Abstract
Nitroxides are stable free radicals that have antioxidant properties. They react with many types of radicals, including alkyl and peroxyl radicals. They act as mimics of superoxide dismutase and stimulate the catalase activity of hemoproteins. In some situations, they may exhibit pro-oxidant activity, mainly due to the formation of oxoammonium cations as products of their oxidation. In this review, the cellular effects of nitroxides and their effects in animal experiments and clinical trials are discussed, including the beneficial effects in various pathological situations involving oxidative stress, protective effects against UV and ionizing radiation, and prolongation of the life span of cancer-prone mice. Nitroxides were used as active components of various types of nanoparticles. The application of these nanoparticles in cellular and animal experiments is also discussed.
1. Introduction
Redox equilibrium is an important element of the functioning of cells and organisms. This somewhat vague term covers an equilibrium between the intensity of undesired side effects of oxidative processes and the activity of antioxidants, or rather a set of dynamic equilibria, different in various cellular compartments and changing depending on the functional state of the cell and the organism. Antioxidants are important players in this dynamic equilibrium. There are numerous antioxidants in the body produced endogenously and ingested in food from external sources. One may wonder if there is any benefit to using synthetic antioxidants not occurring in nature. Nevertheless, the plethora of fully synthetic drugs, with no natural counterparts, are quite effective in treating various diseases, and synthetic antioxidants with new properties may also prove quite useful.
One group of synthetic antioxidants is nitroxides. They are unusual in that they are stable free radicals, with odd electrons on the nitroxyl N-O• group, surrounded by bulky methyl or ethyl groups, thereby considerably limiting the reactivity of the nitroxyl group and conditioning its considerable stability. Nitroxides have found a wide range of applications in biology and medicine. They have been used to monitor intracellular redox reactions, oxygen concentration, and pH, as contrast agents in magnetic resonance imaging, and as probes in EPR imaging. The main biomedical applications of nitroxides are due to their antioxidant properties. Nitroxide has some disadvantages, mainly the short lifetime in the body. To overcome these limitations, several types of nanoparticles containing nitroxide residues have been synthesized and studied. This review is devoted to the antioxidant action of nitroxides and nitroxide-containing nanoparticles in cellular and organismal systems.
2. Structure of Nitroxides
Most nitroxides are derivatives of substituted derivatives of pyrrolidinyl-N-oxyl (6-membered ring) or pyrrolidinyl-N-oxyl (5-membered ring). The structures of the most used nitroxides and some others mentioned in this paper are presented in Figure 1.
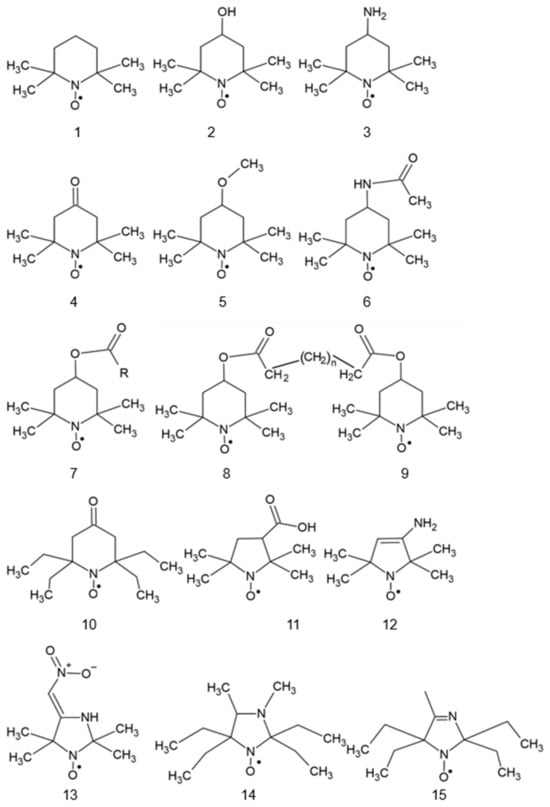
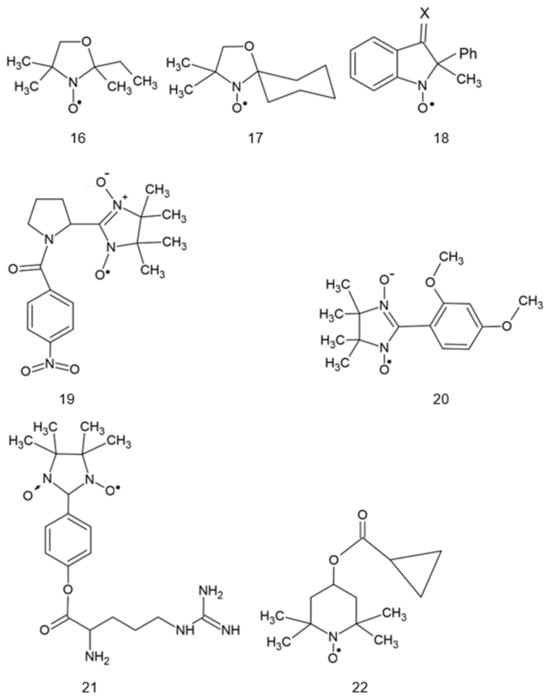
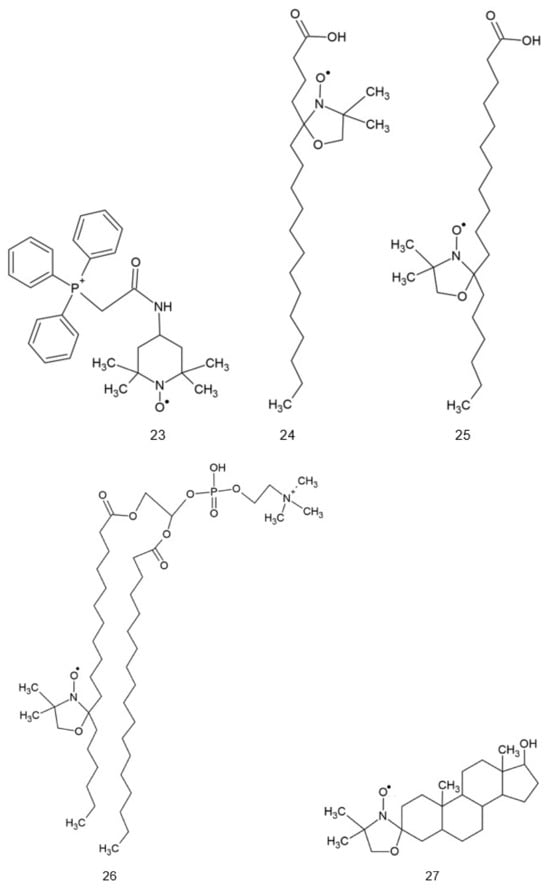
Figure 1.
Structures of various nitroxides. 1: 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO); 2: 4-hydroxy-TEMPO (TEMPOL); 3: 4-amino-TEMPO (TEMPAMINE); 4: TEMPONE; 5: 4-methoxy-TEMPO; 6: 4-acetamido-TEMPO; 7: TEMP: TEMP2, R = CH3CH2; TEMP8, R = (CH2)7CH3; 8: TINO; 9: TEEPONE; 10: 3-carboxy-PROXYL (3-CP); 11: 4-amino-2,2,5,5-tetramethyl-3-imidazoline-1-oxyl (ATI); 12: 3-carbamoyl-proxyl (3-CP); 13: 2,2,5,5-tetramethyl-4-nitromethylene-3-imidazolidine-N-oxyl (NTI); 14: 3,4-dimethyl-2,2,5,5-tetraethylperhydroimidazol-1-oxyl); 15: 4-methyl-2,2,5,5-tetraethyl-2,5-dihydro-1H-imidazol-1-oxyl; 16: OXANO; 17: CHD; 18: 1,2-dihydro-2-methyl-2-phenyl-3H-indole-3-phenylimino-1-oxyl (X = NPh or = CH2CH3); 19: L-N-p-nitrobenzoylpyrrolidinyl(4,5-dihydro-4,4,5,5-tetramethyl-3-oxido-1H-imidazol-3-ium-1-oxyl-2-yl) (L-NNNBP); 20: (2,4-dimetoxyphenyl)-4,4,5,5-tetra-methylimidazoline-1-oxyl-3-oxide (NNR); 21: NNR-arginine conjugate; 22: OT-551; 23: 2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium (Mito-TEMPO); 24: 5-doxylstearic acid; 25: 16-doxylstearic acid; 26: lecithin labeled with 12-doxylstearic acid; 27: 3-doxylcholestane.
Some nitroxides may be targeted to specific sites in the cell. The coupling of antioxidants to a membrane-permeable cation targets them to mitochondria as these organelles, with their inner membrane potential of −180 V, are the most electronegative site in the cell. Thus, synthesized mito-TEMPO (Figure 1, #23) accumulates in the mitochondria and and may exert its antioxidant action at the main site of superoxide generation in the cell [1,2].
Spin-labeled fatty acids, cholestane, and phospholipids (Figure 1, #24–27) locate in cell membranes like their natural precursors [3,4].
3. Reactivity of Nitroxides
Nitroxides are known to enter one-electron reactions with other radicals, oxidize transition metal ions, and stimulate the catalase-like activity of hemoproteins. Nitroxides (both indolinonic and piperidine) effectively scavenge alkyl, aroyloxyl [5], phenoxyl [6], alkoxyl, and peroxyl radicals [7,8].
Nitroxides react rapidly with alkyl and peroxyl radicals. The reaction of TEMPO with alkyl radicals occurs with diffusion-limited rates, with rate constants of ~1–3 × 1010 M−1 s−1 (for TEMPO), thereby enabling this compound to compete with O2 for alkyl radicals [9]. The reactions may consist of electron or hydrogen atom transfer or the addition of a carbon-centered radical to the nitroxide:
where R′ is a carbon-centered radical [10].
R′ + R-N-O● → R-N-O-R′
Genovese et al. [11] compared the reaction rate constants of several TEMPO analogs with peroxyl radicals generated by the thermal decomposition of 4,4′-azobis(4-cyanovaleric acid) (ABCV) and subsequent reaction of so-formed alkyl radicals with oxygen. Their results, as shown in Table 1, exhibit a correlation between the reaction rate constant of the nitroxides studied with peroxyl radicals and their redox potentials. Although the values of the standard redox potentials determined by various authors differ [11,12,13], the effect of substituents on these values is consistent.

Table 1.
Reaction rate constants of several pyrimidine nitroxides with peroxyl radicals. After [11] and others.
Nitroxides are efficient scavengers of peroxyl radicals, so they can be expected to inhibit lipid peroxidation and other reactions induced by peroxyl radicals. TEMPOL protected against the AAPH-induced DNA breakage and the AAPH-induced inactivation of glucose oxidase [14]. TEMPO inhibited lipid peroxidation in soybean phosphatidylcholine liposomes [15]. Carbonylation of bovine serum albumin (BSA) was observed upon UVA illumination in the presence of Parsol 1789, forming free radicals upon irradiation (initially carbon-centered, and then oxygen-centered), apparently due to the scavenging of these radicals. TEMPO and TEMPOL inhibited BSA carbonylation [16]. Lipid peroxidation in phosphatidylcholine liposomes induced by UVA in the presence of Parsol 1789 was inhibited by nitroxides, the order of protection efficiency being: TINO (Figure 1, #8) > TEMP8 > TEMP2 (Figure 1, #7) > hydroxylamine of TINO [17]. Nitroxide radicals (TEMPO, TEMPOL, and indolinonic nitroxides) protected against strand breaks inflicted on DNA when illuminated in vitro in the presence of dibenzoylmethane and Parsol 1789, used as a UVA-absorbing sunscreen. In contrast, vitamin E had no protective effect under the conditions used. These results might have practical implications because of the widespread use of vitamin E as an antioxidant in cosmetics, including sunscreens [10]. When the efficiency of a series of indolinic nitroxides of different lengths of the hydrophobic tail to protect against protein oxidation and lipid peroxidation were compared, 2-dihydro-2-decyl-2-phenyl-3H-indole-3-phenylimino-1-oxyl (Figure 1, #18, R = (CH2)9CH3)) was the most effective in the inhibition of carbonyl formation of BSA but 1,2-dihydro-2-ethyl-2-phenyl-3H-indole-3-phenylimino-1-oxyl) (R = CH2CH3) was the most effective in preventing malondialdehyde formation in rat liver microsomes [18].
Nitroxides are efficient scavengers of •NO2 at physiological pH (k = (3–9) × 108 M−1 s−1) and belong to the most effective metal-independent scavengers of CO3•− radicals (k = (2–6) × 108 M−1 s−1) [19].
Nitroxides also react with peroxynitrite. TEMPOL was found to inhibit the peroxynitrite-mediated nitration of phenolic compounds in the presence of a large molar excess of peroxynitrite over the pH range of 6.5–8.5, suggesting a catalytic-like mechanism of peroxynitrite decomposition. In these experiments, inhibition was specific for nitration and did not affect hydroxylation [20]. TEMPOL was oxidized by peroxynitrite-derived radicals (•OH and CO3•−, in the absence and presence of carbon dioxide, respectively) to the oxoammonium cation, which, in turn, was reduced back to TEMPOL while oxidizing peroxynitrite to oxygen and nitric oxide [21]. However, no correlation was established between the kinetics of nitroxide reactions with HO2•/O2•−, •NO2, and CO3•− and their protective activity against biological oxidative stress [19].
The EPR signal of nitroxides in aqueous solutions is destroyed by ionizing radiation, mainly due to oxidation to oxoammonium cation by the hydroxyl radical; however, the oxoammonium cation can be reduced back by the hydrated electrons of H atoms [22].
Nitroxides, like nitric oxide, are efficient scavengers of protein radicals generated via radical transfer from H2O2-activated horseradish peroxidase, radicals formed on myoglobin via reaction with H2O2, and carbon-centered radicals formed from amino acid hydroperoxides on exposure to Fe2+-EDTA [23]. Nitroxides are considered sterically hindered, structural mimics of NO● because both types of compounds contain an odd electron, which is delocalized over the nitrogen-oxygen bond. The biological activity of nitroxides is often attributed to their NO●-mimetic properties. Nitroxides are more convenient in such applications since they are mostly air-stable crystalline solids, in contrast to NO●, which is unstable and gaseous at room temperature [24].
From a biological point of view, the reactions of nitroxides with the superoxide radical anion are especially interesting. The exposure of five-membered (pyrrolidinyl) nitroxides to superoxide flux results in a decrease in their EPR signal. The signal loss is reversed by the addition of ferricyanide, indicating that superoxide reduces the nitroxide to its respective hydroxylamine [25]. One-electron reduction in stable nitroxides to the corresponding hydroxylamine was proposed to be the primary metabolic pathway [26]. A similar loss of EPR signal was not found for six-membered ring piperidine nitroxides (TEMPO, TEMPOL, and TEMPAMINE); the effect was ascribed to the facile reoxidation of the corresponding hydroxylamine by superoxide [27]. However, the reaction rate of the corresponding hydroxylamines with superoxide is slow; for Tempol-H it was found to be 4 × 102 M−1 s−1 [26]. This value is two orders of magnitude lower than that estimated for the reaction of superoxide with TEMPOL and in the same order of magnitude estimated for TEMPO [28]. On this basis, Krishna et al. concluded that the previously proposed mechanism of superoxide dismutation involving hydroxylamine as an intermediate is not consistent with these observed rates [26]. They proposed that the initial reaction with superoxide is a one-electron oxidation to the oxoammonium cation. Piperidine derivatives such as TEMPO and TEMPOL are readily oxidized by protonated superoxide (perhydroxyl radical) ●OOH to yield oxoammonium cation. The oxoammonium cation may either be reduced back to the nitroxide by superoxide or may react with NADH or NADPH to form the corresponding hydroxylamine [26]. During the dismutation of superoxide, high steady-state levels of piperidinyl and proxyl derivative nitroxides are maintained. This suggests that the reduction in the oxoammonium cation by superoxide is relatively fast. The O2●−/H2O2 couple has a redox potential of +0.89 V (although a value of 1.06 was also reported) [29], so the oxidation rather than reduction in nitroxides is to be expected. The doxyl derivatives, on the other hand, are directly reduced by superoxide to their hydroxylamines [26,27]. The following reaction cycle was proposed [30]:
RR′NO● + O2●− + 2 H+ → RR′NO+ + H2O2
RR′NO+ + O2●− → RR′NO● + O2
The catalytic rate constants for O2●− dismutation, determined for TEMPO and TEMPOL, were found to increase with decreasing pH, indicating that ●OOH rather than O2●− is oxidizing the nitroxide [30]. Direct evidence for the reaction of the oxoammonium cation with superoxide (reaction 2) was not obtained, but in biological systems, this intermediate may be expected to react with many endogenous one- and two-electron reducing agents. Thus, the pathway for superoxide dismutation in vivo would not necessarily involve direct reduction of O2●− (reaction 2) as the major route for regeneration of the cyclic nitroxide. Given the instability of the oxoammonium cation, it would appear that regeneration of the nitroxide would occur predominantly by reaction of the oxoammonium with endogenous substrates other than superoxide. This raises the possibility of deleterious reactions with critical biomolecules if repair (re-reduction) mechanisms are too slow to prevent subsequent irreversible processes [26].
The cellular destruction of persistent spin adduct nitroxides may be facilitated by primary univalent oxidation. The reactions of nitroxides with superoxide reveal that the piperidinyl and the proxyl derivatives were reduced in a superoxide-dependent manner only in the presence of two-electron donors such as NADH and NADPH. The reduction products of these nitroxides are the corresponding hydroxylamines. These results suggest that while the initial reaction with superoxide is one-electron oxidation to the oxoammonium cation, this transient species either may be reduced back to the nitroxide by superoxide or may react with NADH or NADPH to form the corresponding hydroxylamine [26]. Superoxide was demonstrated to reduce nitroxides to their corresponding hydroxylamines in the presence of sulfhydryl-containing compounds (3 nitroxides were reduced per superoxide). Superoxide directly reacts with nitroxide to yield a N-hydroxy-N-hydroperoxyl compound. This product rapidly decomposes, giving a hydroxylamine and an oxidized sulfhydryl compound, postulated to be a sulfenyl hydroperoxide. It was hypothesized that this sulfenyl hydroperoxide reduces two additional nitroxyl free radicals to account for the unusual stoichiometry [31].
Thus, nitroxides catalytically decompose superoxide, showing a pseudoenzymatic superoxide dismutase activity and are superoxide dismutase mimics. They are less efficient than superoxide dismutases, which dismutate superoxide with second-order rate constants exceeding 109 s−1M−1 [32] but are usually membrane-permeable and thus act intracellularly. The therapeutic application of superoxide dismutase (SOD) has been proposed, but exogenously added SOD may be immunogenic, does not penetrate readily into the cells, and unless bound to a protein or polyethylene glycol, has a metabolic half-life of only several minutes as a small protein [33]. Therefore, cell-permeable, low-molecular-weight compounds that mimic the activity of SOD were also proposed. Usually, SOD mimics are chelates of transition metals such as copper, iron, or manganese, which, like the metal center in native SOD, can undergo alternate reduction and oxidation. However, metal-containing SOD mimics are prone to dissociation in the presence of cellular proteins, thus not only causing loss of SOD mimic activity but also mobilization of potentially toxic redox-active metals [30,34]. In contrast, nitroxides do not release metal ions and even oxidize transition metal ions, preventing their participation in the Fenton reaction.
The catalytic dismutation rates were found to be directly related to the midpoint redox potential of the nitroxides. The catalytic rates of O●−2 dismutation for TEMPO. TEMPOL and TEMPAMINE were characterized by rate constants of 1.2 × 105 M−1 s−1, 6.5 × 104 M−1 s−1, and 6.5 × 104 M−1 s−1, respectively [26], although higher values of superoxide dismutation by nitroxides, up to 106 M−1 s−1 to 108 M−1 s−1 were also reported [35].
Nitroxides were found to stimulate the decomposition of hydrogen peroxide by heme proteins. By shuttling between two oxidation states (nitroxide and oxoammonium cation), stable nitroxides (R-NO●) enhance the catalase-mimetic activity of myoglobin (Mb) by reducing MbFeIV to MbFeIII, thus facilitating H2O2 dismutation accompanied by oxygen evolution.
MbFeIV + R-NO● → MbFe III + R-NO+
The oxoammonium species has indeed been isolated as an end product in the hemoglobin/nitroxide system upon the addition of hydrogen peroxide [36]. Myoglobin is more readily activated than oxymyoglobin to the ferryl states, which are strong oxidants capable of inflicting significant damage by oxidizing a number of biological targets, including initiation of lipid peroxidation in biological membranes [37]. Generally, reagents reducing MbFeIV to MbFeIII operate in a stoichiometric manner; nitroxide radicals, which shuttle among three oxidation states, can detoxify hypervalent metals in a catalytic fashion and provide protection against hypervalent heme iron.
The oxoammonium cation R-NO+ formed (3) can oxidize another H2O2 molecule [34]:
H2O2 + R-NO+ → R-NO + O2●− + 2 H+
4. Reduction of Nitroxides
The chemical reduction of nitroxides to EPR-silent hydroxylamines, in many cases, is an unfavorable factor that significantly limits their applications in biological systems. On the other side, EPR-measured rates of nitroxide reduction have been shown to provide information on tissue redox status [38,39,40], and reactive oxygen species (ROS) generation in vivo. The enhanced generation of hydroxyl radicals accelerated the disappearance rate of the EPR signal of nitroxides [41].
In erythrocytes and many other cell types, ascorbate is the predominant component responsible for the reduction of nitroxides [42].
The reduction rates of NR by ascorbate normally correlate with its electrochemical reduction potential and depend on the nature of the radical ring, charge of the radical, and steric shielding of nitroxyl fragment [43]. The bimolecular rate constants of ascorbate-induced reduction are significantly higher for six-membered ring nitroxide of piperidine types (e.g., 3.5 M−1 s−1 for TEMPO [44] and 7 M−1 s−1 for TEMPOL [45] than for the five-membered ring nitroxides of pyrrolidine (0.07–0.3 M−1 s−1) [43,44] and imidazolidine (0.85 M−1 s−1) [43] types. The presence of the double bond at position 3 in the five-membered ring nitroxides of pyrroline and imidazoline types increases their reduction rates by ascorbate. A negative charge is a factor stabilizing NR against reduction by the negatively charged ascorbate anion [43]. The reduction rate of 4-methyl-2,2,5,5-tetraethyl-2,5-dihydro-1H-imidazol-1-oxyl by ascorbate was much lower than that of 3-carboxy-2,2,5,5-tetramethyl-1-pyrrolidine-1-oxyl. A tetraethyl-substituted imidazole nitroxide (Figure 1, #14) [46] was characterized by the lowest rate constant for the reaction with ascorbate (0.02 M−1 s−1) [43].
The reaction of nitroxides with glutathione (GSH) is of particular interest due to the importance of GSH in the regulation of intracellular redox status. The appreciable chemical reduction of nitroxides via GSH does not occur over a few hours [31,47,48]. However, GSH can significantly contribute to the reduction of nitroxides in biological systems indirectly by acting as a secondary source of reducing equivalents [39]. In the presence of ascorbate, the addition of GSH facilitated the reduction of nitroxides by ascorbate, which was attributed to the scavenging of the ascorbate radical by GSH and inhibition of oxidation of the hydroxylamine formed by the ascorbate radical Asc•− formed during nitroxide reduction to hydroxylamine.
Ascorbic acid and reduced glutathione, as well as both compounds together, reduced piperidine nitroxides to corresponding hydroxylamines exclusively. Neither corresponding secondary amines were found [49]. Different substituents at the C2 and C4 positions affect the susceptibility of piperidine nitroxides toward ascorbate reduction [50]. In contrast to methyl-substituted nitroxides, tetraethyl-substituted piperidine nitroxides bearing an exocyclic double bond like TEEPONE (Figure 1, #9) are also substrates to fast P450-induced hydrogen atom abstraction in α-position to the sp2 carbon of the ring, followed by the destruction in vivo [51].
NADH is an obligatory two-electron reductant. No direct reaction of piperidine nitroxides with NADH was observed [26]. In human keratinocytes isolated from foreskin and breast skin, acetamido-2,2,6,6-tetramethylpiperidine-N-oxyl benzyl dimethylammonium bromide (spin-labeled quat) was reduced by thioredoxin reductase localized at the outer plasma membrane but not by glutathione reductase. The reduction was inhibited by pCMB, DTNB, and NADP+, inhibitors of thioredoxin reductase [52]. Five-membered ring nitroxides and α-carboxy α-aryl tert-butyl nitroxides were more resistant toward reduction by liver homogenates than six-membered ring and heterocyclic-substituted nitroxides in all systems. The presence of carboxylate groups tends to enhance the resistance of all the nitroxides toward reduction by liver homogenate, hepatocytes, and subcellular fractions [53]. In the keratinocytes, TEMPO was reduced to hydroxylamine and secondary amine, while for TEMPAMINE, the sole detected metabolite was the corresponding hydroxylamine. No evidence for the formation of glucuronides or sulfates was found in the keratinocyte cell line HaCaT. The reduction of the hydroxylamine was only partially thiol-dependent in keratinocytes. The lost ESR signal of TEMPO was recovered upon the addition of the mild oxidant ferricyanide to 70–80% [49].
In photosynthesizing cells, nitroxides are also reduced by the photosynthetic apparatus. The rate of nitroxide reduction upon illumination of PS II depends on the chemical structure of radicals and the capability of their coming close to low-potential redox centers of photoactive PS II complexes. Nitroxide radicals NTI (2,2,5,5-tetramethyl-4-nitromethylene-3-imidazo-lidine-N-oxyl) and TACET (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl-acetate; Figure 1 #7, R = CH3), containing polar groups, appear to be most efficient acceptors of electrons donated by PS II compared to neutral (TEMPOL) or positively charged (TEMPAMINE) spin labels [54].
Hydrophobic 5-doxyl stearate (Figure 1, #24) localizes to cellular membranes and, in normal erythrocytes, is not reduced, in contrast to TEMPO, TEMPOL or TEMPONE, located in the aqueous phase. However, in erythrocytes infected with the malarial parasite Plasmodium berghei, it is subject to reduction; this effect was ascribed to increased oxidative stress in these cells [55]. Similar behavior can be expected in other cases of intense oxidative stress.
5. Cellular Effects of Nitroxides
Numerous experiments demonstrated various beneficial antioxidant effects of nitroxides, i.e., protection of cells against oxidative stress induced by various factors (some of them as models of clinically relevant situations, e.g., ischemia reoxygenation [56], UV [57], and ionizing radiation [58,59]). Protective effects of nitroxides were also demonstrated in neuroblastoma cells treated with 6-hydroxydopamine (a model of Parkinson’s disease) [60,61,62], cells treated with high concentrations of glucose (a model of diabetes) [63,64], spermatozoa [65,66,67], and oocytes [68].
TEMPOL was found to not be mutagenic and to afford protection against mutagenic effects [69,70,71]. However, nitroxides were also reported to be mutagenic using the Salmonella typhimurium mutagenicity test. Nitroxide mutagenicity was dramatically increased in the presence of the superoxide radical generating system, xanthine oxidase/hypoxanthine [70].
Among the six nitroxides tested, 3-aminomethyl-PROXYL and TEMPAMINE were the most effective in protecting V79 hamster cells against ionizing radiation. These nitroxides possess amine groups which, at intracellular pH, would be partly positively charged, thus allowing for their association with negatively charged DNA. The radioprotection was hypothesized to be conditioned, at least in part, to the ability of the nitroxides to associate with DNA. Indeed, these nitroxides showed the highest extent of association with DNA in comparison with other nitroxides [59].
Coronavirus 2 (SARS-CoV-2), the causal agent of COVID-19, uses an RNA-dependent RNA polymerase (RdRp) for the replication of its genome and the transcription of its genes. The catalytic subunit of the RdRp, nsp12, ligates two iron–sulfur metal cofactors in sites that were modeled as zinc centers of the RdRp complex. These metal binding sites are essential for replication and interaction with viral helicase. Oxidation of the clusters by the stable nitroxide TEMPOL caused their disassembly, potently inhibited the RdRp and blocked SARS-CoV-2 replication in cell culture [72].
Interestingly, in some situations, the protective effects of products of nitroxide reduction, hydroxylamines, were demonstrated. TEMPOL hydroxylamine (OT-674) showed desirable safe and strong protective activities against oxidative damage in the ocular tissue. OT-674 was shown to be an effective singlet oxygen quencher. Pretreatment protected light-irradiated ARPE-19 cells that had accumulated A2E chromophore [73]. However, OT-674 is unable to effectively cross the cornea and is not feasible for applications to the back of the eye. Therefore, it was chemically modified into a prodrug, OT-551 (with a cyclopropyl group and an ester linkage), which is more lipophilic and fully capable of penetrating the cornea and can travel via the scleral route to reach the macula at the back of the eye. In the eye, ocular esterases can convert OT-551 to more water-soluble, less lipophilic, TEMPOL hydroxylamine, which can function in the back of the eye, at the macula and retina, and be helpful in preventing age-related macular degeneration (AMD). OT-551, formulated as a topical daily eye drop for dry AMD patients, is capable of significant preservation of both standard luminance and low luminance visual acuity [74].
An Interesting distant effect of TEMPO was reported due to the volatility of this compound, which is rather unique among antioxidants. Concentration-dependent inhibition of ferroptosis on human fibrosarcoma cells by TEMPO present in a neighbor vessel was reported [75]. This result suggests a possibility of administration of TEMPO in the form of vapor.
Various cellular effects of nitroxides are exemplified in Table 2.

Table 2.
Protective effects of nitroxides in cellular systems or organ culture.
Adverse cellular effects of nitroxides were also reported, attributable mainly to the pro-oxidant action of the oxyammonium cations. Nitroxides, like any compounds, are cytotoxic at appropriately high concentrations. Toxicities of five nitroxides for human HaCaT keratinocytes correlated with the lipid/water partition coefficients of the investigated nitroxides, with IC50 values ranging from 1.1 mM (TEMPOL-benzoate) to 11.1 mM (TEMPOL) [49]. These effects cannot always be conceived as deleterious. The activation of Nrf2 by nitroxides (probably mainly by the oxoammonium cations) may be another mechanism of their antioxidant action at the cellular level [91]. Similarly, cytotoxicity to malignant cells or microbial pathogens is beneficial for the organism. Some adverse cellular effects of nitroxides are presented in Table 3.

Table 3.
Pro-oxidant and adverse effects of nitroxides in cellular systems.
6. Effects of Nitroxides in Animal Experiments
Numerous experiments demonstrated the effects of nitroxides in experimental animals. Listing all of them would be too cruel for the readers, so only a selection is presented in Table 4. A comprehensive review of early experiments concerning the protective effects of nitroxides in oxidative stress and their hypotensive action is presented in reviews by Wilcox and Pearlman [101] and Wilcox [102], while their action on cancer cells was reviewed more recently by Lewandowski and Gwożdziński [103].

Table 4.
Effect of nitroxides in experimental animals.
For TEMPOL injection at a dose of 250 mg/kg, the peak whole blood concentration of TEMPOL was about 600 μg/mL (about 3.5 mM) 5–10 min after injection [59]. Tempol-administered animals in the feed consumed ca. 20–30% less food than control animals, but it did not seem to be due to a lower attractiveness of TEMPOL-containing food. The leptin levels were also significantly (by about one-half) lower in TEMPOL-treated animals when compared to control groups. Increased levels of the uncoupling protein 2 in the skeletal muscle might also contribute to weight loss [104]. For TEMPOL hydroxylamine (TEMPOL-H), the maximal tolerated dose in female CH3 mice was 325 mg/kg. Tempol-H provided protection against the lethality of whole-body radiation in C3H mice at 30 d with a dose modification factor of 1.3, which is similar to the results obtained with TEMPOL. However, TEMPOL-H produced little effect on blood pressure or pulse compared with TEMPOL. Thus, TEMPOL-H is also a systemic in vivo radioprotector of C3H mice associated with less hemodynamic toxicity than TEMPOL [155]. TEMPOL supplementation to old Fischer 344 rats decreased plasma glucose, insulin, and triglycerides, unlike vehicle-supplemented old rats, and alleviated insulin resistance [156]. The amelioration of cardiac hypertrophy induced by hypoxic conditions [138] may suggest a possibility of using nitroxides in the prophylaxis of adverse effects of hypoxic conditions, e.g., in high mountain climbers.
Many reports document the protective effects of nitroxides in the ischemia-reperfusion injury of various organs.
Inflammation generates a multitude of reactive nitrogen and oxygen species, apart from cytokines, chemokines, and growth factors. Nitroxides show anti-inflammatory properties by scavenging these reactive species and many studies demonstrated their anti-inflammatory action. Apart from scavenging ROS and RNS, nitroxides also modulate directly the NF-κB factor considered to be a master regulator of inflammation, as shown for TEMPOL [157]. In prostate cancer progression, TEMPOL reduced inflammation in preclinical models, downregulated the initial inflammatory signaling via Toll-like receptors, and upregulated iκB-αand iκB-β levels, leading to a decrease in NF-κB, TNF-α, and other inflammatory markers [153]. TEMPOL was also reported to inhibit myeloperoxidase, which plays a fundamental role in oxidant production by neutrophils [158].
Neurodegenerative diseases are accompanied by oxidative stress, which has been suggested to contribute to their development [159,160]. Various reports document the potential protective effects of nitroxides in cellular and animal models of neurodegenerative diseases. TEMPOL inhibited lipopolysaccharide-induced β-amyloid (Aβ) formation in mouse hippocampal HT22 cells and mouse hippocampus [161]. In a mouse model of Alzheimer’s disease, a pyrrolyl α-nitronyl nitroxide (Figure 1, #19) attenuated brain Aβ deposition, and tau phosphorylation, decreased astrocyte activation and improved spatial learning and memory, being more effective than TEMPO [89]. TEMPOL was reported to prevent vascular response impairment and normalize astrocyte Ca2+ levels in APP mice, a mouse model of Alzheimer’s disease [162].
Nitroxide-containing nanoparticles administered to mice attenuated cognitive deficits of both spatial and non-spatial memories, reduced oxidative stress, and decreased Aβ(1–40), Aβ(1–42), and gamma (γ)-secretase levels, and Aβ plaque (see below).
The intraperitoneal administration of TEMPOL induced hypotension in C3H mice as well. This vasodilatory effect could contribute to its radioprotective effect in vivo since it can cause relative bone marrow and tissue hypoxia, resulting in decreased sensitivity of the bone marrow to ionizing radiation [116]. Interestingly, vaporized TEMPO was able to protect mice against ischemic damage. Other less volatile piperidine nitroxides were not effective [75].
Apparently, one of the most important results concerning the effects of nitroxides at the organismal level concerns the prolongation of the lifespan of tumor-prone mice [84,104,105]. The increased latency to tumorigenesis in Atm-deficient mice was associated with reduced oxidative stress and damage in cancer-prone tissues, suggesting that the chemopreventive effects of TEMPOL resulted from the reduction in oxidative stress and damage. The increased latency to tumorigenesis was greater in Atm2/2 (100%) than in p532/2 (25%). However, p53-deficient mice, which do not display an oxidative stress phenotype but are cancer-prone, and TEMPOL treatment of p532/2 mice did not have any effect on oxidative stress and damage. TEMPOL directly affected p53, increasing p53 phosphorylation at serine 18. TEMPOL also induced p21 protein expression. It was suggested that the chemopreventive effects of TEMPOL are not only due to modulation of oxidative stress and damage but, at least in part, to activation of the p53 pathway and modulation of redox-mediated signaling [84].
7. Clinical Trials of Nitroxides
By altering the redox status of tissues, nitroxides have the ability to interact with and alter many metabolic processes. Positive results of animal experiments justified clinical trials on nitroxides [163] that have been conducted.
TEMPOL was found to acutely and rapidly (within 30 min) improve the thermal hyperemia response in young adult smokers, returning the response back to that typically observed in healthy non-smokers and effectively reversing their impaired endothelial function observed. This effect was found to be entirely nitric oxide-dependent due to the decomposition of superoxide and protection of nitric oxide from inactivation by superperoxide. The various TEMPOL-based nitroxide drug candidates, which can be best used to improve cutaneous microvascular function or reduce the cardiovascular burden of cigarette smoking in humans, remain to be steadily investigated [164].
TEMPOL-based piperidine nitroxides may have similar effects on the aging microvasculature. TEMPOL was reported to attenuate the reduction in thermal hyperemia caused by infusion of angiotensin-II in young adults. Angiotensin-II is elevated with advanced age as well as in many disease states and induces oxidative stress by activating NADPH oxidase and xanthine oxidase. Hence, infusion of angiotensin-II mimics an aging state [165]. TEMPOL and apocynin, an inhibitor of NADPH oxidase, ameliorated the impaired thermal hyperemia observed in chronic kidney disease, another disease state characterized by high oxidative stress [166].
OT-551 (1-hydroxy-4-cyclopropanecarbonyloxy-2,2,6,6-tetramethylpiperidine hydrochloride, TEMPOL-H prodrug; Figure 1 #22) is a small molecule with antioxidant and anti-inflammatory effects. The protective efficacy of OT-551 and its metabolite TEMPOL-H against AMD was tested. A topical daily eye drop for AMD patients was capable of significant preservation of both standard luminance visual acuity and low luminance visual acuity, which is a measure of impaired night and reduced light vision, as evidenced in human Phase II clinical trials for dry AMD. The initial National Eye Institute (NEI), open-label single center was an NEI Intramural Research Program-sponsored Phase 2 clinical trial for OT-551 in dry AMD, which achieved statistical significance for the primary endpoint of preserving visual acuity [167].
The clinical application of TEMPOL was also evaluated in a pilot study at the University of Pennsylvania in which eleven patients with metastatic cancer to the brain were treated with topical TEMPOL. The nitroxide (70 mg/mL in water, ethanol and hydroxylpropyl cellulose) was applied topically to the scalp 15 min before and washed off immediately after the completion of each of 10 fractions of whole brain radiation. These results demonstrated that topical application of TEMPOL to the scalp before whole brain radiation is safe and well tolerated. The evidence of protection against radiation-induced alopecia was observed. A phase II study that uses a gel formulation to increase the exposure of the scalp to TEMPOL has been initiated [168]. A cyclosporine A—TEMPOL topical gel was proposed to be a highly promising platform for treating alopecia [169].
TEMPOL applied in a topical gel was also found to be tolerable when used to manage dermatotoxicity in patients with localized anal cancer undergoing chemoradiation. A total of 5 patients received topical TEMPOL. Adverse events attributed to TEMPOL included asymptomatic grade 1 hypoglycemia and grade 1–2 diarrhea. Dermatitis within untreated, radiated skin was not more severe than dermatitis in MTS-01-treated, unirradiated skin. Examples of clinical application of nitroxides are shown in Table 5.

Table 5.
Nitroxide effects in humans.
8. Nitroxide-Containing Redox Nanoparticles
Low-molecular-weight nitroxides may pose various problems, including the limited life in the body due to reduction, metabolic transformations their rapid clearance by the kidney. For these reasons, they sometimes cannot fully exert their potent antioxidant capacity in vivo. To overcome these problems, redox polymers with covalently conjugated nitroxides were designed. If their size is in the nanometer range, they are referred to as nanoparticles. Several types of nanoparticles containing nitroxides have been synthesized.
Pluronic silica nanoparticles having nitroxide moieties covalently bound to the silica core protected by poly(ethylene glycol) chains (PluS–NO) via a TEMPO–CONH–R link and coumarin dyes embedded in the silica core reacted with peroxyl radicals with a rate constant of (1.5 ± 0.4) × 105 M−1 s−1. As each PluS−NO particle bears an average of 30 nitroxide units, this yields an overall ≈60-fold larger inhibition of the PluS−NO nanoantioxidant compared to the molecular analog [11].
CdSe quantum dots functionalized with TEMPAMINE were proposed to act as free radical sensors due to efficient fluorescence quenching by TEMPAMINE and restoration of fluorescence upon reaction of TEMPAMINE with free radicals to form non-paramagnetic species [171].
The synthesis of two kinds of amphiphilic block copolymers, which can self-assemble into micelles with nitroxyl radicals-containing segments in the core, was reported [172]. Their diameter was <100 nm.
The synthesis of rotaxane-branched radical dendrimers Gn-TEMPO (generation n = 1–3) with up to 24 TEMPO radicals as termini [173], mannose-TEMPO functionalized G4- polyamidoamine (PAMAM) dendrimers [174] and 3Gc0T zero generation dendrimer with a cyclotriphosphazene core functionalized with nitroxyl radicals [175] was reported. PAMAM dendrimers of G1.0 and 2.0 with over 28% TEMPO loading were synthesized and used to oxidize cellulose in water [176]. TEMPO-terminated polyurethane dendrimers (G0–G4) showed better ABTS● scavenging and hydrogen peroxide-scavenging activity than TEMPOL [177]. However, no cellular effects of such dendrimers, as well as of other nanoparticles mentioned previously, have been reported so far.
Synthesis of TEMPO-coated gold nanoparticles (average diameter of 2.5 nm) was reported [178]. Gold nanoparticles (average size 40 nm) conjugated with TEMPO were effectively taken up by human mesenchymal stem cells, did not significantly impair the cell viability, reduced the ROS level in H2O2-treated cells, and promoted the osteogenic differentiation of these cells [179].
Fluorescently trackable liquid crystal nanoparticles synthesized from a nematic diacrylate liquid crystalline cross-linker, a derivative of the chromophore perylene and a polymerizable monoacrylate amphiphile with a carboxylate headgroup that caps the nanoparticle [180] were taken up by HeLa cells showing limited effect on their viability and reduced intracellular ROS level [181]. pH-sensitive nanoparticles loaded with curcumin protected curcumin against degradation and allowed for the delivery of minimally degraded curcumin to target regions [182].
Nano-sized sterically stabilized liposomes loaded with TEMPAMINE [183] proved to be efficient in inhibiting autoimmune encephalomyelitis in mice, as well as adjuvant-induced arthritis in rats [184,185,186] and exerted an anticancer effect [169].
TEMPAMINE conjugated to poly[oligo(ethylene glycol)methyl ether acrylate] (POEGA) or poly(2-hydroxyethyl acrylate) (PHEA) yielded nanoparticles of molecular weight of 4.5–33.4 kDa and average diameter 31 and 27 nm, respectively. They showed good biocompatibility, not compromising the viability of MRC-5 fibroblasts, not affecting erythrocyte osmotic fragility, and not inducing hemolysis [187].
The group of Nagasaki synthesized redox nanoparticles (RNPs), which are self-assembling polymeric micelles with a diameter of about 40 nm using poly(ethyleneglycol)-b-poly[4-(2,2,6,6-tetramethylpiperidine-1-oxyl)oxymethylstyrene](PEG-b-PMOT) diblock copolymer. In aqueous media, the poly(ethylene glycol) (PEG) segments form an outer layer shielding the hydrophobic core containing the nitroxide residues [188]. By coupling TEMPOL or TEMPAMINE residues, they produced two types of RNPs: RNPO with conjugated TEMPOL residues and RNPN with conjugated TEMPAMINE residues. The latter are pH-sensitive since, at below 7.0, the amide bond becomes ionized, which results in the disintegration of micelles and an improvement in ROS scavenging activity. Thus, RNPNs can exert their action locally, at inflammation sites, or in the acidic tumor environment [189].
A range of beneficial effects of RNP was reported (Table 6 and Table 7), including anticancer effects and protection against pathological conditions involving oxidative stress, including ischemia-reperfusion injury [190,191,192,193,194,195,196]. While low-molecular weight nitroxides show dose-related antihypertensive action accompanied by reflex tachycardia, increased skin temperature, and seizures [120], both RNPN and RNPO did not induce any decrease in the arterial blood pressure.

Table 6.
Cellular effects of nitroxide-containing nanoparticles.

Table 7.
Effects of nitroxide-containing nanoparticles in animal experiments.
9. Safety and Adverse Effects of Nitroxides and Nitroxide-Containing Nanoparticles
The toxicity of nitroxides is generally low. The IC50 values reported for HACaT cells were 2.66 mM for TEMPO, 9.5 mM for TEMPAMINE, and 11.4 mM for TEMPOL [49]. However, lower IC50 values were reported for other cell types. The IC50 values of TEMPOL for normal and malignant lung cells were 1–2 mM [100] and for Leishmania promastigotes to ca. 0.66 mM [100]. The higher sensitivity of malignant cells or parasites may be advantageous, provided that normal cells of the host are more resistant. Nitroxides can be given to animals in drinking water or food. TEMPOL administered in food (10 mg/g food) chronically was well tolerated and prolonged the life span of tumor-prone mice [84,104,105]. TEMPOL, injected intraperitoneally, was tolerated by female C3H mice up to a dose of 275 mg/kg. Above this dose, TEMPOL was lethal to varying degrees [59]. In acute animal experiments, nitroxide doses of 300 mg/kg or even 400 mg/kg [117] were applied. Nitroxides have a short time of circulation in blood. TEMPOL showed a fast decay in blood, and the EPR signal could not be detected approximately 5 min after injection, due to uptake by tissues and reduction. Metabolism of nitroxides takes place mainly in the liver, where TEMPO can be transformed into a five-membered ring in liver microsomes; the metabolites are excreted mainly into the bile [206].
In contrast to low-molecular-weight nitroxides, nitroxides bound to a macromolecular scaffold have much longer circulation time. The half-life of the RNPN was 60 times longer (15 min) than that of TEMPOL. RNPOs show much longer circulation: the half-life of the RNPO was 600 min, i.e., 2400 times longer than that of TEMPOL [190,191]. This effect seems to be partly due to the interaction of RNP with blood plasma proteins [193]. There are obvious conditions that must be fulfilled by an artificial biomaterial to be applied in vivo, including biocompatibility, adequate stability, and safety [207]. The redox PEG-b-PMNT nanoparticles containing nitroxides were reported to be even less cytotoxic than free nitroxides, not affecting cell viability up to a concentration of 8 mmol nitroxide residues/L and no mice toxicity at a dose of 300 mg/kg [190]. They can be given to animals in the food or drinking water [190,191]. The low toxicity of these nanoparticles is attributed to the fact that the outer PEG layer constitutes a stealth shield around the nitroxide moieties in the RNP core. However, the toxicity and safety of nanoparticles depend on their composition as they or cells containing them are ultimately subject to phagocytosis, their components are degraded, and metals, if present, are released.
10. Conclusions and Perspectives
The field of nitroxide research is continuously expanding, from chemical and physical studies of their properties via cellular effects to animal experiments and clinical applications. Their antioxidant properties, allowing them to react with free radicals and other ROS, in particular decompose superoxide radicals and prevent the Fenton reactions, justify their broad use in biology and medicine to alleviate oxidative stress. The decomposition of superoxide by piperidine nitroxides may be useful for the protection of blood nitric oxide and lower blood pressure. These compounds are used as NMR contrasting agents in NMR imaging and for EPR imaging (the latter fields of application were not the subject of this review). They are elements of nanoparticles prolonging their lifetime in vivo, allowing for the targeting of inflammation and tumor areas. There are reasons to expect that nitroxide-containing redox nanoparticles can be next-generation antioxidants. Adverse effects of nitroxides are generally not serious, and their cytotoxic effects may be useful for eliminating malignant cells and parasites. Presently, Pubmed responds to the item “nitroxides”, showing over 6300 publications; this number is expected to grow rapidly with the identification of new possibilities of experimental and therapeutic applications of these compounds.
Author Contributions
Both authors have equally contributed to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This review was performed within the project “Nanomolecular antioxidants: biological basis of targeted therapy of neurodegenerative diseases” (number of the application 2016/22/E/NZ7/00641) financed by the National Science Centre (NCN), Poland, in the program “SONATA-BIS 6”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| A2E | bis-retinoid N-retinyl-N-retinylidene ethanolamine (A2E), a major component of lipofuscin in the retina |
| AMD | age-related macular degeneration |
| Asc | ascorbate |
| t-BHP | tert-butyl hydroperoxide |
| BSA | bovine serum albumin |
| But | butyl |
| DTNB | 5,5-dithio-bis-(2-nitrobenzoic acid) (Ellman’s reagent) |
| EPR | electron paramagnetic resonance |
| GLUT | glucose transporter |
| GSH | glutathione |
| HO-1 | heme oxygenase 1 |
| IC50 | half-inhibitory concentration |
| IL | interleukin |
| IR | ionizing radiation |
| LDH | lactate dehydrogenase |
| MMP | matrix metalloproteinase |
| mtDNA | mitochondrial DNA |
| NHE | normal hydrogen electrode |
| NMR | nuclear magnetic resonance |
| pCMB | para-chloromercuribenzoate |
| PEG | poly(ethylene glycol) |
| PMOT | poly [4-(2,2,6,6-tetramethylpiperidine-1-oxyl)oxymethylstyrene] |
| PROXYL | 2,2,5,5-tetramethylpyrrolidinyl-1-oxyl |
| RNP | redox nanoparticles |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric-acid reactive substances |
| TEMPO | 2,2,6,6-tetramethylpiperidine-1-oxyl |
| TEMPAMINE | 4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl |
| TEMPOL | 4-hydroxyl-2,2,6,6-tetramethylpiperidine-1-oxyl |
| TEMPOL-H | hydroxylamine of TEMPOL |
References
- Dessolin, J.; Schuler, M.; Quinart, A.; De Giorgi, F.; Ghosez, L.; Ichas, F. Selective targeting of synthetic antioxidants to mitochondria: Towards a mitochondrial medicine for neurodegenerative diseases? Eur. J. Pharmacol. 2002, 447, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Trnka, J.; Blaikie, F.H.; Smith, R.A.; Murphy, M.P. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic. Biol. Med. 2008, 44, 1406–1419. [Google Scholar] [CrossRef]
- Zhdanov, R.; Sukhanov, V.; Shvets, V. Synthesis and Properties of Spin-Labeled Phospholipids. In Bioactive Spin Labels; Springer: Berlin/Heidelberg, Germany, 1992; pp. 297–315. [Google Scholar]
- Megli, F.M.; Conte, E.; Russo, L. Comparative 5-doxylstearoyllecithin and 3-doxylcholestane EPR spin labeling study of phospholipid bilayer perturbation by different oxidized lecithin species. Biochim. Biophys. Acta BBA Biomembr. 2010, 1798, 1886–1898. [Google Scholar] [CrossRef] [PubMed]
- Carloni, P.; Greci, L.; Stipa, P.; Eberson, L. Electron-transfer reactions. Oxidation of Grignard reagents in the presence of an aminoxyl as a radical-trapping agent. J. Org. Chem. 1991, 56, 4733–4737. [Google Scholar] [CrossRef]
- Carloni, P.; Greci, L.; Stipa, P.; Rizzoli, C.; Sgarabotto, P.; Ugozzoli, F. Antioxidants and light stabilizers. Part 1. Reactions of an indolinone nitroxide and phenoxy radicals. X-ray crystallographic analysis of 1-[O-(3,5-di-tert-butyl-4-hydroxy)-benzyl]-1,2-dihydro-2-methyl-2-phenyl-3-oxo-3H-indole and 3,5,3′5′-tetra-tert-butylstilbene-4,4′-quinone. Polym. Degrad. Stab. 1993, 39, 73–83. [Google Scholar]
- Cardellini, L.; Carloni, P.; Greci, L.; Stipa, P.; Faucitano, A. Homolytic Substitutions in Indolinone Nitroxide Radicals. Part 5. Reaction with tert.-Butylperoxy radicals. ChemInform 1990, 21, 1. [Google Scholar] [CrossRef]
- Greci, L. Homolytic substitutions in indolinone nitroxide radicals—III: Reactions with terbutoxy and methyl radicals. Tetrahedron 1982, 38, 2435–2439. [Google Scholar] [CrossRef]
- Haidasz, E.A.; Meng, D.; Amorati, R.; Baschieri, A.; Ingold, K.U.; Valgimigli, L.; Pratt, D.A. Acid is key to the radical-trapping antioxidant activity of nitroxides. J. Am. Chem. Soc. 2016, 138, 5290–5298. [Google Scholar] [CrossRef]
- Damiani, E.; Greci, L.; Parsons, R.; Knowland, J. Nitroxide radicals protect DNA from damage when illuminated in vitro in the presence of dibenzoylmethane and a common sunscreen ingredient. Free Radic. Biol. Med. 1999, 26, 809–816. [Google Scholar] [CrossRef]
- Genovese, D.; Baschieri, A.; Vona, D.; Baboi, R.E.; Mollica, F.; Prodi, L.; Amorati, R.; Zaccheroni, N. Nitroxides as building blocks for nanoantioxidants. ACS Appl. Mater. Interfaces 2021, 13, 31996–32004. [Google Scholar] [CrossRef]
- Carloni, P.; Damiani, E.; Greci, L.; Stipa, P.; Marrosu, G.; Petrucci, R.; Trazza, A. Chemical and electrochemical study on the interactions of aminoxyls with superoxide anion. Tetrahedron 1996, 52, 11257–11264. [Google Scholar] [CrossRef]
- Blinco, J.P.; Hodgson, J.L.; Morrow, B.J.; Walker, J.R.; Will, G.D.; Coote, M.L.; Bottle, S.E. Experimental and theoretical studies of the redox potentials of cyclic nitroxides. J. Org. Chem. 2008, 73, 6763–6771. [Google Scholar] [CrossRef] [PubMed]
- Offer, T.; Samuni, A. Nitroxides inhibit peroxyl radical-mediated DNA scission and enzyme inactivation. Free Radic. Biol. Med. 2002, 32, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Cimato, A.N.; Piehl, L.L.; Facorro, G.B.; Torti, H.B.; Hager, A.A. Antioxidant effects of water-and lipid-soluble nitroxide radicals in liposomes. Free Radic. Biol. Med. 2004, 37, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Carloni, P.; Biondi, C.; Greci, L. Increased oxidative modification of albumin when illuminated in vitro in the presence of a common sunscreen ingredient: Protection by nitroxide radicals. Free Radic. Biol. Med. 2000, 28, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Castagna, R.; Greci, L. The effects of derivatives of the nitroxide tempol on UVA-mediated in vitro lipid and protein oxidation. Free Radic. Biol. Med. 2002, 33, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Antosiewicz, J.; Damiani, E.; Jassem, W.; Wozniak, M.; Orena, M.; Greci, L. Influence of structure on the antioxidant activity of indolinic nitroxide radicals. Free Radic. Biol. Med. 1997, 22, 249–255. [Google Scholar] [CrossRef]
- Goldstein, S.; Samuni, A.; Hideg, K.; Merenyi, G. Structure−activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J. Phys. Chem. A 2006, 110, 3679–3685. [Google Scholar] [CrossRef]
- Carroll, R.T.; Galatsis, P.; Borosky, S.; Kopec, K.K.; Kumar, V.; Althaus, J.S.; Hall, E.D. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) inhibits peroxynitrite-mediated phenol nitration. Chem. Res. Toxicol. 2000, 13, 294–300. [Google Scholar] [CrossRef]
- Bonini, M.G.; Mason, R.P.; Augusto, O. The mechanism by which 4-hydroxy-2,2,6,6-tetramethylpiperidene-1-oxyl (tempol) diverts peroxynitrite decomposition from nitrating to nitrosating species. Chem. Res. Toxicol. 2002, 15, 506–511. [Google Scholar] [CrossRef]
- Deffner, U.; Schimmack, W. Radiation effects on aqueous solutions of the nitroxyl free radical TMPN (2,2,6,6-tetramethyl-4-piperidinol-N-oxyl). Int. J. Radiat. Biol. 1976, 29, 71–75. [Google Scholar] [CrossRef]
- Lam, M.A.; Pattison, D.I.; Bottle, S.E.; Keddie, D.J.; Davies, M.J. Nitric oxide and nitroxides can act as efficient scavengers of protein-derived free radicals. Chem. Res. Toxicol. 2008, 21, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Dhouib, R.; Fairfull-Smith, K.E.; Totsika, M. Nitroxide functionalized antibiotics are promising eradication agents against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2019, 64, e01685-19. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Krishna, C.M.; Riesz, P.; Finkelstein, E.; Russo, A. Superoxide reaction with nitroxide spin-adducts. Free Radic. Biol. Med. 1989, 6, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Grahame, D.A.; Samuni, A.; Mitchell, J.B.; Russo, A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc. Natl. Acad. Sci. USA 1992, 89, 5537–5541. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Krishna, C.M.; Mitchell, J.B.; Collins, C.R.; Russo, A. Superoxide reaction with nitroxides. Free Radic. Res. Commun. 1990, 9, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.M.; Finkelstein, E.; Rauckman, E.J. A method for the detection of superoxide in biological systems. Arch. Biochem. Biophys. 1982, 215, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.T.; Valentine, J.S. How super is superoxide? Acc. Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Krishna, M.C.; Russo, A.; Mitchell, J.B.; Goldstein, S.; Dafni, H.; Samuni, A. Do nitroxide antioxidants act as scavengers of O2−˙ or as SOD mimics? J. Biol. Chem. 1996, 271, 26026–26031. [Google Scholar] [CrossRef]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Superoxide-dependent reduction of nitroxides by thiols. Biochim. Biophys. Acta BBA Gen. Subj. 1984, 802, 90–98. [Google Scholar] [CrossRef]
- Abreu, I.A.; Cabelli, D.E. Superoxide dismutases—A review of the metal-associated mechanistic variations. Biochim. Biophys. Acta BBA Proteins Proteom. 2010, 1804, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Caliceti, P.; Schiavon, O.; Sergi, M. Polyethylene glycol–superoxide dismutase, a conjugate in search of exploitation. Adv. Drug Deliv. Rev. 2002, 54, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Samuni, A.; Taira, J.; Goldstein, S.; Mitchell, J.B.; Russo, A. Stimulation by nitroxides of catalase-like activity of hemeproteins: Kinetics and mechanism. J. Biol. Chem. 1996, 271, 26018–26025. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.-I.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Nakano, T.; Kimoto, E. Oxidation of nitroxide radicals by the reaction of hemoglobin with hydrogen peroxide. Biochem. Biophys. Res. Commun. 1984, 120, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Rice-Evans, C.; Davies, M.; Newman, E. The formation of free radicals by cardiac myocytes under oxidative stress and the effects of electron-donating drugs. Biochem. J. 1991, 277, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.-I.; Krishna, M.C.; Mitchell, J.B. Novel pharmacokinetic measurement using electron paramagnetic resonance spectroscopy and simulation of in vivo decay of various nitroxyl spin probes in mouse blood. J. Pharmacol. Exp. Ther. 2004, 310, 1076–1083. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Li, H.; Ilangovan, G.; Cardounel, A.J.; Zweier, J.L.; Yamada, K.; Krishna, M.C.; Mitchell, J.B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002, 62, 307–312. [Google Scholar]
- Yamada, K.-I.; Inoue, D.; Matsumoto, S.; Utsumi, H. In vivo measurement of redox status in streptozotocin-induced diabetic rat using targeted nitroxyl probes. Antioxid. Redox Signal. 2004, 6, 605–611. [Google Scholar] [CrossRef]
- Kasazaki, K.; Yasukawa, K.; Sano, H.; Utsumi, H. Non-invasive analysis of reactive oxygen species generated in NH4OH-induced gastric lesions of rats using a 300 MHz in vivo ESR technique. Free Radic. Res. 2003, 37, 757–766. [Google Scholar] [CrossRef]
- Zhang, Y.; Fung, L.-M. The roles of ascorbic acid and other antioxidants in the erythrocyte in reducing membrane nitroxide radicals. Free Radic. Biol. Med. 1994, 16, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bobko, A.A.; Kirilyuk, I.A.; Grigor’ev, I.A.; Zweier, J.L.; Khramtsov, V.V. Reversible reduction of nitroxides to hydroxylamines: Roles for ascorbate and glutathione. Free Radic. Biol. Med. 2007, 42, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Keana, J.; Van Nice, F. Influence of structure on the reduction of nitroxide MRI contrast-enhancing agents by ascorbate. Physiol. Chem. Phys. Med. NMR 1984, 16, 477–480. [Google Scholar] [PubMed]
- Lin, Y.; Liu, W.; Ohno, H.; Ogata, T. Determination of ascorbate concentration in a raw leaf with electron spin resonance spectroscopy. Anal. Sci. 1999, 15, 973–977. [Google Scholar] [CrossRef]
- Kirilyuk, I.A.; Bobko, A.A.; Grigor’ev, I.A.; Khramtsov, V.V. Synthesis of the tetraethyl substituted pH-sensitive nitroxides of imidazole series with enhanced stability towards reduction. Org. Biomol. Chem. 2004, 2, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Khramtsov, V.; Yelinova, V.; Weiner, L.; Berezina, T.; Martin, V.; Volodarsky, L. Quantitative determination of SH groups in low-and high-molecular-weight compounds by an electron spin resonance method. Anal. Biochem. 1989, 182, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Głębska, J.; Skolimowski, J.; Kudzin, Z.; Gwoździński, K.; Grzelak, A.; Bartosz, G. Pro-oxidative activity of nitroxides in their reactions with glutathione. Free Radic. Biol. Med. 2003, 35, 310–316. [Google Scholar] [CrossRef]
- Kroll, C.; Langner, A.; Borchert, H.H. Nitroxide metabolism in the human keratinocyte cell line HaCaT. Free Radic. Biol. Med. 1999, 26, 850–857. [Google Scholar] [CrossRef]
- Azuma, R.; Yamasaki, T.; Emoto, M.C.; Sato-Akaba, H.; Sano, K.; Munekane, M.; Fujii, H.G.; Mukai, T. Effect of relative configuration of TEMPO-type nitroxides on ascorbate reduction. Free Radic. Biol. Med. 2023, 194, 114–122. [Google Scholar] [CrossRef]
- Babić, N.; Orio, M.; Peyrot, F. Unexpected rapid aerobic transformation of 2,2,6,6-tetraethyl-4-oxo (piperidin-1-yloxyl) radical by cytochrome P450 in the presence of NADPH: Evidence against a simple reduction of the nitroxide moiety to the hydroxylamine. Free Radic. Biol. Med. 2020, 156, 144–156. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Pittelkow, M.R.; Wood, J.M. Free radical reduction by thioredoxin reductase at the surface of normal and vitiliginous human keratinocytes. J. Investig. Dermatol. 1986, 87, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Keana, J.F.; Pou, S.; Rosen, G.M. Nitroxides as potential contrast enhancing agents for MRI application: Influence of structure on the rate of reduction by rat hepatocytes, whole liver homogenate, subcellular fractions, and ascorbate. Magn. Reson. Med. 1987, 5, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Trubitsin, B.; Milanovsky, G.; Mamedov, M.; Semenov, A.Y.; Tikhonov, A. The interaction of water-soluble nitroxide radicals with Photosystem II. Appl. Magn. Reson. 2022, 53, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, R.; Butler, K.; Smith, I.C. Oxidant stress in malaria as probed by stable nitroxide radicals in erythrocytes infected with Plasmodium berghei. The effects of primaquine and chloroquine. Biochim. Biophys. Acta BBA Mol. Cell Res. 1987, 931, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845. [Google Scholar] [CrossRef]
- Yan, S.-X.; Hong, X.-Y.; Hu, Y.; Liao, K.-H. Tempol, one of nitroxides, is a novel ultraviolet-A1 radiation protector for human dermal fibroblasts. J. Dermatol. Sci. 2005, 37, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; DeGraff, W.; Kaufman, D.; Krishna, M.C.; Samuni, A.; Finkelstein, E.; Ahn, M.S.; Hahn, S.M.; Gamson, J.; Russo, A. Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch. Biochem. Biophys. 1991, 289, 62–70. [Google Scholar] [CrossRef]
- Hahn, S.M.; Tochner, Z.; Krishna, C.M.; Glass, J.; Wilson, L.; Samuni, A.; Sprague, M.; Venzon, D.; Glatstein, E.; Mitchell, J.B. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992, 52, 1750–1753. [Google Scholar]
- Leathem, A.; Simone, M.; Dennis, J.M.; Witting, P.K. The cyclic nitroxide TEMPOL ameliorates oxidative stress but not inflammation in a cell model of Parkinson’s disease. Antioxidants 2022, 11, 257. [Google Scholar] [CrossRef]
- Liang, Q.; Smith, A.D.; Pan, S.; Tyurin, V.A.; Kagan, V.E.; Hastings, T.G.; Schor, N.F. Neuroprotective effects of TEMPOL in central and peripheral nervous system models of Parkinson’s disease. Biochem. Pharmacol. 2005, 70, 1371–1381. [Google Scholar] [CrossRef]
- Pichla, M.; Pulaski, Ł.; Kania, K.D.; Stefaniuk, I.; Cieniek, B.; Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Nitroxide radical-containing redox nanoparticles protect neuroblastoma SH-SY5Y cells against 6-hydroxydopamine toxicity. Oxidative Med. Cell. Longev. 2020, 2020, 9260748. [Google Scholar] [CrossRef] [PubMed]
- Alpert, E.; Altman, H.; Totary, H.; Gruzman, A.; Barnea, D.; Barash, V.; Sasson, S. 4-Hydroxy tempol-induced impairment of mitochondrial function and augmentation of glucose transport in vascular endothelial and smooth muscle cells. Biochem. Pharmacol. 2004, 67, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, Z.; Zhang, C.; Shi, Y.; Han, W.; Song, S.; Mu, L.; Du, C.; Shi, Y. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism 2021, 118, 154748. [Google Scholar] [CrossRef]
- Zarei, F.; Daghigh-Kia, H.; Masoudi, R. Supplementation of ram’s semen extender with Mito-TEMPO II: Quality evaluation and flow cytometry study of post-thawed spermatozoa. Andrologia 2022, 54, e14299. [Google Scholar] [CrossRef] [PubMed]
- Zarei, F.; Kia, H.D.; Masoudi, R.; Moghaddam, G.; Ebrahimi, M. Supplementation of ram’s semen extender with Mito-TEMPO I: Improvement in quality parameters and reproductive performance of cooled-stored semen. Cryobiology 2021, 98, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar Ghosh, S.; Katiyar, R.; Gemeda, A.E.; Rautela, R.; Bisla, A.; Srivastava, N.; Kumar Bhure, S.; Devi, H.L.; Chandra, V. Supplementation of Mito TEMPO and acetovanillone in semen extender improves freezability of buffalo spermatozoa. Andrology 2022, 10, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, I.; Zare-Shahneh, A.; Goodarzi, A.; Baghshahi, H.; Fouladi-Nashta, A. The effect of Tempo and MitoTEMPO on oocyte maturation and subsequent embryo development in bovine model. Theriogenology 2021, 176, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Degraff, W.G.; Krishna, M.C.; Russo, A.; Mitchell, J.B. Antimutagenicity of a low molecular weight superoxide dismutase mimic against oxidative mutagens. Environ. Mol. Mutagen. 1992, 19, 21–26. [Google Scholar] [CrossRef]
- Sies, H.; Mehlhorn, R. Mutagenicity of nitroxide-free radicals. Arch. Biochem. Biophys. 1986, 251, 393–396. [Google Scholar] [CrossRef]
- Lewinska, A.; Wnuk, M.; Slota, E.; Bartosz, G. The nitroxide antioxidant Tempol affects metal-induced cyto-and genotoxicity in human lymphocytes in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 649, 7–14. [Google Scholar] [CrossRef]
- Maio, N.; Lafont, B.A.; Sil, D.; Li, Y.; Bollinger, J.M., Jr.; Krebs, C.; Pierson, T.C.; Linehan, W.M.; Rouault, T.A. Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets. Science 2021, 373, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jang, Y.P.; Chang, S.; Sparrow, J.R. OT-674 Suppresses Photooxidative Processes Initiated by an RPE Lipofuscin Fluorophore. Photochem. Photobiol. 2008, 84, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Zarling, J.A.; Brunt, V.E.; Vallerga, A.K.; Li, W.; Tao, A.; Zarling, D.A.; Minson, C.T. Nitroxide pharmaceutical development for age-related degeneration and disease. Front. Genet. 2015, 6, 325. [Google Scholar] [CrossRef]
- Mizuno, H.; Kubota, C.; Takigawa, Y.; Shintoku, R.; Kannari, N.; Muraoka, T.; Obinata, H.; Yoshimoto, Y.; Kanazawa, M.; Koshiishi, I. 2,2,6,6-Tetramethylpiperidine-1-oxyl acts as a volatile inhibitor of ferroptosis and neurological injury. J. Biochem. 2022, 172, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pinson, A.; Samuni, A. Both hydroxylamine and nitroxide protect cardiomyocytes from oxidative stress. Free Radic. Biol. Med. 1998, 24, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, D.; Damiani, E.; Greci, L.; Littarru, G.P.; Falcioni, G. Nitroxide radicals protect against DNA damage in rat epithelial cells induced by nitric oxide, nitroxyl anion and peroxynitrite. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 535, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Czepas, J.; Koceva-Chyła, A.; Gwoździński, K.; Jóźwiak, Z. Different effectiveness of piperidine nitroxides against oxidative stress induced by doxorubicin and hydrogen peroxide. Cell Biol. Toxicol. 2008, 24, 101–112. [Google Scholar] [CrossRef]
- Castagna, R.; Davis, P.; Vasu, V.; Soucek, K.; Cross, C.; Greci, L.; Valacchi, G. Nitroxide radical TEMPO reduces ozone-induced chemokine IL-8 production in lung epithelial cells. Toxicol. In Vitro 2009, 23, 365–370. [Google Scholar] [CrossRef]
- He, S.-M.; Lei, Y.-H.; Wang, J.-M.; Geng, L.-N.; Wang, S.-P.; Zhao, J.; Hou, Y.-F. The protective effect of nitronyl nitroxide radical on peroxidation of A549 cell damaged by iron overload. Mater. Sci. Eng. C 2020, 108, 110189. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; McDonald, M.; Mazzon, E.; Siriwardena, D.; Costantino, G.; Fulia, F.; Cucinotta, G.; Gitto, E.; Cordaro, S.; Barberi, I. Effects of tempol, a membrane-permeable radical scavenger, in a gerbil model of brain injury. Brain Res. 2000, 875, 96–106. [Google Scholar] [CrossRef]
- Zigler, J.S., Jr.; Qin, C.; Kamiya, T.; Krishna, M.C.; Cheng, Q.; Tumminia, S.; Russell, P. Tempol-H inhibits opacification of lenses in organ culture. Free Radic. Biol. Med. 2003, 35, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; Wilson, L.; Krishna, C.M.; Liebmann, J.; DeGraff, W.; Gamson, J.; Samuni, A.; Venzon, D.; Mitchell, J.B. Identification of nitroxide radioprotectors. Radiat. Res. 1992, 132, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Erker, L.; Schubert, R.; Yakushiji, H.; Barlow, C.; Larson, D.; Mitchell, J.B.; Wynshaw-Boris, A. Cancer chemoprevention by the antioxidant tempol acts partially via the p53 tumor suppressor. Hum. Mol. Genet. 2005, 14, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G.; Pieńkowska, N.; Kut, K.; Cieniek, B.; Stefaniuk, I.; Sadowska-Bartosz, I. Effect of Low Concentration of Nitroxides on SH-SY5Y Cells Transfected with the Tau Protein. Int. J. Mol. Sci. 2023, 24, 16675. [Google Scholar] [CrossRef]
- Santos, G.B.; Ribeiro, A.C.; Lima, S.N.; Trostchansky, A.; Cerdeira, C.D.; Brigagão, M.R. Nitroxide Tempol down-regulates kinase activities associated with NADPH oxidase function in phagocytic cells and potentially decreases their fungicidal response. Chem. Biol. Int. 2018, 279, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mołoń, M.; Szlachcikowska, D.; Stępień, K.; Kielar, P.; Galiniak, S. Two faces of TEMPO (2,2,6,6-tetramethylpiperidinyl-1-oxyl)–An antioxidant or a toxin? Biochim. Biophys. Acta BBA Mol. Cell Res. 2023, 1870, 119412. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Stylianou, M.; Lopes, J.P.; Müller, D.C.; Häggman, A.; Holmberg, S.; Grumaz, C.; Johansson, A.; Sohn, K.; Dieterich, C. Stable redox-cycling nitroxide tempol has antifungal and immune-modulatory properties. Front. Microbiol. 2019, 10, 1843. [Google Scholar] [CrossRef]
- Shi, T.Y.; Zhao, D.Q.; Wang, H.B.; Feng, S.; Liu, S.B.; Xing, J.H.; Qu, Y.; Gao, P.; Sun, X.L.; Zhao, M.G. A new chiral pyrrolyl α-nitronyl nitroxide radical attenuates β-amyloid deposition and rescues memory deficits in a mouse model of Alzheimer disease. Neurotherapeutics 2013, 10, 340–353. [Google Scholar] [CrossRef]
- Du, F.; Yu, Q.; Kanaan, N.M.; Yan, S.S. Mitochondrial oxidative stress contributes to the pathological aggregation and accumulation of tau oligomers in Alzheimer’s disease. Hum. Mol. Genet. 2022, 31, 2498–2507. [Google Scholar] [CrossRef]
- Greenwald, M.B.Y.; Anzi, S.; Sasson, S.B.; Bianco-Peled, H.; Kohen, R. Can nitroxides evoke the Keap1–Nrf2–ARE pathway in skin? Free Radic. Biol. Med. 2014, 77, 258–269. [Google Scholar] [CrossRef]
- Balcerczyk, A.; Łuczak, K.; Soszyński, M.; Bartosz, G. Prooxidative effects of TEMPO on human erythrocytes. Cell Biol. Int. 2004, 28, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Bujak-Pietrek, S.; Pieniazek, A.; Gwozdzinski, K.; Gwozdzinski, L. The Effect of Piperidine Nitroxides on the Properties of Metalloproteins in Human Red Blood Cells. Molecules 2023, 28, 6174. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, M.; Ravizza, R.; Petterino, C.; Castagnaro, M.; Finocchiaro, G.; Monti, E. Study of in vitro and in vivo effects of the piperidine nitroxide Tempol—A potential new therapeutic agent for gliomas. Eur. J. Cancer 2003, 39, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Suy, S.; Mitchell, J.B.; Samuni, A.; Mueller, S.; Kasid, U. Nitroxide tempo, a small molecule, induces apoptosis in prostate carcinoma cells and suppresses tumor growth in athymic mice. Cancer 2005, 103, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Braunhut, S.; Medeiros, D.; Lai, L.; Bump, E. Tempol prevents impairment of the endothelial cell wound healing response caused by ionising radiation. Br. J. Cancer 1996, 27, S157. [Google Scholar]
- Czepas, J.; Matczak, K.; Koceva-Chyła, A.; Grobelski, B.; Jóźwiak, Z.; Gwoździński, K. Doxyl Nitroxide Spin Probes Can Modify Toxicity of Doxorubicin towards Fibroblast Cells. Molecules 2020, 25, 5138. [Google Scholar] [CrossRef] [PubMed]
- Sultani, H.N.; Morgan, I.; Hussain, H.; Roos, A.H.; Haeri, H.H.; Kaluđerović, G.N.; Hinderberger, D.; Westermann, B. Access to new cytotoxic triterpene and steroidal acid-TEMPO conjugates by Ugi multicomponent-reactions. Int. J. Mol. Sci. 2021, 22, 7125. [Google Scholar] [CrossRef]
- Park, W.H. Tempol Inhibits the growth of lung cancer and normal cells through apoptosis accompanied by increased O2•− levels and glutathione depletion. Molecules 2022, 27, 7341. [Google Scholar] [CrossRef]
- Oliveira, L.B.; Celes, F.S.; Paiva, C.N.; de Oliveira, C.I. The paradoxical leishmanicidal effects of superoxide dismutase (SOD)-mimetic tempol in Leishmania braziliensis infection in vitro. Front. Cell. Infect. Microbiol. 2019, 9, 23. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as antioxidants and anticancer drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Xavier, S.; DeLuca, A.M.; Sowers, A.L.; Cook, J.A.; Krishna, M.C.; Hahn, S.M.; Russo, A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic. Biol. Med. 2003, 34, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Erker, L.; Barlow, C.; Yakushiji, H.; Larson, D.; Russo, A.; Mitchell, J.B.; Wynshaw-Boris, A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum. Mol. Genet. 2004, 13, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D.; Karmeli, F.; Okon, E.; Samuni, A. A novel antiulcerogenic stable radical prevents gastric mucosal lesions in rats. Gut 1994, 35, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Gelvan, D.; Saltman, P.; Powell, S.R. Cardiac reperfusion damage prevented by a nitroxide free radical. Proc. Natl. Acad. Sci. USA 1991, 88, 4680–4684. [Google Scholar] [CrossRef]
- Behringer, W.; Safar, P.; Kentner, R.; Wu, X.; Kagan, V.E.; Radovsky, A.; Clark, R.S.; Kochanek, P.M.; Subramanian, M.; Tyurin, V.A. Antioxidant Tempol enhances hypothermic cerebral preservation during prolonged cardiac arrest in dogs. J. Cereb. Blood Flow Metab. 2002, 22, 105–117. [Google Scholar] [CrossRef]
- Berber, I.; Aydin, C.; Cevahir, N.; Yenisey, C.; Gumrukcu, G.; Kocbil, G.; Tellioglu, G.; Tekin, K. Tempol reduces bacterial translocation after ischemia/reperfusion injury in a rat model of superior mesenteric artery occlusion. Surg. Today 2009, 39, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Cai, J.; Xue, P.; Zhang, Y.; Liu, S.; Gao, X.; Li, M.; Wang, Z.; Baudy-Floch, M.; Green, S.A. Protective effect of nitronyl nitroxide–amino acid conjugates on liver ischemia–reperfusion-induced injury in rats. Bioorg. Med. Chem. Lett. 2008, 18, 1788–1794. [Google Scholar] [CrossRef]
- Castro, M.M.; Rizzi, E.; Rodrigues, G.J.; Ceron, C.S.; Bendhack, L.M.; Gerlach, R.F.; Tanus-Santos, J.E. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic. Biol. Med. 2009, 46, 1298–1307. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; Cuzzocrea, S.; Brown, P.A.; Zacharowski, K.; Stewart, K.N.; Mota-Filipe, H.; Thiemermann, C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000, 58, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Takaoka, M.; Ohkita, M.; Matsumura, Y. Tempol protects against ischemic acute renal failure by inhibiting renal noradrenaline overflow and endothelin-1 overproduction. Biol. Pharm. Bull. 2005, 28, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Li, F.; Anderson, R.E. Protection of retinal pigment epithelium by OT-551 and its metabolite TEMPOL-H against light-induced damage in rats. Exp. Eye Res. 2010, 91, 111–114. [Google Scholar] [CrossRef]
- Tanito, M.; Li, F.; Elliott, M.H.; Dittmar, M.; Anderson, R.E. Protective effect of TEMPOL derivatives against light-induced retinal damage in rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; DeLuca, A.M.; Coffin, D.; Krishna, C.M.; Mitchell, J.B. In vivo radioprotection and effects on blood pressure of the stable free radical nitroxides. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; Sullivan, F.J.; DeLuca, A.M.; Krishna, C.M.; Wersto, N.; Venzon, D.; Russo, A.; Mitchell, J.B. Evaluation of tempol radioprotection in a murine tumor model. Free Radic. Biol. Med. 1997, 22, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, A.P.; Hyodo, F.; Matsumoto, K.-I.; Sowers, A.L.; Cook, J.A.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin. Cancer Res. 2007, 13, 4928–4933. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yamasaki, T.; Ueno, M.; Shibata, S.; Ozawa, Y.; Kamada, T.; Nakanishi, I.; Yamada, K.-I.; Aoki, I.; Matsumoto, K.-I. Radiation-induced redox alteration in the mouse brain. Free Radic. Biol. Med. 2019, 143, 412–421. [Google Scholar] [CrossRef]
- Hahn, S.M.; Sullivan, F.J.; DeLuca, A.M.; Bacher, J.D.; Liebmann, J.; Krishna, M.C.; Coffin, D.; Mitchell, J.B. Hemodynamic effect of the nitroxide superoxide dismutase mimics. Free Radic. Biol. Med. 1999, 27, 529–535. [Google Scholar] [CrossRef]
- Adeagbo, A.S.; Joshua, I.G.; Falkner, C.; Matheson, P.J. Tempol, an antioxidant, restores endothelium-derived hyperpolarizing factor-mediated vasodilation during hypertension. Eur. J. Pharmacol. 2003, 481, 91–100. [Google Scholar] [CrossRef]
- Banday, A.A.; Marwaha, A.; Tallam, L.S.; Lokhandwala, M.F. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor–G-protein coupling and function in obese Zucker rats. Diabetes 2005, 54, 2219–2226. [Google Scholar] [CrossRef]
- Ebenezer, P.J.; Mariappan, N.; Elks, C.M.; Haque, M.; Francis, J. Diet-induced renal changes in Zucker rats are ameliorated by the superoxide dismutase mimetic TEMPOL. Obesity 2009, 17, 1994–2002. [Google Scholar] [CrossRef]
- DeRubertis, F.R.; Craven, P.A.; Melhem, M.F. Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: Effects of tempol. Metabolism 2007, 56, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, R.; Sowers, A.L.; Chandramouli, G.; Gamson, J.; Krishna, M.C.; Mitchell, J.B.; Cook, J.A. The antioxidant tempol transforms gut microbiome to resist obesity in female C3H mice fed a high fat diet. Free Radic. Biol. Med. 2022, 178, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Mitchell, J.B.; Bursill, C.A.; Sowers, A.L.; Thetford, A.; Cook, J.A.; van Reyk, D.M.; Davies, M.J. The nitroxide radical TEMPOL prevents obesity, hyperlipidaemia, elevation of inflammatory cytokines, and modulates atherosclerotic plaque composition in apoE−/− mice. Atherosclerosis 2015, 240, 234–241. [Google Scholar] [CrossRef]
- Beswick, R.A.; Zhang, H.; Marable, D.; Catravas, J.D.; Hill, W.D.; Webb, R.C. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 2001, 37, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Lan, C.; Chen, C.; Xu, Z.; Luo, H.; Zheng, S.; Gong, X.; Ren, H.; Li, Z.; Qu, S. Prenatal lipopolysaccharides exposure induces transgenerational inheritance of Hypertension. Circulation 2022, 146, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Chess, D.J.; Xu, W.; Khairallah, R.; O’Shea, K.M.; Kop, W.J.; Azimzadeh, A.M.; Stanley, W.C. The antioxidant tempol attenuates pressure overload-induced cardiac hypertrophy and contractile dysfunction in mice fed a high-fructose diet. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2223–H2230. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; McDonald, M.C.; Filipe, H.M.; Costantino, G.; Mazzon, E.; Santagati, S.; Caputi, A.P.; Thiemermann, C. Effects of tempol, a membrane-permeable radical scavenger, in a rodent model of carrageenan-induced pleurisy. Eur. J. Pharmacol. 2000, 390, 209–222. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; McDonald, M.C.; Mazzon, E.; Dugo, L.; Lepore, V.; Fonti, M.T.; Ciccolo, A.; Terranova, M.L.; Caputi, A.P.; Thiemermann, C. Tempol, a membrane-permeable radical scavenger, reduces dinitrobenzene sulfonic acid-induced colitis. Eur. J. Pharmacol. 2000, 406, 127–137. [Google Scholar] [CrossRef]
- Chami, B.; San Gabriel, P.T.; Kum-Jew, S.; Wang, X.; Dickerhof, N.; Dennis, J.M.; Witting, P.K. The nitroxide 4-methoxy-tempo inhibits the pathogenesis of dextran sodium sulfate-stimulated experimental colitis. Redox Biol. 2020, 28, 101333. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; McDonald, M.C.; Mazzon, E.; Filipe, H.M.; Lepore, V.; Terranova, M.L.; Ciccolo, A.; Caputi, A.P.; Thiemermann, C. Beneficial effects of tempol, a membrane-permeable radical scavenger, on the multiple organ failure induced by zymosan in the rat. Crit. Care Med. 2001, 29, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; McDonald, M.C.; Mota-Filipe, H.; Mazzon, E.; Costantino, G.; Britti, D.; Mazzullo, G.; Caputi, A.P.; Thiemermann, C. Beneficial effects of tempol, a membrane-permeable radical scavenger, in a rodent model of collagen-induced arthritis. Arthritis Rheum. 2000, 43, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Deng-Bryant, Y.; Singh, I.N.; Carrico, K.M.; Hall, E.D. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. 2008, 28, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Mazzon, E.; Zito, D.; Maiere, D.; Britti, D.; Genovese, T.; Cuzzocrea, S. Effects of Tempol, a membrane-permeable radical scavenger, in a rodent model periodontitis. J. Cin. Periodontol. 2005, 32, 1062–1068. [Google Scholar] [CrossRef]
- Duann, P.; Datta, P.K.; Pan, C.; Blumberg, J.B.; Sharma, M.; Lianos, E.A. Superoxide dismutase mimetic preserves the glomerular capillary permeability barrier to protein. J. Pharmacol. Exp. Ther. 2006, 316, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Elmedal, B.; De Dam, M.Y.; Mulvany, M.J.; Simonsen, U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br. J. Pharmacol. 2004, 141, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Black, H.D.; Xu, W.; Hortle, E.; Robertson, S.I.; Britton, W.J.; Kaur, A.; New, E.J.; Witting, P.K.; Chami, B.; Oehlers, S.H. The cyclic nitroxide antioxidant 4-methoxy-TEMPO decreases mycobacterial burden in vivo through host and bacterial targets. Free Radic. Biol. Med. 2019, 135, 157–166. [Google Scholar] [CrossRef]
- Li, T.; Zhang, T.; Gao, H.; Liu, R.; Gu, M.; Yang, Y.; Cui, T.; Lu, Z.; Yin, C. Tempol ameliorates polycystic ovary syndrome through attenuating intestinal oxidative stress and modulating of gut microbiota composition-serum metabolites interaction. Redox Biol. 2021, 41, 101886. [Google Scholar] [CrossRef]
- Li, F.; Jiang, C.; Krausz, K.W.; Li, Y.; Albert, I.; Hao, H.; Fabre, K.M.; Mitchell, J.B.; Patterson, A.D.; Gonzalez, F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 2013, 4, 2384. [Google Scholar] [CrossRef]
- Spejo, A.B.; Teles, C.B.; Zuccoli, G.D.S.; Oliveira, A.L.R. Synapse preservation and decreased glial reactions following ventral root crush (VRC) and treatment with 4-hydroxy-tempo (TEMPOL). J. Neurosci. Res. 2019, 97, 520–534. [Google Scholar] [CrossRef]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T. Oxidative stress mediates the fetal programming of hypertension by glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef]
- Shetty, S.; Kumar, V.; Ramesh, V.; Bharati, S. Mito-TEMPO protects against bisphenol-A-induced testicular toxicity: An in vivo study. Free Radic. Res. 2022, 56, 427–435. [Google Scholar] [CrossRef]
- Andrade, M.R.; Azeez, T.A.; Montgomery, M.M.; Caldwell, J.T.; Park, H.; Kwok, A.T.; Borg, A.M.; Narayanan, S.A.; Willey, J.S.; Delp, M.D. Neurovascular dysfunction associated with erectile dysfunction persists after long-term recovery from simulations of weightlessness and deep space irradiation. FASEB J. 2023, 37, e23246. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Xie, K.; Cao, Y.-X.; Zhang, A. Hepatoprotective effect of mitochondria-targeted antioxidant mito-TEMPO against lipopolysaccharide-induced liver injury in mouse. Med. Inflamm. 2022, 2022, 6394199. [Google Scholar] [CrossRef]
- Assayag, M.; Goldstein, S.; Samuni, A.; Kaufman, A.; Berkman, N. The nitroxide/antioxidant 3-carbamoyl proxyl attenuates disease severity in murine models of severe asthma. Free Radic. Biol. Med. 2021, 177, 181–188. [Google Scholar] [CrossRef]
- Assayag, M.; Goldstein, S.; Samuni, A.; Berkman, N. 3-Carbamoyl-proxyl nitroxide radicals attenuate bleomycin-induced pulmonary fibrosis in mice. Free Radic. Biol. Med. 2021, 171, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Fan, S.; Zheng, D.; Wang, G.; Yu, Y.; Chen, R.; Song, L.-S.; Fan, G.-C.; Zhang, Z.; Peng, T. Increased calpain-1 in mitochondria induces dilated heart failure in mice: Role of mitochondrial superoxide anion. Basic Res. Cardiol. 2019, 114, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.C.; Ferreira, V.F.; Vaz, M.G.; Cassaro, R.A.A.; Resende, J.A.; Sacramento, C.Q.; Costa, J.; Abrantes, J.L.; Souza, T.M.L.; Jordão, A.K. Chemistry and anti-herpes simplex virus type 1 evaluation of 4-substituted-1 H-1, 2, 3-triazole-nitroxyl-linked hybrids. Mol. Divers. 2021, 25, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Perumal, E.; Bi, X.; Wang, Y.; Ding, W. Potential mechanisms of uremic muscle wasting and the protective role of the mitochondria-targeted antioxidant Mito-TEMPO. Int. Urol. Nephrol. 2020, 52, 1551–1561. [Google Scholar] [CrossRef]
- Shetty, S.; Kumar, R.; Bharati, S. Mito-TEMPO, a mitochondria-targeted antioxidant, prevents N-nitrosodiethylamine-induced hepatocarcinogenesis in mice. Free Radic. Biol. Med. 2019, 136, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, I.; Santos, F.; Kido, L.; Lamas, C.; Montico, F.; Cagnon, V. Tempol differential effect on prostate cancer inflammation: In vitro and in vivo evaluation. Prostate 2023, 83, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Chen, Y.; Dennehy, K.; Blau, J.; Connors, S.; Mendonca, M.; Tarpey, M.; Krishna, M.; Mitchell, J.B.; Welch, W.J.; et al. Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R37–R43. [Google Scholar] [CrossRef]
- Hahn, S.M.; Krishna, M.C.; DeLuca, A.M.; Coffin, D.; Mitchell, J.B. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic. Biol. Med. 2000, 28, 953–958. [Google Scholar] [CrossRef]
- Asghar, M.; Lokhandwala, M.F. Antioxidant tempol lowers age-related increases in insulin resistance in Fischer 344 rats. Clin. Exp. Hypertens. 2006, 28, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Pisano, B.; Dugo, L.; Ianaro, A.; Patel, N.S.; Caputi, A.P.; Thiemermann, C. Tempol reduces the activation of nuclear factor-kappaB in acute inflammation. Free Radic. Res. 2004, 38, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, R.F.; Vaz, S.M.; Augusto, O. Inhibition of the chlorinating activity of myeloperoxidase by tempol: Revisiting the kinetics and mechanisms. Biochem. J. 2011, 439, 423–431. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Sun, Z.; Li, X.; Yang, L.; Dong, X.; Han, Y.; Li, Y.; Luo, J.; Li, W. SOCE-mediated NFAT1-NOX2-NLRP1 inflammasome involves in lipopolysaccharide-induced neuronal damage and Aβ generation. Mol. Neurobiol. 2022, 59, 3183–3205. [Google Scholar] [CrossRef]
- Li, L.; Tong, X.K.; Hosseini Kahnouei, M.; Vallerand, D.; Hamel, E.; Girouard, H. impaired hippocampal neurovascular coupling in a mouse model of Alzheimer’s disease. Front. Physiol. 2021, 12, 715446. [Google Scholar] [CrossRef] [PubMed]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. Therapeutic and clinical applications of nitroxide compounds. Antioxid. Redox Signal. 2007, 9, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Brunt, V.E.; Minson, C.T. Tempol improves cutaneous thermal hyperemia through increasing nitric oxide bioavailability in young smokers. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1507–H1511. [Google Scholar] [CrossRef] [PubMed]
- Medow, M.S.; Bamji, N.; Clarke, D.; Ocon, A.J.; Stewart, J.M. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J. Appl. Physiol. 2011, 111, 20–26. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; Ramick, M.G.; Farquhar, W.B.; Townsend, R.R.; Edwards, D.G. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2014, 306, F1499–F1506. [Google Scholar] [CrossRef]
- Wong, W.T.; Kam, W.; Cunningham, D.; Harrington, M.; Hammel, K.; Meyerle, C.B.; Cukras, C.; Chew, E.Y.; Sadda, S.R.; Ferris, F.L. Treatment of geographic atrophy by the topical administration of OT-551: Results of a phase II clinical trial. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6131–6139. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.M.; Smith, D.; Mick, R.; Lustig, R.; Mitchell, J.; Cherakuri, M.; Glatstein, E.; Hahn, S.M. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin. Cancer Res. 2004, 10, 6411–6417. [Google Scholar] [CrossRef]
- Palakkal, S.; Cortial, A.; Frušić-Zlotkin, M.; Soroka, Y.; Tzur, T.; Nassar, T.; Benita, S. Effect of cyclosporine A—Tempol topical gel for the treatment of alopecia and anti-inflammatory disorders. Int. J. Pharm. 2023, 642, 123121. [Google Scholar] [CrossRef]
- Citrin, D.; Valle, L.; Camphausen, K.; Cooley-Zgela, T.; Smart, D.; Yao, M.; Mitchell, J.B.; Thompson, W.; Sereti, I.; Uldrick, T. Pilot trial of topical MTS-01 application to reduce dermatitis in patients receiving chemoradiotherapy for stage I-III carcinoma of the anal canal. Int. J. Oncol. 2022, 60, 68. [Google Scholar] [CrossRef]
- Maurel, V.; Laferrière, M.; Billone, P.; Godin, R.; Scaiano, J. Free radical sensor based on CdSe quantum dots with added 4-amino-2, 2, 6, 6-tetramethylpiperidine oxide functionality. J. Phys. Chem. B 2006, 110, 16353–16358. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiao, C.; Oyaizu, K.; Chikushi, N.; Chen, X.; Nishide, H. Synthesis of amphiphilic block copolymers bearing stable nitroxyl radicals. J. Polym. Sci. A Polym. Chem. 2010, 48, 5404–5410. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, X.-Q.; Wang, X.-Q.; Shi, X.; Wang, W.; Yang, H.-B. Rotaxane-branched radical dendrimers with TEMPO termini. Chem. Commun. 2022, 58, 2006–2009. [Google Scholar] [CrossRef]
- Samuelson, L.E.; Sebby, K.B.; Walter, E.D.; Singel, D.J.; Cloninger, M.J. EPR and affinity studies of mannose–TEMPO functionalized PAMAM dendrimers. Org. Biomol. Chem. 2004, 2, 3075–3079. [Google Scholar] [CrossRef] [PubMed]
- Badetti, E.; Lloveras, V.; Wurst, K.; Sebastián, R.M.; Caminade, A.-M.; Majoral, J.-P.; Veciana, J.; Vidal-Gancedo, J. Synthesis and structural characterization of a dendrimer model compound based on a cyclotriphosphazene core with TEMPO radicals as substituents. Org. Lett. 2013, 15, 3490–3493. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liang, H.; Sun, T.; Yang, D.; Cao, M. A recoverable dendritic polyamidoamine immobilized TEMPO for efficient catalytic oxidation of cellulose. Carbohydr. Polym. 2018, 202, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; Boothapandi, M.; Nasar, A.S. Nitric oxide, DPPH and hydrogen peroxide radical scavenging activity of TEMPO terminated polyurethane dendrimers: Data supporting antioxidant activity of radical dendrimers. Data Brief 2020, 28, 104972. [Google Scholar] [CrossRef] [PubMed]
- Swiech, O.; Bilewicz, R.; Megiel, E. TEMPO coated Au nanoparticles: Synthesis and tethering to gold surfaces. RSC Adv. 2013, 3, 5979–5986. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Chen, Y.; Kawazoe, N.; Chen, G. TEMPO-conjugated gold nanoparticles for reactive oxygen species scavenging and regulation of stem cell differentiation. ACS Appl. Mater. Interfaces 2017, 9, 35683–35692. [Google Scholar] [CrossRef]
- Spillmann, C.M.; Naciri, J.; Algar, W.R.; Medintz, I.L.; Delehanty, J.B. Multifunctional liquid crystal nanoparticles for intracellular fluorescent imaging and drug delivery. ACS Nano 2014, 8, 6986–6997. [Google Scholar] [CrossRef]
- Nag, O.K.; Naciri, J.; Lee, K.; Oh, E.; Almeida, B.; Delehanty, J.B. Liquid crystal nanoparticle conjugates for scavenging reactive oxygen species in live cells. Pharmaceuticals 2022, 15, 604. [Google Scholar] [CrossRef]
- Thangavel, S.; Yoshitomi, T.; Sakharkar, M.K.; Nagasaki, Y. Redox nanoparticles inhibit curcumin oxidative degradation and enhance its therapeutic effect on prostate cancer. J. Control. Release 2015, 209, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Avnir, Y.; Turjeman, K.; Tulchinsky, D.; Sigal, A.; Kizelsztein, P.; Tzemach, D.; Gabizon, A.; Barenholz, Y. Fabrication principles and their contribution to the superior in vivo therapeutic efficacy of nano-liposomes remote loaded with glucocorticoids. PLoS ONE 2011, 6, e25721. [Google Scholar] [CrossRef]
- Wasserman, V.; Kizelsztein, P.; Garbuzenko, O.; Kohen, R.; Ovadia, H.; Tabakman, R.; Barenholz, Y. The antioxidant tempamine: In vitro antitumor and neuroprotective effects and optimization of liposomal encapsulation and release. Langmuir 2007, 23, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Kizelsztein, P.; Ovadia, H.; Garbuzenko, O.; Sigal, A.; Barenholz, Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J. Nneuroimmunol. 2009, 213, 20–25. [Google Scholar] [CrossRef]
- Turjeman, K.; Bavli, Y.; Kizelsztein, P.; Schilt, Y.; Allon, N.; Katzir, T.B.; Sasson, E.; Raviv, U.; Ovadia, H.; Barenholz, Y. Nano-drugs based on nano sterically stabilized liposomes for the treatment of inflammatory neurodegenerative diseases. PLoS ONE 2015, 10, e0130442. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, I.; Pieńkowska, N.; Chmielarz, P.; Bartosz, G.; Dziedzic, A.; Sadowska-Bartosz, I. Nitroxide-containing amphiphilic polymers prepared by simplified electrochemically mediated ATRP as candidates for therapeutic antioxidants. Polymer 2023, 273, 125885. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Miyamoto, D.; Nagasaki, Y. Design of core− shell-type nanoparticles carrying stable radicals in the core. Biomacromolecules 2009, 10, 596–601. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Ozaki, Y.; Thangavel, S.; Nagasaki, Y. Redox nanoparticle therapeutics to cancer—Increase in therapeutic effect of doxorubicin, suppressing its adverse effect. J. Control. Release 2013, 172, 137–143. [Google Scholar] [CrossRef]
- Nagasaki, Y. Nitroxide radicals and nanoparticles: A partnership for nanomedicine radical delivery. Ther. Deliv. 2012, 3, 165–179. [Google Scholar] [CrossRef]
- Nagasaki, Y. Design and application of redox polymers for nanomedicine. Polym. J. 2018, 50, 821–836. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Redox nanoparticles: Synthesis, properties and perspectives of use for treatment of neurodegenerative diseases. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, C.P.; Nagasaki, Y. Oral nanotherapeutics: Redox nanoparticles attenuate ultraviolet B radiation-induced skin inflammatory disorders in Kud: Hr-hairless mice. Biomaterials 2017, 142, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Shiota, K.; Hama, S.; Yoshitomi, T.; Nagasaki, Y.; Kogure, K. Prevention of UV-induced melanin production by accumulation of redox nanoparticles in the epidermal layer via iontophoresis. Biol. Pharm. Bull. 2017, 40, 941–944. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, A.; Yonemoto, C.; Feliciano, C.P.; Shashni, B.; Nagasaki, Y. Antioxidant nanomedicine significantly enhances the survival benefit of radiation cancer therapy by mitigating oxidative stress-induced side effects. Small 2021, 17, 2008210. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Uto, Y.; Kawasaki, A.; Noguchi, C.; Tanaka, R.; Yoshitomi, T.; Nagasaki, Y.; Endo, Y.; Hori, H. Evaluation of the in vivo antioxidative activity of redox nanoparticles by using a developing chicken egg as an alternative animal model. J. Control. Release 2014, 182, 67–72. [Google Scholar] [CrossRef] [PubMed]
- DeJulius, C.R.; Dollinger, B.R.; Kavanaugh, T.E.; Dailing, E.; Yu, F.; Gulati, S.; Miskalis, A.; Zhang, C.; Uddin, J.; Dikalov, S. Optimizing an antioxidant tempo copolymer for reactive oxygen species scavenging and anti-inflammatory effects in vivo. Bioconjug. Chem. 2021, 32, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Dao, N.V.; Ercole, F.; Li, Y.; Davis, T.P.; Kaminskas, L.M.; Sloan, E.K.; Quinn, J.F.; Whittaker, M.R. Nitroxide-functional PEGylated nanostars arrest cellular oxidative stress and exhibit preferential accumulation in co-cultured breast cancer cells. J. Mater. Chem. B 2021, 9, 7805–7820. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Krist, B.; Schroeder, G.; Kurczewska, J.; Bluyssen, H.A. ESR method in monitoring of nanoparticle endocytosis in cancer cells. Int. J. Mol. Sci. 2020, 21, 4388. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J. ESR as a monitoring method of the interactions between TEMPO-functionalized magnetic nanoparticles and yeast cells. Sci. Rep. 2019, 9, 18733. [Google Scholar] [CrossRef]
- Pala, R.; Barui, A.K.; Mohieldin, A.M.; Zhou, J.; Nauli, S.M. Folate conjugated nanomedicines for selective inhibition of mTOR signaling in polycystic kidneys at clinically relevant doses. Biomaterials 2023, 302, 122329. [Google Scholar] [CrossRef]
- Shashni, B.; Tamaoki, J.; Kobayashi, M.; Nagasaki, Y. Design of a new self-assembling antioxidant nanomedicine to ameliorate oxidative stress in zebrafish embryos. Acta Biomater. 2023, 159, 367–381. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Hirayama, A.; Nagasaki, Y. The ROS scavenging and renal protective effects of pH-responsive nitroxide radical-containing nanoparticles. Biomaterials 2011, 32, 8021–8028. [Google Scholar] [CrossRef]
- Asanuma, H.; Sanada, S.; Yoshitomi, T.; Sasaki, H.; Takahama, H.; Ihara, M.; Takahama, H.; Shinozaki, Y.; Mori, H.; Asakura, M. Novel synthesized radical-containing nanoparticles limit infarct size following ischemia and reperfusion in canine hearts. Cardiovasc. Drugs Ther. 2017, 31, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Boonruamkaew, P.; Chonpathompikunlert, P.; Vong, L.B.; Sakaue, S.; Tomidokoro, Y.; Ishii, K.; Tamaoka, A.; Nagasaki, Y. Chronic treatment with a smart antioxidative nanoparticle for inhibition of amyloid plaque propagation in Tg2576 mouse model of Alzheimer’s disease. Sci. Rep. 2017, 7, 3785. [Google Scholar] [CrossRef] [PubMed]
- Okajo, A.; Matsumoto, K.; Mitchell, J.B.; Krishna, M.C.; Endo, K. Competition of nitroxyl contrast agents as an in vivo tissue redox probe: Comparison of pharmacokinetics by the bile flow monitoring (BFM) and blood circulating monitoring (BCM) methods using X-band EPR and simulation of decay profiles. Magn. Reason. Med. 2006, 56, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M. Advanced biomedical applications of multifunctional natural and synthetic biomaterials. Processes 2023, 11, 2696. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).