Abstract
We aimed to provide a summary of the existing published knowledge on the association between adverse birth outcomes and the development of wheezing during the first two years of life. We carried out a systematic review of epidemiological studies within the MEDLINE database. Epidemiological studies on human subjects, published in English, were included in the review. A comprehensive literature search yielded 72 studies for further consideration. Following the application of the eligibility criteria we identified nine studies. A positive association and an excess risk of wheezing during the first two years of life were revealed for adverse birth outcomes.
1. Introduction
A number of maternal and lifestyle factors have been shown to be associated with the occurrence of wheezing or diagnosed asthma in childhood and adolescence, such as maternal age [], maternal smoking before birth and exposure to secondhand smoke (SHS) [,]. Maternal smoking, low maternal age and early bottle feeding are also associated with lower respiratory tract illness in the first 2 years of life, along with environmental exposures such as dampness [,] which, in turn, are linked with the development of asthma in childhood []. Consequently this evidence suggests that exposures occurring during pregnancy and early childhood can influence the risk of respiratory signs, such as wheezing in early childhood, or asthma later on in life.
Furthermore, low birth weight or very low birth weight infants are known to develop decreased respiratory function and have been found to be at an increased risk for the development of chronic respiratory symptoms during childhood [–]. Prematurely born children are known to have more respiratory symptoms during their first years of life than children born full-term [–] and their lung function at school age has been shown to be significantly reduced [–] especially among the children who had bronchopulmonary dysplasia at birth [,,].Taking the above into account both prematurity and low birth weight are considered significant risk factors for the development of childhood wheezing and asthma, even if the aetiology and the mechanism by which adverse birth outcomes predispose to wheezing is not known [–]. Although the above causal relationships are of significant importance to both pediatrics and public health, the international literature remarkably lacks information on the respiratory symptoms that are experienced by infants born growth restricted or premature. Taking the above into account, this systematic review aims to summarize the existing published scientific knowledge regarding the association between adverse birth outcomes and the development of wheezing during the first two years of life.
2. Methodology
A systematic review of the existing literature on adverse birth outcomes related and wheezing was carried out. We posed the following review question: “Given the existing epidemiological evidence, is there a link between adverse birth outcomes and the occurrence of wheezing during the first two years of life?”. We drew up a review protocol in advance following standards outlined in the MOOSE Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies []. We carried out a systematic, comprehensive bibliographic search using the US National Library of Medicine Medline database for the years 1990–2009, using the PubMed interface due to its free access to abstracts.
Search terms used were chosen from the USNLM Institutes of Health list of Medical Subject Headings (MeSH) for 2007. These were: “Infant, Low Birth Weight” OR “Infant, Very Low Birth Weight” OR “Premature Birth” OR “Fetal Growth Retardation” OR “Infant, Extremely Low Birth Weight” AND “Respiratory Sounds”, OR “Signs and Symptoms, Respiratory” OR “Wheezing”. Although not officially MeSH terms, “Wheezing” and “Small for gestational age” was also added as key terms so as to broaden the scope of the search. Retrieved studies were checked against a list of eligibility criteria, while the references of each retrieved study were also checked by hand for additional studies that met the eligibility criteria.
We defined a priori eligibility criteria to restrict the studies included. Studies were only included if they referred to humans, were published in English after 1990, were epidemiological studies (of any study design) and they examined the presence of wheezing up to two years old. Studies not meeting these criteria were excluded from the review. Data were extracted systematically from each included study by two researchers separately using a standardized data extraction form. The following data were extracted from each study: study main characteristics, study population, study topic, and measures of effect and confidence intervals for each outcome.
3. Results
Figure 1 demonstrates the numbers of studies identified and selected/excluded in each phase of the search. Manual searching of bibliographies provided some additional studies (two) that met the broad eligibility criteria. Ultimately, nine studies were deemed suitable for inclusion in the review although one study included two sub-studies [] and as a result we finally considered ten studies as the final number included in the systematic review.
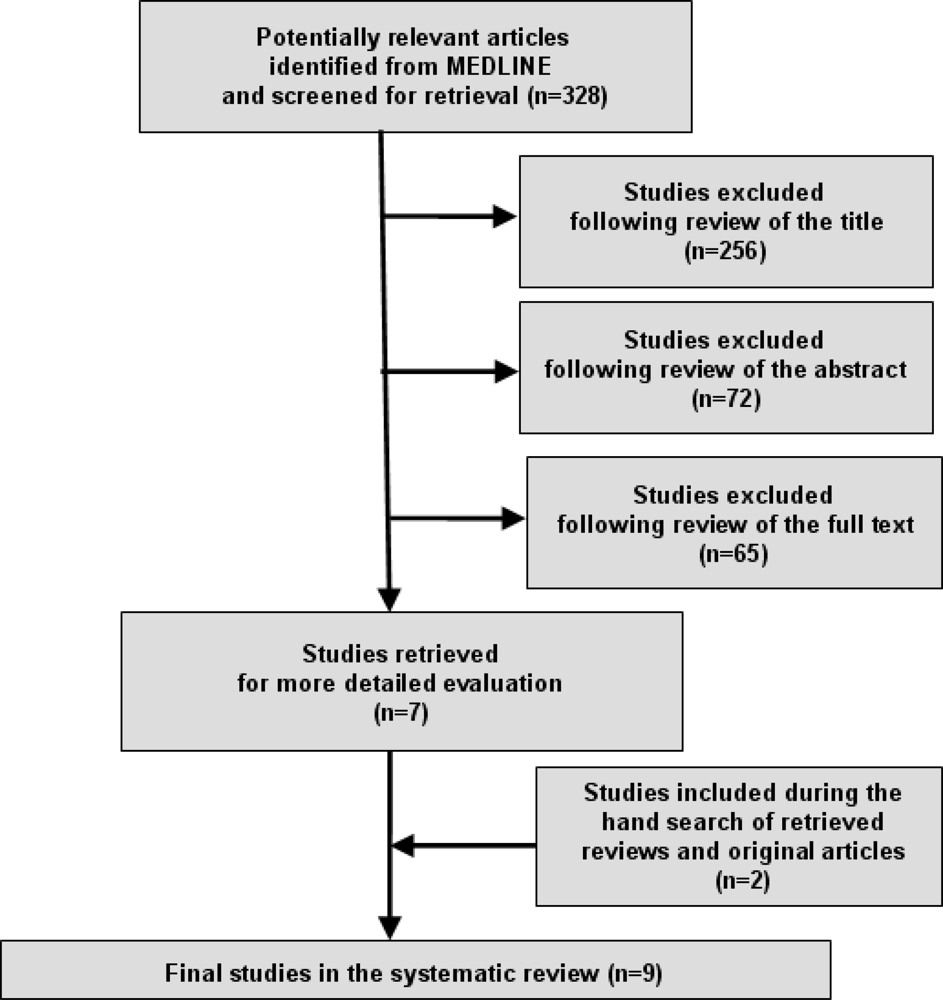
Figure 1.
Literature search and strategy outcomes.
The main characteristics of the studies included in the analysis are given in Table 1. The systematic review included nine prospective cohort studies [–] and one case control study []. Four studies were conducted in the USA, four in the UK, one in Italy and one in Poland.

Table 1.
Summary of characteristics of studies included in the systematic review.
Table 2 summarizes the main findings of the ten studies included in the systematic review. Four studies reported their results as odds ratios [,,,] one as relative risk ratio [] and five as prevalence [,,]. There were also some disparities in the definitions of adverse birth outcomes between studies. Two studies examined LBW which is commonly defined as birthweight <2500 g. PD was examined by six studies and was generally defined as a birth <37 weeks of gestation [,], although Lewis et al. [] used gestational age <36 weeks and Holditch-Davis et al. [] gestational age <35 weeks as the cut off for PD definition. The definition of VPD varied between the three studies by using as a cut off the <29 gestational weeks [], the <34 gestational weeks [] and the <33 gestational weeks [], respectively. VLBW was studied in four studies [,,]. Halterman et al. [] used the criterion of birth weight <1,500 g, Lewis et al. [] defined VLBW as birth weight <2,000 g while the definition for VLBW by Grenough et al. [] was not available. One study examined the infant length at birth as an outcome [] while term low birth weight and small for gestational age was not studied in any of the previously mentioned studies.

Table 2.
Main findings of studies included in the systematic review.
All studies examined wheezing as an outcome although the time period of wheezing presence was different. Four studies examined wheezing at first year of life [,,], four studies examined wheezing up to two years of life [,,,], one study examined wheezing up to five years old [] and one included children with a mean age of 2.2 years old [] (the last two studies remained in the review due to the fact that they also examined wheezing presence at the age of 2 years old).
Four studies measured the outcome as an odds ratio [,,,]. Galli et al. [] underlined an excess risk of wheezing presence for LBW infants (OR 11.88 95%CI 6.01–23.47) and Kumar et al. [] underlined the clear association of wheezing and both preterm and very preterm delivery (OR 1.7 95%CI 1.2–2.6 and OR 2.7 95%CI 1.3–5.5, respectively). Additionally, Lewis et al. [] although referring to the wheezing presence up to five years old reported a risk for LBW infants but it was not statistically significant (OR 1.22 95%CI 0.96–1.54). No statistically significant associations were also revealed for wheezing and PD, VPD, VLBW by Lewis et al. []. Taveras et al. [] also did not find any correlation between PD and wheezing at first two years of life. Additionally, Jedrychowski et al. [] reported results as a relative risk ratio but did not reveal any correlation between infant length at birth and wheezing presence.
The prevalence of wheezing was also estimated by five studies [,,,]. Grenough et al. [] in the two separate studies reported similar findings for wheezing prevalence during the first year of life (65% in the case-control and 53% in the cohort study) for infants that were both PD and VLBW. Additionally, VLBW infants were found to present wheezing, cough or heavy breathing in a percentage of 26% [] while Grenough et al. [] reported 42% prevalence of wheezing among VPD infants. Finally, Holditch-Davis et al. [] reported wheezing at different ages (2, 6, 9, 13, 18 and 22 months) in a population of preterm infants with wheezing prevalence ranging from 8% (at two months) up to 26% (at 18 months).
4. Discussion
As indicated through this systematic review, we have gathered the existing epidemiological evidence in order to examine the possible association between adverse birth outcomes and the development of wheezing during early childhood. Furthermore we identified a positive association between adverse birth outcomes such as LBW, VLBW, PD, VPD and the development of wheezing during early childhood. No studies examining the association of term low birth weight and small for gestational age infants with wheezing were revealed and thus these outcomes were not further investigated into.
Considerable variation in the prevalence of wheezing has been observed in previous studies. A prevalence increase has been noticed between countries and over time specifically from the 1970s up to the early 1990s []. These differences can be partially explained by the studies’ geographical variation and the deficiency of a common and strict definition of wheezing []. Prevalence of wheezing symptoms in children has been studied extensively while there is gap in the literature concerning wheezing prevalence during early childhood.
There are many potential causes of wheezing including genetic/familial or environmental factors, and viral respiratory infections []. Generally, exposure to a number of toxicants during lung development has the potential to significantly affect the function of the respiratory system of children []. Among other factors, maternal malnutrition during pregnancy, smoking both active and passive, chemicals and particles are only some of the potential hazards. A strong link also exists between the prenatal PM2.5 exposure, moldy/ damp house, maternal atopy and presence of older siblings with the severity of wheezing and respiratory illness []. However, low birth weight, preterm birth, low maternal age, household size, maternal smoking, infant feeding practices, and socioeconomic status are all interrelated and as a result it is difficult to distinguish which of these factors consist the independent determinants of wheezing during early childhood [].
The majority of children who develop wheeze in early childhood are free of symptoms by adolescence or early adulthood [,]. In addition, it is not clear whether wheezing that resolves in early childhood and wheezing which persists into adolescence represent the same disease, or they consist manifestations of fundamentally separate disease processes [,]. Wheezing is often followed up with asthma and lung function abnormalities since a significant proportion of children who develop asthma wheezed in early life []. Abnormalities in lung function after early childhood wheezing seem to continue at least until teenage years. Most children with early childhood wheezing outgrow their symptoms during school years, but asthma relapses are common in young adults as childhood wheezing is associated with permanent changes in the airways that continue until adulthood []. Further investigation is needed so as to conclude whether an increased prevalence of wheezing during early childhood is correlated with an increased prevalence of asthma and related health outcomes in late childhood and adolescence.
Early life events are important since the origin of airway abnormalities occurs early in infancy. Prospective birth cohort studies clarify the incidence of an illness by evaluating the risk factors and the possible confounders and/or modifiers related to the disease. This kind of studies may allow us to shed light on the primary factors that initiate wheezing and its correlation to long term implications such as chronic obstructive lung disease.
Conclusively, this study area is of high importance due to the fact that long term implications are unknown. It is therefore desirable to determine the correlation between adverse birth outcomes and wheezing during early childhood and identify whether there are preventable or treatable risk factors. For the purpose of this study, review was restricted to wheezing as a health outcome while other important causes of wheezing during early childhood other than adverse birth outcomes (e.g., genetic/familial or environmental factors) were not examined. As mentioned above, well designed epidemiological studies which will evaluate the relevant confounders and possible exposures and risk factors during pregnancy are needed. This estimation of summary should be considered by epidemiologists, health care specialists and research community as the most interesting areas for further research work.
References
- Schwartz, J; Gold, D; Dockery, DW; Weiss, ST; Speizer, FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Am. Rev. Respir. Dis 1990, 142, 555–562. [Google Scholar]
- Martinez, FD; Cline, M; Burrows, B. Increased incidence of asthma in children of smoking mothers. Pediatr 1992, 89, 21–26. [Google Scholar]
- Pattenden, S; Antova, T; Neuberger, M; Nikiforov, B; de Sario, M; Grize, L; Heinrich, J; Hruba, F; Janssen, N; Luttmann-Gibson, H; Privalova, L; Rudnai, P; Splichalova, A; Zlotkowska, R; Fletcher, T. Parental smoking and children’s respiratory health: independent effects of prenatal and postnatal exposure. Tob. Control 2006, 15, 294–301. [Google Scholar]
- Fergusson, DM; Horwood, LJ; Shannon, FT. Parental smoking and respiratory illness in infancy. Arch. Dis. Child 1980, 55, 358–361. [Google Scholar]
- Pirastu, R; Bellu, C; Greco, P; Pelosi, U; Pistelli, R; Accetta, G; Biggeri, A. Indoor exposure to environmental tobacco smoke and dampness: respiratory symptoms in Sardinian children—DRIAS study. Environ. Res 2009, 109, 59–65. [Google Scholar]
- Pullan, CR; Hey, EN. Wheezing, asthma and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br. Med. J 1982, 284, 1665–1669. [Google Scholar]
- Speer, CP; Silverman, M. Issues relating to children born prematurely. Eur. Respir. J 1998, 27, 13–16. [Google Scholar]
- Chan, KN; Noble-Jamieson, CM; Elliman, A; Bryan, EM; Silverman, M. Lung function in children of low birth weight. Arch. Dis. Child 1989, 64, 1284–1293. [Google Scholar]
- Wjst, M; Popescu, M; Trepka, MJ; Heinrich, J; Wichmann, HE. Pulmonary function in children with initial low birth weight. Pediatr. Allergy Immunol 1998, 9, 80–90. [Google Scholar]
- Kitchen, WH; Olinsky, A; Doyle, LW; Ford, GW; Murton, LJ; Slonim, L; Callanan, C. Respiratory health and lung function in 8-year-old children of very low birth weight: a cohort study. Pediatrics 1992, 89, 1151–1158. [Google Scholar]
- Chan, KN; Wong, YC; Silverman, M. Relationship between infant lung mechanics and childhood lung function in children of very low birth weight. Pediatr. Pulmonol 1990, 8, 74–81. [Google Scholar]
- Elder, DE; Hagan, R; Evans, SF; Benninger, HR; French, NP. Recurrent wheezing in very preterm infants. Arch. Dis. Child Fetal Neonatal Ed 1996, 74, 165–171. [Google Scholar]
- Rona, RJ; Gulliford, MC; Chinn, S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. Br. Med. J 1993, 306, 817–820. [Google Scholar]
- Northway, WH, Jr; Moss, RB; Carlisle, KB; Parker, BR; Popp, RL; Pitlick, PT; Eichler, I; Lamm, RL; Brown, BW, Jr. Late pulmonary sequelae of bronchopulmonary dysplasia. N. Engl. J. Med 1990, 323, 1793–1799. [Google Scholar]
- Parat, S; Moriette, G; Delaperche, MF; Escourrou, P; Denjean, A; Gaultier, C. Long-term pulmonary functional outcome of bronchopulmonary dysplasia and premature birth. Pediatr. Pulmonol 1995, 20, 289–296. [Google Scholar]
- Hakulinen, AL; Jarvenpaa, AL; Turpeinen, M; Sovijarvi, A. Diffusing capacity of the lung in school-aged children born very preterm, with and without bronchopulmonary dysplasia. Pediatr. Pulmonol 1996, 21, 353–360. [Google Scholar]
- Pelkonen, AS; Hakulinen, AL; Turpeinen, M. Bronchial lability and responsiveness in school children born very preterm. Am. J. Respir. Crit. Care Med 1997, 156, 1178–1184. [Google Scholar]
- Giacoia, GP; Venkataraman, PS; West-Wilson, KI; Faulkner, MJ. Follow-up of school-age children with bronchopulmonary dysplasia. J. Pediatr 1997, 130, 400–408. [Google Scholar]
- Koumbourlis, AC; Motoyama, EK; Mutich, RL; Mallory, GB; Walczak, SA; Fertal, K. Longitudinal follow-up of lung function from childhood to adolescence in prematurely born patients with neonatal chronic lung disease. Pediatr. Pulmonol 1996, 21, 28–34. [Google Scholar]
- Seidman, DS; Laor, A; Gale, R; Stevenson, DK; Danon, YL. Is low birth weight a risk factor for asthma during adolescence? Arch. Dis. Child 1991, 66, 584–587. [Google Scholar]
- von Mutius, E; Nicolai, T; Martinez, FD. Prematurity as a risk factor for asthma in preadolescent children. J. Pediatr 1993, 123, 223–229. [Google Scholar]
- Kuehr, J; Frischer, T; Karmaus, W; Meinert, R; Barth, R; Urbanek, R. Clinical atopy and associated factors in primary-school pupils. Allergy 1992, 47, 650–655. [Google Scholar]
- Stroup, DF; Berlin, JA; Morton, SC; Olkin, I; Williamson, GD; Rennie, D; Moher, D; Becker, BJ; Sipe, TA; Thacker, SB. Meta-analysis of observational studies in epidemiology a proposal for reporting. Am. Med. Assoc 2000, 283, 2008–2012. [Google Scholar]
- Greenough, A; Maconochie, I; Yuksel, B. Recurrent respiratory symptoms in the first year of life following preterm delivery. J. Perinat. Med 1990, 18, 489–494. [Google Scholar]
- Jedrychowski, W; Perera, FP; Maugeri, U; Mrozek-Budzyn, D; Mroz, E; Flak, E; Edwards, S; Spengler, JD; Jacek, R; Sowa, A; Musiał, A. Early wheezing phenotypes and severity of respiratory illness in very early childhood: study on intrauterine exposure to fine particle matter. Environ. Int 2009, 35, 877–884. [Google Scholar]
- Halterman, JS; Lynch, KA; Conn, KM; Hernandez, TE; Perry, TT; Stevens, TP. Environmental exposures and respiratory morbidity among very low birth weight infants at 1 year of life. Arch. Dis. Child 2009, 94, 28–32. [Google Scholar]
- Holditch-Davis, D; Merrill, P; Schwartz, T; Scher, M. Predictors of wheezing in prematurely born children. J. Obstet. Gynecol. Neonatal Nurs 2008, 37, 262–273. [Google Scholar]
- Kumar, R; Yu, Y; Story, RE; Pongracic, JA; Gupta, R; Pearson, C; Ortiz, K; Bauchner, HC; Wang, X. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J. Allergy Clin. Immunol 2008, 121, 878–884. [Google Scholar]
- Taveras, EM; Camargo, CA, Jr; Rifas-Shiman, SL; Oken, E; Gold, DR; Weiss, ST; Gillman, MW. Association of birth weight with asthma-related outcomes at age 2 years. Pediatr. Pulmonol 2006, 41, 643–648. [Google Scholar]
- Greenough, A; Limb, E; Marston, L; Marlow, N; Calvert, S; Peacock, J. Risk factors for respiratory morbidity in infancy after very premature birth. Arch. Dis. Child. Fetal Neonatal Ed 2005, 90, 320–323. [Google Scholar]
- Galli, L; Sabatino, G; Zappa, M; Barbante, E; Chiappini, E; de Martino, M. Reduced frequency of wheezing respiratory illness in infants with perinatal human immunodeficiency virus-type 1 infection: a model for immunologic and inflammatory mechanisms of airway obstruction? Pediatr. Allergy Immunol 2003, 14, 42–49. [Google Scholar]
- Lewis, S; Richards, D; Bynner, J; Butler, N; Britton, J. Prospective study of risk factors for early and persistent wheezing in childhood. Eur. Respir. J 1995, 8, 349–356. [Google Scholar]
- Patel, SP; Järvelin, MR; Little, MP. Systematic review of worldwide variations of the prevalence of wheezing symptoms in children. Environ. Health 2008, 10, 57. [Google Scholar]
- Stein, RT. Long-term airway morbidity following viral LRTI in early infancy: recurrent wheezing or asthma? Paediatr. Respir. Rev 2009, 10, 29–31. [Google Scholar]
- Wang, L; Pinkerton, KE. Detrimental effects of tobacco smoke exposure during development on postnatal lung function and asthma. Birth Defects Res. C. Embryo Today 2008, 84, 54–60. [Google Scholar]
- Anderson, HR; Bland, JM; Peckham, CS. Risk factors for asthma up to 16 years of age. Chest 1987, 91, 127–130. [Google Scholar]
- Park, ES; Golding, J; Carswell, F; Stewart-Brown, S. Preschool wheezing and prognosis at 10. Arch. Dis. Child 1986, 61, 642–646. [Google Scholar]
- Williams, H; Mcnicol, KN. Prevalence, natural history, and relationship of wheezy bronchitis and asthma in children. Br. Med. J 1969, 4, 321–325. [Google Scholar]
- Wilson, NM. Wheezy bronchitis revisited. Arch. Dis. Child 1989, 64, 1194–1199. [Google Scholar]
- Piippo-Savolainen, E; Korppi, M. Long-term outcomes of early childhood wheezing. Curr. Opin. Allergy Clin. Immunol 2009, 9, 190–196. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).