Abstract
In the present review we have discussed the occurrence of β-N-methylamino-L-alanine (BMAA) and its natural isomers, and the organisms and sample types in which the toxin(s) have been detected. Further, the review discusses general pathogenic mechanisms of neurodegenerative diseases, and how modes of action of BMAA fit in those mechanisms. The biogeography of BMAA occurrence presented here contributes to the planning of epidemiological research based on the geographical distribution of BMAA and human exposure. Analysis of BMAA mechanisms in relation to pathogenic processes of neurodegeneration is used to critically assess the potential significance of the amino acid as well as to identify gaps in our understanding. Taken together, these two approaches provide the basis for the discussion on the potential role of BMAA as a secondary factor in neurodegenerative diseases, the rationale for further research and possible directions the research can take, which are outlined in the conclusions.
1. Introduction
Some strains of cyanobacteria are known to produce potent hepato-, cyto-, neuro- and/or dermatotoxins and other bioactive compounds []. Blooms of cyanobacteria and cyanobacteria-associated health problems have been documented throughout the world [,]. Many cyanobacterial poisonings can be traced back to microcystins, cyanobacterial peptide hepatotoxins, tumor promoters and possible carcinogens, which may pose a serious threat to health through contaminated drinking water []. Other cyanobacterial toxins (cyanotoxins) include compounds from the groups of cylindrospermopsins (cytotoxins), anatoxins and saxitoxins (neurotoxins) and nodularins (hepatotoxins, tumor promoters and possible carcinogens). All of these toxins have been implicated in cyanobacterial poisonings of either humans or animals.
One interesting compound in the spectrum of cyanobacterial metabolites is the non-proteinogenic amino acid β-N-methylamino-L-alanine, abbreviated BMAA. This compound, according to systematic chemical nomenclature (S)-2-amino-3-methylaminopropanoic acid (L-BMAA), has the natural isomers (S)-2,4-diaminobutyric acid (L-DAB), N-(2-aminoethyl)glycine (AEG) and β-amino-N-methylalanine (BAMA) []. This review concentrates on BMAA which is the best characterised of the compounds.
BMAA was first observed in cycad seeds some 55 years ago [], and its occurrence and properties continue to inspire scientists of various disciplines []. BMAA has been suggested to play a causal role in amyotrophic lateral sclerosis/Parkinsonism-dementia complex (ALS/PDC), found at an elevated incidence in the Chamorro people of the Pacific island of Guam []. The Chamorro people are thought to become exposed to the neurotoxin through the use of cycad seed flour contaminated by BMAA originating from symbiotic cyanobacteria. As BMAA can be found or even biomagnified in certain food web compartments [,,] it is also possible that a major share of the BMAA exposure on Guam occurs through the traditional consumption of cycad-feeding bats.
Early research pointed to a wide occurrence of BMAA in free-living and symbiotic cyanobacteria in many parts of the world, at up to mg/g DW levels []. Another paper from South Africa confirmed the taxonomic ubiquity of BMAA in freshwater cyanobacteria, but the reported toxin concentrations were lower []. BMAA was found in combination with additional cyanotoxins in British waterbodies []. In contrast, a study involving 62 cyanobacterial samples of worldwide origin found no BMAA in any of the cyanobacterial samples []. BMAA has also been reported in other groups of microalgae (Supplementary Table S1). The extent of BMAA production by cyanobacteria and other organisms may not thus be fully understood, but it seems a widely observed phenomenon. Out of 74 publications dealing with BMAA detection and quantification in samples of cyanobacteria or human tissues, only 12 failed to detect a BMAA signal in environmental samples []. Less is known about the transfer and possible biomagnification of BMAA in food webs. Further, it is not fully understood how much of the toxin occurs in free, protein-bound or soluble-bound forms [] and whether such a bound toxin is bioavailable.
Some of the BMAA analytical protocols have been shown to suffer from methodological problems or at least from poor reporting [], and therefore, especially older literature must be treated with caution. Analytical methods relying on liquid chromatography and fluorescence detection of derivatized BMAA seem to be more prone to report either false positives or an overestimation of BMAA concentration in the studied samples. More selective and sufficiently validated analytical protocols involving appropriate sample preparation and relying on liquid chromatography and tandem mass spectrometry (MS/MS) for the detection and quantification of (derivatized) BMAA have overcome most, but not all, of the methodological challenges [,]. The AOAC-accepted method for BMAA in a cyanobacterial matrix is based on ultra-performance liquid chromatography of AQC-derivatized BMAA and tandem mass spectrometry [].
As will be shown in this review, the importance of BMAA in neurodegenerative disease continues to be controversial. Shortcomings in exposure assessment and the slow onset of disease (i.e., a delay between low-level exposure and observed pathological effects) obscure the understanding of the role and significance of BMAA in neurodegenerative diseases. While there is strong circumstantial evidence connecting BMAA exposure and ALS/PDC disease in the Chamorro people, there is much less evidence of the toxico-hygienic relevance of BMAA in other environments and settings. BMAA has been reported in the brain tissues of patients dying from ALS/PDC or Alzheimer’s disease [] but it was not found in the brains of patients with confirmed Alzheimer’s disease in another study []. For instance, it is not known whether BMAA should be seen as a causative or a contributing factor in the neurodegenerative process. A critical review concluded that a causal relationship between BMAA and neurodegenerative disease is not supported by existing data [] but some of the arguments in the review were later refuted []. According to the precautionary principle and knowing the possibly grave outcome of exposure to the toxin, it would be logical to try to avoid all exposure to BMAA until a comprehensive exposure assessment, full toxicological data and a medical consensus about the risks are available.
This review first discusses the biogeography of BMAA occurrence in environmental samples and the organisms in which BMAA has been detected. The review then describes general pathogenic mechanisms of neurodegenerative diseases and how BMAA fits into these. Based on the exposure assessment and a medical understanding of the neurodegenerative processes, some conclusions about the role and significance of BMAA in neurodegeneration will be drawn, and possible avenues for further research proposed.
2. Occurrence of BMAA
2.1. Data Search Strategy
An extensive literature search was performed on the Scopus, PubMed and Web of Science databases to explore the presence of BMAA in ecosystems worldwide, as well as cases of associated human and animal poisonings, and experimental toxicological publications. Searches were performed using the following key words: (“BMAA”, “DAB” or “AEG”) and (“poisoning”, “intoxication”, “incident”, “death”, “mortality”, “health”, “health effects”, “ALS”, “ALS/PDC”, “Alzheimer’s”, “neurodegenerative diseases”, “adverse effects”, “exposure”, “environment”, “cyanobacteria”, “microalgae”, “phytoplankton”, “zooplankton”, “fish”, “bivalvia”, “crustacea”, “gastropoda”, “mammals” or “plants”). Papers found through these searches contained some additional relevant references.
2.2. Occurrence of BMAA in Cyanobacteria and Microalgae
Based on the published papers listed in Supplementary Table S1 (Occurrence of BMAA and its isomers in environmental samples and organisms), BMAA and its isomers (DAB and AEG) were found to be produced by cyanobacteria belonging to 29 genera: Anabaena [,,,,,,], Anabaenopsis [], Aphanizomenon [,,,,,], Calothrix [,,], Chlorogloeopsis [], Chroococcidiopsis [,], Cyanobium [,], Cylindrospermopsis [,], Fischerella [,], Gomphosphaeria [], Leptolyngbya [,,,,,], Lyngbya [,,], Microcoleus [,], Microcystis [11,13,14,23–25, 34], Myxosarcina [,], Nodularia [,,,,,,], Nostoc [,,,,,,,,], Oscillatoria [,], Phormidium [,,,], Planktothrix [,,,], Plectonema [], Prochlorococcus [], Pseudanabaena [], Scytonema [,], Symploca [,], Synechococcus [,,,,,], Synechocystis [,,], Trichodesmium [] and Woronichinia []. These cyanobacteria represented: (a) a biomass of cyanobacteria collected from: freshwater (40 reports from different localities), marine (5 reports) and brackish (1 report) environments and (b) cultures of cyanobacterial strains originating from freshwaters (28 strains), marine habitats (14 strains), brackish waters (33 strains), symbiotic plants (10 strains) and terrestrial environments (4 strains). For 32 strains, the origin could not be found in the corresponding publications. There are also some reports about the presence of BMAA and/or its isomers in the biomass of collected cyanobacteria, but the producers were not identified [,,,,,,,,,].
BMAA and its isomers have been found in 22 supplements made of biomass of the cyanobacteria Spirulina [,,] and Aphanizomenon [,] (Table S1). The compounds have been also detected in cyanobacterial biocrust samples from Qatar [,,,] and aerosols (air-filters from the lakeshore) [].
In total, the presence of BMAA and/or its isomers was linked to more than 200 findings related to cyanobacteria from nature (freshwaters, marine and brackish environment, terrestrial habitats and plant symbionts), market samples and specimens from culture collections. Although BMAA and its isomers are found in many ecosystems, the occurrence of these compounds is not ubiquitous. A total of 387 environmental and biological samples (water, fish, aquatic plants) taken from Nebraska (USA) were analysed for BMAA, DABA and anatoxin-a (a compound unrelated to BMAA and DABA). Measurable levels of BMAA, DABA and anatoxin-a were found in 18%, 17% and 12% of the samples, respectively []. In a study involving bloom-impacted lakes and reservoirs of Brazil, Canada, France, Mexico and the United Kingdom, 390 samples were taken from 45 lake sampling sites. AEG and DAB isomers were detected in 30% and 43% of the samples, respectively, while BAMA was found in less than 8% of the samples and BMAA was not observed in any sample []. No BMAA was found in any of the analyzed biological loess crusts (BLCs, terrestrial samples) taken from various locations in Serbia, China and Iran []. These results indicate that while BMAA occurs in many ecosystems, it is not present in every ecosystem. The results also underline the need to look for the isomers of BMAA (DAB, AEG, BAMA) in order to get a more complete picture of the occurrence of the BMAA family of compounds.
Due to the inconsistency in the methods, the measured concentrations of BMAA have not been reported in this review, but it is only indicated whether BMAA and its isomers have been detected in the samples. It can also be noted that the BMAA and isomer concentrations are not stable in one ecosystem but vary temporally and spatially. They depend on when (daily, monthly and annual variations), where and how the samples have been taken from a waterbody, and how the samples have been analyzed. Further, BMAA can be found in either free or bound forms which necessitate due attention during the analysis and reporting []. Comparisons are therefore more meaningful within one publication with a consistent methodology. The physico-chemical environment also seems to have an influence on the BMAA concentration. BMAA in environmental phytoplankton samples ranged from 1 µg/g to 276 μg/g DW, while cultures had higher values ranging from 20 µg/g to 6.4 mg/g DW []. One of the most extensive analytical studies on the occurrence of BMAA isomers in bloom-impacted lakes and reservoirs analyzed environmental water samples from five countries []. The study did not detect BMAA in any of the samples but observed isomers at the following min-max concentrations: 10–1100 ng/L (DAB), 5–19,000 ng/L (AEG) and 15–56 ng/L (BAMA). The same paper [] further presented widely varying BMAA isomer concentrations in environmental water samples reported in previously published studies. For instance, the reported BMAA concentrations in environmental waters in the paper [] and four earlier papers cited therein varied dramatically: not detected, 6.5–7 ng/L (mean values from two years), 10–300 ng/L, 110 ng/L, 1800–25,300 ng/L. The interested reader is advised to consult the papers listed in Table S1 for the reported individual BMAA/isomer concentrations in various sample types and many organisms, but it should be noted that the absolute values reported may not be comparable between the papers.
There are reports that planktonic diatoms (Bacillariophyta), dinoflagellates (Pyrrhophyta), green algae (Chlorophyta), euglenas (Euglenophyta), red algae (Rhodophyta), Haptophyta and Cryptophyta also produce BMAA and/or its isomers (Supplementary Table S1). Diatoms are represented by 15 genera: Achnanthes [], Asterionellopsis [], Aulacoseira [], Chaetoceros [,], Cyclotella [], Fragilaria [], Halamphora [], Navicula [,], Odontella [], Phaeodactylum [,], Proboscia [], Pseudo-nitzschia [], Skeletonema [,], Tabellaria [] and Thalassiosira [,,]; dinoflagellates by seven genera: Alexandrium [,], Gymnodinium [], Heterocapsa [], Prorocentrum [], Pyrocystis [], Scrippsiella [] and Symbiodinium []; green algae by four genera: Ostreococcus [], Chlamydomonas [], Chlorella [] and Dunaliella []; Euglenophyta and Rhodophyta by one genus each: Eutreptiella [] and Porphyridium [], respectively; Haptophyta by two genera: Tisochrysis [] and Emiliana []; and Cryptophyta by four genera: Hemiselmis [], Proteomonas [], Rhinomonas [] and Rhodomonas [] isolated from marine and freshwater environments.
2.3. Occurrence of BMAA in Animals
BMAA has also been detected in the zooplankton community in the Baltic Sea [,]. The presence of BMAA and its isomers in zooplankton organisms, molluscs, crustaceans, fish, birds, mammals and other animals is a consequence of the bioaccumulation of BMAA in food webs [,,].
In the group of molluscs, BMAA was found in bivalvia Anodonta woodiana [], Antigona lamellaris [], Arca inflate [], Atrina pectinate [], Cerastoderma edule [], Chlamys farreri [], Corbicula fluminea [], Crassosstrea sp. [], Crassosstrea gigas [,,], Crassostrea virginica [], Gafrarium tumidum [], Mactra chinensis [], Mercenaria mercenaria [], Moerella iridescens [], Mytilus coruscus [], Mytilus edulis [,,,], Mytilus edulis platensis [], Mytilus galloprovincialis [,,,], Ostrea edulis [,,], Periglypta petechialis [], Perna canaliculus [], Perna viridis [], Placopecten magellanicus [], Ruditapes philippinarum [], Scapharca subcrenata [], Sinonovacula constricta [], Solen strictus [], Tegillarca granosa [] and an unidentified mussel []. BMAA was also found in gastropods Bellamya aeruginosa [], Neverita didyma [], Neptunea cumingii [], Natica maculosa [], Haliotis discus hannai [], Volutharpa ampullaceal [] and Rapana venosa [].
The arthropods (Crustacea) in which BMAA was found are represented by ten species: Callinectes sapidus [,,], Cancer pagurus [], Heterocarpus ensifer [], Eriocheir sinensis [], Macrobrachium nipponense [], Mysis mixta [], Neomysis integer [], Palaemon modestus [], Panulirus sp. [] and Procambarus clarkia [].
In the tissues of the fish, there was evidence of accumulation of BMAA after the consumption of BMAA producers, mostly cyanobacteria. A total of 39 species of fish showed the presence of BMAA in their tissues: Abramis brama [], Anguilla anguilla [], Aristichthys nobilis [], Carassius auratus [], Carcharhinus acronotus [,], Carcharhinus leucas [,], Carcharhinus limbatus [,], Clupea harengus [,], Coilia ectenes taihuensis [], Coregonus lavaretus [,], Cyprinus carpio [,], Erythroculter ilishaeformis [], Esox lucius [], Galeocerdo cuvier [], Ginglymostoma cirratum [,], Gymnocephalus cernua [], Hemiramphus kurumeus [], Hypophthalmichthys molitrix [], Neosalanx taihuensis [], Negaprion brevirostris [,], Osmerus eperlanus [], Parabramis pekinensis [], Parasilurus asotus [], Pelteobagrus fulvidraco [], Perca fluviatilis [], Pleuronectes platessa [], Protosalanx hyalocranius [], Pseudorasbora parva [], Rhizoprionodon terraenovae [], Rhodeus sinensis [], Rutilus rutilus [], Salvelinus alpinus [], Sander lucioperca [], Scophthalmus maximus [], Sphyrna mokarran [,], Sphyrna tiburo [,], Sphyrna zygaena [], Tinca tinca [] and Triglopsis quadricornis []. Fish samples from Nebraska reservoirs (carp, white crappie, bass, shad, walleye, catfish, wiper and bluegill) showed the presence of BMAA, and in many samples, also the presence of DAB []. BMAA, DAB and/or AEG were found in 16 fish-based dietary supplements (shark cartilage powders) from seven manufacturers [].
The reports about mammals showed that BMAA was detected in flying foxes [,], in dolphins [] and in human brain tissue from some patients who died from ALS [,,]. It was also found in human hair [].
2.4. Occurrence of BMAA in Plants
BMAA and/or its isomers were found in parts of the following symbiotic and other plants: Azolla filiculoides [], Brassica oleracea [], Cycas micronesica [,,,], Cycas revoluta [,,], Cycas debaoensis [], Gunnera kauaiensi [], Lathyrus latifolius [] and aquatic plants from Nebraska []. BMAA was found in flour prepared from the gametophyte of cycad seeds [].
2.5. Exposure to BMAA
The reports presented in Table S1 suggest that exposure to BMAA can occur through the same routes of exposure that are known for other cyanotoxins: through drinking water (e.g., in a case when a cyanobacterial mass development occurs in a drinking water reservoir), recreational activities (e.g., swimming, canoeing or bathing), the aquatic food web, terrestrial plants and animals and food supplements [,,,]. Synthesized by cyanobacteria and microalgae, BMAA is transported through some food webs in aquatic ecosystems from zooplankton and benthos invertebrates, planktivorous fish, shellfish, snails and crustaceans to carnivorous fish and mammals. Human contact with BMAA is possible through all these food web compartments in aquatic ecosystems. In terrestrial ecosystems, BMAA can be found in some symbioses of cyanobacteria with higher plants, but it can also be transferred from aquatic ecosystems into terrestrial ecosystems through irrigation. As a consequence of irrigation with BMAA-contaminated water, BMAA can be accumulated in plant tissues and thus reach animals and humans. The potential collective exposure burden through different groups of organisms shown in Table S1 might be one part of the explanation behind significant concentrations of BMAA (up to 350 µg/g) in the brain tissues of some patients who died from ALS/PDC and Alzheimer’s disease [,,].
As presented in Figure 1, BMAA and/or its isomers have been found throughout the world and exposure scenarios either through contaminated drinking water or consumption of contaminated foodstuffs are present in most parts of the world. There are clearly some hotspots of BMAA occurrence: parts of Europe, the United States and China as well as some islands including Guam. The largest number of reports deal with European countries which could be an indication of active research on the topic there. The absence of reports from, e.g., most African, Asian and South American countries probably does not mean the absence of BMAA and its isomers in these parts of the world, but that those territories were not as thoroughly investigated as for instance Western European countries. The situation with BMAA is similar to that of cylindrospermopsin which was first thought to be a tropical toxin. Generally speaking, cylindrospermopsin has been found in most countries where it has been looked for carefully enough and this is the likely scenario with BMAA, too.
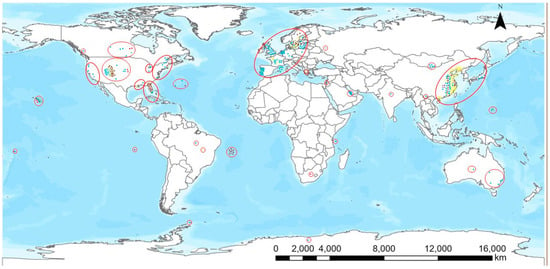
Figure 1.
Geographical distribution of the occurrence of BMAA and its isomers. Color coding of the dots: phytoplankton and zooplankton, blue; plants, green; bivalvia, yellow; gastropoda, orange; crustacea, purple; fish, brown; mammals, red.
According to the map shown, BMAA and also its isomers are frequently recorded in regions where analyses have been performed. Assuming methodological robustness, BMAA and its isomers are thus found in most parts of the world, and therefore there are potential exposure scenarios in many countries. This exposure would be reflected in the incidence of neurodegenerative diseases if BMAA is regarded as a causative factor for such diseases and the exposure levels are high enough. Likewise, the incidence of neurodegenerative diseases would increase upon BMAA exposure even if BMAA is only regarded as a contributing factor in the presence of other risk factors present in the investigated areas. Whether exposure to BMAA in natural conditions really has an effect on the incidence of neurodegenerative diseases has to be proven and confirmed by epidemiological research [,].
3. BMAA and the Pathogenic Mechanisms of Neurodegenerative Diseases
3.1. General Mechanisms of Neurodegeneration
Although neurodegenerative diseases (NDDs) are a heterogeneous group of disorders, each of them affecting distinct anatomical or functional units within the nervous system, many share common pathogenic processes: protein misfolding and aggregation; disorders of proteostasis; mitochondrial damage and oxidative stress; microglial activation and chronic inflammation; and disorders of synaptic function (Figure 2).
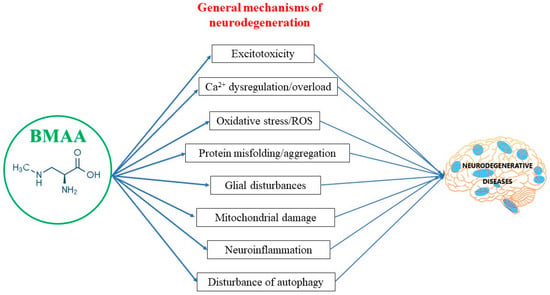
Figure 2.
BMAA and general mechanisms of neurodegeneration.
The process of protein misfolding and aggregation is one of the hallmarks of NDDs. Protein aggregates are almost invariably present in affected tissues, and often constitute classical pathological criteria for these diseases, even though the proteins involved may differ. In Alzheimer’s disease they are amyloid beta (Aβ), that forms senile plaques, and microtubule-associated protein tau (MAPT, often referred to as just tau) which assembles neurofibrillary tangles; in Parkinson’s disease it is alpha-synuclein which is the primary structural component of Lewy bodies; in amyotrophic lateral sclerosis the main aggregate constituent is TAR DNA-binding protein 43 (TDP-43), etc. Apart from being valuable diagnostic tools, aggregates are also very important in terms of pathogenesis since they often exhibit neurotoxicity and/or trigger other pathogenic processes that lead to neuronal death. For instance, Aβ oligomers may induce mitochondrial dysfunction and oxidative stress, bind to a variety of receptors causing synaptic dysfunction, induce calcium dysregulation, activate intracellular signalling to induce apoptosis, and induce activation of microglia and production of proinflammatory cytokines []. They also promote tau phosphorylation, thus fostering tau aggregation into neurofibrillary tangles, which can themselves be neurotoxic. Alpha-synuclein has been shown to have detrimental effects on several intracellular organelles and pathways, including mitochondria, lysosomes, synapses, and microtubules []. Finally, aggresomes, aggregates of misfolded TDP-43, may adversely affect neuronal function and viability by causing numerous disturbances such as cellular stress, inhibition of axonal transport, mitochondrial dysfunction, inhibition of endocytosis, and reduced autophagy [].
Proteostasis is the balance between protein synthesis and degradation in the cell. It is crucial for the maintenance of cellular homeostasis, especially in post-mitotic cells such as neurons. Two main proteostasis mechanisms in charge of altered protein removal are autophagy and the ubiquitin-proteasome system (UPS). Both of these pathways are dysfunctional in most NDDs [,]. Since autophagy carries out the degradation of long-lived protein aggregates, and crucially depends on lysosomal function, defects in lysosomal membrane stability, enzymatic content, and activity or acidification of the lysosomal lumen can result in reduced aggregate removal or accumulation of altered degradation pathway intermediates. Autophagy is also involved in organelle dynamics. Therefore, disorders of autophagy affect the neuron’s capacity to maintain proper mitochondrial quality control [].
In NDDs mitochondria are not affected only by altered autophagy. Protein aggregates can interfere with numerous mitochondrial enzymes, induce disturbances in the respiratory chain complex, and promote free radical generation. This leads to lower mitochondrial ATP production, reduced capacity of mitochondria for calcium handling, excessive production of reactive oxygen species and mitochondrial membrane permeabilization (MMP). All these have deleterious effects on neurons. Reduced energy production interferes with numerous cellular processes in neurons including synaptic assembly and neurotransmission, thus altering synaptic function. Calcium can cause both necrosis and apoptosis, and also damages mitochondrial function, which will be discussed further with excitotoxicity. Excessive mitochondrial ROS generation affects diverse signalling pathways including apoptosis, oxygen sensing, protein kinases, phosphatases, and transcription factors, leading to cell damage and death. Oxidative stress can also stimulate protein misfolding. For instance, in Alzheimer’s disease oxidative stress increases tau phosphorylation. In NDDs, in addition to increased ROS production, the oxidative stress defenses are reduced as well. For example, the altered enzymatic activity of superoxide dismutase has been shown in both PD and ALS. Finally, MMP induces loss of the mitochondrial membrane potential, subsequent respiratory chain uncoupling, reduced ATP synthesis, lysosome disintegration, cell swelling and finally, cell death. As mentioned above, altered lysosome function will affect autophagy which may hinder aggregate removal. In addition, MMP elicits the release of proteins that induce apoptosis and of mitochondrial DNA that plays an important role in neuroinflammation [,,].
The role of microglial activation and neuroinflammation was long considered to be secondary in NDDs, i.e., a reaction to the tissue and cellular damage. Recent research is changing this stance in favour of a more primary pathogenic involvement. The pivotal role in this is attributed to Triggering Receptor Expressed on Myeloid cells 2 (TREM2). TREM2 receptors are selectively expressed in myeloid cells (which are microglia in the brain). They attenuate inflammatory reactions and promote phagocytosis. Mutations of TREM2 have been associated with increased risk for late-onset AD and linked to frontotemporal dementia and ALS. In NDDs, altered TREM2 activation, as well as other causes such as the presence of extracellular mitochondrial DNA secondary to mitochondrial damage and increased mitochondrial membrane permeability, could provoke pathological microglial activation and disturbance of microglial function []. Since microglia serve multiple roles in the nervous system, including induction of programmed cell death of neurons, phagocytosis, neuronal plasticity and synaptic pruning, disruption of microglial function could lead to neurodegeneration, reduced removal of aggregates and synaptic malfunction. Aberrant activation of microglia also gives rise to the uncontrolled release of proinflammatory mediators resulting in chronic neuroinflammation []. Chronic inflammation in NDDs can be provoked by mitochondrial damage and oxidative stress too. As a consequence of chronic inflammation, there is dysfunction in detecting or responding to the accumulation of protein aggregates, morphological and functional disturbances of the mitochondria, dysregulation of adult neurogenesis, loss of synapses and altered synaptic plasticity [].
Disturbances of synaptic function on the circuit level result in network instability and dysfunction, while on the single-cell level lead to excitotoxicity. Excitotoxicity is a mechanism of neuronal cell death that is induced by the overactivation of glutamate receptors. The exaggerated activation of glutamate receptors may be a consequence of excessive excitatory neurotransmitter release, reduced excitatory neurotransmitter removal, the presence of exogenous excitatory amino acids, and altered expression or kinetics of glutamate receptors. Whatever the cause, the result is an accumulation of Ca2+ in the neuron. This leads to cellular damage and death due to excess stimulation of Ca2+-sensitive targets, many of which control key cellular functions. For example, Ca2+-dependant activation of calpains is an essential component of necrosis; lipoxygenases are Ca2+-activated enzymes that can induce inflammatory response and apoptosis; and a set of DNA-ses involved in apoptosis are Ca2+-activated enzymes as well. Furthermore, taking up the excess of cytosolic Ca2+ by the mitochondria damages these organelles with consequences that have already been discussed. Finally, many enzymes involved in protein phosphorylation, folding and proteostasis are Ca2+ sensitive, which indicates the role that Ca2+ overload may have in the formation of protein aggregates [,].
All these pathogenic processes in NDDs are inextricably linked. Activation or disturbance of any of them may undermine proper function or promote activation of many others in a feedforward manner so that the effects amplify and the vicious circle of neurodegeneration is formed.
3.2. Involvement of BMAA in Pathogenic Mechanisms of Neurodegeneration
Reports from numerous researchers, utilizing various models and routes of administration indicate the potential of BMAA to trigger virtually all of the pathogenic mechanisms associated with primary neurodegeneration (Table 1).

Table 1.
Summary of studies on the effects/mechanisms of BMAA neurotoxicity.
BMAA has been shown to produce a host of synaptic disturbances related to neurotransmitters crucial for neurodegenerative diseases—acetylcholine, which is a key player in Alzheimer’s disease; dopamine, which has a central role in Parkinson’s disease; and glutamate, a neurotransmitter in central motor neurons which are affected by the amyotrophic lateral sclerosis. Rakonczay et al. have reported a significant decrease in acetylcholine esterase activity and glutamate binding in brain homogenates of BMAA-treated rats []. Spencer et al. [] have demonstrated that macaques treated with oral L-dopa, a dopamine replacement therapy, showed selective recovery of marked neurological disturbances induced by BMAA. Interestingly, Lindstrom et al., although reporting dose-dependent lesions of substantia nigra produced by BMAA in rats, have not found any effects on dopamine levels []. However, Carion et al. provide evidence that BMAA induces altered expression of genes for the dopamine D4 receptor, indicating that a disturbance in the dopaminergic network may not be transmitter-dependent only but receptor-related as well []. Finally, de Munck and colleagues have shown that levels of glutamate are increased and levels of GABA decreased in the motor cortex of rats upon intraperitoneal application of BMAA [].
Significant as these findings are, from a mechanistic point of view, a more interesting property of BMAA is its potential to trigger excitotoxicity. Indeed, excitotoxicity was the first mechanism of action of BMAA to be hypothesised and investigated. The ability of BMAA to mimic glutamate and act at the glutamate receptors was demonstrated both via reduction or blockade of BMAA-induced subcellular effects and neurotoxicity by glutamate receptor antagonists and in electrophysiological studies.
Attenuation of subcellular and cellular BMAA-induced damage by NMDA ionotropic glutamate receptor (iGluR) antagonists was demonstrated in mice mixed cortical cultures by Ross et al. []. Weiss and Choi went on to show that BMAA causes signs of both the acute phase of excitotoxicity (neuronal swelling) and the late phase of excitotoxicity (neurodegeneration), in dissociated mouse cortical cultures. Effects were attenuated by NMDA iGluR antagonists and produced only in presence of bicarbonate ions []. Blockade of NMDA iGluRs was also shown to be protective against vacuolisation and swelling of the neuronal somas in chick retina [].
An early indication that non-NMDA ionotropic glutamate receptors may be involved in the action of BMAA as well came from Weiss et al. They have reported that neuronal cell death in murine mixed cortical cultures induced by BMAA can be prevented by non-NMDA receptor antagonists at lower doses of BMAA (300 µM), and by NMDA receptor antagonists at higher doses of the amino acid (3 mM, []). Similarly, and more recently, Liu and co-workers [] have shown in cultured septal and mesencephalic neurons of mice that NMDA receptor antagonists are protective against neurotoxic effects at higher BMAA doses (500 µM), while at lower BMAA doses (300 µM) the death of neurons is attenuated by non-NMDA receptor antagonists. Activation of non-NMDA iGluRs has also been shown to mitigate BMAA-induced neurological disturbance in mice [], and neurotoxicity in murine cell cultures [,], while NMDA, non-NMDA and metabotropic glutamate receptors (mGluR) were protective against BMAA neurotoxicity in human embryonal and neuroblastoma cell cultures [].
Electrophysiological studies have provided further evidence of the excitatory nature and excitotoxic potential of BMAA. Weiss and Choi have reported evidence of bicarbonate-dependant rapid membrane depolarisation and increased membrane conductance which were attenuated by NMDA receptor antagonists [], Wilson et al. have shown that BMAA-elicited depolarisation of field potentials were blocked by NMDA receptor antagonists in rat neocortical slices [], while Lobner et al. provide evidence that NMDA receptor antagonists attenuate the BMAA induced inward current []. Non-NMDA receptors have been implicated in electrophysiological effects too. BMAA-induced membrane depolarisation, increase in intracellular Na+ and decrease of intracellular K+ concentration of leech Retzius neurons have been partially blocked by non-NMDA receptor antagonists []. Additionally, Cucchiaroni et al. conclude that reversible membrane depolarization and inward current of dopaminergic and GABAergic neurons of rat midbrain slices are mediated by the AMPA subtype of non-NMDA iGluRs.
Finally, the previously mentioned finding of de Munck et al. that illustrates an imbalance between glutamate and GABA-favouring excitation [] indicates that BMAA could induce excitotoxicity not only by direct action on glutamate receptors but also by perturbing the balance between excitatory and inhibitory neurotransmission.
Since Ca2+ overload is the principal mechanism by which excitotoxicity induces neuronal injury and death, it is no surprise that in many studies the disturbance of cellular Ca2+ homeostasis was investigated together with activation of glutamate receptors. It has been shown that BMAA administration leads to Ca2+ influx into the cell and intracellular Ca2+ accumulation in neurons [,,,,] and glia [,]. As mentioned before, in most of these studies Ca2+ dysregulation has been linked to the activation of glutamate receptors, but other mechanisms have also been proposed, such as the activation of system Xc− [] and the sodium/calcium exchanger [].
BMAA has been shown to induce mitochondrial vacuolisation [,], fragmentation [], and reduced mitochondrial viability []. BMAA can also produce endoplasmic reticulum disassembly [,]. This is important because the endoplasmic reticulum and mitochondria form mitochondria-associated ER membranes (MAMs), which are involved in calcium homeostasis, mitochondrial dynamics, autophagy, inflammation, and apoptosis. The levels of glutamate dehydrogenase 1 [], an enzyme implicated in glutamate metabolism and energy homeostasis, as well as glycogen synthase kinase-3 beta (GSK-3β) [], an enzyme with a role in glucose homeostasis and energy metabolism, inflammation, mitochondrial dysfunction, and apoptotic pathways, are altered as a result of BMAA application too.
As a result of organelle and enzymatic disturbances, BMAA affects numerous cell critical processes. Energy metabolism in neurons is reduced with diminished oxygen consumption, reduced glycolysis and ATP production []. Increased production of reactive oxygen species (ROS), and oxidative stress are induced by BMAA in murine neurons from the spinal cord [], cortex [,], neuroblastoma [] and neuronal stem cell cultures [], in rat midbrain slices [] and human cell lines []. As indirect evidence of oxidative stress induction and ROS-mediated cellular damage, ROS scavengers have been shown to be protective in BMAA-induced neuronal cell death [,]. It has also been demonstrated that BMAA induces ROS generation in the rat [,] and human [] glial cells.
Another process linked to mitochondrial damage is the induction of apoptosis. BMAA induces apoptosis and neuronal loss in the cingulate cortex and the hippocampus of mice [,] and rat motoneurons [] and alters caspases 3 and 9 levels and activity in the rat motoneurons [,], cortex, hippocampus, substantia nigra and spinal cord []. BMAA can also influence the apoptosis regulator Bax and trigger the unfolded protein response (UPR) mediated apoptosis [].
As discussed above, a crucial mechanism for the sustainment of healthy mitochondria and the maintenance of mitochondrial dynamics is autophagy. BMAA alters autophagy in rat cerebellar Purkinje cells [] and affects factors with prominent roles in autophagy such as TRPML1 (transient receptor potential cation channel, mucolipin subfamily, member 1), lysosomal function and sequestosome-1/ubiquitin-binding protein p62 (SQSTM1/p62) in rat motoneurons [].
Autophagy is significant not only for mitochondrial dynamics, but also for aggregate removal, and therefore disturbances of autophagy favour the accumulation of misfolded protein aggregates. BMAA contributes to this process through aggregate formation as well. It induces enzymes involved in protein misfolding and aggregation [,,,] and can lead to the accumulation of all relevant protein aggregates—amyloid-beta [,,], tau protein [,,,,,], alpha-synuclein [] and TDP-43 [,,,,,,].
Neuroinflammation and microglial activation are common to many neurodegenerative diseases. BMAA stimulates the NLR family pyrin domain containing 3 (NLRP3) protein, a component of inflammasomes that trigger an inflammatory response, increases the activity of caspase-1, an enzyme that initiates inflammation and activates interleukin 1β, an important mediator of inflammation that can promote the spread of inflammation and is also elevated as a result of BMAA application []. BMAA causes overexpression of other pro-inflammatory factors as well, such as cyclooxygenase-2 (COX-2), nuclear factor kappa B (NF-κB) and tumor necrosis factor alpha (TNF-α) [] and can lead to activation of microglia [].
Finally, BMAA has been shown to alter gene expression and induce genotoxicity in nerve cells [,,,,,,,], thus changing the expression of proteins related to mitochondrial dysfunction, apoptosis, ROS handling and proteostasis and creating a milieu that facilitates neurodegeneration.
4. Discussion and Conclusions
In the present review we have discussed the biogeography of BMAA occurrence in environmental samples representing different organisms worldwide, and how BMAA fits into general pathogenic mechanisms of neurodegenerative diseases.
BMAA is a widely occurring compound both taxonomically and geographically (Supplementary Table S1). It is present in cyanobacteria from nature (freshwater, marine and brackish environments, terrestrial habitats and plant symbionts), market samples and specimens from culture collections. It has been shown to bioaccumulate or even biomagnify in certain organisms consumed as food. Exposure scenarios are thus present in many parts of the world. As cyanobacteria are generally favoured by the continued discharge of nutrients into waterbodies, rising CO2 levels and warmer water temperature [] it is likely that the frequency and magnitude of cyanobacterial problems will increase in the future.
An analysis of the biomedical literature on BMAA shows that although this review is based on references that are only examples of the indicated mechanisms of action of BMAA (Table 1), and not a comprehensive presentation of all references that show the action of BMAA (according to our records more than 200 papers), it can be easily seen that pure synthetic BMAA was used in most experiments (more than 92% of selected papers). In addition to pure synthetic BMAA, purified BMAA from cycad material, extracts of cyanobacteria, flour of cycads and extracts of cycads were used in BMAA research. Although the toxicity of a substance can be best studied when analysing it in a pure form, there are very rare occasions when people are exposed to pure cyanobacterial toxins, including BMAA. In natural conditions, BMAA is found in a combination with other (secondary) metabolites of cyanobacteria or plants, the potential synergistic action of which is neglected when working with the pure toxin. In the natural sources of BMAA, other compounds that either increase or decrease the toxicity or influence the onset of disease can be found. The importance of these compounds is completely ignored when pure BMAA is utilised in the experiments []. Due to such potential interaction of cyanobacteria and cycad biomass extracts, one concentration of BMAA in the extract can be either more or less toxic than the same concentration of pure BMAA.
In the surveyed studies, the natural route of human exposure was also partially neglected. It is known that the sources of BMAA are the biomass of cyanobacteria (highest during mass occurrence), the biomass of plants (mainly cycads), and animals (to which BMAA has been transferred through food chains). In natural conditions, humans can be exposed to BMAA present in drinking and recreational waters and through food such as aquatic and terrestrial animals, edible plants, and cyanobacteria-based food supplements [,,]. Intended ingestion and accidental ingestion are more relevant for exposure than dermal contact or inhalation. For these reasons, it would be reasonable that the most common route of BMAA exposure would be oral, which was not applied in the majority of analysed papers (Table 1). The oral route of exposure was followed in only 20% of experiments which is equal to the percentage of the subcutaneous injection route. Other exposure routes were also applied in the surveyed investigations: intracerebroventricular (14%), immersion (11%), intraperitoneal (11%), intravenous (9%), inhalation (6%), intracranial (3%), intranasal (3%) and intrathecal (3%).
The majority of the tested organisms (Table 1) were mammals in the in vivo studies (37%), which is the expected scenario when looking for a more realistic picture of the BMAA role in neurodegenerative diseases. In addition to experiments with mammals, in vivo experiments were done with fish (5%) and arthropods (4%). The remaining 54% of the papers were in vitro studies where cell lines were dominant (34%), then in vitro experiments with mammals (7%), annelids (1%), birds (1%) and enzyme kits (1%).
The usually chronic nature of BMAA exposure and the delayed mechanisms of BMAA action were typically underestimated in the experiments. Most experiments were done under acute exposure (84% of the surveyed papers) while the emphasis should be on oral, chronic BMAA exposure to environmentally observed concentrations.
However, even though there are some shortcomings, the research spanning more than thirty years described in this review illustrates that the neurotoxicity of BMAA has been established in a multitude of animal and cellular models, through various routes of administration and involving multiple mechanisms of neuronal damage and death. Yet, the role of this amino acid in the pathogenesis of neurodegenerative diseases remains a matter of controversy and dispute. This dissent can be attributed in large part to the fact that relatively high concentrations of BMAA were required to produce the effects. This indeed is a problem if BMAA is viewed as a sole, or even principal causative agent of neurodegeneration, a stance that has sometimes been taken or proposed. However, this approach may be problematic for two reasons.
Firstly, it is considered that the aetiology and pathogenesis of primary neurodegeneration are multifactorial. The causes are believed to involve genetic, environmental and factors related to aging. These causes trigger multiple mechanisms that are interrelated, and that may affect and amplify each other, forming complex and overlapping vicious circles which finally result in cell dysfunction and death []. As an illustration, therapeutic approaches targeting a single one of the pathogenic pathways have mainly shown limited success [].
The papers analyzed in this review provide evidence that BMAA can initiate and/or facilitate most of the mechanisms related to neurodegeneration. In this regard, even without the potency to cause the disease, by altering the intensity and kinetics of intertwined processes and pathways central to neurodegeneration, BMAA may act as a secondary or contributing factor. In favour of this, Lobner et al. have shown that BMAA at concentrations of 10–100 µmol potentiates neurotoxicity induced by NMDA, Fe, amyloid-β and MPP+ []. In other words, in genetically or otherwise susceptible individuals that would develop the disease regardless of exposure, exposure to BMAA would lead to the earlier onset and faster progression of the illness.
The second point is that, as discussed above, BMAA has almost exclusively been used in research alone and as a synthetic compound, even though in nature it often appears together with other environmental factors including, but not limited to, BMAA isomers and metals. Compounds isomeric to BMAA have been shown to be neurotoxic themselves [], while some metals, such as iron, mercury, lead and aluminium, are also known to contribute to neurodegeneration []. It is noteworthy that the presence of metals related to neurotoxicity has been reported in soil and water in the regions of the Western Pacific where a cluster of a high incidence of neurodegenerative diseases, the so-called amyotrophic lateral sclerosis/Parkinsonism dementia complex (ALS/PDC), has first spurred the interest for BMAA as a factor in neurotoxicity []. Additionally, the use of the seeds of the Cycas palm is epidemiologically linked to the occurrence of ALS/PDC in affected populations, and BMAA isomers were detected in the seeds of at least one species []. Therefore, BMAA could act in concert with other neurotoxic compounds and environmental factors in an additive or synergistic ways.
Although some of these issues have been addressed (e.g., Nunn et al. [] and Takser et al. []), more research is needed to further elucidate the matter. We propose that this further research could be directed at the interactions of BMAA with other environmental factors, as well as the possibility that BMAA acts as a contributing factor that speeds up rather than initiates the pathogenic processes of neurodegeneration, thus not causing the neurodegenerative diseases but leading to their earlier onset and faster progression. In conclusion, the biogeographic and biomedical data in the literature strongly suggest global distribution and risk of human exposure to BMAA, as well as neurotoxicity of this amino acid. Even though this does not mean that BMAA is in any way linked to neurodegenerative diseases, it does justify further research. Risk of human exposure prompts the need for the matter to be resolved, while BMAA remains relevant even as a secondary factor since delayed onset and slower progression of the neurodegenerative diseases would be a significant benefit not only to the patients and their families but also to health and social services. In order to establish whether the presence and neurotoxic properties of BMAA pose a risk to human health, continuing research efforts into the epidemiology and potential role of BMAA in the pathogenesis of neurodegeneration are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10122418/s1, Table S1: Occurrence of BMAA and its isomers in environmental samples and organisms.
Author Contributions
S.L., Z.S. and J.M. conceptualized the manuscript. All authors contributed to the data search and writing. S.L. and A.K. delivered medical expertise, Z.S. and T.P.M. biological expertise, and J.M. and A.I. biochemical and chemical expertise. Section 1 was authored by J.M.; Section 2 and Table S1 by Z.S. and T.P.M.; Section 3 by S.L. and A.K.; Figure 1 and Figure 2 by A.I. and S.L.; Table 1 by S.L. and A.I.; Abstract and Section 4 by all authors together with strongest input by S.L. and J.M. Technical support was delivered by A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Science Fund of the Republic of Serbia, #7726976, Integrated Strategy for Rehabilitation of Disturbed Land Surfaces and Control of Air Pollution–RECAP. The authors acknowledge financial support of the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-68/2022-14/200125, 451-03-68/2022-14/200287).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Aβ | amyloid beta |
| AchE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| AEG | N-(2-aminoethyl)glycine |
| AIF | apoptosis inducing factor |
| ALS | amyotrophic lateral sclerosis |
| ALS/PDC | amyotrophic lateral sclerosis/Parkinsonism dementia complex |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AOAC | Association of Official Analytical Chemists |
| AQC | 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate |
| BDNF | brain derived neurotrophic factor |
| BLC | biological loess crust |
| BMAA | β-N-methylamino-L-alanine |
| BSO | buthionine-S-R-sulfoximine |
| CaM | calmodulin |
| CDC-42 | cell division control protein 42 homolog |
| CD50 | half curative dose |
| COX-2 | cyclooxygenase-2 |
| cyt-c | cytochrome-c |
| DA | dopamine |
| DAB | 2,4-diaminobutyric acid |
| DRD4 | dopamine D4 receptor |
| DW | dry weight |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| GD | gestation day |
| GLT-1 | glutamate transporter 1 |
| GRP78 | 78 kDa glucose-regulated protein |
| GSK-3β | glycogen synthase kinase-3 beta |
| iGluR | ionotropic glutamate receptor |
| i.c.v. | intracerebroventricular |
| IL-1β | interleukin 1 beta |
| i.p. | intraperitoneal |
| i.v. | intravenous |
| JNK | c-Jun N-terminal kinase |
| LC3-II | microtubule-associated protein 1 light chain 3, |
| LDH | lactate dehydrogenase |
| MAMs | mitochondria-associated ER membranes |
| MAOA | monoamine oxidase A |
| MAPT | microtubule associated protein tau |
| mGluR | metabotropic glutamate receptor |
| MMP | mitochondrial membrane permeabilization |
| MN | motoneuron |
| MPP+ | 1-methyl-4-phenylpyridinium |
| MS/MS | tandem mass spectrometry |
| NA | noradrenaline |
| NDD | neurodegenerative disease |
| NF-κB | nuclear factor kappa B |
| NFT | neurofibrillary tangle |
| NLRP3 | NLR family pyrin domain containing 3 protein |
| NMDA | N-methyl-D-aspartate |
| PD | Parkinson’s disease |
| PND | postnatal day |
| PP2A | protein phosphatase 2 |
| ROS | reactive oxygen species |
| s.c. | subcutaneous |
| SNpc | substantia nigra pars compacta |
| SQSTM1/p62 | lysosomal function and sequestosome-1/ubiquitin-binding protein p62 |
| TDP-43 | TAR DNA-binding protein 43 |
| TH | tyrosine hydroxylase |
| TLR | Toll-like receptor |
| TNF-α | tumour necrosis factor alpha |
| TREM2 | triggering receptor expressed on myeloid cells 2 |
| TRPML1 | transient receptor potential cation channel, mucolipin subfamily, member 1 |
| UPR | unfolded protein response |
| UPS | ubiquitin-proteasome system |
| Xc− | cystine/glutamate antiporter |
References
- Meriluoto, J.; Spoof, L.; Codd, G.A. (Eds.) Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Wiley: Chichester, UK, 2017; p. 548. [Google Scholar]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Đenić, D.; Simeunović, J.; Hiskia, A.; Kaloudis, T.; Mijović, B.; Šušak, S.; Protić, M.; et al. Lessons from the Užice case: How to complement analytical data. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; Wiley: Chichester, UK, 2017; pp. 298–308. [Google Scholar]
- Ploux, O.; Combes, A.; Eriksson, J.; Metcalf, J.S. β-N-methylamino-L-alanine and (S)-2,4-diaminobutyric acid. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; Wiley: Chichester, UK, 2017; pp. 160–164. [Google Scholar]
- Vega, A.; Bell, E.A. α-Amino-β-methylaminopropionic acid, a new amino acid from seeds of Cycas circinalis. Phytochemistry 1967, 6, 759–762. [Google Scholar] [CrossRef]
- Nunn, P.B. 50 years of research on α-amino-β-methylaminopropionic acid (β-methylaminoalanine). Phytochemistry 2017, 144, 271–281. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef]
- Jonasson, S.; Eriksson, J.; Berntzon, L.; Spáčcil, Z.; Ilag, L.L.; Ronnevi, L.-O.; Rasmussen, U.; Bergman, B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 9252–9257. [Google Scholar] [CrossRef]
- Réveillon, D.; Abadie, E.; Séchet, V.; Masseret, E.; Hess, P.; Amzil, Z. β-N-methylamino-l-alanine (BMAA) and isomers: Distribution in different food web compartments of Thau lagoon, French Mediterranean Sea. Mar. Environ. Res. 2015, 110, 8–18. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Esterhuizen, M.; Downing, T.G. β-N-methylamino-L-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol. Environ. Saf. 2008, 71, 309–313. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Lindsay, J.; Morrison, L.F.; Cox, P.A.; Codd, G.A. Co-occurrence of β-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ. Microbiol. 2008, 10, 702–708. [Google Scholar] [CrossRef]
- Krüger, T.; Mönch, B.; Oppenhäuser, S.; Luckas, B. LC-MS/MS determination of the isomeric neurotoxins BMAA (β-N-methylamino-L-alanine) and DAB A mechanism for slow release (2,4-diaminobutyric acid) in cyanobacteria and seeds of Cycas revoluta and Lathyrus latifolius. Toxicon 2010, 55, 547–557. [Google Scholar] [CrossRef]
- Banack, S.A.; Murch, S.J. Methods for the chemical analysis of β-N-methylamino-L-alanine: What is known and what remains to be determined. Neurotox. Res. 2018, 33, 184–191. [Google Scholar] [CrossRef]
- Faassen, E.J.; Antoniou, M.G.; Beekman-Lukassen, W.; Blahova, L.; Chernova, E.; Christophoridis, C.; Combes, A.; Edwards, C.; Fastner, J.; Harmsen, J.; et al. A collaborative evaluation of LC-MS/MS based methods for BMAA analysis: Soluble bound BMAA found to be an important fraction. Mar. Drugs 2016, 14, 45. [Google Scholar] [CrossRef]
- Faassen, E.J. Presence of the neurotoxin BMAA in aquatic ecosystems: What do we really know? Toxins 2014, 6, 1109–1138. [Google Scholar] [CrossRef]
- Bishop, S.L.; Murch, S.J. A systematic review of analytical methods for the detection and quantification of β-N-methylamino-L-alanine (BMAA). Analyst 2020, 145, 13–28. [Google Scholar] [CrossRef]
- Glover, W.B.; Baker, T.C.; Murch, S.J.; Brown, P.N. Determination of β-N-methylamino-L-alanine, N-(2-aminoethyl)glycine, and 2,4-diaminobutyric acid in food products containing cyanobacteria by ultra-performance liquid chromatography and tandem mass spectrometry: Single-laboratory validation. J. AOAC Int. 2015, 98, 1559–1565. [Google Scholar] [CrossRef]
- Meneely, J.P.; Chevallier, O.P.; Graham, S.; Greer, B.; Green, B.D.; Elliot, C.T. β-methylamino-L-alanine (BMAA) is not found in the brains of patients with confirmed Alzheimer’s disease. Sci. Rep. 2016, 6, 36363. [Google Scholar] [CrossRef]
- Chernoff, N.; Hill, D.J.; Diggs, D.L.; Faison, B.D.; Francis, B.M.; Lang, J.R.; Larue, M.M.; Le, T.-T.; Loftin, K.A.; Lugo, J.N.; et al. A critical review of the postulated role of the non-essential amino acid, β-N-methylamino-L-alanine, in neurodegenerative disease in humans. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 183–229. [Google Scholar] [CrossRef]
- Dunlop, R.A.; Banack, S.A.; Bishop, S.L.; Metcalf, J.S.; Murch, S.J.; Davis, D.A.; Stommel, E.W.; Karlsson, O.; Brittebo, E.B.; Chatziefthimiou, A.D.; et al. Is exposure to BMAA a risk factor for neurodegenerative diseases? A response to a critical review of the BMAA hypothesis. Neurotox. Res. 2021, 39, 81–106. [Google Scholar]
- Fan, H.; Qiu, J.; Fan, L.; Li, A. Effects of growth conditions on the production of neurotoxin 2,4-diaminobutyric acid (DAB) in Microcystis aeruginosa and its universal presence in diverse cyanobacteria isolated from freshwater in China. Environ. Sci. Pollut. Res. Int. 2015, 22, 5943–5951. [Google Scholar] [CrossRef]
- Craighead, D.; Metcalf, J.S.; Banack, S.A.; Amgalan, L.; Reynolds, H.V.; Batmunkh, M. Presence of the neurotoxic amino acids beta-N-methylamino-L-alanine (BMAA) and 2,4-diamino-butyric acid (DAB) in shallow springs from the Gobi Desert. Amyotroph. Lateral Scler. 2009, 10 (Suppl. 2), 96–100. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J.; Gillissen, F.; Zweers, H.A.; Lürling, M. Determination of the neurotoxins BMAA (β-N-methylamino-L-alanine) and DAB (alpha-,gamma-diaminobutyric acid) by LC-MSMS in Dutch urban waters with cyanobacterial blooms. Amyotroph. Lateral Scler. 2009, 2, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Metcalf, J.S.; Jiang, L.; Craighead, D.; Ilag, L.L.; Cox, P.A. Cyanobacteria produce N-(2-aminoethyl)glycine, a backbone for peptide nucleic acids which may have been the first genetic molecules for life on Earth. PLoS ONE 2012, 7, e49043. [Google Scholar] [CrossRef] [PubMed]
- Spáčil, Z.; Eriksson, J.; Jonasson, S.; Rasmussen, U.; Ilag, L.L.; Bergmana, B. Analytical protocol for identification of BMAA and DAB in biological samples. Analyst 2010, 135, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J.; Gillissen, F.; Lürling, M. A comparative study on three analytical methods for the determination of the neurotoxin BMAA in cyanobacteria. PLoS ONE 2012, 7, e36667. [Google Scholar] [CrossRef]
- Réveillon, D.; Abadie, E.; Séchet, V.; Brient, L.; Savar, V.; Bardouil, M.; Hess, P.; Amzil, Z. Beta-N-methylamino-L-alanine: LC-MS/MS optimization, screening of cyanobacterial strains and occurrence in shellfish from Thau, a French Mediterranean lagoon. Mar. Drugs 2014, 12, 5441–5467. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cianca, R.C.; Lopes, V.R.; Almeida, C.M.; Vasconcelos, V.M. Determination of the non protein amino acid β-N-methylamino-l-alanine in estuarine cyanobacteria by capillary electrophoresis. Toxicon 2011, 58, 410–414. [Google Scholar] [CrossRef]
- Cervantes Cianca, R.C.; Baptista, M.S.; Lopes, V.R.; Vasconcelos, V.M. The non-protein amino acid β-N-methylamino-L-alanine in Portuguese cyanobacterial isolates. Amino Acids 2012, 42, 2473–2479. [Google Scholar] [CrossRef]
- Jiang, L.; Johnston, E.; Aberg, K.M.; Nilsson, U.; Ilag, L.L. Strategy for quantifying trace levels of BMAA in cyanobacteria by LC/MS/MS. Anal. Bioanal. Chem. 2013, 405, 1283–1292. [Google Scholar] [CrossRef]
- Jiang, L.; Eriksson, J.; Lage, S.; Jonasson, S.; Shams, S.; Mehine, M.; Ilag, L.L.; Rasmussen, U. Diatoms: A novel source for the neurotoxin BMAA in aquatic environments. PLoS ONE 2014, 9, e84578. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, Q.; Chen, X.; Wang, X.; Liao, X.; Jiang, L.; Wu, J.; Yang, L. Occurrence and transfer of a cyanobacterial neurotoxin β-methylamino-L-alanine within the aquatic food webs of Gonghu Bay (Lake Taihu, China) to evaluate the potential human health risk. Sci. Total Environ. 2014, 468–469, 457–463. [Google Scholar] [CrossRef]
- Monteiro, M.; Costa, M.; Moreira, C.; Vasconcelos, V.M.; Baptista, M.S. Screening of BMAA-producing cyanobacteria in cultured isolates and in in situ blooms. J. Appl. Phycol. 2017, 29, 879–888. [Google Scholar] [CrossRef]
- Baker, T.C.; Tymm, F.J.M.; Murch, S.J. Assessing environmental exposure to β-N-methylamino-L-alanine (BMAA) in complex sample matrices: A comparison of the three most popular LC-MS/MS methods. Neurotox. Res. 2018, 33, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Abbes, S.; Vo Duy, S.; Munoz, G.; Dinh, Q.T.; Simon, D.F.; Husk, B.; Baulch, H.M.; Vinçon-Leite, B.; Fortin, N.; Greer, C.W.; et al. Occurrence of BMAA isomers in bloom-impacted lakes and reservoirs of Brazil, Canada, France, Mexico, and the United Kingdom. Toxins 2022, 14, 251. [Google Scholar] [CrossRef]
- Al-Sammak, M.A.; Hoagland, K.D.; Cassada, D.; Snow, D.D. Co-occurrence of the cyanotoxins BMAA, DABA and anatoxin-a in Nebraska reservoirs, fish, and aquatic plants. Toxins 2014, 6, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Jungblut, A.D.; Wilbraham, J.; Banack, S.A.; Metcalf, J.S.; Codd, G.A. Microcystins, BMAA and BMAA isomers in 100-year-old Antarctic cyanobacterial mats collected during Captain R.F. Scott’s Discovery Expedition. Eur. J. Phycol. 2018, 53, 115–121. [Google Scholar] [CrossRef]
- Lage, S.; Annadotter, H.; Rasmussen, U.; Rydberg, S. Biotransfer of β-N-methylamino-L-alanine (BMAA) in a eutrophicated freshwater lake. Mar. Drugs 2015, 13, 1185–1201. [Google Scholar] [CrossRef]
- Murch, S.; Cox, P.A.; Banack, S.A. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc. Natl. Acad. Sci. USA 2004, 101, 12228–12231. [Google Scholar] [CrossRef]
- Pip, E.; Munford, K.; Bowman, L. Seasonal Nearshore Occurrence of the Neurotoxin β N methylamino L alanine (BMAA) in Lake Winnipeg, Canada. Environ. Pollut. 2016, 5, 110–118. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Sauvé, S. Determination of BMAA and three alkaloid cyanotoxins in lake water using dansyl chloride derivatization and high-resolution mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5487–5501. [Google Scholar] [CrossRef]
- Zguna, N.; Karlson, A.M.L.; Ilag, L.L.; Garbaras, A.; Gorokhova, E. Insufficient evidence for BMAA transfer in the pelagic and benthic food webs in the Baltic Sea. Sci. Rep. 2019, 9, 10406. [Google Scholar] [CrossRef]
- McCarron, P.; Logan, A.C.; Giddings, S.D.; Quilliam, M.A. Analysis of β-N-methylamino-L-alanine (BMAA) in spirulina-containing supplements by liquid chromatography-tandem mass spectrometry. Aquat. Biosyst. 2014, 10, 5. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of cyanotoxins in algae dietary supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.D.; Banack, S.A.; Cox, P.A. Biocrust-produced cyanotoxins are found vertically in the desert soil profile. Neurotox. Res. 2021, 39, 42–48. [Google Scholar] [CrossRef]
- Cox, P.A.; Richer, R.; Metcalf, J.S.; Banack, S.A.; Codd, G.A.; Bradley, W.G. Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans. Amyotroph. Lateral Scler. 2009, 10, 109–117. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Richer, R.; Cox, P.A. Neurotoxic amino acids and their isomers in desert environments. J. Arid Environ. 2015, 112, 140–144. [Google Scholar] [CrossRef]
- Richer, R.; Banack, S.A.; Metcalf, J.S.; Cox, P.A. The persistence of cyanobacterial toxins in desert soils. J. Arid Environ. 2015, 112, 134–139. [Google Scholar] [CrossRef]
- Banack, S.A.; Caller, T.; Henegan, P.; Haney, J.; Murby, A.; Metcalf, J.S.; Powell, J.; Cox, P.A.; Stommel, E. Detection of cyanotoxins, β-N-methylamino-L-alanine and microcystins, from a lake surrounded by cases of amyotrophic lateral sclerosis. Toxins 2015, 29, 322–336. [Google Scholar] [CrossRef]
- Dulić, T.; Svirčev, Z.; Palanački Malešević, T.; Faassen, E.J.; Savela, H.; Hao, Q.; Meriluoto, J. Assessment of common cyanotoxins in cyanobacteria of biological loess crusts. Toxins 2022, 14, 215. [Google Scholar] [CrossRef]
- Manolidi, K.; Triantis, T.M.; Kaloudis, T.; Hiskia, A. Neurotoxin BMAA and its isomeric amino acids in cyanobacteria and cyanobacteria-based food supplements. J. Hazard Mater. 2018, 365, 345–365. [Google Scholar] [CrossRef]
- Réveillon, D.; Séchet, V.; Hess, P.; Amzil, Z. Systematic detection of BMAA (β-N-methylamino-l-alanine) and DAB (2,4-diaminobutyric acid) in mollusks collected in shellfish production areas along the French coasts. Toxicon 2016, 110, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Violi, J.P.; Facey, J.A.; Mitrovic, S.M.; Colville, A.; Rodgers, K.J. Production of β-methylamino-L-alanine (BMAA) and its Isomers by freshwater diatoms. Toxins 2019, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Réveillon, D.; Séchet, V.; Hess, P.; Amzil, Z. Production of BMAA and DAB by diatoms (Phaeodactylum tricornutum, Chaetoceros sp., Chaetoceros calcitrans and, Thalassiosira pseudonana) and bacteria isolated from a diatom culture. Harmful Algae 2016, 58, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lage, S.; Costa, P.R.; Moita, T.; Eriksson, J.; Rasmussen, U.; Rydberg, S.J. BMAA in shellfish from two Portuguese transitional water bodies suggests the marine dinoflagellate Gymnodinium catenatum as a potential BMAA source. Aquat. Toxicol. 2014, 152, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Koksharova, O.A.; Safronova, N.A. Non-proteinogenic amino acid β-N-methylamino-L-alanine (BMAA): Bioactivity and ccological significance. Toxins 2022, 14, 539. [Google Scholar] [CrossRef]
- Li, A.; Song, J.; Hu, Y.; Deng, L.; Ding, L.; Li, M. New typical vector of neurotoxin β-N-methylamino-l-alanine (BMAA) in the marine benthic ecosystem. Mar. Drugs 2016, 14, 202. [Google Scholar] [CrossRef]
- Jiang, L.; Kiselova, N.; Rosén, J.; Ilag, L. Quantification of neurotoxin BMAA (b-N-methylamino-L-alanine) in seafood from Swedish markets. Sci. Rep. 2014, 4, 6931. [Google Scholar] [CrossRef]
- Christensen, S.J.; Hemscheidt, T.K.; Trapido-Rosenthal, H.; Laws, E.A.; Bidigare, R.R. Detection and quantification of β-methylamino-L-alanine in aquatic invertebrates. Limnol. Oceanogr.-Method 2012, 10, 891–898. [Google Scholar] [CrossRef]
- Salomonsson, M.L.; Fredriksson, E.; Alfjorden, A.; Hedeland, M.; Bondesson, U. Seafood sold in Sweden contains BMAA: A study of free and total concentrations with UHPLC–MS/MS and dansyl chloride derivatization. Toxicol. Rep. 2015, 2, 1473–1481. [Google Scholar] [CrossRef]
- Salomonsson, M.L.; Hanssonab, A.; Bondesson, U. Development and in-house validation of a method for quantification of BMAA in mussels using dansyl chloride derivatization and ultra performance liquid chromatography tandem mass spectrometry. Anal. Methods-UK 2013, 18, 4865–4874. [Google Scholar] [CrossRef]
- Field, N.C.; Metcalf, J.S.; Caller, T.A.; Banack, S.A.; Cox, P.A.; Stommel, E.W. Linking β-methylamino-L-alanine exposure to sporadic amyotrophic lateral sclerosis in Annapolis, MD. Toxicon 2013, 70, 179–183. [Google Scholar] [CrossRef]
- Banack, S.A.; Metcalf, J.S.; Bradley, W.G.; Cox, P.A. Detection of cyanobacterial neurotoxin β-N-methylamino-L-alanine within shellfish in the diet of an ALS patient in Florida. Toxicon 2014, 90, 167–173. [Google Scholar] [CrossRef]
- Mondo, K.; Hammerschlag, N.; Basile, M.; Pablo, J.; Banack, S.A.; Mash, D.C. Cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) in shark fins. Mar. Drugs 2012, 10, 509–520. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Davis, D.A.; Mondo, K.; Seely, M.S.; Murch, S.J.; Glover, W.B.; Divoll, T.; Evers, D.C.; Mash, D.C. Cyanobacterial neurotoxin BMAA and mercury in sharks. Toxins 2016, 8, 238. [Google Scholar] [CrossRef]
- Mondo, K.; Broc Glover, W.; Murch, S.J.; Liu, G.; Cai, Y.; Davis, D.A.; Mash, D.C. Environmental neurotoxins β-N-methylamino-L-alanine (BMAA) and mercury in shark cartilage dietary supplements. Food Chem. Toxicol 2014, 70, 26–32. [Google Scholar] [CrossRef]
- Banack, S.A.; Cox, P.A. Biomagnification of cycad neurotoxins in flying foxes: Implications for ALS-PDC in Guam. Neurology 2003, 61, 387–389. [Google Scholar] [CrossRef]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.M.; Murch, S.J.; Coyne, T.M.; Brand, L.M.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.G.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar] [CrossRef]
- Downing, S.; Scott, L.L.; Zguna, N.; Downing, T.G. Human scalp hair as an indicator of exposure to the environmental toxin β-N-methylamino-l-alanine. Toxins 2018, 10, 14. [Google Scholar] [CrossRef]
- Rosén, J.; Hellenäs, K.-E. Determination of the neurotoxin BMAA (β-N-methylamino-l-alanine) in cycad seed and cyanobacteria by LC-MS/MS (liquid chromatography tandem mass spectrometry). Analyst 2008, 12, 1785–1789. [Google Scholar] [CrossRef]
- Courtier, A.; Potheret, D.; Giannoni, P. Environmental bacteria as triggers to brain disease: Possible mechanisms of toxicity and associated human risk. Life Sci. 2022, 304, 120689. [Google Scholar] [CrossRef] [PubMed]
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human exposure to cyanotoxins and their effects on health. Arh. Hig. Rada Toksikol. 2013, 64, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Lance, E.; Arnich, N.; Maignien, T.; Biré, R. Occurrence of β-N-methylamino-l-alanine (BMAA) and isomers in aquatic environments and aquatic food sources for humans. Toxins 2018, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Iadanza, M.G.; Jackson, M.P.; Hewitt, E.W.; Ranson, N.A.; Radford, S.E. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Villar-Piqué, A.; Lopes da Fonseca, T.; Outeiro, T.F. Structure, function and toxicity of alpha-synuclein: The Bermuda triangle in synucleinopathies. J. Neurochem. 2016, 139 (Suppl. 1), 240–255. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Huntley, M.L.; Perry, G.; Wang, X. Pathomechanisms of TDP-43 in neurodegeneration. J. Neurochem. 2018, 146, 7–20. [Google Scholar] [CrossRef]
- Boland, B.; Yu, W.H.; Corti, O.; Mollereau, B.; Henriques, A.; Bezard, E.; Pastores, G.M.; Rubinsztein, D.C.; Nixon, R.A.; Duchen, M.R.; et al. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2018, 17, 660–688. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, T.; Zhang, L.; Zhou, Y.; Luo, H.; Xu, H.; Wang, X. Dysregulation of ubiquitin-proteasome system in neurodegenerative diseases. Front. Aging Neurosci. 2016, 8, 303. [Google Scholar] [CrossRef]
- Kumar, A.; Dhawan, A.; Kadam, A.; Shinde, A. Autophagy and mitochondria: Targets in neurodegenerative disorders. CNS Neurol. Disord. Drug Targets 2018, 17, 696–705. [Google Scholar] [CrossRef]
- Correia, S.C.; Santos, R.X.; Perry, G.; Zhu, X.; Moreira, P.I.; Smith, M.A. Mitochondrial importance in Alzheimer’s, Huntington’s and Parkinson’s diseases. Adv. Exp. Med. Biol. 2012, 724, 205–221. [Google Scholar]
- Tarafdar, A.; Pula, G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Kazim, S.N.; Islam, A. An Insight Into Mitochondrial Dysfunction and its Implications in Neurological Diseases. Curr. Drug Targets 2021, 22, 1585–1595. [Google Scholar] [CrossRef]
- Filipello, F.; Goldsbury, C.; You, S.F.; Locca, A.; Karch, C.M.; Piccio, L. Soluble TREM2: Innocent bystander or active player in neurological diseases? Neurobiol. Dis. 2022, 165, 105630. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Bano, D.; Ankarcrona, M. Beyond the critical point: An overview of excitotoxicity, calcium overload and the downstream consequences. Neurosci. Lett. 2018, 663, 79–85. [Google Scholar] [CrossRef]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, J.; Zhao, M.; Li, F.; Yu, R.; Li, A. Accumulation and distribution of neurotoxin BMAA in aquatic animals and effect on the behavior of zebrafish in a T-maze test. Toxicon 2020, 173, 39–47. [Google Scholar] [CrossRef]
- Muñoz-Sáez, E.; de Munck García, E.; Arahuetes Portero, R.M.; Martínez, A.; Solas Alados, M.T.; Miguel, B.G. Analysis of β-N-methylamino-L-alanine (L-BMAA) neurotoxicity in rat cerebellum. Neurotoxicology 2015, 48, 192–205. [Google Scholar] [CrossRef]
- Lopicic, S.; Nedeljkov, V.; Cemerikic, D. Augmentation and ionic mechanism of effect of β-N-methylamino-L-alanine in presence of bicarbonate on membrane potential of Retzius nerve cells of the leech Haemopis sanguisuga. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 284–292. [Google Scholar] [CrossRef]
- Carion, A.; Markey, A.; Hétru, J.; Carpentier, C.; Suarez-Ulloa, V.; Denoël, M.; Earley, R.L.; Silvestre, F. Behavior and gene expression in the brain of adult self-fertilizing mangrove rivulus fish (Kryptolebias marmoratus) after early life exposure to the neurotoxin β-N-methylamino-l-alanine (BMAA). Neurotoxicology 2020, 79, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Buenz, E.J.; Howe, C.L. Beta-methylamino-alanine (BMAA) injures hippocampal neurons in vivo. Neurotoxicology 2007, 28, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Pai, K.S.; Shankar, S.K.; Ravindranath, V. Billionfold difference in the toxic potencies of two excitatory plant amino acids, L-BOAA and L-BMAA: Biochemical and morphological studies using mouse brain slices. Neurosci. Res. 1993, 17, 241–248. [Google Scholar] [CrossRef]
- Martin, R.M.; Bereman, M.S.; Marsden, K.C. BMAA and MCLR interact to modulate behavior and exacerbate molecular changes related to neurodegeneration in larval zebrafish. Toxicol. Sci. 2021, 179, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Laugeray, A.; Oummadi, A.; Jourdain, C.; Feat, J.; Meyer-Dilhet, G.; Menuet, A.; Plé, K.; Gay, M.; Routier, S.; Mortaud, S.; et al. Perinatal exposure to the cyanotoxin β-N-méthylamino-L-alanine (BMAA) results in long-lasting behavioral changes in offspring-potential involvement of DNA damage and oxidative stress. Neurotox. Res. 2018, 33, 87–112. [Google Scholar] [CrossRef]
- Karlsson, O.; Roman, E.; Berg, A.L.; Brittebo, E.B. Early hippocampal cell death, and late learning and memory deficits in rats exposed to the environmental toxin BMAA (β-N-methylamino-L-alanine) during the neonatal period. Behav. Brain Res. 2011, 219, 310–320. [Google Scholar] [CrossRef]
- Rakonczay, Z.; Matsuoka, Y.; Giacobini, E. Effects of L-beta-N-methylamino-L-alanine (L-BMAA) on the cortical cholinergic and glutamatergic systems of the rat. J. Neurosci. Res. 1991, 29, 121–126. [Google Scholar] [CrossRef]
- Purdie, E.L.; Samsudin, S.; Eddy, F.B.; Codd, G.A. Effects of the cyanobacterial neurotoxin β-N-methylamino-L-alanine on the early-life stage development of zebrafish (Danio rerio). Aquat. Toxicol. 2009, 95, 279–284. [Google Scholar] [CrossRef]
- Xie, X.; Basile, M.; Mash, D.C. Cerebral uptake and protein incorporation of cyanobacterial toxin β-N-methylamino-L-alanine. Neuroreport 2013, 24, 779–784. [Google Scholar] [CrossRef]
- Tian, K.W.; Jiang, H.; Wang, B.B.; Zhang, F.; Han, S. Intravenous injection of l-BMAA induces a rat model with comprehensive characteristics of amyotrophic lateral sclerosis/Parkinson-dementia complex. Toxicol. Res.-UK 2016, 5, 79–96. [Google Scholar] [CrossRef]
- Karlsson, O.; Roman, E.; Brittebo, E.B. Long-term cognitive impairments in adult rats treated neonatally with β-N-methylamino-L-alanine. Toxicol. Sci. 2009, 112, 185–195. [Google Scholar] [CrossRef]
- Chiu, A.S.; Braidy, N.; Marçal, H.; Welch, J.H.; Gehringer, M.M.; Guillemin, G.J.; Neilan, B.A. Global cellular responses to β-methyl-amino-l-alanine (BMAA) by olfactory ensheathing glial cells (OEC). Toxicon 2015, 99, 136–145. [Google Scholar] [CrossRef]
- de Munck, E.; Muñoz-Sáez, E.; Miguel, B.G.; Solas, M.T.; Martínez, A.; Arahuetes, R.M. Morphometric and neurochemical alterations found in l-BMAA treated rats. Environ. Toxicol. Pharmacol. 2015, 39, 1232–1245. [Google Scholar] [CrossRef]
- Potjewyd, G.; Day, P.J.; Shangula, S.; Margison, G.P.; Povey, A.C. L-β-N-methylamino-l-alanine (BMAA) nitrosation generates a cytotoxic DNA damaging alkylating agent: An unexplored mechanism for neurodegenerative disease. Neurotoxicology 2017, 59, 105–109. [Google Scholar] [CrossRef][Green Version]
- Dawson, R., Jr.; Marschall, E.G.; Chan, K.C.; Millard, W.J.; Eppler, B.; Patterson, T.A. Neurochemical and neurobehavioral effects of neonatal administration of β-N-methylamino-L-alanine and 3,3’-iminodipropionitrile. Neurotoxicol. Teratol. 1998, 20, 181–192. [Google Scholar] [CrossRef]
- Tan, V.X.; Lassus, B.; Lim, C.K.; Tixador, P.; Courte, J.; Bessede, A.; Guillemin, G.J.; Peyrin, J.M. Neurotoxicity of the cyanotoxin BMAA through axonal degeneration and intercellular spreading. Neurotox. Res. 2018, 33, 62–75. [Google Scholar] [CrossRef]
- Shen, H.; Kim, K.; Oh, Y.; Yoon, K.S.; Baik, H.H.; Kim, S.S.; Ha, J.; Kang, I.; Choe, W. Neurotoxin β-N-methylamino-L-alanine induces endoplasmic reticulum stress-mediated neuronal apoptosis. Mol. Med. Rep. 2016, 14, 4873–4880. [Google Scholar] [CrossRef]
- Weiss, J.H.; Koh, J.Y.; Choi, D.W. Neurotoxicity of β-N-methylamino-L-alanine (BMAA) and β-N-oxalylamino-L-alanine (BOAA) on cultured cortical neurons. Brain Res. 1989, 497, 64–71. [Google Scholar] [CrossRef]
- Andersson, H.; Lindqvist, E.; Olson, L. Plant-derived amino acids increase hippocampal BDNF, NGF, c-fos and hsp70 mRNAs. Neuroreport 1997, 27, 1813–1817. [Google Scholar] [CrossRef]
- Lindström, H.; Luthman, J.; Mouton, P.; Spencer, P.; Olson, L. Plant-derived neurotoxic amino acids (β-N-oxalylamino-l-alanine and β-N-methylamino-l-alanine): Effects on central monoamine neurons. J. Neurochem. 1990, 55, 941–949. [Google Scholar] [CrossRef]
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. Biol. Sci. 2016, 283, 20152397. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Rush, T.; Ciske, J.; Lobner, D. Selective death of cholinergic neurons induced by beta-methylamino-L-alanine. Neuroreport 2010, 21, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Meldrum, B.S. Receptor site specificity for the acute effects of β-N-methylamino-alanine in mice. Eur. J. Pharmacol. 1990, 187, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.M.; Seelig, M.; Spencer, P.S. Specific antagonism of excitotoxic action of ‘uncommon’ amino acids assayed in organotypic mouse cortical cultures. Brain Res. 1987, 425, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.M.; Mabry, T.J.; Leslie, S.W. The cycad neurotoxic amino acid, β-N-methylamino-L-alanine (BMAA), elevates intracellular calcium levels in dissociated rat brain cells. J. Ethnopharmacol. 2002, 82, 159–167. [Google Scholar] [CrossRef]
- Purdie, E.L.; Metcalf, J.S.; Kashmiri, S.; Codd, G.A. Toxicity of the cyanobacterial neurotoxin beta-N-methylamino-L-alanine to three aquatic animal species. Amyotroph. Lateral Scler. 2009, 10 (Suppl. 2), 67–70. [Google Scholar] [CrossRef]
- Liu, X.Q.; Rush, T.; Zapata, J.; Lobner, D. β-N-methylamino-L-alanine induces oxidative stress and glutamate release through action on system Xc−. Exp. Neurol. 2009, 217, 429–433. [Google Scholar] [CrossRef]
- Lobner, D.; Piana, P.M.; Salous, A.K.; Peoples, R.W. β-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 2007, 25, 360–366. [Google Scholar] [CrossRef]
- Zhou, X.; Escala, W.; Papapetropoulos, S.; Zhai, R.G. β-N-methylamino-L-alanine induces neurological deficits and shortened life span in Drosophila. Toxins 2010, 2, 2663–2679. [Google Scholar] [CrossRef]
- Nunn, P.B.; Ponnusamy, M. β-N-Methylaminoalanine (BMAA): Metabolism and metabolic effects in model systems and in neural and other tissues of the rat in vitro. Toxicon 2009, 54, 85–94. [Google Scholar] [CrossRef]
- Scott, L.L.; Downing, T.G. β-N-methylamino-L-alanine (BMAA) toxicity is Gender and exposure-age dependent in rats. Toxins 2017, 10, 16. [Google Scholar] [CrossRef]
- Tedeschi, V.; Petrozziello, T.; Sisalli, M.J.; Boscia, F.; Canzoniero, L.M.T.; Secondo, A. The activation of Mucolipin TRP channel 1 (TRPML1) protects motor neurons from L-BMAA neurotoxicity by promoting autophagic clearance. Sci. Rep. 2019, 9, 10743. [Google Scholar] [CrossRef]
- Scott, L.L.; Downing, S.; Downing, T.G. The evaluation of BMAA inhalation as a potential exposure route using a rat model. Neurotox. Res. 2018, 33, 6–14. [Google Scholar] [CrossRef]
- Lee, M.; McGeer, P.L. Weak BMAA toxicity compares with that of the dietary supplement beta-alanine. Neurobiol. Aging 2012, 33, 1440–1447. [Google Scholar] [CrossRef]
- Waidyanatha, S.; Ryan, K.; Sanders, J.M.; McDonald, J.D.; Wegerski, C.J.; Doyle-Eisle, M.; Garner, C.E. Disposition of β-N-methylamino-l-alanine (L-BMAA), a neurotoxin, in rodents following a single or repeated oral exposure. Toxicol. Appl. Pharmacol. 2018, 15, 151–160. [Google Scholar] [CrossRef]
- Perry, T.L.; Bergeron, C.; Biro, A.J.; Hansen, S. β-N-methylamino-L-alanine: Chronic oral administration is not neurotoxic to mice. J. Neurol. Sci. 1989, 94, 173–180. [Google Scholar] [CrossRef]
- Wilson, J.M.; Khabazian, I.; Wong, M.C.; Seyedalikhani, A.; Bains, J.S.; Pasqualotto, B.A.; Williams, D.E.; Andersen, R.J.; Simpson, R.J.; Smith, R.; et al. Behavioral and neurological correlates of ALS-parkinsonism dementia complex in adult mice fed washed cycad flour. Neuromolecular. Med. 2002, 1, 207–221. [Google Scholar]
- Rao, S.D.; Banack, S.A.; Cox, P.A.; Weiss, J.H. BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Exp. Neurol. 2006, 201, 244–252. [Google Scholar] [CrossRef]
- Takser, L.; Benachour, N.; Husk, B.; Cabana, H.; Gris, D. Cyanotoxins at low doses induce apoptosis and inflammatory effects in murine brain cells: Potential implications for neurodegenerative diseases. Toxicol. Rep. 2016, 15, 180–189. [Google Scholar] [CrossRef]
- D’Mello, F.; Braidy, N.; Marçal, H.; Guillemin, G.; Rossi, F.; Chinian, M.; Laurent, D.; Teo, C.; Neilan, B.A. Cytotoxic effects of environmental toxins on human glial cells. Neurotox. Res. 2017, 31, 245–258. [Google Scholar] [CrossRef]
- Muñoz-Saez, E.; de Munck, E.; Arahuetes, R.M.; Solas, M.T.; Martínez, A.M.; Miguel, B.G. β-N-methylamino-L-alanine induces changes in both GSK3 and TDP-43 in human neuroblastoma. J. Toxicol. Sci. 2013, 38, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Zeevalk, G.D.; Nicklas, W.J. Acute excitotoxicity in chick retina caused by the unusual amino acids BOAA and BMAA: Effects of MK-801 and kynurenate. Neurosci. Lett. 1989, 102, 284–290. [Google Scholar] [CrossRef] [PubMed]
- van Onselen, R.; Downing, T.G. BMAA-protein interactions: A possible new mechanism of toxicity. Toxicon 2018, 143, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.H.; Choi, D.W. Beta-N-methylamino-L-alanine neurotoxicity: Requirement for bicarbonate as a cofactor. Science 1988, 19, 973–975. [Google Scholar] [CrossRef] [PubMed]
- de Munck, E.; Muñoz-Sáez, E.; Miguel, B.G.; Solas, M.T.; Ojeda, I.; Martínez, A.; Gil, C.; Arahuetes, R.M. β-N-methylamino-l-alanine causes neurological and pathological phenotypes mimicking Amyotrophic Lateral Sclerosis (ALS): The first step towards an experimental model for sporadic ALS. Environ. Toxicol. Pharmacol. 2013, 36, 243–255. [Google Scholar] [CrossRef]
- Spencer, P.S.; Nunn, P.B.; Hugon, J.; Ludolph, A.C.; Ross, S.M.; Roy, D.N.; Robertson, R.C. Guam amyotrophic lateral sclerosis-Parkinsonism-dementia linked to a plant excitant neurotoxin. Science 1987, 237, 517–522. [Google Scholar] [CrossRef]
- Pierozan, P.; Cattani, D.; Karlsson, O. Hippocampal neural stem cells are more susceptible to the neurotoxin BMAA than primary neurons: Effects on apoptosis, cellular differentiation, neurite outgrowth, and DNA methylation. Cell Death Dis. 2020, 11, 910. [Google Scholar] [CrossRef]
- Chiu, A.S.; Gehringer, M.M.; Braidy, N.; Guillemin, G.J.; Welch, J.H.; Neilan, B.A. Gliotoxicity of the cyanotoxin, β-methyl-amino-L-alanine (BMAA). Sci. Rep. 2013, 3, 1482. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Zhu, Y.; Diaz-Perez, Z.; Sheridan, M.; Royer, H.; Leibensperger, R., 3rd; Maizel, D.; Brand, L.; Popendorf, K.J.; et al. Exposure to aerosolized algal toxins in South Florida increases short- and long-term health risk in Drosophila model of aging. Toxins 2020, 11, 787. [Google Scholar] [CrossRef]
- Yin, H.Z.; Yu, S.; Hsu, C.I.; Liu, J.; Acab, A.; Wu, R.; Tao, A.; Chiang, B.J.; Weiss, J.H. Intrathecal infusion of BMAA induces selective motor neuron damage and astrogliosis in the ventral horn of the spinal cord. Exp. Neurol. 2014, 261, 1–9. [Google Scholar] [CrossRef]
- Karlsson, O.; Berg, A.L.; Hanrieder, J.; Arnerup, G.; Lindström, A.K.; Brittebo, E.B. Intracellular fibril formation, calcification, and enrichment of chaperones, cytoskeletal, and intermediate filament proteins in the adult hippocampus CA1 following neonatal exposure to the nonprotein amino acid BMAA. Arch. Toxicol. 2015, 89, 423–436. [Google Scholar] [CrossRef]
- Silva, D.F.; Candeias, E.; Esteves, A.R.; Magalhães, J.D.; Ferreira, I.L.; Nunes-Costa, D.; Rego, A.C.; Empadinhas, N.; Cardoso, S.M. Microbial BMAA elicits mitochondrial dysfunction, innate immunity activation, and Alzheimer’s disease features in cortical neurons. J. Neuroinflamm. 2020, 17, 332. [Google Scholar] [CrossRef]
- Cruz-Aguado, R.; Winkler, D.; Shaw, C.A. Lack of behavioral and neuropathological effects of dietary β-methylamino-L-alanine (BMAA) in mice. Pharmacol. Biochem. Behav. 2006, 84, 294–299. [Google Scholar] [CrossRef]
- Cucchiaroni, M.L.; Viscomi, M.T.; Bernardi, G.; Molinari, M.; Guatteo, E.; Mercuri, N.B. Metabotropic glutamate receptor 1 mediates the electrophysiological and toxic actions of the cycad derivative β-N-Methylamino-L-alanine on substantia nigra pars compacta DAergic neurons. J. Neurosci. 2010, 14, 5176–5188. [Google Scholar] [CrossRef]
- Petrozziello, T.; Boscia, F.; Tedeschi, V.; Pannaccione, A.; de Rosa, V.; Corvino, A.; Severino, B.; Annunziato, L.; Secondo, A. Na+/Ca2+ exchanger isoform 1 takes part to the Ca2+-related prosurvival pathway of SOD1 in primary motor neurons exposed to beta-methylamino-L-alanine. Cell Commun. Signal. 2022, 20, 8. [Google Scholar] [CrossRef]
- Myhre, O.; Eide, D.M.; Kleiven, S.; Utkilen, H.C.; Hofer, T. Repeated five-day administration of L-BMAA, microcystin-LR, or as mixture, in adult C57BL/6 mice—Lack of adverse cognitive effects. Sci. Rep. 2018, 8, 2308. [Google Scholar] [CrossRef]
- Arif, M.; Kazim, S.F.; Grundke-Iqbal, I.; Garruto, R.M.; Iqbal, K. Tau pathology involves protein phosphatase 2A in Parkinsonism-dementia of Guam. Proc. Natl. Acad. Sci. USA 2014, 111, 1144–1149. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Bire, R.; Fessard, V.; Sialehaamoa, A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2016, 13, 998e. [Google Scholar] [CrossRef]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef]
- Jensen, M.P.; Barker, R.A. Disease-modification in Huntington’s Disease: Moving away from a single-target approach. J. Huntingtons Dis. 2019, 8, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Simpson, C.; Desai, P.; Tucker, M.; Lobner, D. Neurotoxicity of isomers of the environmental toxin L-BMAA. Toxicon 2020, 184, 175–179. [Google Scholar] [CrossRef]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and neurodegenerative diseases. A systematic review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Garruto, R.M.; Yase, Y. Neurodegenerative disorders of the western Pacific: The search for mechanisms of pathogenesis. Trends Neurosci. 1986, 9, 368–374. [Google Scholar] [CrossRef]
- Nunn, P.B.; O’Brien, P.; Pettit, L.D.; Pyburn, S.I. Complexes of zinc, copper, and nickel with the nonprotein amino acid L-α-amino-methylaminopropionic acid: A naturally occurring neurotoxin. J. Inorg. Biochem. 1989, 37, 175–183. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).