Micronutrients at Supplemental Levels, Tight Junctions and Epithelial Barrier Function: A Narrative Review
Abstract
1. The Singular Importance of Fluid Compartmentation and Epithelial Barriers
2. The near Universal Occurrence of Barrier Leak in Disease
3. Micronutrient Reduction of Barrier Leak
4. The Case for Adjuvant Therapy
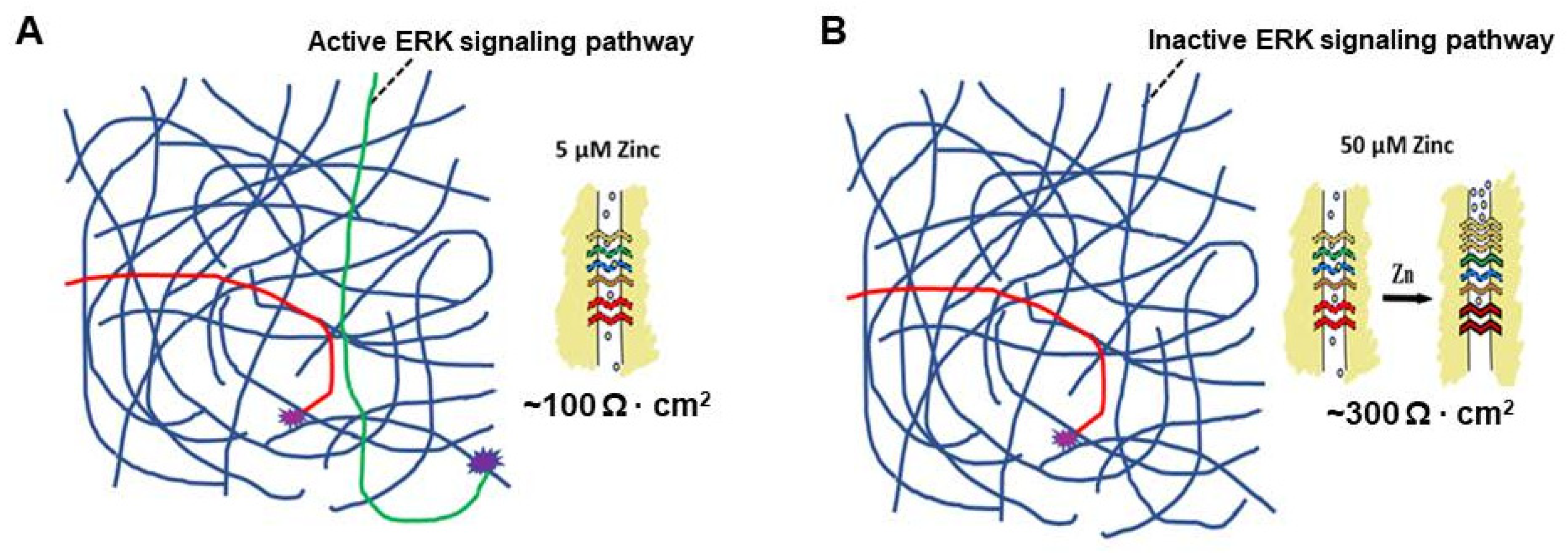
5. A Hypothesis for How Micronutrients at Elevated Levels Are Effective in Supporting Barrier Function
6. The Economic Realities
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–Brain Barrier |
| GI | Gastrointestinal |
| GRAS | Generally Recognized as Safe |
| IBD | Inflammatory Bowel Disease |
| ICU | Intensive Care Unit |
| PKC | Protein Kinase C |
| RDA | Recommended Daily Allowance |
| TJ | Tight Junction |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Sawada, N. Tight junction-related human diseases. Pathol. Int. 2013, 63, 1–12. [Google Scholar] [CrossRef]
- Guttman, J.A.; Finlay, B.B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 2009, 1788, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Bergelson, J.M. Intercellular junctional proteins as receptors and barriers to virus infection and spread. Cell Host Microbe 2009, 5, 517–521. [Google Scholar] [CrossRef]
- Shrestha, A.; Uzal, F.A.; McClane, B.A. The interaction of Clostridium perfringens enterotoxin with receptor claudins. Anaerobe 2016, 41, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Schumann, M.; Siegmund, B.; Schulzke, J.D.; Fromm, M. Celiac Disease: Role of the Epithelial Barrier. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 150–162. [Google Scholar] [CrossRef]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113 Pt 24, 4435–4440. [Google Scholar] [CrossRef]
- Riley, M. Pump and leak in regulation of fluid transport in rabbit cornea. Curr. Eye Res. 1985, 4, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Wang, W.; Giebisch, G.; Welling, P.A. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc. Natl. Acad. Sci. USA 1992, 89, 6418–6422. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Fromm, M. Tight junctions: Molecular structure meets function. Ann. N. Y. Acad. Sci. 2009, 1165, 1–6. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef]
- Sugimoto, K.; Chiba, H. The claudin-transcription factor signaling pathway. Tissue Barriers 2021, 9, 1908109. [Google Scholar] [CrossRef]
- DiGuilio, K.M.; Mercogliano, C.M.; Born, J.; Ferraro, B.; To, J.; Mixson, B.; Smith, A.; Valenzano, M.C.; Mullin, J.M. Sieving characteristics of cytokine- and peroxide-induced epithelial barrier leak: Inhibition by berberine. World J. Gastrointest. Pathophysiol. 2016, 7, 223–234. [Google Scholar] [CrossRef]
- Foerster, E.G.; Mukherjee, T.; Cabral-Fernandes, L.; Rocha, J.D.B.; Girardin, S.E.; Philpott, D.J. How autophagy controls the intestinal epithelial barrier. Autophagy 2022, 18, 86–103. [Google Scholar] [CrossRef]
- Soler, A.P.; Marano, C.W.; Bryans, M.; Miller, R.D.; Garulacan, L.A.; Mauldin, S.K.; Stamato, T.D.; Mullin, J.M. Activation of NF-kappaB is necessary for the restoration of the barrier function of an epithelium undergoing TNF-alpha-induced apoptosis. Eur. J. Cell Biol. 1999, 78, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Samarin, S.; Nusrat, A. Inflammatory bowel disease and the apical junctional complex. Ann. N. Y. Acad. Sci. 2006, 1072, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial tight junctions in intestinal inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef]
- Fries, W.; Mazzon, E.; Squarzoni, S.; Martin, A.; Martines, D.; Micali, A.; Sturniolo, G.C.; Citi, S.; Longo, G. Experimental colitis increases small intestine permeability in the rat. Lab. Investig. 1999, 79, 49–57. [Google Scholar] [PubMed]
- Nusrat, A.; Turner, J.R.; Madara, J.L. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: Nutrients, cytokines, and immune cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G851–G857. [Google Scholar] [CrossRef] [PubMed]
- Heyman, M.; Desjeux, J.F. Cytokine-induced alteration of the epithelial barrier to food antigens in disease. Ann. N. Y. Acad. Sci. 2000, 915, 304–311. [Google Scholar] [CrossRef]
- Frey, T.; Antonetti, D.A. Alterations to the blood-retinal barrier in diabetes: Cytokines and reactive oxygen species. Antioxid. Redox Signal. 2011, 15, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- John, L.J.; Fromm, M.; Schulzke, J.D. Epithelial barriers in intestinal inflammation. Antioxid. Redox Signal. 2011, 15, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- DiGuilio, K.M.; Rybakovsky, E.; Abdavies, R.; Chamoun, R.; Flounders, C.A.; Shepley-McTaggart, A.; Harty, R.N.; Mullin, J.M. Micronutrient Improvement of Epithelial Barrier Function in Various Disease States: A Case for Adjuvant Therapy. Int. J. Mol. Sci. 2022, 23, 2995. [Google Scholar] [CrossRef] [PubMed]
- McClane, B.A. The complex interactions between Clostridium perfringens enterotoxin and epithelial tight junctions. Toxicon 2001, 39, 1781–1791. [Google Scholar] [CrossRef]
- Mailly, L.; Baumert, T.F. Hepatitis C virus infection and tight junction proteins: The ties that bind. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183296. [Google Scholar] [CrossRef] [PubMed]
- Javier, R.T.; Rice, A.P. Emerging theme: Cellular PDZ proteins as common targets of pathogenic viruses. J. Virol. 2011, 85, 11544–11556. [Google Scholar] [CrossRef] [PubMed]
- Shepley-McTaggart, A.; Sagum, C.A.; Oliva, I.; Rybakovsky, E.; DiGuilio, K.; Liang, J.; Bedford, M.T.; Cassel, J.; Sudol, M.; Mullin, J.M.; et al. SARS-CoV-2 Envelope (E) protein interacts with PDZ-domain-2 of host tight junction protein ZO1. PLoS ONE 2021, 16, e0251955. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Robinson, C. Cellular and Molecular Events in the Airway Epithelium Defining the Interaction between House Dust Mite Group 1 Allergens and Innate Defences. Int. J. Mol. Sci. 2018, 19, 3549. [Google Scholar] [CrossRef]
- Soler, A.P.; Miller, R.D.; Laughlin, K.V.; Carp, N.Z.; Klurfeld, D.M.; Mullin, J.M. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 1999, 20, 1425–1431. [Google Scholar] [CrossRef]
- Martin, T.A.; Mason, M.D.; Jiang, W.G. Tight junctions in cancer metastasis. Front. Biosci. (Landmark Ed.) 2011, 16, 898–936. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Yamaguchi, H.; Ito, T.; Kyuno, D.; Kono, T.; Konno, T.; Sawada, N. Tight junctions in human pancreatic duct epithelial cells. Tissue Barriers 2013, 1, e24894. [Google Scholar] [CrossRef] [PubMed]
- Mullin, J.M.; Laughlin, K.V.; Ginanni, N.; Marano, C.W.; Clarke, H.M.; Peralta Soler, A. Increased tight junction permeability can result from protein kinase Cactivation/translocation and act as a tumor promotional event in epithelial cancers. Ann. N. Y. Acad. Sci. 2000, 915, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Mullin, J.M. Epithelial barriers, compartmentation, and cancer. Sci. STKE 2004, 2004, pe2. [Google Scholar] [CrossRef] [PubMed]
- Barmeyer, C.; Fromm, M.; Schulzke, J.D. Active and passive involvement of claudins in the pathophysiology of intestinal inflammatory diseases. Pflugers Arch. 2017, 469, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Massier, L.; Blüher, M.; Kovacs, P.; Chakaroun, R.M. Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front. Endocrinol. 2021, 12, 616506. [Google Scholar] [CrossRef]
- Horani, M.H.; Mooradian, A.D. Effect of diabetes on the blood brain barrier. Curr. Pharm. Des. 2003, 9, 833–840. [Google Scholar] [CrossRef]
- Robles-Osorio, M.L.; Sabath, E. Tight junction disruption and the pathogenesis of the chronic complications of diabetes mellitus: A narrative review. World J. Diabetes 2023, 14, 1013–1026. [Google Scholar] [CrossRef]
- Amasheh, M.; Andres, S.; Amasheh, S.; Fromm, M.; Schulzke, J.D. Barrier effects of nutritional factors. Ann. N. Y. Acad. Sci. 2009, 1165, 267–273. [Google Scholar] [CrossRef]
- Vargas-Robles, H.; Castro-Ochoa, K.F.; Citalán-Madrid, A.F.; Schnoor, M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo. World J. Gastroenterol. 2019, 25, 4181–4198. [Google Scholar] [CrossRef]
- Mullin, J.M.; Skrovanek, S.M.; Ramalingam, A.; DiGuilio, K.M.; Valenzano, M.C. Methionine restriction fundamentally supports health by tightening epithelial barriers. Ann. N. Y. Acad. Sci. 2016, 1363, 59–67. [Google Scholar] [CrossRef]
- Lobo de Sá, F.D.; Schulzke, J.D.; Bücker, R. Diarrheal Mechanisms and the Role of Intestinal Barrier Dysfunction in Campylobacter Infections. Curr. Top. Microbiol. Immunol. 2021, 431, 203–231. [Google Scholar] [CrossRef]
- Bücker, R.; Zakrzewski, S.S.; Wiegand, S.; Pieper, R.; Fromm, A.; Fromm, M.; Günzel, D.; Schulzke, J.D. Zinc prevents intestinal epithelial barrier dysfunction induced by alpha-hemolysin-producing Escherichia coli 536 infection in porcine colon. Vet. Microbiol. 2020, 243, 108632. [Google Scholar] [CrossRef] [PubMed]
- Rybakovsky, E.; DiGuilio, K.M.; Valenzano, M.C.; Geagan, S.; Pham, K.; Harty, R.N.; Mullin, J.M. Calcitriol modifies tight junctions, improves barrier function, and reduces TNF-α-induced barrier leak in the human lung-derived epithelial cell culture model, 16HBE 14o. Physiol. Rep. 2023, 11, e15592. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, X.; Liu, W.; Xiao, Y.; Tang, H.; Wu, Y.; Xiong, Y.; Gao, S. The role of vitamin D in the prevention and treatment of SARS-CoV-2 infection: A meta-analysis of randomized controlled trials. Clin. Nutr. 2023, 42, 2198–2206. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Juber, N.F.; Al-Naseri, H.; Heumann, C.; Ali, R.; Oliver, T. Association between Average Vitamin D Levels and COVID-19 Mortality in 19 European Countries-A Population-Based Study. Nutrients 2023, 15, 4818. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Abdel-Wadood, Y.A.; Thabet, R.H.; Gomaa, G.A. Pharmacological evaluation of vitamin D in COVID-19 and long COVID-19: Recent studies confirm clinical validation and highlight metformin to improve VDR sensitivity and efficacy. Inflammopharmacology 2024, 32, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Field, H.P.; Whitley, A.J.; Srinivasan, T.R.; Walker, B.E.; Kelleher, J. Plasma and leucocyte zinc concentrations and their response to zinc supplementation in an elderly population. Int. J. Vitam. Nutr. Res. 1987, 57, 311–317. [Google Scholar] [PubMed]
- Wang, X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Mullin, J.M. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig. Dis. Sci. 2013, 58, 77–87. [Google Scholar] [CrossRef]
- Wang, X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Flounders, C.J.; Mullin, J.M. Zinc enhancement of LLC-PK1 renal epithelial barrier function. Clin. Nutr. 2014, 33, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Sturniolo, G.C.; Di Leo, V.; Ferronato, A.; D’Odorico, A.; D’Incà, R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm. Bowel Dis. 2001, 7, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Sturniolo, G.C.; Fries, W.; Mazzon, E.; Di Leo, V.; Barollo, M.; D’Incà, R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J. Lab. Clin. Med. 2002, 139, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, P.J.; Ferrick, B.; Rybakovsky, E.; Thomas, S.; Mullin, J.M. Epithelial barrier function properties of the 16HBE14o- human bronchial epithelial cell culture model. Biosci. Rep. 2020, 40, BSR20201532. [Google Scholar] [CrossRef] [PubMed]
- Daneshkhah, A.; Agrawal, V.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin. Exp. Res. 2020, 32, 2141–2158. [Google Scholar] [CrossRef]
- Maigoro, A.Y.; An, D.; Lee, S. Exploring the Link between Vitamin D Deficiency and Cytokine Storms in COVID-19 Patients: An In SilicoAnalysis. J. Med. Food. 2022, 25, 130–137. [Google Scholar] [CrossRef]
- Saponaro, F.; Franzini, M.; Okoye, C.; Antognoli, R.; Campi, B.; Scalese, M.; Neri, T.; Carrozzi, L.; Monzani, F.; Zucchi, R.; et al. Is There a Crucial Link between Vitamin D Status and Inflammatory Response in Patients with COVID-19? Front. Immunol. 2022, 12, 745713. [Google Scholar] [CrossRef]
- DiGuilio, K.M.; Rybakovsky, E.; Baek, Y.; Valenzano, M.C.; Mullin, J.M. The m ultiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function. Exp. Lung Res. 2023, 49, 72–85. [Google Scholar] [CrossRef]
- DiGuilio, K.M.; Rybakovsky, E.; Valenzano, M.C.; Nguyen, H.H.; Del Rio, E.A.; Newberry, E.; Spadea, R.; Mullin, J.M. Quercetin improves and protects Calu-3 airway epithelial barrier function. Front. Cell Dev. Biol. 2023, 11, 1271201. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Bishop, W.R.; Kirschmeier, P.; George, S.J.; Cramer, S.P.; Hendrickson, W.A. Identification and characterization of zinc binding sites in protein kinase C. Science 1991, 254, 1776–1779. [Google Scholar] [CrossRef]
- Murakami, K.; Whiteley, M.K.; Routtenberg, A. Regulation of protein kinase Cactivity by cooperative interaction of Zn2+ and Ca2+. J. Biol. Chem. 1987, 262, 13902–13906. [Google Scholar] [CrossRef]
- Slepchenko, K.G.; Holub, J.M.; Li, Y.V. Intracellular zinc increase affects phosphorylation state and subcellular localization of protein kinase C delta (δ). Cell Signal. 2018, 44, 148–157. [Google Scholar] [CrossRef]
- Zalewski, P.D.; Forbes, I.J.; Giannakis, C.; Cowled, P.A.; Betts, W.H. Synergy between zinc and phorbol ester in translocation of protein kinase C to cytoskeleton. FEBS Lett. 1990, 273, 131–134. [Google Scholar] [CrossRef]
- Speizer, L.A.; Watson, M.J.; Kanter, J.R.; Brunton, L.L. Inhibition of phorbol ester binding and protein kinase C activity by heavy metals. J. Biol. Chem. 1989, 264, 5581–5585. [Google Scholar] [CrossRef]
- Rosson, D.; O’Brien, T.G.; Kampherstein, J.A.; Szallasi, Z.; Bogi, K.; Blumberg, P.M.; Mullin, J.M. Protein kinase C-alpha activity modulates transepithelial permeability and cell junctions in the LLC-PK1 epithelial cell line. J. Biol. Chem. 1997, 272, 14950–14953. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Soler, A.P.; Mullin, J.M. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J. Cell Sci. 2000, 113 Pt 18, 3187–3196. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Raduka, A.; Rezaee, F. Vitamin D3 protects against respiratory syncytial virus-induced barrier dysfunction in airway epithelial cells via PKA signaling pathway. Eur. J. Cell Biol. 2023, 102, 151336. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Saha, T.; Sheikh, I.A.; Chakraborty, S.; Aoun, J.; Chakrabarti, M.K.; Rajendran, V.M.; Ameen, N.A.; Dutta, S.; Hoque, K.M. Zinc ameliorates intestinal barrier dysfunctions in shigellosis by reinstating claudin-2 and -4 on the membranes. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G229–G246. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.B. Food and Evolution. In An Overview of Trends in Dietary Variation from Hunter-Gatherer to Modern Capitalist Societies; Temple University Press: Philadelphia, PA, USA, 1989. [Google Scholar]
- Atkinson. The Prehistoric Peoples of Scotland. In Fisherman and Farmers; Routledge: New York, NY, USA, 2015. [Google Scholar]
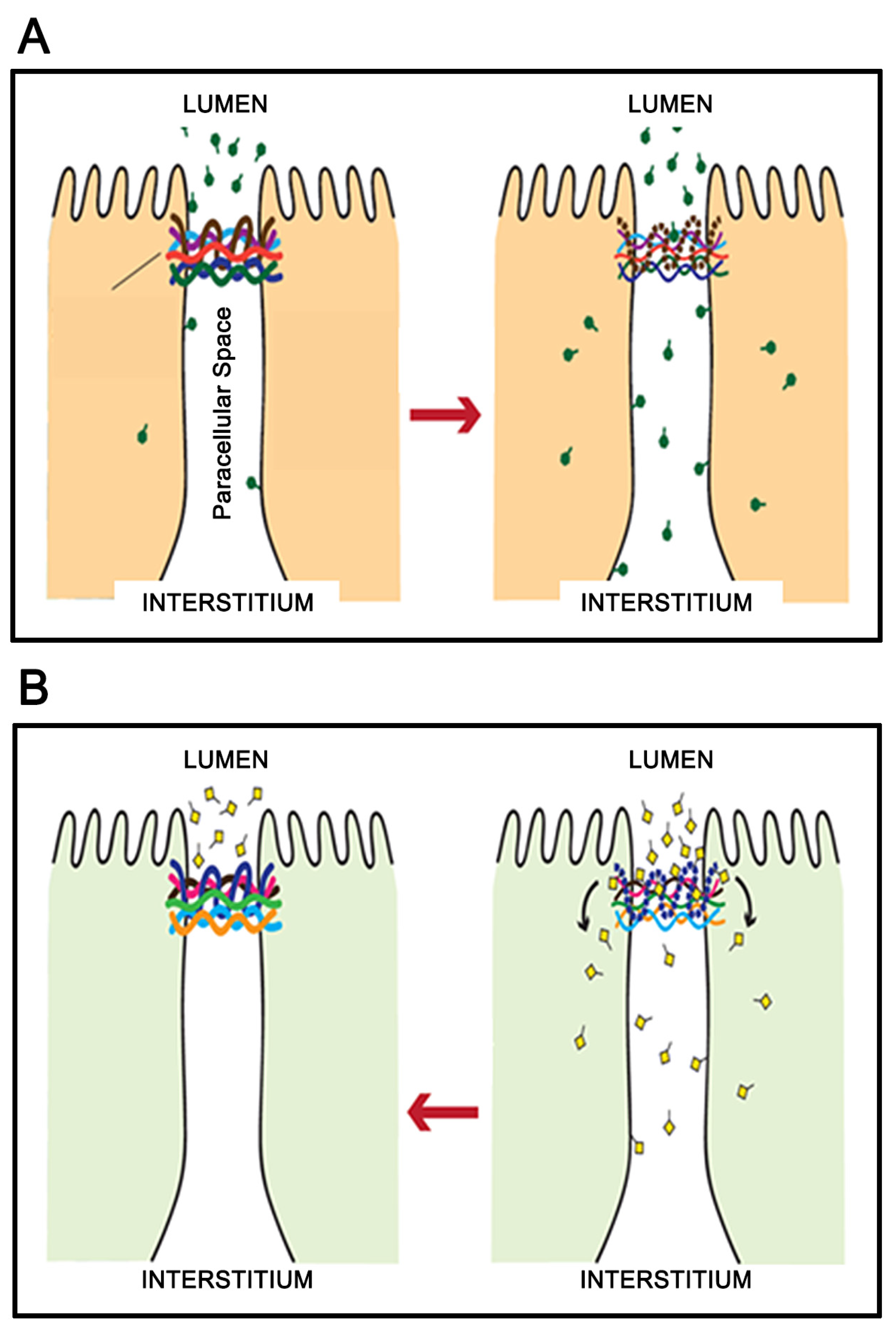
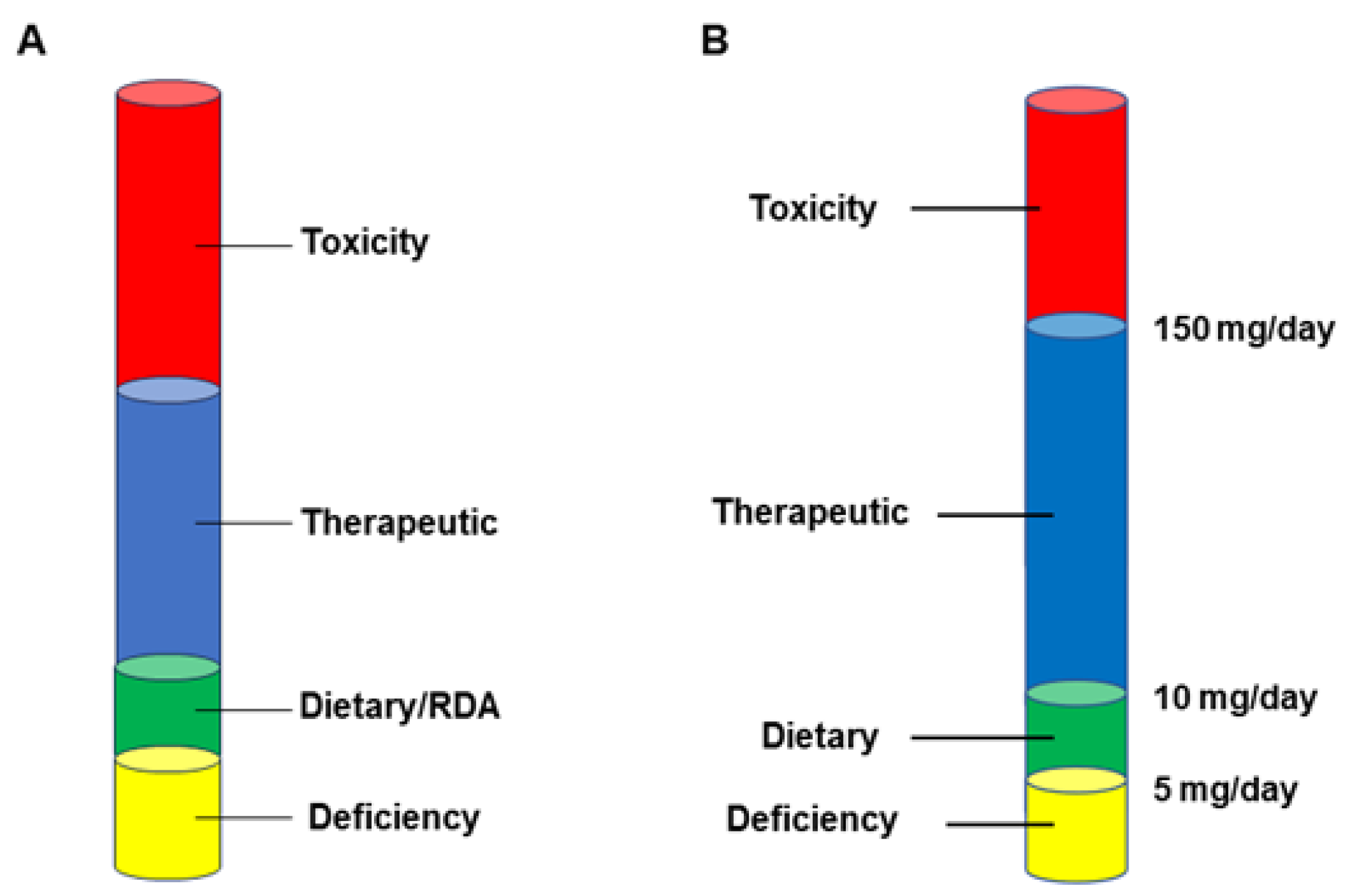
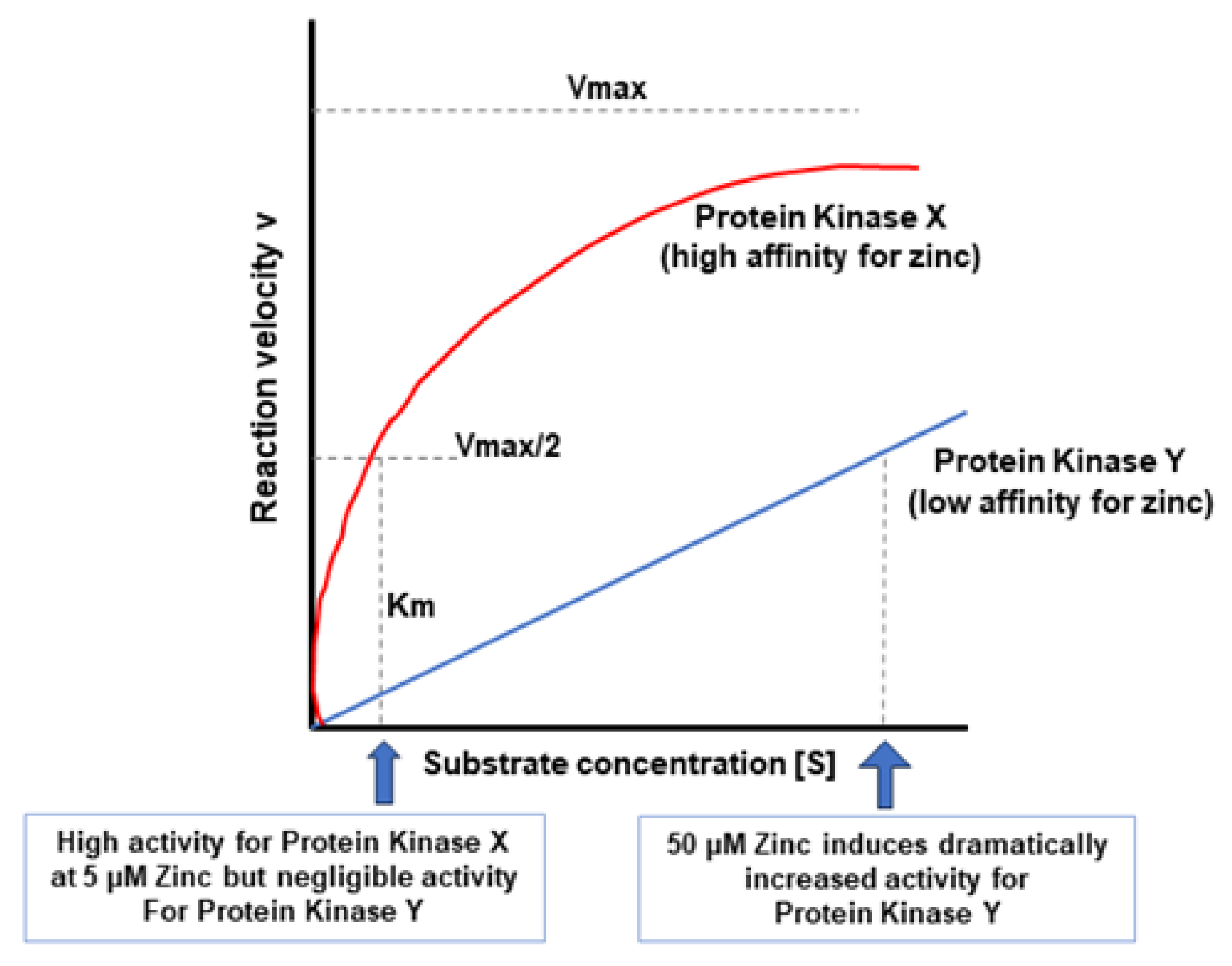
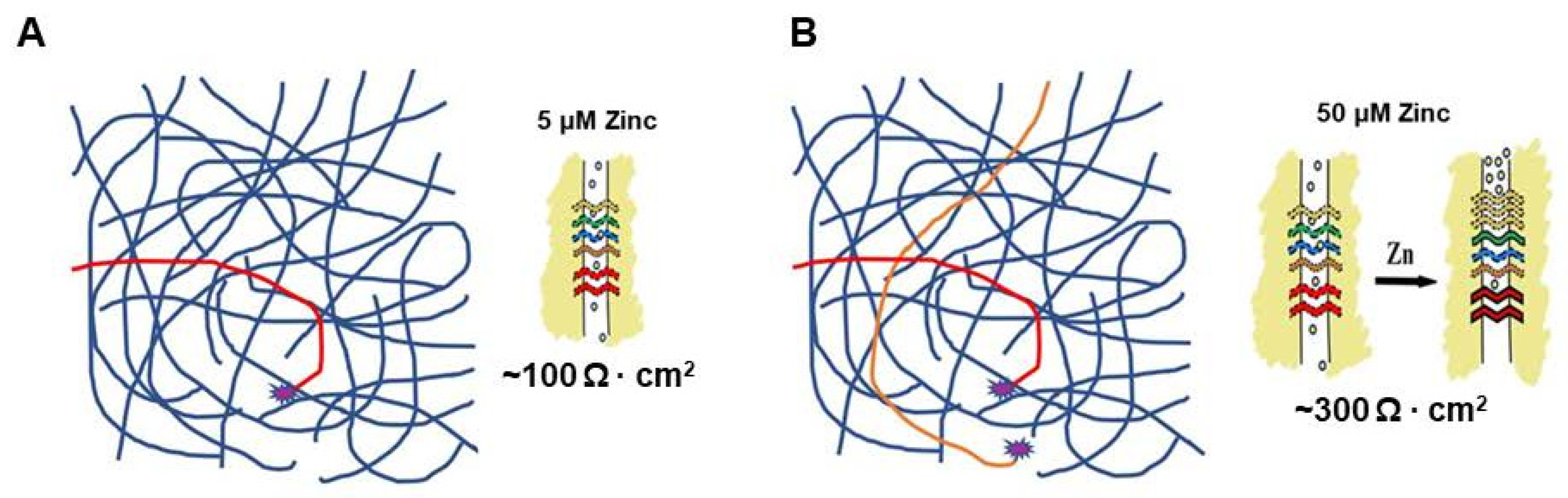
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiGuilio, K.M.; Del Rio, E.A.; Harty, R.N.; Mullin, J.M. Micronutrients at Supplemental Levels, Tight Junctions and Epithelial Barrier Function: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 3452. https://doi.org/10.3390/ijms25063452
DiGuilio KM, Del Rio EA, Harty RN, Mullin JM. Micronutrients at Supplemental Levels, Tight Junctions and Epithelial Barrier Function: A Narrative Review. International Journal of Molecular Sciences. 2024; 25(6):3452. https://doi.org/10.3390/ijms25063452
Chicago/Turabian StyleDiGuilio, Katherine M., Elizabeth A. Del Rio, Ronald N. Harty, and James M. Mullin. 2024. "Micronutrients at Supplemental Levels, Tight Junctions and Epithelial Barrier Function: A Narrative Review" International Journal of Molecular Sciences 25, no. 6: 3452. https://doi.org/10.3390/ijms25063452
APA StyleDiGuilio, K. M., Del Rio, E. A., Harty, R. N., & Mullin, J. M. (2024). Micronutrients at Supplemental Levels, Tight Junctions and Epithelial Barrier Function: A Narrative Review. International Journal of Molecular Sciences, 25(6), 3452. https://doi.org/10.3390/ijms25063452








