2015, 6, 83. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Frampton, C.M.; Gorman, B.; Boggis, A. Vitamin–mineral treatment of attention-deficit hyperactivity disorder in adults: Double-blind randomised placebo-controlled trial. Br. J. Psychiatry 2014, 204, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidullah, J.D.; Carlson, J.S.; Haggerty, D.; Lancaster, B.M. Integrated care models for ADHD in children and adolescents: A systematic review. Fam. Syst. Health 2018, 36, 233–247. [Google Scholar] [CrossRef]
- Sultan, M.A.; Pastrana, C.S.; Pajer, K.A. Shared Care Models in the Treatment of Pediatric Attention-Deficit/Hyperactivity Disorder (ADHD): Are They Effective? Health Serv. Res. Manag. Epidemiol. 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Boland, H.; DiSalvo, M.; Fried, R.; Woodworth, K.Y.; Wilens, T.; Faraone, S.V.; Biederman, J. A literature review and meta-analysis on the effects of ADHD medications on functional outcomes. J. Psychiatr. Res. 2020, 123, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Weyandt, L.; Oster, D.; Marraccini, M.E.; Gudmundsdottir, B.; Munro, B.; Zavras, B.M.; Kuhar, B. Pharmacological interventions for adolescents and adults with ADHD: Stimulant and nonstimulant medications and misuse of prescription stimulants. Psychol. Res. Behav. Manag. 2014, 7, 223–249. [Google Scholar] [CrossRef] [Green Version]
- Tsujii, N.; Okada, T.; Usami, M.; Kuwabara, H.; Fujita, J.; Negoro, H.; Kawamura, M.; Iida, J.; Saito, T. Effect of Continuing and Discontinuing Medications on Quality of Life After Symptomatic Remission in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis. J. Clin. Psychiatry 2020, 81, 19r13015. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz, L.F.; Simonoff, E.; McGough, J.J.; Halperin, J.M.; Arnold, L.E.; Stringaris, A. Treatment of Children with Attention-Deficit/Hyperactivity Disorder (ADHD) and Irritability: Results from the Multimodal Treatment Study of Children with ADHD (MTA). J. Am. Acad. Child Adolesc. Psychiatry 2014, 54, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Weiss, M.D.; Salpekar, J. Sleep Problems in the Child with Attention-Deficit Hyperactivity Disorder: Defining Aetiology and Appropriate Treatments. CNS Drugs 2010, 24, 811–828. [Google Scholar] [CrossRef]
- Chamorro, M.; Lara, J.P.; Insa, I.; Espadas, M.; Alda-Diez, J.A. Evaluacion y tratamiento de los problemas de sueño en niños diagnosticados de trastorno por deficit de atencion/hiperactividad: Actualizacion de la evidencia [Evaluation and treatment of sleep problems in children diagnosed with attention deficit hyperactivity disorder: An update of the evidence]. Rev. Neurol. 2017, 64, 413–421. [Google Scholar]
- DelRosso, L.M.; Mogavero, M.P.; Baroni, A.; Bruni, O.; Ferri, R. Restless Legs Syndrome in Children and Adolescents. Child Adolesc. Psychiatr. Clin. North Am. 2020, 30, 143–157. [Google Scholar] [CrossRef]
- Baxter, A.J.; Patton, G.; Scott, K.M.; Degenhardt, L.; Whiteford, H.A. Global Epidemiology of Mental Disorders: What Are We Missing? PLoS ONE 2013, 8, e65514. [Google Scholar] [CrossRef]
- Polanczyk, G.V.; Willcutt, E.G.; Salum, G.A.; Kieling, C.; Rohde, L.A. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014, 43, 434–442. [Google Scholar] [CrossRef]
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-analysis. Pediatrics 2015, 135, e994–e1001. [Google Scholar] [CrossRef] [Green Version]
- Logan, A.C.; Jacka, F.N. Nutritional psychiatry research: An emerging discipline and its intersection with global urbanization, environmental challenges and the evolutionary mismatch. J. Physiol. Anthr. 2014, 33, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Nobile, M.; Ciappolino, V.; DelVecchio, G.; Tesei, A.; Turolo, S.; Crippa, A.; Mazzocchi, A.; Altamura, C.A.; Brambilla, P.; et al. The Role of Omega-3 Fatty Acids in Developmental Psychopathology: A Systematic Review on Early Psychosis, Autism, and ADHD. Int. J. Mol. Sci. 2017, 18, 2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigg, J.T.; Lewis, K.; Edinger, T.; Falk, M. Meta-Analysis of Attention-Deficit/Hyperactivity Disorder or Attention-Deficit/Hyperactivity Disorder Symptoms, Restriction Diet, and Synthetic Food Color Additives. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Torp, N.M.U.; Thomsen, P.H. The use of diet interventions to treat symptoms of ADHD in children and adolescents—A systematic review of randomized controlled trials. Nord. J. Psychiatry 2020, 74, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Rytter, M.J.H.; Andersen, L.B.B.; Houmann, T.; Bilenberg, N.; Hvolby, A.; Mølgaard, C.; Michaelsen, K.F.; Lauritzen, L. Diet in the treatment of ADHD in children—A systematic review of the literature. Nord. J. Psychiatry 2014, 69, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloch, M.H.; Mulqueen, J. Nutritional Supplements for the Treatment of ADHD. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 883–897. [Google Scholar] [CrossRef]
- Verlaet, A.A.J.; Maasakkers, C.M.; Hermans, N.; Savelkoul, H.F.J. Rationale for Dietary Antioxidant Treatment of ADHD. Nutrients 2018, 10, 405. [Google Scholar] [CrossRef] [Green Version]
- Del-Ponte, B.; Quinte, G.C.; Cruz, S.; Grellert, M.; Santos, I.S. Dietary patterns and attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. J. Affect. Disord. 2019, 252, 160–173. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Avcil, S.; Uysal, P.; Yenisey, Ç.; Abas, B.I. Elevated Melatonin Levels in Children With Attention Deficit Hyperactivity Disorder: Relationship to Oxidative and Nitrosative Stress. J. Atten. Disord. 2019, 25, 693–703. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med Sci. Isfahan Univ. Med Sci. 2014, 19, 164–174. [Google Scholar]
- Robberecht, H.; Verlaet, A.A.J.; Breynaert, A.; De Bruyne, T.; Hermans, N. Magnesium, Iron, Zinc, Copper and Selenium Status in Attention-Deficit/Hyperactivity Disorder (ADHD). Molecules 2020, 25, 4440. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Eggleston, M.J.; Darling, K.A.; Stevens, A.J.; Kennedy, M.A.; Frampton, C.M. Can we predict treatment response in children with ADHD to a vitamin-mineral supplement? An investigation into pre-treatment nutrient serum levels, MTHFR status, clinical correlates and demographic variables. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 89, 181–192. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Eggleston, M.J.F.; Boggis, A.; Darling, K.; Gorman, B.; Frampton, C.M. Do Changes in Blood Nutrient Levels Mediate Treatment Response in Children and Adults with ADHD Consuming a Vitamin–Mineral Supplement? J. Atten. Disord. 2019, 25, 1107–1119. [Google Scholar] [CrossRef]
- Fanjiang, G.; Kleinman, E.R. Nutrition and performance in children. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 342–347. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Johnstone, J.; Kaplan, B.J. Nutrient supplementation approaches in the treatment of ADHD. Expert Rev. Neurother. 2009, 9, 461–476. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med Res. Methodol. 2007, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. Available online: https://handbook.cochrane.org (accessed on 15 July 2021).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-R; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Welch, R.W.; Antoine, J.-M.; Berta, J.-L.; Bub, A.; De Vries, J.; Guarner, F.; Hasselwander, O.; Hendriks, H.; Jäkel, M.; Koletzko, B.V.; et al. Guidelines for the design, conduct and reporting of human intervention studies to evaluate the health benefits of foods. Br. J. Nutr. 2011, 106, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence, NICE. Methods for the Development of NICE Public Health Guidance. 2012. Available online: https://www.nice.org.uk/process/pmg4/resources/methods-for-the-development-of-nice-public-health-guidance-third-edition-pdf-2007967445701 (accessed on 15 July 2021).
- Critical Appraisal Skills Programme, CASP. Randomized Controlled Trials Checklist. 2020. Available online: https://casp-uk.net/casp-tools-checklists (accessed on 15 July 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Noorazar, S.G.; Malek, A.; Aghaei, S.M.; Yasamineh, N.; Kalejahi, P. The efficacy of zinc augmentation in children with attention deficit hyperactivity disorder under treatment with methylphenidate: A randomized controlled trial. Asian J. Psychiatry 2019, 48, 101868. [Google Scholar] [CrossRef] [PubMed]
- Zamora, J.; Velásquez, A.; Troncoso, L.; Barra, P.; Guajardo, K.; Castillo-Duran, C. Zinc en la terapia del sindrome de déficit de atención e hiperactividad en niños. Un estudio controlado aleatorio preliminar [Zinc in the therapy of the atten-tion-deficit/hyperactivity disorder in children. A preliminar randomized controlled trial]. Arch. Latinoam. Nutr. 2011, 61, 242–246. [Google Scholar] [PubMed]
- Arnold, L.E.; DiSilvestro, R.A.; Bozzolo, D.; Bozzolo, H.; Crowl, L.; Fernandez, S.; Ramadan, Y.; Thompson, S.; Mo, X.; Abdel-Rasoul, M.; et al. Zinc for Attention-Deficit/Hyperactivity Disorder: Placebo-Controlled Double-Blind Pilot Trial Alone and Combined with Amphetamine. J. Child Adolesc. Psychopharmacol. 2011, 21, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Akhondzadeh, S.; Mohammadi, M.-R.; Khademi, M. Zinc sulfate as an adjunct to methylphenidate for the treatment of attention deficit hyperactivity disorder in children: A double blind and randomized trial [ISRCTN64132371]. BMC Psychiatry 2004, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bilici, M.; Yıldırım, F.; Kandil, S.; Bekaroğlu, M.; Yıldırmış, S.; Değer, O.; Ülgen, M.; Yıldıran, A.; Aksu, H. Double-blind, placebo-controlled study of zinc sulfate in the treatment of attention deficit hyperactivity disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 181–190. [Google Scholar] [CrossRef]
- Konofal, E.; Lecendreux, M.; Deron, J.; Marchand, M.; Cortese, S.; Zaïm, M.; Mouren, M.C.; Arnulf, I. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatr. Neurol. 2008, 38, 20–26. [Google Scholar] [CrossRef]
- Safavi, P.; Panahandeh, G.; Vatani, B.; Khoshdel, A. The effect of adding ferrous sulfate to methylphenidate on attention-deficit/hyperactivity disorder in children. J. Adv. Pharm. Technol. Res. 2017, 8, 138–142. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Eggleston, M.J.; Johnstone, J.M.; Darling, K.; Frampton, C.M. Vitamin-mineral treatment improves aggression and emotional regulation in children with ADHD: A fully blinded, randomized, placebo-controlled trial. J. Child Psychol. Psychiatry 2017, 59, 232–246. [Google Scholar] [CrossRef]
- Cortese, S.; Azoulay, R.; Castellanos, F.; Chalard, F.; Lecendreux, M.; Chechin, D.; Delorme, R.; Sebag, G.; Sbarbati, A.; Mouren, M.-C.; et al. Brain iron levels in attention-deficit/hyperactivity disorder: A pilot MRI study. World J. Biol. Psychiatry 2011, 13, 223–231. [Google Scholar] [CrossRef]
- Lozoff, B. Early Iron Deficiency Has Brain and Behavior Effects Consistent with Dopaminergic Dysfunction. J. Nutr. 2011, 141, 740S–746S. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Mech, A.W.; Farah, A. Correlation of Clinical Response with Homocysteine Reduction During Therapy with Reduced B Vitamins in Patients with MDD Who Are Positive for MTHFR C677T or A1298C Polymorphism: A randomized, double-blind, place-bo-controlled study. J. Clin. Psychiatry 2016, 77, 668–671. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.D.; Smith, S.; De Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-Lowering by B Vitamins Slows the Rate of Accelerated Brain Atrophy in Mild Cognitive Impairment: A Randomized Controlled Trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Vazir, S.; Nagalla, B.; Thangiah, V.; Kamasamudram, V.; Bhattiprolu, S. Effect of micronutrient supplement on health and nutritional status of schoolchildren: Mental function. Nutrition 2006, 22, S26–S32. [Google Scholar] [CrossRef]
- Arnold, L.E.; Bozzolo, H.; Hollway, J.; Cook, A.; DiSilvestro, R.A.; Bozzolo, D.R.; Crowl, L.; Ramadan, Y.; Williams, C. Serum Zinc Correlates with Parent- and Teacher- Rated Inattention in Children with Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2005, 15, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Cortese, S.; Konofal, E.; Bernardina, B.D.; Mouren, M.-C.; Lecendreux, M. Sleep disturbances and serum ferritin levels in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2009, 18, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.H.; Ahlberg, C.D.; Chow, C.A.; Shah, D.R.; Koo, B.B. Iron, dopamine, genetics, and hormones in the pathophysiology of restless legs syndrome. J. Neurol. 2017, 264, 1634–1641. [Google Scholar] [CrossRef]
- Oner, O.; Oner, P.; Bozkurt, H.; Odabas, E.; Keser, N.; Karadag, H.; Kizilgun, M. Effects of Zinc and Ferritin Levels on Parent and Teacher Reported Symptom Scores in Attention Deficit Hyperactivity Disorder. Child Psychiatry Hum. Dev. 2010, 41, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Swanson, J.M.; Kinsbourne, M.; Nigg, J.; Lanphear, B.; Stefanatos, G.A.; Volkow, N.; Taylor, E.; Casey, B.J.; Castellanos, F.; Wadhwa, P.D. Etiologic Subtypes of Attention-Deficit/Hyperactivity Disorder: Brain Imaging, Molecular Genetic and Environmental Factors and the Dopamine Hypothesis. Neuropsychol. Rev. 2007, 17, 39–59. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Newcorn, J.; Telang, F.; Solanto, M.V.; Fowler, J.S.; Logan, J.; Ma, Y.; Schulz, K.; Pradhan, K.; et al. Depressed Dopamine Activity in Caudate and Preliminary Evidence of Limbic Involvement in Adults With Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 2007, 64, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Bonaventura, J.; Quiroz, C.; Cai, N.-S.; Rubinstein, M.; Tanda, G.; Ferré, S. Key role of the dopamine D 4 receptor in the modulation of corticostriatal glutamatergic neurotransmission. Sci. Adv. 2017, 3, e1601631. [Google Scholar] [CrossRef] [Green Version]
- Unger, E.L.; Bianco, L.E.; Jones, B.C.; Allen, R.P.; Earley, C.J. Low brain iron effects and reversibility on striatal dopamine dynamics. Exp. Neurol. 2014, 261, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Huss, M.; Völp, A.; Stauss-Grabo, M. Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems—An observational cohort study. Lipids Health Dis. 2010, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bourre, J.M. Effects of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain. Part 1: Micronutrients. J. Nutr. Health Aging 2006, 10, 377–385. [Google Scholar]
- Sinn, N.; Wilson, C. Dietary Supplementation with Highly Unsaturated Fatty Acids: Implications for Interventions with Persons with Mental Retardation from Research on Infant Cognitive Development, ADHD, and Other Developmental Disabilities. Int. Rev. Res. Ment. Retard. 2006, 159–196. [Google Scholar] [CrossRef]
- Arnold, L.E.; Pinkham, S.M.; Votolato, N. Does Zinc Moderate Essential Fatty Acid and Amphetamine Treatment of Attention-Deficit/Hyperactivity Disorder? J. Child Adolesc. Psychopharmacol. 2000, 10, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Calarge, C.; Farmer, C.; DiSilvestro, R.; Arnold, L.E. Serum Ferritin and Amphetamine Response in Youth with Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2010, 20, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.A.; Xie, D.; Zimmerman, B.M. Iron Status in Toddlerhood Predicts Sensitivity to Psychostimulants in Children. J. Atten. Disord. 2010, 16, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [Green Version]
| Study | Refer. | N | Supp. | Dose | Age (Years) | Sex | Duration | Measures for ADHD | Results | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Noorazar et al. (2020) | [69] | 60 | Zinc | 10 mg zinc/day | Range = 7–12 Mean = 9.67 | 20% Women 80% Men | 6 weeks | ● Conners Parent’s Questionnaire. | Zinc related with improvement, but only in the inattention factor |
| 2. | Zamora et al. (2011) | [70] | 40 | Zinc | 10 mg zinc/day | Range = 7–14 Mean = 9.8 | 22.5% Women 77.5% Men | 6 weeks | ● Conners Rating Scales-Revised. | Improvement in the Conner’s score, but only for the teacher version |
| 3. | Arnold et al. (2011) | [71] | 52 | Zinc | 15 mg zinc/day | Range = 6–14 Mean = 9.8 | 17.3% Women 82.7% Men | 13 weeks | ● Childen’s Interview for Psychiatric Syndromes (parent version). ● Parent and teacher behavioral ratings. ● Clinical Global Impressions (CGI). ● Conners’ Parent Rating Scale ● Neuropsychological cognitive-motor test battery. | Equivocal results for most measures. Only neuropsychological measures mostly favored zinc |
| 4. | Akhondzadeh et al. (2004) | [72] | 44 | Zinc | 15 mg zinc/day | Range = 5–11 Mean = 7.9 | 40.9% Women 59.1% Men | 6 weeks | ● ADH Rating Scale (ADHD-RS) | Parent and Teacher rating scale scores improved with zinc |
| 5. | Bilici et al. (2004) | [73] | 400 | Zinc | 40 mg zinc/day | Range = 6–14 Mean = 9.6 | 18% Women 82% Men | 12 weeks | ● Attention Deficit Hyperactivity Disorder Scale (ADHDS) ● Conners Teacher Questionnaire ● DuPaul Parent Ratings of ADHD | Zinc related with improvements in hyperactive, impulsive and socialization symptoms. No impact of zinc was observed for the attention deficit levels. A moderator effect with age and BMI was observed |
| 6. | Konofal et al. (2008) | [74] | 22 | Iron | 80 mg/day (ferrous sulfate, Tardyferon) | Range = 5–8 Mean = 5.9 | 21.7% Women 78.3% Men | 12 weeks | ● Conners’ Parent Rating Scale ● Conners’ Teacher Rating Scale ● Attention Deficit Hyperactivity Disorder Rating Scale (ADHD RS). ● Clinical Global Impression-Severity (CGI-S). | Iron related to improvement on the ADHD RS scale and the CGI-S score. Iron did not achieved improvements on the Conner’s tests |
| 7. | Panahandeh et al. (2017) | [75] | 42 | Iron | 5 mg/kg/day (ferrous sulfate) | Range = 5–15 Mean = 8.9 | 9.5% Women 90.5% Men | 8 weeks | ● Child Symptom Inventory-4 (CSI-4). | Iron related with higher decreases on the CSI-4 total and factor scores |
| 8. | Rucklidge et al. (2018) | [76] | 93 | Zinc- Iron | Zinc (3.2 mg/capsule) Iron (0.9 mg/capsule). Dose: starting 3 and increasing to 12 capsules/day | Range = 5–15 Mean = 9.7 | 23.7% Women 76.3% Men | 10 weeks | ● Conners’ Parent Rating Scale (CPRS-R) ● Strengths and Difficulties Questionnaire (SDQ, Parents version) ● Strengths and Difficulties Questionnaire (SDQ, Teachers version) | Supplements related with improvements in inattentive levels. No contribution were observed on hyperactive-impulsive symptoms. |
| 9. | Rucklidge et al. (2021) | [58] | 38 | Zinc- Iron | Zinc (3.2 mg/capsule) Iron (0.9 mg/capsule). Dose: starting 3 and increasing to 12 capsules/day | Range = 7–13 Mean = 10.1 | 21% Women 79% Men | 10 weeks | ● ADH Rating Scale IV (ADHD-RS-IV). ● Children’s Depression Rating Scale (CDRS) ● Children’s Globabl Assessment Scale (CGAS) | Differences in the ferritin levels achieved an interaction role for improving the ADHD severity levels. |
| Title: Includes Design Type | Abstract: Structured-Complete | Introduction: Background | Introduction: Objectives-Hypothesis | Methods: Design Described | Methods: Participants | Methods: Interventions | Methods: Outcomes | Methods: Sample-Size Calculation—Power | Methods: Randomization | Methods: Implementation | Methods: Statistical Procedure | Results: participants Flow | Results: Numbers Analyzed | Results: Outcomes-Estimates | Discussion: Limitations | Discussion: Generalization | Discussion: Interpretation | Other Registration-Protocol-Funding | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Noorazar et al. (2020) | [69] | (+) | (+) | (+) | (P) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (P) | (+) | (P) | (+) | (+) | |
| 2. | Zamora et al. (2011) | [70] | (+) | (+) | (+) | (P) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (P) | (?) | (P) | (+) | (+) | |
| 3. | Arnold et al. (2011) | [71] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (P) | (+) | (+) | |
| 4. | Akhondzadeh et al. (2004) | [72] | (+) | (+) | (+) | (P) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (P) | (+) | (+) | |
| 5. | Bilici et al. (2004) | [73] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (P) | (P) | (?) | (P) | (+) | (+) | |
| 6. | Konofal et al. (2008) | [74] | (?) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (P) | (+) | (+) | |
| 7. | Panahandeh et al. (2017) | [75] | (?) | (+) | (+) | (P) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (P) | (+) | (+) | |
| 8. | Rucklidge et al. (2018) | [76] | (+) | (+) | (+) | (P) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (P) | (+) | (+) | |
| 9. | Rucklidge et al. (2021) | [58] | (?) | (+) | (+) | (P) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (P) | (+) | (+) |
| A1. Adequate Randomization Method | A2. Adequate Concealment of Allocation | A3. Groups Comparable at Baseline | B1. Comparison Groups Received Same Care | B2. Participants Receiving Care Blind to Treatment | B3. Individuals Administering Care Blind to Allocation | C1. All Groups Followed Up for Equal Length of Time | C2a. How Many Participants Did Not Complete Treatment | C2b. Groups Comparable for Treatment Completion | C3. Participants in Each Were No Outcome Data Available | C3b. Groups Comparable Respect Availability of Outcome Data | D1. Adequate Length of Follow-Up | D2. Precise Definition of Outcome | D3. Reliable Method Used to Determine the Outcome | D4. Investigators Kept Blind to Participants Exposure | D5. Investigators Were Kept Blind to Confounding-Predictors | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Noorazar et al. (2020) | [69] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) |
| 2. | Zamora et al. (2011) | [70] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) |
| 3. | Arnold et al. (2011) | [71] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) |
| 4. | Akhondzadeh et al. (2004) | [72] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) |
| 5. | Bilici et al. (2004) | [73] | (+) | (+) | (?) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) |
| 6. | Konofal et al. (2008) | [74] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) |
| 7. | Panahandeh et al. (2017) | [75] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) |
| 8. | Rucklidge et al. (2018) | [76] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) |
| 9. | Rucklidge et al. (2021) | [58] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) |
| A1.Clearly Focused Question | A2. Use of Randomization Method | A3. Participants Accounted for | B4. Use of “Blinded” Methods | B5. Groups Similar at the Start of Randomization | B6. Each Study Group Received the Same Care | C7. Effects of Intervention Adequately Reported | C8. Precision Estimates Reported (CI or Other Effect Sizes) | C9. Cost-Effectiveness Analysis Was Done | D10. Applicability of the Results | D11. Intervention Provides Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Noorazar et al. (2020) | [69] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) | (+) | (+) |
| 2. | Zamora et al. (2011) | [70] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) | (+) | (+) |
| 3. | Arnold et al. (2011) | [71] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (+) | (+) |
| 4. | Akhondzadeh et al. (2004) | [72] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) | (+) | (+) |
| 5. | Bilici et al. (2004) | [73] | (+) | (+) | (+) | (+) | (+) | (?) | (+) | (?) | (?) | (+) | (+) |
| 6. | Konofal et al. (2008) | [74] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (+) | (+) |
| 7. | Panahandeh et al. (2017) | [75] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (?) | (+) | (+) |
| 8. | Rucklidge et al. (2018) | [76] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (+) | (+) |
| 9. | Rucklidge et al. (2021) | [58] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (?) | (+) | (+) |
| Randomization | Deviations from the Intended Intervention/s | Missing Outcome Data | Outcome Measurements | Selective Reporting | Incomplete Reporting | Study Power Calculation/Sample Size Justification | |||
|---|---|---|---|---|---|---|---|---|---|
| 1. | Noorazar et al. (2020) | [69] | (?) | (+) | (+) | (+) | (+) | (+) | (−) |
| 2. | Zamora et al. (2011) | [70] | (?) | (+) | (+) | (+) | (+) | (+) | (−) |
| 3. | Arnold et al. (2011) | [71] | (?) | (+) | (+) | (+) | (+) | (+) | (−) |
| 4. | Akhondzadeh et al. (2004) | [72] | (?) | (+) | (+) | (+) | (+) | (+) | (−) |
| 5. | Bilici et al. (2004) | [73] | (?) | (+) | (+) | (+) | (+) | (+) | (−) |
| 6. | Konofal et al. (2008) | [74] | (?) | (+) | (+) | (+) | (+) | (+) | (−) |
| 7. | Panahandeh et al. (2017) | [75] | (?) | (+) | (+) | (+) | (+) | (+) | (−) |
| 8. | Rucklidge et al. (2018) | [76] | (?) | (+) | (+) | (+) | (+) | (+) | (−) |
| 9. | Rucklidge et al. (2021) | [58] | (?) | (+) | (+) | (+) | (+) | (+) | (−) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granero, R.; Pardo-Garrido, A.; Carpio-Toro, I.L.; Ramírez-Coronel, A.A.; Martínez-Suárez, P.C.; Reivan-Ortiz, G.G. The Role of Iron and Zinc in the Treatment of ADHD among Children and Adolescents: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 4059. https://doi.org/10.3390/nu13114059
Granero R, Pardo-Garrido A, Carpio-Toro IL, Ramírez-Coronel AA, Martínez-Suárez PC, Reivan-Ortiz GG. The Role of Iron and Zinc in the Treatment of ADHD among Children and Adolescents: A Systematic Review of Randomized Clinical Trials. Nutrients. 2021; 13(11):4059. https://doi.org/10.3390/nu13114059
Chicago/Turabian StyleGranero, Roser, Alfred Pardo-Garrido, Ivonne Lorena Carpio-Toro, Andrés Alexis Ramírez-Coronel, Pedro Carlos Martínez-Suárez, and Geovanny Genaro Reivan-Ortiz. 2021. "The Role of Iron and Zinc in the Treatment of ADHD among Children and Adolescents: A Systematic Review of Randomized Clinical Trials" Nutrients 13, no. 11: 4059. https://doi.org/10.3390/nu13114059
APA StyleGranero, R., Pardo-Garrido, A., Carpio-Toro, I. L., Ramírez-Coronel, A. A., Martínez-Suárez, P. C., & Reivan-Ortiz, G. G. (2021). The Role of Iron and Zinc in the Treatment of ADHD among Children and Adolescents: A Systematic Review of Randomized Clinical Trials. Nutrients, 13(11), 4059. https://doi.org/10.3390/nu13114059







